Abstract
Context:
Visceral adiposity is associated with increased cardiometabolic risk and decreased GH secretion.
Objective:
Our objective was to determine the effects of GH administration in abdominally obese young men on body composition, including liver fat, mitochondrial function, and cardiovascular (CV) risk markers.
Design and Participants:
This was a 6-month, randomized, double-blind, placebo-controlled study with 62 abdominally obese men (IGF-1 below the mean, no exclusion based on GH level), 21 to 45 years of age.
Main Outcome Measures:
We evaluated abdominal fat depots, thigh muscle and fat (computed tomography), fat and lean mass (dual-energy x-ray absorptiometry), intramyocellular and intrahepatic lipids (proton magnetic resonance spectroscopy), mitochondrial function (dynamic phosphorous magnetic resonance spectroscopy), CV risk markers, carotid intimal-medial thickness, and endothelial function.
Results:
GH administration resulted in a mean IGF-1 SD score increase from −1.9 ± 0.08 to −0.2 ± 0.3 in the GH group and a decrease in visceral adipose tissue (VAT), VAT/sc adipose tissue, trunk/extremity fat, intrahepatic lipids, high-sensitivity C-reactive protein and apolipoprotein B/low-density lipoprotein vs placebo after controlling for the increase in weight observed in both groups. There were inverse associations between change in IGF-1 levels and change in VAT, VAT/sc adipose tissue, trunk fat, trunk/extremity fat, high-sensitivity C-reactive protein, and apolipoprotein B. Mitochondrial function improved in the GH group compared with placebo after controlling for change in glucose. There was no change in thigh fat, muscle mass, intramyocellular lipids, cholesterol, fibrinogen, intimal-medial thickness, or endothelial function. There was no increase in fasting glucose or hemoglobin A1c in the GH vs placebo group, although glucose during the 2-hour oral glucose tolerance test increased slightly.
Conclusion:
GH replacement in abdominally obese men improves body composition, including liver fat, mitochondrial function, and markers of CV risk. Although fasting glucose was unchanged, a slight increase in 2-hour glucose during an oral glucose tolerance test was noted.
Visceral obesity is associated with increased cardiometabolic risk (1), whereas lower-body sc fat is relatively protective (2). In addition, accumulation of liver fat resulting in disease, including nonalcoholic fatty liver disease, is an increasing public health problem with the increase in the prevalence of obesity (3). In individuals with visceral obesity, physiologic GH secretion and peak stimulated GH response are impaired (4–6), and reduced GH secretion in men and women with visceral obesity is associated with increased cardiovascular (CV) risk markers (4, 6). GH plays an important role in regulation of body composition, insulin resistance, inflammation, and atherogenesis. GH stimulates lipolysis and regulates lipid deposition (7) and intracellular cholesterol metabolism in the liver (8). We have previously shown an inverse association of stimulated GH and both intrahepatic lipids (IHL) and intramyocellular lipids (IMCL) in obese premenopausal women (9), suggesting that low GH results in ectopic lipid deposits, which are thought to play an etiologic role in obesity-induced insulin resistance. Moreover, a small study in healthy men and women demonstrated increased mitochondrial oxidative capacity in muscle biopsies after a single infusion of GH (10). Decreased oxidative capacity due to mitochondrial dysfunction has been implicated in the pathogenesis of insulin resistance through accumulation of IMCL, which then interferes with insulin signaling (11, 12). GH also decreases inflammation and atherogenesis (6). IGF-1, which is secreted by the liver and other organs in response to GH, is also an important modulator of body composition, especially muscle mass (13).
GH replacement in patients with GH deficiency due to hypopituitarism improves body composition with a decrease in visceral adipose tissue (VAT) and an increase in lean mass and reduces inflammatory CV risk markers (14–17). There are few studies administering low-dose GH to obese, but otherwise healthy, men and women (18–22). We have previously shown improvement in body composition and CV risk markers in obese premenopausal women after 6 months of low-dose GH administration (18). Johannsson et al (20) demonstrated a decrease in VAT and improvement in serum lipids and glucose homeostasis in obese men aged 48 to 66 years after 9 months of GH administration. No study has studied the effects of low-dose GH administration on CV risk markers, including inflammatory markers, detailed measures of body composition including ectopic lipid deposits, and mitochondrial function in obese young men. We hypothesized that GH administration for 6 months to obese young men with relative IGF-1 deficiency due to abdominal obesity would reduce high-sensitivity C-reactive protein (hsCRP) (primary endpoint) and other CV risk markers and improve body composition (reduce VAT and ectopic lipid deposits [IHL and IMCL] and increase muscle mass) and mitochondrial function.
Subjects and Methods
The study was approved by the Partners HealthCare Inc (Boston, Massachusetts) institutional review board, and written informed consent was obtained from all subjects.
Subjects
Subjects were recruited from the community through advertisements. Inclusion criteria were ages 18 to 45 years, male gender, body mass index (BMI) ≥25 kg/m2, waist circumference >102 cm (per the National Cholesterol Education Program Adult Treatment Panel III definition of central obesity and based on epidemiologic data demonstrating an increase in cardiovascular risk with waist circumference >102 cm) (23, 24), IGF-1 level below normal mean for age, stable weight (defined as weight loss or weight gain ≤5 pounds in the preceding 3 months), and willingness to maintain current activity level and diet for the study duration. Exclusion criteria included smoking, hypothalamic or pituitary disorders, diabetes mellitus (either history of diabetes mellitus or screening-visit fasting glucose ≥126 mg/dL or 75-g, 2-hour oral glucose tolerance test [OGTT] glucose ≥200 mg/dL) or other chronic illnesses, testosterone or glucocorticoid use, use of statins or antihypertensives, or regular use of aspirin.
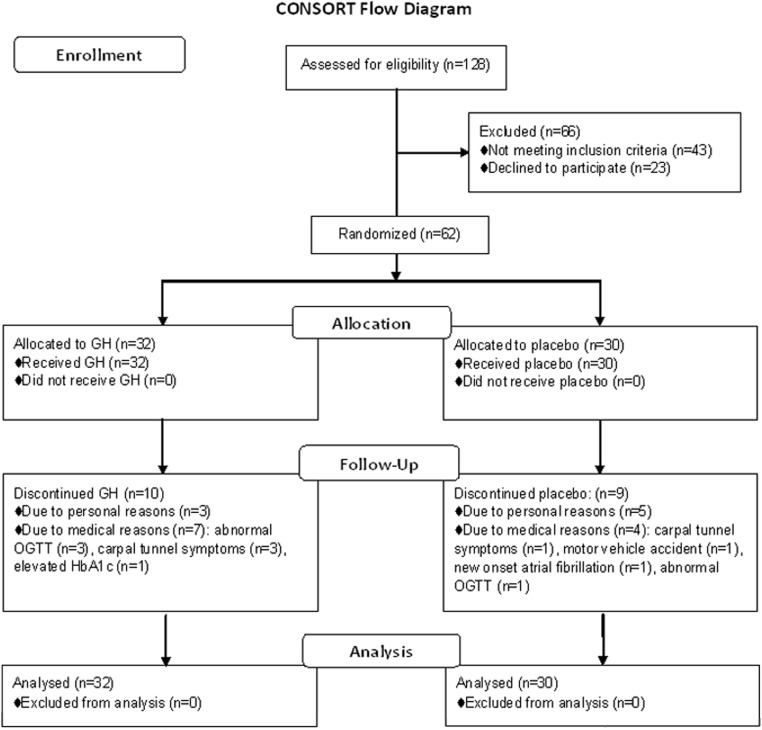
A total of 128 obese men were screened for participation. Sixty-two men met the criteria and were enrolled in the study. Sixty-two subjects completed the baseline visit, 62 subjects completed the 3-week visit, 58 subjects completed the 6-week visit, 55 subjects completed the 9-week visit, 50 subjects completed the 3-month visit, and 43 subjects completed the 6-month visit (Figure 1).
Figure 1.
Flow diagram of randomized trial of GH vs placebo according to Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Eight subjects withdrew for personal reasons, and 11 subjects were discontinued from the study for medical reasons (Figure 1).
Baseline clinical characteristics and body composition have been previously reported on a subset of these study subjects (25), but no longitudinal data have been previously published.
Protocol
The study was a 6-month, double-blind, randomized, placebo-controlled trial performed at the Massachusetts General Hospital Clinical Research Center.
After baseline evaluation, subjects were randomized to receive daily sc recombinant human GH (Genentech, Inc) or placebo, which was identical in appearance to the GH, for 6 months. Starting GH dose was 2 μg/kg/d. Subjects were instructed to inject study medication at bedtime.
Fasting blood samples for IGF-1 were drawn at 3, 6, and 9 weeks and 3 and 6 months, and GH doses were adjusted based on IGF-1 levels at these visits, by a physician not involved in the study, using an algorithm based on pretreatment IGF-1 level and an IGF-1 level target in the upper normal age-appropriate range. Participants in the placebo group were sham dose adjusted to maintain study-subject and investigator blinding to randomization assignment.
Fasting blood samples for CV risk markers (hsCRP, apolipoprotein B [ApoB], fibrinogen, and tissue plasminogen activator [tPA]), serum lipids (triglycerides and total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] cholesterol), a 75-g, 2-hour OGTT, and hemoglobin A1c (HbA1c) in 36 subjects were drawn at baseline, 6 weeks and 6 months. The following tests were performed at baseline, 6 weeks, and 6 months: computed tomography (CT) of the abdomen and midthigh for body composition, bioelectric impedance analysis for total body water, proton magnetic resonance spectroscopy (1H-MRS) of soleus muscle and liver in 44 subjects (21 GH, 23 placebo) for IMCL and IHL, dynamic phosphorous MRS (31P-MRS) of calf muscles in 33 subjects (16 GH, 17 placebo) for mitochondrial function as previously described (26–28). Dual-energy x-ray absorptiometry (DXA) for fat and lean mass was performed at baseline and 3 and 6 months. Resting energy expenditure was calculated from substrate oxidation rates obtained by indirect calorimetry (Vmax29N Sensor Medics; Vyasis Healthcare) at baseline and 3 and 6 months. Carotid intima-media thickness (IMT) (n = 50: 26 GH, 21 placebo) and endothelial function were determined at baseline and 6 months. A glucagon GH stimulation test was performed at baseline as follows: after im administration of glucagon (GlucaGen; Novo Nordisk), 1 mg (if <90 kg) or 1.5 mg (if >90 kg), blood was drawn every 30 minutes for 4 hours for assessment of GH (29). All other radiologic and biochemical methods used were as reported in our study of GH administration to obese women (18). Compliance was assessed by self-report. All study subjects were asked to fill out daily drug administration diaries, and those subjects who did not complete the drug diaries were asked how many injections they had missed since the last study visit.
Physical activity
Subjects were asked to refrain from modifying their exercise levels throughout the duration of the study. Level of activity, including exercise, was assessed using the Paffenbarger questionnaire (30). The Modified Activity Questionnaire (31) was used to assess activity level over the previous year.
Statistical analysis
The study was powered based on a study by Sesmilo et al (17). With 22 evaluable study subjects in each study arm, we had greater than 90% power for detecting a difference of 1.8 mg/L in hsCRP at a 2-sided .05 significance level, assuming a SD of the difference from baseline of 1.79.
The data were analyzed using repeated-measures ANOVA with the treatment difference at 6 months as the primary contrast of interest (SAS Proc Mixed; SAS Institute). This analysis included all data collected on all study subjects irrespective of whether the subject completed the 6-month follow-up period and follows the Institute of Medicine suggestion for analysis of data with missing observations (http://books.nap.edu/openbook.php?record_id=12955). Repeated-measures analyses of covariance were also performed with change in weight as a covariate because weight changes are known to affect body composition and CV risk markers. Baseline testosterone was entered as a covariate in repeated-measures analyses of covariance. Glucose area under the curve (AUC) was entered as a covariate for analysis of change in mitochondrial function due to the known effects of glucose homeostasis on mitochondrial dysfunction. Univariate regression models were constructed to determine hormonal and body composition predictors of endpoints studied, and Spearman ρ values are reported. Within-group comparisons were made using a paired t test. Statistical significance was defined as a 2-tailed P ≤ .05, and trend was defined as 2-tailed P ≤ .1.
Results
Baseline characteristics
Thirty-two subjects were randomized to receive GH and 30 subjects to receive placebo. The groups had comparable mean age, IGF-1 level, CV risk marker levels, BMI, and body composition, except for lean mass, which was higher in the placebo group (73.7 ± 1.6 vs 78.6 ± 1.9 kg, P = .05). Subjects ranged in age from 21 to 45 years, with a mean of 33.3 ± 0.8 years and in BMI from 27.9 to 53.7 kg/m2, with a mean of 37.0 ± 0.8 kg/m2 (Table 1). Serum testosterone ranged from 147 to 728 ng/dL with a mean of 385 ± 17 ng/dL. Nineteen men were hypogonadal with testosterone levels <300 ng/dL. Peak stimulated GH ranged from 0.02 to 30.0 ng/mL with a mean of 4.8 ± 0.7 ng/mL. There was no difference in resting energy expenditure at baseline or 3 or 6 months between the groups (P = .6). Baseline physical activity, determined using the Paffenbarger questionnaire, was higher in the GH group (P = .01); however, there was no difference between the groups at 6 months (P = .4). There was no difference in baseline activity history as determined by the Modified Activity Questionnaire between the groups (P = .7). Self-reported compliance was 94.9%.
Table 1.
Baseline Characteristicsa
| GH Group (n = 32) | Placebo Group (n = 30) | P | |
|---|---|---|---|
| Age, y | 32.4 ± 1.2 | 34.3 ± 1.1 | .3 |
| BMI, kg/m2 | 36.4 ± 0.9 | 37.7 ± 1.2 | .4 |
| Largest waist circumference, cm | 118.3 ± 2.2 | 121.8 ± 2.9 | .3 |
| Peak stimulated GH, ng/mL | 4.6 ± 0.9 | 5.0 ± 1.1 | .8 |
| IGF-1, ng/mL | 131 ± 8 (range, 52–239 | 131 ± 7 (range, 60–216) | 1.0 |
| IGF-1 SDS | −1.9 ± 0.1 | −1.7 ± 0.1 | .3 |
| Testosterone, ng/dL | 401 ± 26 | 369 ± 23 | .4 |
Data are presented as mean ± SEM.
The mean GH dose for the GH treatment group was 0.2 ± 0.007 mg/d at 3 weeks, 0.5 ± 0.01 mg/d at 6 weeks, 0.8 ± 0.04 mg/d at 9 weeks, 1.0 ± 0.08 mg/d at 3 months, and 1.1 ± 0.08 mg/d at 6 months. These doses resulted in a mean increase in IGF-1 SD score (SDS) from −1.9 ± 0.08 at baseline to 0.2 ± 0.3 at 6 months (P < .0001 compared with placebo). Mean IGF-1 level was 264 ± 11 ng/mL, and mean IGF-1 SDS was −0.4 ± 0.1 at 6 weeks.
Effects of GH administration on body composition
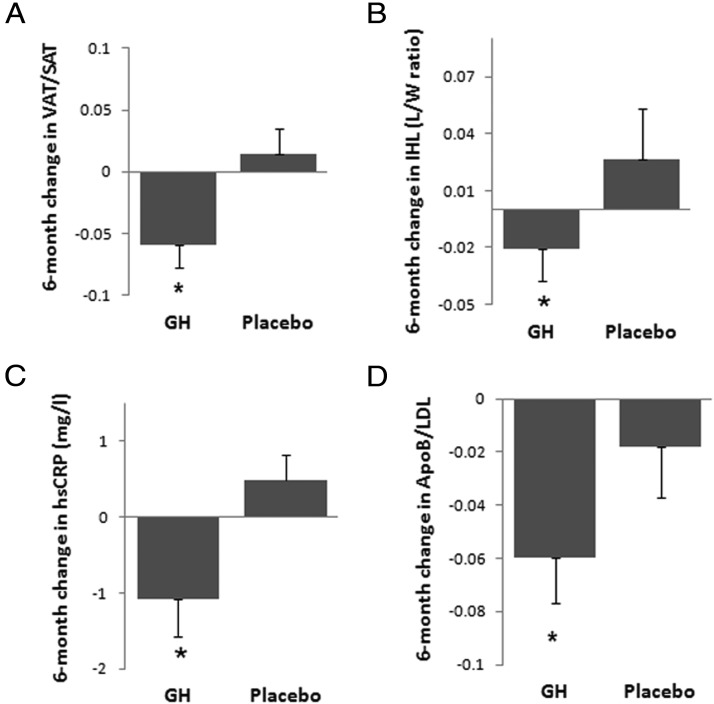
The group as a whole gained weight over 6 months (BMI, 37.0 ± 0.8 kg/m2 at baseline vs 38.3 ± 1.1 at 6 months, P = .05). However, there was no significant difference in change in weight between the GH and placebo groups over the treatment period. Body composition parameters at baseline, 6 weeks, and 6 months are summarized in Table 2. There was a decrease in VAT/sc adipose tissue (SAT) measured by CT (Figure 2A) and trunk/extremity fat measured by DXA in the GH group compared with placebo. After controlling for the increase in weight experienced by the cohort, the decrease in VAT/SAT and trunk/extremity fat remained significant, and in addition, there was a significant decrease in VAT in the GH group compared with placebo. There was a trend toward a decrease in IHL between the GH and placebo group, which became significant after controlling for change in weight (P = .002) (Figure 2B). There was no significant change in total lean mass or total body fat (as measured by DXA) or thigh muscle or thigh SAT (as measured by CT) between the GH and placebo groups. No change in lean or muscle mass was observed after controlling for baseline free or total testosterone or baseline lean mass (P = .3–.8). Controlling for baseline free or total testosterone did not modify the results of GH effect on other body composition parameters. The improvement in mitochondrial function in the GH group compared with placebo, with a decreased phosphocreatine (PCr) recovery time constant τ, a marker of mitochondrial dysfunction that is determined by plotting the PCr peak integrated area vs time during exercise recovery and fitting recovery curves to a monoexponential function, became significant after controlling for change in glucose AUC from the OGTT (P = .04).
Table 2.
Body Composition in Men With Abdominal Obesity Treated With GH vs Placebo for 6 Monthsa
| Variable | Treatment | Baseline (n = 62) | 6 wk (n = 58) | 6 mo (n = 43) | Effect Size ± SE | Pb |
|---|---|---|---|---|---|---|
| n | GH | 32 | 31 | 22 | ||
| Placebo | 30 | 27 | 21 | |||
| Weight, kg | GH | 115 ± 3 | 116 ± 3 | 116 ± 4 | 1.2 ± 1.1 | .3 |
| Placebo | 122 ± 4 | 123 ± 5 | 128 ± 6 | |||
| BMI, kg/m2 | GH | 36.4 ± 0.9 | 36.6 ± 1.0 | 36.8 ± 1.3 | 0.4 ± 0.3 | .2 |
| Placebo | 37.7 ± 1.2 | 38.0 ± 1.3 | 40.0 ± 1.8 | |||
| VAT/SAT | GH | 0.38 ± 0.04 | 0.34 ± 0.03 | 0.31 ± 0.05 | −0.06 ± 0.02 | .01c |
| Placebo | 0.35 ± 0.03 | 0.36 ± 0.03 | 0.36 ± 0.04 | |||
| Abdomen VAT, cm2 | GH | 157.5 ± 12.2 | 149.2 ± 11.1 | 134.6 ± 13.9 | −12.4 ± 9.4 | .2c |
| Placebo | 158.3 ± 10.5 | 158.2 ± 12.7 | 161.4 ± 15.8 | |||
| Abdomen SAT, cm2 | GH | 450.1 ± 23.8 | 453.6 ± 22.0 | 443.8 ± 30.5 | 12.2 ± 13.2 | .4 |
| Placebo | 464.9 ± 28.4 | 454.3 ± 29.8 | 467.6 ± 36.2 | |||
| Thigh SAT, cm2 | GH | 143.7 ± 9.2 | 141.8 ± 9.2 | 142.1 ± 12.5 | 2.3 ± 5.0 | .6 |
| Placebo | 146.1 ± 11.6 | 153.8 ± 14.6 | 165.0 ± 19.6 | |||
| Thigh muscle, cm2 | GH | 207.0 ± 5.1 | 209.8 ± 5.7 | 212.6 ± 6.5 | 1.0 ± 2.6 | .7 |
| Placebo | 219.7 ± 6.2 | 218.7 ± 5.6 | 220.0 ± 7.1 | |||
| Total body fat, kge | GH | 40.1 ± 2.0 | 40.3 ± 2.6 | 40.4 ± 2.7 | −2.4 ± 3.0 | .9 |
| Placebo | 42.2 ± 2.9 | 44.2 ± 3.5 | 46.7 ± 4.6 | |||
| Trunk/extremity fate | GH | 1.24 ± 0.04 | 1.21 ± 0.04 | 1.19 ± 0.04 | −0.07 ± 0.02 | .005c |
| Placebo | 1.17 ± 0.04 | 1.18 ± 0.04 | 1.24 ± 0.04 | |||
| Total lean mass, kge | GH | 73.7 ± 1.6 | 74.7 ± 1.8 | 74.4 ± 2.0 | 0.3 ± 1.2 | .8 |
| Placebo | 78.6 ± 1.9 | 78.3 ± 1.8 | 78.6 ± 2.0 | |||
| Total body water/lean | GH | 59.8 ± 1.4 | 61.0 ± 1.5 | 61.3 ± 1.9 | 0.3 ± 1.2 | .8 |
| body mass | Placebo | 63.7 ± 1.9 | 64.4 ± 1.9 | 65.8 ± 2.3 | ||
| IMCL-SOL (lipid/water) | GH | 0.05 ± 0.007 | 0.04 ± 0.008 | 0.04 ± 0.01 | 0.005 ± 0.008 | .5 |
| Placebo | 0.04 ± 0.005 | 0.04 ± 0.006 | 0.03 ± 0.005 | |||
| IHL (lipid/water) | GH | 0.19 ± 0.05 | 0.25 ± 0.06 | 0.11 ± 0.03 | −0.04 ± 0.03 | .1c |
| Placebo | 0.23 ± 0.1 | 0.19 ± 3.0 | 0.26 ± 0.08 | |||
| PCr recovery constant τ (sec) (mitochondrial function) | GH | 35.1 ± 2.2 | 30.9 ± 2.4 | 33.2 ± 2.3 | −6.1 ± 4.4 | .2d |
| Placebo | 29.5 ± 2.5 | 31.5 ± 2.5 | 36.8 ± 3.9 |
Abbreviation: IMCL-SOL, IMCL concentration of soleus.
Data are presented as mean ± SEM.
P between groups (0–6 months) determined by repeated-measures ANOVA without controlling for change in weight.
significant (P ≤ .05) after controlling for 6-month change in weight
significant (P ≤ .05) after controlling for 6-month change in glucose area under the curve
DXA was performed at baseline and 3 and 6 months.
Figure 2.
Mean (SEM) change in VAT/SAT (A), IHL (B), hsCRP (C), and ApoB/LDL (D) over 6 months of GH administration vs placebo. *, P < .05 vs placebo after controlling for change in weight.
Effects of GH on CV risk markers
There was a significant decrease in hsCRP in the GH group compared with placebo (−1.1 ± 0.5 vs 0.5 ± 0.3 mg/L, P = .008) (Figure 2C), which remained significant after controlling for change in weight (P = .005). There was a trend of decrease in tPA and ApoB in the GH group compared with placebo (P = .1). After controlling for change in weight, there was a significant decrease in ApoB/LDL in the GH group compared with placebo (−0.06 ± 0.02 vs −0.02 ± 0.02, P = .05) (Figure 2D). There was no significant difference in change in triglycerides, total and LDL cholesterol, HDL cholesterol, carotid IMT, and reactive hyperemia index between the groups. Controlling for baseline free or total testosterone did not modify the results outcome of GH effect on CV risk markers.
Effects of GH on glucose tolerance
Measures of glucose tolerance at baseline, 6 weeks, and 6 months are summarized in Table 3. There was no significant difference between fasting glucose levels, HbA1c, glucose AUC from OGTT, or homeostasis model assessment of insulin resistance in the GH group vs placebo at baseline or 6 months. Two-hour glucose increased in the GH group vs placebo over the course of the study. GH dose at 6 months correlated with HbA1c (r = 0.55, P = .03). There was no association between increase in IGF-1 levels and change in any measure of glucose tolerance. Two subjects in the GH group had fasting glucose levels ≥126 mg/dL, 2 subjects (1 in the GH and 1 in the placebo group) had 2-hour glucose >200 mg/mL, and 1 subject in the GH group had an HbA1c level ≥6.5, prespecified drop criteria, and were discontinued from the study.
Table 3.
Measures of Glucose Tolerance in Men With Abdominal Obesity Treated With GH vs Placebo for 6 Monthsa
| Variable | Treatment | Baseline (n = 62) | 6 wk (n = 58) | 6 mo (n = 43) | Effect Size ± SE | Pb |
|---|---|---|---|---|---|---|
| n | GH | 32 | 31 | 22 | ||
| Placebo | 30 | 27 | 21 | |||
| Fasting glucose, mg/dL | GH | 83.2 ± 1.2 | 90.3 ± 1.3 | 84.3 ± 2.0 | 1.9 ± 2.5 | .4 |
| Placebo | 85.1 ± 1.1 | 87.1 ± 1.3 | 83.6 ± 1.9 | |||
| 2-h glucose, mg/dL | GH | 114.2 ± 4.1 | 124.8 ± 5.6 | 116.0 ± 6.0 | 14.9 ± 7.1 | .04 |
| Placebo | 113.4 ± 5.0 | 107.3 ± 5.6 | 105.4 ± 5.6 | |||
| Glucose AUC, mg/dL/120 min | GH | 15953 ± 454 | 17090 ± 601 | 16184 ± 624 | 1025 ± 609 | .1 |
| Placebo | 14811 ± 399 | 15316 ± 457 | 14651 ± 514 | |||
| Hemoglobin A1c, % | GH | 5.6 ± 0.07 | 5.6 ± 0.09 | 5.7 ± 0.08 | 0.04 ± 0.09 | .6 |
| Placebo | 5.6 ± 0.05 | 5.3 ± 0.09 | 5.6 ± 0.07 | |||
| HOMA-IR | GH | 3.2 ± 0.3 | 4.8 ± 0.6 | 3.7 ± 0.7 | 0.4 ± 1.0 | .7 |
| Placebo | 2.5 ± 0.3 | 3.3 ± 0.5 | 3.2 ± 0.8 |
Abbreviation: HOMA-IR, homeostasis model assessment of insulin resistance.
Data are presented as mean ± SEM and include all men who underwent the tests.
P between groups (0–6 months) determined by repeated-measures ANOVA.
Predictors of endpoints
There were inverse associations between change in IGF-1 levels and change in VAT (r = −0.33, P = .03), VAT/SAT (r = −0.42, P = .01), trunk fat (r = −0.40, P = .01), and trunk/extremity fat (r = −0.43, P = .006) in the entire group that remained significant after controlling for change in weight (P = .001–.03). Change in IGF-1 level was also inversely associated with hsCRP (r = −0.46, P = .003) and ApoB (r = −0.31, P = .05) over the 6-month treatment period, suggesting that those subjects experiencing the greatest increases in IGF-1 levels experienced the greatest decreases in VAT, trunk fat, hsCRP, and ApoB. GH doses at 6 weeks and 6 months were inversely associated with the changes in SAT over the 6-month treatment period (r = −0.57, P = .01; and r = −0.58, P = .009, respectively). GH dose at 6 months was inversely associated with change in PCr recovery time τ (r = −0.58, P = .06), suggesting that subjects with higher GH doses had improved mitochondrial function.
Baseline free testosterone was inversely associated with 6-month change in VAT (r = −0.28, P = .03). Baseline VAT was positively associated with the 6-month change in τ (r = 0.71, P = .0002), suggesting that subjects with high baseline VAT were less likely to improve mitochondrial function.
Adverse events
There was a significant difference between the GH group and the placebo group in reported incidence of known adverse events related to GH treatment: paresthesias in the median and ulnar nerve distributions (GH group, 37.5%; placebo, 3.3%; P = .001). There was no significant difference between the GH group and the placebo group in reported incidences at any visit of mild edema (GH group, 25.0%; placebo, 40.0%; P = .3), mild joint discomfort (GH group, 34.4%; placebo 30.0%; P = .8), muscle aches (GH group, 3.1%; placebo, 13.3%; P = .2), nasal congestion (GH group, 6.3%; placebo, 6.7%; P = 1), back pain (GH group, 3.2%; placebo, 20.0%; P = .05), or headache (GH group, 25.0%; placebo, 10.0%; P = .2). One subject (in the placebo group) reported onset of radicular symptoms in the setting of known disc disease at his 6-month visit. There were 3 serious unrelated adverse events: inpatient admission for lower gastrointestinal bleed in a study subject who was receiving placebo, inpatient admission for trauma sustained in a motor vehicle accident in a subject who was receiving placebo, and new-onset atrial fibrillation in a subject who was receiving placebo. No other serious related or unrelated adverse events occurred during this study. There was no association between GH dose at 6 months and presence of side effects, controlling for 6-month change in weight. There was an association between 6-month change in IGF-1 levels in the whole group and the presence of paresthesias, controlling for 6-month change in weight (P = .01). There were no associations between change in IGF-1 level and any other side effects.
Discussion
Our study in obese young men demonstrated that GH administration that increased IGF-1 levels to the normal mean for age resulted in improvements in CV risk markers hsCRP and ApoB/LDL, a validated measure of LDL atherogenicity. It also improved body composition with preferential reduction of VAT and reduction of liver fat after controlling for the increase in weight observed in both groups. Our study is the first to show a relative improvement in mitochondrial function after GH administration using dynamic 31P-MRS. Mitochondrial function, as assessed by PCr kinetics, improved in the GH group vs placebo, after controlling for change in glucose homeostasis, suggesting that GH has direct effects on mitochondrial function, independent of glucose.
This is the first study to focus on young obese men, because previous studies on GH administration in obesity included women (18, 19) and only men from 40 to 70 years of age (20, 21). We hypothesized that younger obese men would demonstrate a greater improvement in body composition with fewer side effects than the previously studied older population, because younger individuals may respond more significantly to weight-loss interventions. Consistent with this hypothesis, VAT decreased by 15% in our study compared with an 8.8% reduction in older obese men. However, we did not observe a more substantial increase in muscle mass than that observed in older individuals (21). Although the 2 studies cannot be directly compared, our data suggest a robust response in the decrease in VAT in the younger population. The lack in increase in muscle mass was not explained by the presence of hypogonadism in a subset of our patient population, nor were effects on other endpoints significantly affected by testosterone levels, despite the fact that testosterone is known to be an important modulator of GH action. We did not measure muscle quality, muscle fat content (other than IMCL), or strength. It is possible that muscle quality was affected by GH administration, and this would be an interesting area of future investigation. Compared with a study administering a GHRH analog for 12 months to obese men and women, the decrease in VAT by 22.9 cm2 in our study in the GH group was slightly higher than that of the GHRH analog (16 cm2) (32). The GHRH analog also resulted in a decrease in hsCRP, carotid IMT, and serum triglycerides compared with placebo (32). It must be noted that GHRH and GH are not equivalent and therefore not directly comparable, because, for example, GHRH may have effects other than simply stimulating GH release.
In our study, GH administration did not affect thigh SAT, which may be important. Although abdominal fat and liver fat accumulation are associated with adverse outcomes, extremity SAT appears to be relatively protective (2). The increased cardiometabolic risk of VAT vs extremity SAT has been attributed in part to higher expression of inflammatory cytokines within VAT and elevated portal vein free fatty acid levels (33, 34). Furthermore, lower-body SAT acts as a buffer for dietary lipid influx, protecting other tissues from lipid overflow, thereby acting as a protective metabolic sink (2).
This is the first study to demonstrate a reduction in hepatic fat, using 1H-MRS, independent of weight gain in obese men. This may be important because of the growing public health problem of hepatic fat accumulation. The prevalence of nonalcoholic fatty liver disease has been reported to be 57% to 74% in obesity and is now the most common cause of chronic liver disease in North America (3, 35). Potential mechanisms for the relative decrease in IHL by GH include reduction in VAT with resultant decrease in delivery of free fatty acids to the liver, increased hepatic very-low-density lipoprotein production and subsequent increase in hepatic fat output, and increased biliary lipid output (19). The effects of GH on fat distribution, with preferential reduction in VAT and liver fat and preservation in lower-body SAT, may be important given the lack of many known therapeutic modulators of body fat distribution.
Our most robust results were an improvement in CV risk markers, namely hsCRP and ApoB/LDL, a marker of atherogenicity, with GH vs placebo administration. We also observed a trend toward a decrease in serum ApoB and tPA. These results are concordant with our study administering GH to obese premenopausal women (18), and the effects on hsCRP are concordant with studies in patients with hypopituitarism (15, 17). This is the first study showing improvement in CV risk markers in obese young men after GH administration that raises the mean IGF-1 level within the normal range. The consistently demonstrated beneficial effects of GH on hsCRP across different study populations are likely attributable to the known cytokine-like effects of GH.
Despite the younger age of our study subjects, we observed a small decrease in glucose tolerance in a minority of men, resulting in discontinuation from the study of 4 men in the GH group compared with 1 who was randomized to placebo, which could limit its therapeutic use in a young obese population. Two-hour glucose on OGTT increased significantly in the GH group, but within the normal range from a mean of 114 mg/dL pretreatment, to 124.8 mg/dL after 6 weeks of treatment, and 116 mg/dL after 6 months. The effect of GH on glucose metabolism is complex. An increase in insulin resistance has been reported after acute GH administration with normalization or improvement in some but not all studies with chronic administration, which may be attributable to favorable body composition effects with long-term GH administration (7, 36, 37). Of note, acute GH-induced insulin resistance is transient and reverses rapidly (38). Other than the mild hyperglycemia noted above and an elevated risk of median and ulnar nerve paresthesias, we observed no other signal of an increased risk of side effects to GH in this study. Of interest, the rate of side effects in the placebo group was rather high. This may reflect a certain suggestibility, in that study subjects were exposed to consent forms and questions at every visit regarding potential GH side effects, and highlights the importance of placebo-controlled trials to determine side effects as well as efficacy of medications.
We did not observe significant change in IMCL but observed an improvement in mitochondrial function after accounting for increases in glucose, which may be important. Impaired mitochondrial function has been implicated in the pathogenesis of insulin resistance through accumulation of IMCL, which then interfere with insulin signaling (11, 12). Impaired mitochondrial function with similar levels of IMCL has been found in women with type 2 diabetes compared with BMI-matched obese subjects, suggesting that impaired mitochondrial function is more strongly associated with insulin resistance than a high IMCL content (39). It is possible that the improvement in mitochondrial function after GH administration precedes reduction in IMCL, and longer-term studies are necessary to investigate this hypothesis.
A limitation of our study was the relatively high discontinuation rate, which, however, was lower than or comparable to other placebo-controlled obesity studies (18, 40) and similar in the GH and placebo groups. It should be noted that most of the discontinuations were for unavoidable medical reasons. Of importance, we followed the Institute of Medicine guidelines for analysis of data with missing observations to ensure proper statistical treatment of missing data. Another limitation was that certain tests, such as 31P-MRS and 1H-MRS, were available only in a subset of subjects. We might have been able to detect GH effects on these endpoints with a larger number, as is suggested by our observed association between GH dose and improved mitochondrial function. We also might have had more power to observe GH effects if our study subject population had been more homogeneous in terms of age and weight, and a larger study size might have allowed us to determine whether such variables were important determinants of effects. A larger study might have also allowed us to determine whether duration of obesity was an important determinant of GH effect. In addition, although compliance appeared to be high based on self-report, we suspect that study medication administration compliance was likely underreported by study subjects. This is the most likely explanation for the relatively high GH doses that were required to raise mean IGF-1 levels for the group as a whole to the midnormal range. Therefore, caution is advised when interpreting the GH doses reported.
In conclusion, GH administration in young men with abdominal adiposity improves body composition, including a preferential reduction in VAT without a decrease in protective lower-body fat, and relative improvement in both IHL and mitochondrial function compared with placebo. Moreover, GH administration exerted beneficial effects on 2 important CV risk markers, hsCRP and ApoB/LDL. Because there are few therapies that improve body fat distribution, and such an effect may have important implications for cardiometabolic health, further studies regarding the mechanisms responsible for these effects are warranted.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 HL-077674, K24 HL092902–03, UL1 RR025758, and K23 RR-23090. Study medication (GH and placebo) only was provided by Genentech, Inc, South San Francisco, California.
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- ApoB
- apolipoprotein B
- AUC
- area under the curve
- BMI
- body mass index
- CT
- computed tomography
- CV
- cardiovascular
- DXA
- dual-energy x-ray absorptiometry
- HDL
- high-density lipoprotein
- hsCRP
- high-sensitivity C-reactive protein
- IHL
- intrahepatic lipids
- IMCL
- intramyocellular lipids
- IMT
- intima-media thickness
- LDL
- low-density lipoprotein
- 1H-MRS
- proton magnetic resonance spectroscopy
- 31P-MRS
- dynamic phosphorous MRS
- OGTT
- oral glucose tolerance test
- PCr
- phosphocreatine
- SAT
- sc adipose tissue
- SDS
- SD score
- tPA
- tissue plasminogen activator
- VAT
- visceral adipose tissue.
References
- 1. Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848 [DOI] [PubMed] [Google Scholar]
- 2. Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308 [DOI] [PubMed] [Google Scholar]
- 3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231 [DOI] [PubMed] [Google Scholar]
- 4. Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90:768–774 [DOI] [PubMed] [Google Scholar]
- 5. Pijl H, Langendonk JG, Burggraaf J, et al. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515 [DOI] [PubMed] [Google Scholar]
- 6. Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab. 2008;93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 8. Rudling M, Angelin B. Loss of resistance to dietary cholesterol in the rat after hypophysectomy: importance of the presence of growth hormone for hepatic low density lipoprotein-receptor expression. Proc Natl Acad Sci U S A. 1993;90:8851–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bredella MA, Torriani M, Thomas BJ, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94:3995–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387 [DOI] [PubMed] [Google Scholar]
- 12. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013 [DOI] [PubMed] [Google Scholar]
- 14. Baum HB, Biller BM, Finkelstein JS, et al. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med. 1996;125:883–890 [DOI] [PubMed] [Google Scholar]
- 15. Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bengtsson BA, Johannsson G. Effect of growth-hormone therapy on early atherosclerotic changes in GH-deficient adults. Lancet. 1999;353:1898–1899 [DOI] [PubMed] [Google Scholar]
- 17. Sesmilo G, Biller BM, Llevadot J, et al. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111–122 [DOI] [PubMed] [Google Scholar]
- 18. Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franco C, Brandberg J, Lönn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–1474 [DOI] [PubMed] [Google Scholar]
- 20. Johannsson G, Mårin P, Lönn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–734 [DOI] [PubMed] [Google Scholar]
- 21. Pasarica M, Zachwieja JJ, Dejonge L, Redman S, Smith SR. Effect of growth hormone on body composition and visceral adiposity in middle-aged men with visceral obesity. J Clin Endocrinol Metab. 2007;92:4265–4270 [DOI] [PubMed] [Google Scholar]
- 22. Tomlinson JW, Crabtree N, Clark PM, et al. Low-dose growth hormone inhibits 11 beta-hydroxysteroid dehydrogenase type 1 but has no effect upon fat mass in patients with simple obesity. J Clin Endocrinol Metab. 2003;88:2113–2118 [DOI] [PubMed] [Google Scholar]
- 23. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421 [PubMed] [Google Scholar]
- 24. Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab. 2009;94:4923–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosseini Ghomi R, Bredella MA, Thomas BJ, Miller KK, Torriani M. Modular MR-compatible lower leg exercise device for whole-body scanners. Skeletal Radiol. 2011;40:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyerspeer M, Krssák M, Moser E. Relaxation times of 31P-metabolites in human calf muscle at 3 T. Magn Reson Med. 2003;49:620–625 [DOI] [PubMed] [Google Scholar]
- 29. Gomez JM, Espadero RM, Escobar-Jimenez F, et al. Growth hormone release after glucagon as a reliable test of growth hormone assessment in adults. Clin Endocrinol (Oxf). 2002;56:329–334 [DOI] [PubMed] [Google Scholar]
- 30. Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70 [DOI] [PubMed] [Google Scholar]
- 31. Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201 [DOI] [PubMed] [Google Scholar]
- 32. Makimura H, Feldpausch MN, Rope AM, et al. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:4769–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496 [PubMed] [Google Scholar]
- 34. Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1–R7 [DOI] [PubMed] [Google Scholar]
- 35. Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152 [DOI] [PubMed] [Google Scholar]
- 36. Fowelin J, Attvall S, Lager I, Bengtsson BA. Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism. 1993;42:1443–1447 [DOI] [PubMed] [Google Scholar]
- 37. Svensson J, Fowelin J, Landin K, Bengtsson BA, Johansson JO. Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab. 2002;87:2121–2127 [DOI] [PubMed] [Google Scholar]
- 38. Krusenstjerna-Hafstrøm T, Clasen BF, Møller N, et al. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab. 2011;96:2548–2557 [DOI] [PubMed] [Google Scholar]
- 39. Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120 [DOI] [PubMed] [Google Scholar]
- 40. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077 [DOI] [PubMed] [Google Scholar]