Abstract
Background:
Supraphysiological “stress dosing” is generally given to adrenally insufficient patients undergoing operative procedures and/or general anesthesia. However, the normal responses of cortisol to surgery are poorly documented, especially in small children. Recent studies in adults suggest that massive glucocorticoid dosing is not needed, especially in minimally invasive surgery.
Objective:
We sought to characterize the normal cortisol secretion rate in healthy children undergoing minimally and moderately invasive urological procedures.
Design and Setting:
This was a prospective observational study conducted at a tertiary referral center.
Patients:
Thirty healthy children, ages 5 months to 6 years, were studied undergoing elective urological procedures.
Methods:
Procedures were performed by a single surgeon; anesthesia was by a standard protocol. Sera were obtained at 5 points: iv catheter placement, intubation, 50% completion of surgery, anesthesia reversal, and 1 hour postoperative. Cortisol and cortisone were quantitated by liquid chromatography-tandem mass spectrometry.
Results:
Group mean cortisol values ranged from 4.21 to 5.71 μg/dL across the 5 time points; none of these mean values differed significantly (P < .05). There were no differences according to age, time of procedure, caudal anesthesia, and moderate vs minimally invasive procedures; 3 patients had higher values. There was a modest diminution in cortisone across the 5 time points.
Conclusions:
Minimal and moderately invasive urological procedures do not result in a cortisol stress response in healthy children. Peak cortisol levels were seen 1 hour postoperatively. These data suggest that current guidelines for stress dosing in adrenally insufficient patients substantially exceed physiological requirements during minimally invasive procedures.
Early studies of adrenal function and steroidogenesis led to a general understanding of adrenally insufficient states (1, 2). In the 1950s, patients were reported with lethal intraoperative circulatory shock after withdrawal from pharmacological glucocorticoid therapy before surgery (3, 4). Postmortem examination of 1 patient revealed diffuse atrophy and hemorrhage in the adrenal; the cause of death was listed as adrenal insufficiency. Guidelines were developed to prevent such future occurrences in patients with both primary and secondary adrenal insufficiency. Recommendations were generalized to quadruple a patient's current glucocorticoid dose in the perioperative period to cover the “stress” of procedures and anesthesia (4). In the decades following these case reports, studies of the hormonal responses to stress showed that ACTH, cortisol, urinary free cortisol, and urinary cortisol metabolites all increase in response to surgery (5–9).
Surgery stimulates the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system through at least 3 different mechanisms: hypovolemic stimulation of baroreceptors, direct afferent stimulation, and cytokine release from traumatized tissue. In addition, psychological stress from postoperative pain and fear can contribute to a rise in cortisol levels (10). A 1975 review of the extensive literature concerning cortisol responses to surgery in adults suggested that the cortisol secretion rate was 75–150 mg/d in response to major surgery and 50 mg/d for minor surgery (11). However, in the 1980s the need for massive stress glucocorticoid dosing during surgery was called into question. A study evaluating cortisol responses to graded surgical stress found no significant alterations in cortisol secretion 1 hour postoperatively with minimally invasive hernia repair (12). A 1987 study of adrenally intact adults undergoing invasive neck surgery showed stable cortisol levels throughout surgery, measured at 10-minute intervals, only to peak at anesthesia reversal (13).
Most authorities base recommendations for “stress dosing” on the normal secretory rate of cortisol, but this value has been controversial. Three studies from 1966 to 1970 established the widely cited value for the secretory rate of cortisol as 12.5 ± 3 mg/m2 of body surface area per day in children and adults (14–16), although others reported values ranging from 2–60 mg/d (17). Re-examination of this question in the early 1990s using stable isotope dilution with mass spectrometry yielded a value of 5.7 mg/m2/d in normal adults (18), and the same technology and deconvolution analysis both yielded values of 5.3–6.8 mg/m2/d in 8 to 17 year olds (19, 20). Because the absorption of orally administered hydrocortisone is over 90% (21, 22), in our center, we regard physiological replacement as 6–8 mg/m2/d. Thus, some recommendations for stress dosing of 100 mg/m2/d in adrenally insufficient children correspond to more than 12 times the normal physiological secretory rate (23).
Few studies have evaluated the HPA axis in children undergoing anesthesia and surgery. A study of preoperative and 1-hour postoperative cortisol responses to surgery based on age found that all children had a statistically significant rise in cortisol levels (24). Intra-operative data were not collected, so that it is unclear whether the observed rise was due to stress of surgery or stress of recovery and postoperative pain. In a study of healthy children undergoing anesthesia without surgery, cortisol levels remained stable during administration of anesthesia, but there was a statistically significant rise during the recovery period; the authors concluded there was no consistent classic stress response to anesthesia alone (25). A study evaluating salivary cortisol levels in response to varying levels of sedation in healthy children found 25% of patients had a four-fold increase in salivary cortisol levels, which was consistent with a stress response, and mean cortisol values increased more than 3-fold from baseline (26). However, most patients had their highest cortisol levels in the recovery phase after their procedure was completed. Based on these conflicting data, we sought to define the glucocorticoid responses of healthy children undergoing minimally and moderately invasive surgery.
Patients and Methods
Surgical procedures
Thirty patients undergoing elective urological procedures at the University of California, San Francisco, were enrolled on the day of their surgical procedure; 27 had minimally invasive surgery—circumcision (10), orchiopexy (7), hypospadias repair (4), hernia repair (2), phimosis repair (1), hydrocele (1), ureteral stent removal (1), and penile torsion (congenital rotational defect of the penile shaft (1); and 3 patients underwent moderately invasive surgery— pyeloplasty (1), nephrectomy (1), and ureter repair (1). All patients who met the inclusion criteria were approached; only 1 patient declined to participate in the study, and 1 patient with Down syndrome was deemed ineligible. Patients' ages ranged from 5 months to 6 years (mean, 2.7 y). Because these were urological procedures, only 3 subjects were female, and 27 were male. All subjects were otherwise healthy without known adrenal or pituitary disease. Patients were excluded if they had received glucocorticoids in the 6 weeks before surgery. The study was approved by the Committee on Human Research, University of California, San Francisco. Written informed consent was obtained from 1 parent for each child enrolled. Samples were obtained from May through June 2012.
Anesthesia
Twenty-eight subjects had airway management with a laryngeal mask airway; 2 subjects received intubation. All subjects received sevoflurane with nitrous oxide and oxygen for induction and maintenance of general anesthesia. Twenty subjects received caudal anesthesia; 19 received bupivacaine 0.25%, and 1 received morphine. Eighteen subjects received 1–4 doses of fentanyl during the procedure, and 5 subjects received 1 dose of midazolam. Two patients received propofol for induction, and 2 patients received morphine during their procedures. No hypotensive episodes occurred, and no patients required additional fluids. All patients had an uneventful postoperative course with pain controlled with acetaminophen if requested.
Samples
Blood samples (1 ml) were collected without anticoagulant at the following 5 time points: 1) insertion of iv catheter; 2) intubation; 3) approximately halfway through the procedure, as estimated by the surgeon; 4) reversal of anesthesia; and 5) 1 hour after completion of the surgery. Blood was allowed to clot for 30 minutes and was spun at 1500–2000 × g for 15 minutes, and the sera were removed and stored at −80°C until batch analysis. All 5 samples were obtained from 28 of the 30 patients. The sample from time point 5 in 1 patient and the samples from time points 2–4 from a second patient were not obtained because of iv catheter malfunction.
Steroid assays
A 10-μL aliquot of serum was diluted with 90 μL of water in a 1.5-mL centrifuge tube. Protein was precipitated by the sequential addition of 0.2 mL acetonitrile and 0.1 mL methanol. After the addition of 0.1 mL of 1000 pg/mL internal standard [2H4]cortisol (C/D/N Isotopes) in acetonitrile, the suspension was vortex mixed and centrifuged for 5 minutes at 13 000 rpm. The supernatant was transferred to a 2-mL tube with 0.5 mL of water, and steroids were extracted with 1 mL of methyl-t-butyl ether. The organic phase was transferred to a clean 2-mL tube and concentrated under nitrogen, and the dried extract was reconstituted with 0.1 mL of 50% aqueous methanol and transferred to a 0.25-mL vial insert. Samples were analyzed with an Agilent 1290 HPLC and 6490 triple quadrupole liquid chromatography-tandem mass spectrometer (Agilent Technologies) using electrospray ionization in positive ionization mode. Steroids were resolved on a Kinetex 50 × 2.1 mm, 2.6-μm particle size C8 column (Phenomenex) using gradient elution with 10 mm ammonium acetate and methanol. Quantitation was achieved by external standardization with a 9-point calibration curve using multiple reaction monitoring mode. Precursor and product ions were 363.2/121.1 and 361.2/163.1 for cortisol and cortisone at retention times of 5.4 and 5.1 minutes, respectively (27). Immunoassays and mass spectrometry assays perform very comparably for serum cortisol and cortisone (28).
Statistical analysis
Data are expressed as means ± SD unless otherwise specified. Paired t tests were used to compare means from time point 1 to each other time point. Signed rank tests, which exclude outlying values, were used to compare the change in cortisol levels from the first time point to each other time point as well as subgroup comparison. Statistical significance was considered to be a P value of < .05. The choice of statistical tools and their application to the data were done in collaboration with a professional medical statistician from the University of California, San Francisco Clinical and Translational Science Institute.
Results
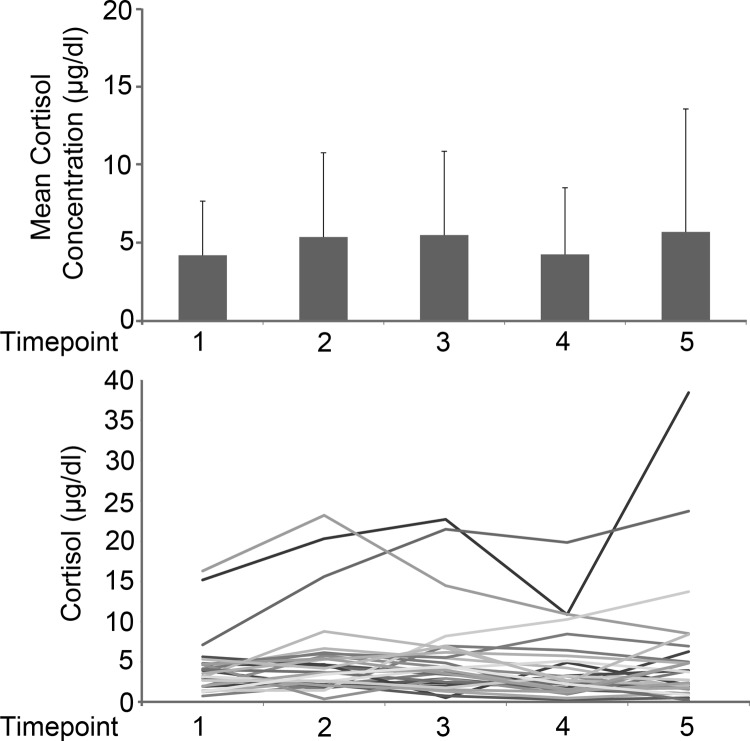
The mean serum cortisol levels were in the normal range at all time points, and the values did not change significantly from the baseline value at any of the 4 subsequent time points (Figure 1A). Mean cortisol levels at each time point were: 1) iv insertion, 4.21 μg/dL; 2) intubation, 5.38 μg/dL; 3) approximately 50% surgical completion, 5.50 μg/dL; 4) anesthesia reversal, 4.26 μg/dL; and 5) 1 hour postoperatively, 5.71 μg/dL (Table 1). Subanalysis showed no statistically significant changes in cortisol levels at any time point based on age (> 2 y vs < 2 y), time of day of the procedure (7–10 am, 10 am to 1 pm, and 1–4 pm), presence of caudal anesthesia, and minimally vs moderately invasive procedures. The change in mean cortisol in minimally and moderately invasive procedures differed from time points 1 to 4 (P = .02) and 1 to 5 (P = .01), suggesting an effect of moderately invasive procedures (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The trend in cortisol values from the time of iv insertion to 1 hour postoperatively for all subjects was flat, although the values of 3 patients were higher (Figure 1B). The patients with higher cortisol values were not the 3 having moderately invasive procedures, but instead underwent minimally invasive circumcisions with laryngeal mask anesthesia. There were no complications or need for additional fluid therapy during any of these procedures and no note of significant postoperative pain.
Figure 1.
Cortisol concentrations. A, Mean cortisol concentrations (μg/dL) with SD values for time points 1–5. Mean values were compared using paired t tests; no statistically significant change was found. B, Cortisol concentrations (μg/dL) over time for all subjects. Three subjects had outlying values, all of whom underwent minimally invasive procedures. To convert to nmol/L, multiply values by 27.78.
Table 1.
Cortisol Concentrations and Change in Cortisol Over Time Compared to Time Point 1
| Time Point |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Mean | 4.206 | 5.376 | 5.496 | 4.263 | 5.706 |
| SD | 3.472 | 5.395 | 5.367 | 4.268 | 7.872 |
| Median | 3.392 | 3.714 | 3.976 | 2.537 | 3.746 |
| Range | 0.76–16.81 | 0.39–23.22 | 0.55–22.72 | 0.29–19.85 | 0.24–38.47 |
| Mean change | 1.079 | 1.199 | −0.0331 | 0.381 | |
| P value | .07 | .19 | .37 | .96 | |
Signed rank test was used to compare the mean change in cortisol from time point 1 to each other time point with corresponding P value.
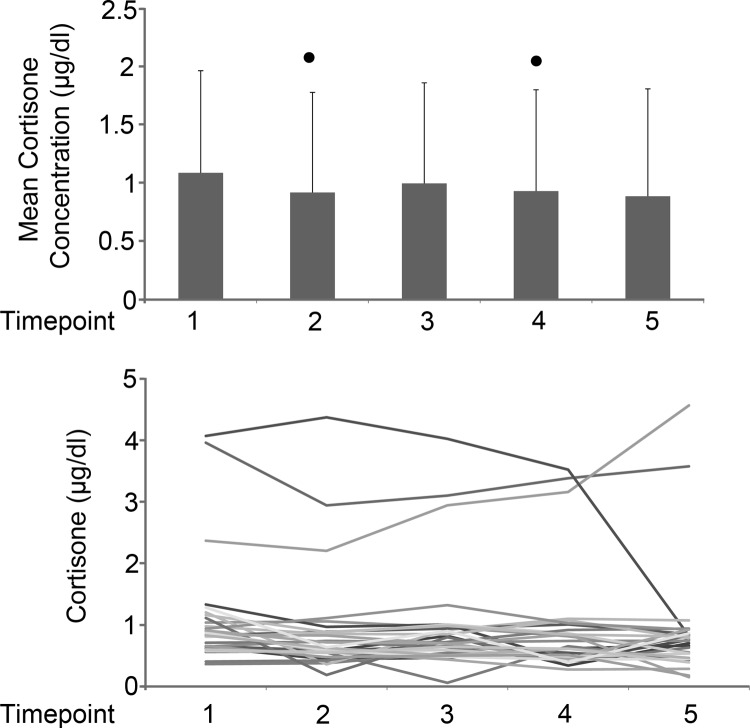
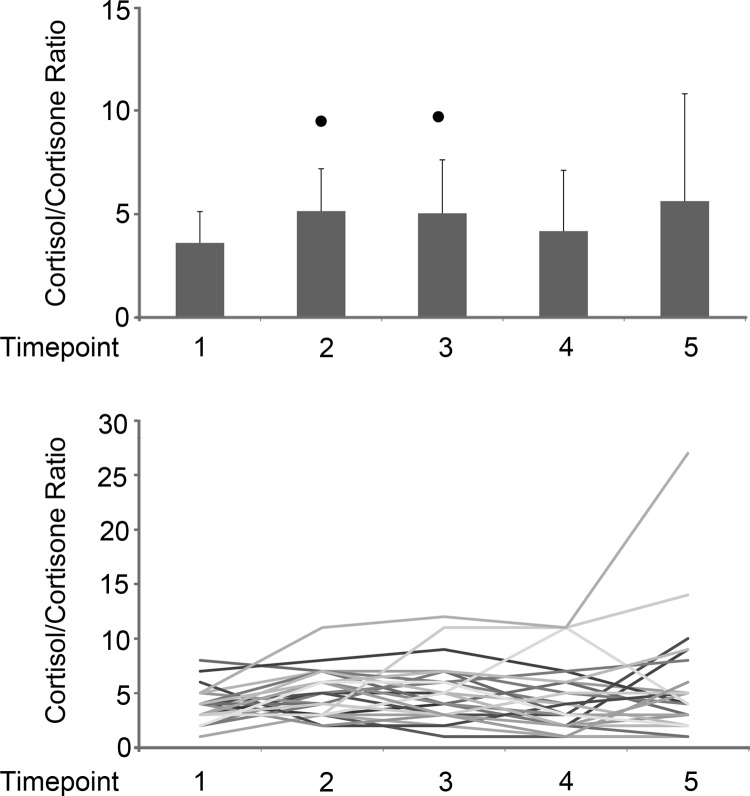
To determine whether there were changes in cortisol metabolism, we also measured cortisone. Mean cortisone was statistically decreased from time point 1 to time points 2 (P = .006) and 4 (P = .02) (Figure 2). The change in mean cortisone differed significantly from time points 1 to 2 (P = .009), 1 to 4 (P = .022), and 1 to 5 (P = .004) (Table 2). Each individual cortisol/cortisone ratio was calculated at each time point, with a mean range of 3.6 to 5.6. There was a significant increase in cortisol/cortisone ratios from time points 1 to 2 (P = .004) and 1 to 3 (P = .019) (Figure 3). The cortisol/cortisone ratios for the 3 patients having high cortisol values were relatively stable.
Figure 2.
Cortisone concentrations. A, Mean cortisone concentrations (μg/dL) with SD values for time points 1–5. Mean values were compared using paired t tests; values that differed from time point 1 by P < .05 are indicated by the dots. B, Cortisone concentrations (μg/dL) over time for all subjects. Three subjects had outlying values; all underwent minimally invasive procedures. To convert to nmol/L, multiply values by 27.63.
Table 2.
Cortisone Concentrations and Change in Cortisone Over Time Compared to Time Point 1
| Time Point |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Mean | 1.086 | 0.917 | 0.996 | 0.930 | 0.886 |
| SD | 0.877 | 0.859 | 0.863 | 0.870 | 0.921 |
| Median | 0.832 | 0.636 | 0.722 | 0.619 | 0.741 |
| Range | 0.36–4.07 | 0.19–4.37 | 0.44–4.02 | 0.42–3.52 | 0.18–4.56 |
| Mean change | −0.179 | −1.01 | −0.167 | −0.225 | |
| P value | .009 | .078 | .022 | .004 | |
Signed rank test was used to compare the mean change in cortisol from time point 1 to each other time point with corresponding P value.
Figure 3.
Cortisol/cortisone ratios. A, Mean cortisol/cortisone ratios with SD values for time points 1–5. Mean values were compared using paired t tests; values that differed from time point 1 by P < .05 are indicated by the dots. B, Cortisol/cortisone ratios over time for all subjects. Two subjects had outlying values, 1 having minimally invasive surgery and 1 having moderately invasive surgery.
Discussion
Our results show that healthy children undergoing minimally invasive urological surgical procedures with general anesthesia do not increase their serum cortisol concentrations significantly. Consistent with prior studies in adults and children (13, 25), the mean cortisol level peaked 1 hour postoperatively, but there was no statistically significant change in serum cortisol levels from time point 1 compared to all other time points. Subgroup analysis did not show statistically significant differences in mean cortisol levels according to patient age, time of day of procedure, presence of caudal anesthesia, or invasiveness of procedure.
Cortisol/cortisone ratios suggested a slight decrease in cortisol metabolism at time points 2 and 3 compared to time point 1. There was no evidence of increased cortisol metabolism at any time point. Critically ill adults have increased cortisol production (apparently in response to cytokines) and decreased cortisol conversion to cortisone (apparently due to suppression of 11β-hydroxysteroid dehydrogenase 2 and A-ring reductases) (29). A doubling in the serum cortisol/cortisone ratio has been observed in adults after major cardiothoracic surgery (30), a much greater stress than our patients experienced.
Some guidelines for stress dosing in adrenally insufficient patients range from 4–12 times the 24-hour physiological cortisol secretion rate (23), and the American College of Critical Care Medicine recommends 200 mg hydrocortisone per day in patients with sepsis (31); assuming that these are adult patients that are approximately 2 m2, this dose is approximately 14 times physiological. In adults, a stress response may be defined as a cortisol of > 20 μg/dL 1 hour after administration of iv cosyntropin (32), but normative data in young children are not available. The mean cortisol level from our cohort was < 6 μg/dL, well below a maximal stress response defined by ACTH stimulation, and only 4 measurements in 2 patients exceeded 20 μg/dL; these values appear to be outliers. Furthermore, the highest mean cortisol value occurred 1 hour postoperatively, not during the surgical procedure; thus, the time of maximal risk to an adrenally insufficient patient would appear to be just after surgery, not during surgery.
Patients with adrenal insufficiency require daily replacement dosing with hydrocortisone, and many patients receive frequent stress dosing, resulting in chronic exposure to supraphysiological hydrocortisone therapy. Excess glucocorticoid exposure can lead to insulin resistance, increased gluconeogenesis, immunosuppression, fluid retention, adipogenesis, reduced collagen synthesis in the skin, inhibition of osteoblast function, and suppression of the thyroid axis (23, 33–36). In the perioperative period, patients are at risk for hyperglycemia, increased rates of infection, reduced wound healing, and hypertension. In children, long-term side effects include osteopenia, reduced final height, and metabolic alterations that lead to increased risk of cardiovascular disease in adulthood. Consequently, in 1 form of adrenal insufficiency, steroid 21-hydroxylase deficiency, current guidelines are to use stress dosing cautiously and briefly (37).
Our results are comparable to 2 recent studies evaluating the cortisol response to sedation with or without invasive procedures in healthy children. In a study evaluating the effect of anesthesia alone on cortisol secretion, there was no significant change in cortisol values from the time of induction to emergence from anesthesia, with the authors concluding that anesthesia alone prompted no consistent classic stress response. However, 52% of those subjects had a stress response defined as a cortisol of > 19.8 μg/dL at the time of recovery, many of these children were extremely distressed or vomited in the recovery phase (25). By contrast, none of our subjects reported significant pain or fear in the postoperative period, possibly accounting for the lower mean cortisol values seen in our patients. In a study of salivary cortisol responses to sedation and anesthesia in healthy children, 25% of patients had a stress response, defined as a statistically significant, 4-fold increase in salivary cortisol levels. However, most of these patients were undergoing moderate or deep sedation, and only 25% received general anesthesia; the relative change in cortisol among those receiving general anesthesia was not different, which is consistent with our findings. Among those patients who had samples collected at sedation reversal and recovery, 75% who met criteria for a stress response had their highest level at the time of recovery and not during the surgical procedure (26).
Our study adds to the growing literature indicating that minimally invasive procedures do not result in activation of the HPA axis in healthy children. We can use these data as a guide for glucocorticoid dosing in children with primary and secondary adrenal insufficiency who are undergoing similar procedures. Based on our data for minimally invasive procedures, we recommend no more than 3 times physiological replacement (6–8 mg/m2/d orally) in the 24-hour perioperative period, with the increase dosing to follow but not precede the surgical procedure. Because multiple studies indicate an approximately 3-fold rise in cortisol in response to severe physiological stress (1, 11, 12), this dosing is more than sufficient for minor procedures.
Our study is limited by the small number of patients studied; our conclusions are statistically and clinically significant for the group undergoing minimally invasive procedures, but a larger group of patients undergoing moderately invasive procedures will be needed to draw conclusions from this group. The peak mean cortisol value was in the last data point collected; hence, it is possible that cortisol levels continued to rise, but were not captured in our protocol because patients were discharged home on the same day as the procedure. Our study is also limited to patients undergoing urological procedures; results may not be generalizable to other common pediatric procedures. Most patients enrolled in our study were male; however, studies in children with equal numbers of males and females have found no significant differences in cortisol response based on sex (24–26). Further studies are needed of pediatric patients undergoing abdominal, cardiac, and neurological surgery to guide glucocorticoid dosing in children with adrenal insufficiency so as to avoid adverse effects of unwarranted glucocorticoid exposure while maintaining safety in the perioperative period.
Supplementary Material
Acknowledgments
We thank Robert Chromic for performing mass spectrometry assays, the Pediatric Anesthesia Service at the University of California, San Francisco (UCSF) Children's Hospital for facilitating phlebotomy, and the Clinical Translational Science Institute for assistance with statistical analysis.
This work was supported in part by funds from the UCSF Divisions of Pediatric Endocrinology and Pediatric Urology. L.K.T. was supported by the UCSF National Institutes of Health Training Grant in Pediatric Endocrinology (Grant T32DK07161). Mass spectrometry was supported by a Clinician-Scientist Award in Translational Research from the Burroughs-Wellcome Fund (no. 1005954; to R.J.A.) and grants for the Michigan Nutrition Obesity Center (DK089503) and the Michigan Regional Comprehensive Metabolomics Research Core (U24DK097153).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- HPA
- hypothalamic-pituitary-adrenal.
References
- 1. Salem M, Tainsh RE, Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage: A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219:416–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller WL. A brief history of adrenal research: steroidogenesis—the soul of the adrenal. Mol Cell Endocrinol. 2013;371:5–14 [DOI] [PubMed] [Google Scholar]
- 3. Fraser CG, Preuss FS, Bigford WD. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc. 1952;149:1542–1543 [DOI] [PubMed] [Google Scholar]
- 4. Lewis L, Robinson RF, Yee J, Hacker LA, Eisen G. Fatal adrenal cortical insufficiency precipitated by surgery during prolonged continuous cortisone treatment. Ann Intern Med. 1953;39:116–126 [DOI] [PubMed] [Google Scholar]
- 5. Sandberg AA, Eik-Nes K, Samuels LT, Tyler FH. The effects of surgery on the blood levels and metabolism of 17-hydroxycorticosteroids in man. J Clin Invest. 1954;33:1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore FD, Steenburg RW, Ball MR, Wilson GM, Myrden JA. Studies in surgical endocrinology. I. The urinary excretion of 17-hydroxycorticosteroids, and associated metabolic changes, in cases of soft tissue trauma of varying severity and in bone trauma. Ann Surg. 1955;141:145–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hume DM, Bell CC, Bartter FC. Direct measurement of adrenal secretion during operative trauma and convalescence. Surgery. 1962;52:174–187 [PubMed] [Google Scholar]
- 8. Gann DS, Egdahl RH. Responses of adrenal corticosteroid secretion to hypotension and hypovolemia. J Clin Invest. 1965;44:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skillman JJ, Lauler DP, Hickler RB, et al. Hemorrhage in normal man: effect on renin, cortisol, aldosterone, and urine composition. Ann Surg. 1967;166:865–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Udelsman R, Holbrook NJ. Endocrine and molecular response to surgical stress. Curr Probl Surg. 1994;31:653–720 [PubMed] [Google Scholar]
- 11. Kehlet H. A rational approach to dosage and preparation of parenteral glucocorticoid substitution therapy during surgical procedures. A short review. Acta Anaesthesiol Scand. 1975;19:260–264 [DOI] [PubMed] [Google Scholar]
- 12. Chernow B, Alexander HR, Smallridge RC, et al. Hormonal response to graded surgical stress. Arch Intern Med. 1987;147:1273–1278 [PubMed] [Google Scholar]
- 13. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64:986–994 [DOI] [PubMed] [Google Scholar]
- 14. Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. II. Normal infants, children, and adults. Pediatrics. 1966;37:34–42 [PubMed] [Google Scholar]
- 15. New MI, Seaman MP, Peterson RE. A method for the simultaneous determination of the secretion rates of cortisol, 11-deoxycortisol, corticosterone, 11-deoxycorticosterone and aldosterone. J Clin Endocrinol Metab. 1969;29:514–522 [DOI] [PubMed] [Google Scholar]
- 16. Kenny FM, Taylor FH, Richard C. Reference standards for cortisol production and 17-hydroxycorticosteroid extraction during growth: variation in the pattern of excretion of radiolabeled cortisol metabolites. Metabolism. 1970;19:280–290 [DOI] [PubMed] [Google Scholar]
- 17. Zumoff B, Fukushima DK, Hellman L. Intercomparison of four methods for measuring cortisol production. J Clin Endocrinol Metab. 1974;38:169–175 [DOI] [PubMed] [Google Scholar]
- 18. Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rates in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991;72:39–45 [DOI] [PubMed] [Google Scholar]
- 19. Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr. 1990;117:892–896 [DOI] [PubMed] [Google Scholar]
- 20. Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76:1505–1510 [DOI] [PubMed] [Google Scholar]
- 21. Charmandari E, Johnston A, Brook CG, Hindmarsh PC. Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. J Endocrinol. 2001;169:65–70 [DOI] [PubMed] [Google Scholar]
- 22. Derendorf H, Möllman H, Barth J, Möllmann C, Tunns S, Krieg M. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol. 1991;31:473–476 [DOI] [PubMed] [Google Scholar]
- 23. Shulman DI, Palmert MR, Kemp SF. Adrenal insufficiency: still a cause of morbidity and death in childhood. Pediatrics. 2007;119:e484–e494 [DOI] [PubMed] [Google Scholar]
- 24. Khilnani P, Munoz R, Salem M, Gelb C, Todres ID, Chernow B. Hormonal responses to surgical stress in children. J Pediatr Surg. 1993;28:1–4 [DOI] [PubMed] [Google Scholar]
- 25. Rains PC, Rampersad N, De Lima J, et al. Cortisol response to general anaesthesia for medical imaging in children. Clin Endocrinol (Oxf). 2009;71:834–839 [DOI] [PubMed] [Google Scholar]
- 26. Hsu AA, von Elten K, Chan D, et al. Characterization of the cortisol stress response to sedation and anesthesia in children. J Clin Endocrinol Metab. 2012;97:E1830–E1835 [DOI] [PubMed] [Google Scholar]
- 27. Wooding KM, Auchus RJ. Mass spectrometry theory and application to adrenal disease. Mol Cell Endocrinol. 2013;371:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogeser M, Briegal J, Jacob K. Determination of serum cortisol by isotope-dilution liquid-chromatography electrospray ionization tandem mass spectrometry with on-line extraction. Clin Chem Lab Med. 2001;39:944–947 [DOI] [PubMed] [Google Scholar]
- 29. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol production during critical illness. N Engl J Med. 2013;368:1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogeser M, Groetzner J, Küpper C, Briegel J. The serum cortisol:cortisone ratio in the postoperative acute-phase response. Horm Res. 2003;59:293–296 [DOI] [PubMed] [Google Scholar]
- 31. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949 [DOI] [PubMed] [Google Scholar]
- 32. Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol (Oxf). 1998;49:287–292 [DOI] [PubMed] [Google Scholar]
- 33. Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and anti-macrophage serum. Am J Pathol. 1975;78:71–100 [PMC free article] [PubMed] [Google Scholar]
- 34. Canalis E. Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–3447 [DOI] [PubMed] [Google Scholar]
- 35. Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoid and bone mineral content in childhood glucocorticoid-sensitive nephritic syndrome. N Engl J Med. 2004;351:868–875 [DOI] [PubMed] [Google Scholar]
- 36. Strickland AL, Underwood LE, Voina SJ, French FS, Van Wyk JJ. Growth retardation in Cushing's syndrome. Am J Dis Child. 1972;123:207–213 [DOI] [PubMed] [Google Scholar]
- 37. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.