Abstract
Male subfertility is common, and it causes significant duress to couples. Although the most common cause of male subfertility is idiopathic failure of spermatogenesis, a significant percentage of male subfertility is medically treatable. Compared to reproductive specialists, endocrinologists may see a population of men that have a higher prevalence of treatable causes of subfertility including sexual disorders, endocrinopathies, obesity, drugs, and ejaculatory dysfunction. Seminal fluid analysis is the most important diagnostic study, and at least 2 samples should be analyzed. All patients with sperm concentrations < 10 million/mL due to idiopathic spermatogenic defects should be referred for genetic counseling and karyotyping; most experts also recommend that these patients be tested for Y chromosomal microdeletions. For most men with low sperm concentrations due to gonadotropin deficiency, gonadotropin therapy effectively increases spermatogenesis. The endocrinologist must recognize when to use medical therapy to stimulate spermatogenesis and when to refer for consideration of assisted reproductive technology.
Accreditation and Credit Designation Statements.
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this JCEM Journal-based CME activity for a maximum of 1 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to:
perform an appropriately focused evaluation for the common causes of male infertility
interpret the results of serum gonadotropins and seminal fluid analysis to determine the likely cause of male infertility in most men
initiate gonadotropin therapy to stimulate spermatogenesis in men with infertility due to hypothalamopituitary disease
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this JCEM Journal-based CME activity are required to disclose to The Endocrine Society and to learners any relevant financial relationship(s) of the individual or spouse/partner that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved all identified conflicts of interest.
The following author reported no relevant financial relationships:
Bradley D. Anawalt, M.D., has no relevant financial relationships.
The following JCEM Editors reported relevant financial relationships:
The Editor-in-Chief, Leonard Wartofsky, M.D., is a Consultant for Asurogen, Genzyme, and IBSA, and is on the Speaker's Bureau for Genzyme. Kenneth Burman, M.D., is a Consultant for Medscape and UpToDate; a Reviewer for the Endocrine Fellows Foundation; and has received Institutional Grants for Research from Amgen, Eisei, and Pfizer. Samuel Dagogo-Jack, M.D., is a Consultant for Merck and Novo Nordisk; a Grantee for the American Diabetes Association, AstraZeneca, Boehringer Ingelheim, National Institutes of Health, and Novo Nordisk; and a Grant Reviewer for the American Diabetes Association and National Institutes of Health. Silvio Inzucchi, M.D., is a Consultant/Advisor for Boehringer Ingelheim, Genentech, Janssen, Merck, and Takeda; has DSMB Activity with Amgen, Esai, and Gilead; and receives CME support from Abbott, Amylin, Boeringher-Ingelheim, Merck, and Takeda. Kieren Mather, M.D., received an Investigator-initiated Grant from Novo Nordisk. Lynnette Nieman, M.D., is an Author/Editor for UpToDate, and receives Research Support from HRA-Pharmaceutical.
The following JCEM Editors reported no relevant financial relationships: Paolo Beck-Peccoz, M.D.; David Ehrmann, M.D.; David Handelsman, Ph.D.; Michael Kleerekoper, M.D.; Merrily Poth, M.D.; Constantine Stratakis, M.D.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
JCEM Journal-based CME activities are not supported by grants, other funds, or in-kind contributions from commercial supporters.
Instructions
The estimated time to complete each JCEM Journal-based CME activity, including review of material, is 1 hour. Instructions for completing this activity can be found at https://www.endocrine.org/education-and-practice-management/continuing-medical-education/journal-cme.
If you have questions about this JCEM Journal-based CME activity, please direct them to education@endocrine.org.
Activity release date: September 2013
Activity expiration date: September 2015
The Case
A 29-year-old man is referred for evaluation of male infertility. He and his 29-year-old wife have been trying to conceive for 2 years. His wife has had a complete evaluation that revealed regular ovulatory cycles and normal reproductive anatomy. The couple has had unprotected vaginal intercourse at least twice weekly. His past medical history is remarkable for hypertension. His medications include a beta blocker and a multivitamin. He has no family history of hypogonadism, cleft palate, or infertility; he has 3 brothers who have fathered children. He works as a software developer. He has smoked a pack of cigarettes daily for at least 10 years, and he occasionally drinks a cocktail. He does not smoke marijuana or use other drugs of abuse. He has never fathered a child. His physical examination is normal (including a normal male voice and normal torso-limb proportions), except for a body mass index of 35 kg/m2, bilateral nontender gynecomastia, a normal genitourinary examination with normally descended testes that are 12 cc bilaterally (measured by Prader orchidometer), and easily palpable vasa deferentia. His laboratory tests (performed on early morning blood samples) include a total T that was 6 nmol/L (normal, 10–32), calculated free T of 87 pmol/L (normal, 220–640), SHBG of 16 pmol/L (normal, 13–90), estradiol < 20 pg/mL (normal, <50), LH of 2 mIU/mL (normal, 2–14), and FSH of 6 mIU/mL (normal, 1–12). Serum prolactin and iron studies are normal. Repeat blood testing yields similar results. Seminal fluid analysis by computer-assisted semen analysis revealed no sperm, but manual inspection under high-power microscopy revealed several immature sperm. Seminal fluid volume is 2.5 cc, with a normal pH (≥7.2) and fructose. Repeat seminal fluid analysis yielded similar results. Sella imaging revealed no hypothalamic or pituitary abnormalities.
Background
About 15% of couples have difficulty conceiving, and in approximately 30% of these couples only the man has reproductive dysfunction. In another 20% of the subfertile couples, both partners have a reproductive abnormality. Thus, male reproductive dysfunction contributes to about half of all cases of subfertile couples.
Infertility is classically defined as the inability for a couple to conceive after 12 months of frequent vaginal intercourse without the use of contraception (1, 2). Subfertility is a term used to describe reduced fertility that might require therapy for successful conception. Absolute infertility cannot be treated even with modern assisted reproductive technology (ART) such as surgical extraction (“harvesting”) of sperm and ova followed by intracytoplasmic sperm injection (ICSI) into an ovum. Many couples previously defined as infertile are subfertile in the modern era. The concept of subfertility also describes couples that take a longer time to conceive. Up to 50% of young, healthy couples that fail to conceive in the first 12 months will conceive in the following 12 months (1). Many of these couples are subfertile; treatment would, at best, only hasten conception.
The most important question that the endocrinologist faces in the evaluation and management of male subfertility is to determine whether the patient has a medically treatable cause of low sperm concentration. Optimization of spermatogenesis and delivery of sperm to the egg are essential for the treatment of male subfertility. In subfertile men, successful delivery of sperm to the egg often is most effectively accomplished by use of ART. However, the endocrinologist plays an important role in identifying and treating men whose spermatogenesis might be increased by hormonal therapy or treatment of endocrinopathies. The endocrinologist should have a basic understanding of the application and success rates of ART for treatment of male subfertility, and the endocrinologist should know when to refer men with subfertility to a specialist in ART.
Although the focus of this review is on male subfertility, it is important to recognize that both members of a subfertile couple must be evaluated for reproductive dysfunction. In this review, I focus on the endocrinologist's approach to the subfertile man. The most common cause of male subfertility is primary testicular dysfunction and a defect in spermatogenesis that is generally due to irreversible damage, and these men typically require treatment with ART (3). However, the endocrinologist sees a very different population than ART centers where men with primary spermatogenic defects predominate. In addition to evaluating subfertile men with primary testicular defects (eg, Klinefelter syndrome) that will likely need ART for treatment, endocrinologists will see men with subfertility due to sexual disorders, endocrinopathies that affect spermatogenesis, or defects in sperm transportation (eg, ejaculatory disorders).
Common Causes of Male Subfertility
Unassisted successful procreation requires intravaginal ejaculation, production of sperm, and normal transportation of sperm. Male subfertility may be broken into broad categories including sexual disorders that prevent or decrease the frequency of intravaginal ejaculation, primary testicular defects, endocrinopathies that reduce spermatogenesis, and defects in sperm transportation (Table 1).
Table 1.
Common Causes of Male Subfertility
| Sexual disorders |
| Erectile dysfunction |
| Failure to have intercourse |
| Lack of libido |
| Relationship dysfunction |
| Anorgasmia |
| Primary testicular defect in sperm production |
| Idiopathic |
| Chemotherapy |
| Klinefelter syndrome |
| Genetic mutations |
| Pelvic irradiation or surgery |
| Orchidectomy |
| Testicular cancer |
| Trauma |
| Large varicoceles |
| Cryptorchidism |
| Infection (eg, mumps orchitis in nonvaccinated men) |
| Autoimmune |
| Drugs |
| Endocrinopathies that affect spermatogenesis |
| Hypothalamopituitary disease |
| Hyperprolactinemia |
| Thyroid dysfunction |
| Obesity |
| Cushing syndrome |
| Defects in sperm transportation |
| Obstruction |
| Congenital absence of the vasa deferens |
| Acquired ejaculatory duct obstruction (eg, recurrent infection, vasectomy) |
| Ejaculatory dysfunction |
| Anejaculation |
| Retrograde ejaculation |
Sexual disorders
Male subfertility may be due to sexual disorders including erectile dysfunction and anorgasmia (leading to decreased sexual pleasure and decreased frequency of sexual intercourse). Although erectile dysfunction is less common in younger men, it occasionally contributes to male subfertility. A common cause of anorgasmia is serotonin reuptake inhibitor therapy (4).
Although lack of sexual intercourse might not be a sexual disorder per se, it is a treatable cause of subfertility. The frequency of sexual intercourse should be 2 to 3 times weekly in order to optimize the likelihood of conception (5). Infrequent sexual intercourse might be due to relationship discord, lack of opportunity (eg, due to work hours or travel that results in separation between the couple), or lack of libido. Difficulty with conception may reduce sexual pleasure and result in a further decrease in the frequency of sexual intercourse.
Primary testicular dysfunction
The most common cause of male infertility is idiopathic primary testicular dysfunction with abnormal spermatogenesis, but other common causes include testicular damage due to systemic chemotherapy for cancer, Klinefelter syndrome, genetic mutations, testicular cancer, pelvic irradiation or surgery, trauma, cryptorchidism, infection, autoimmune destruction, and drugs. Idiopathic spermatogenic failure is usually associated with sperm concentrations < 10 million/mL, and it is usually also associated with abnormal sperm morphology and motility. Men with normal sperm concentrations but abnormal sperm morphology and motility are relatively uncommon; they should be referred to an ART specialist.
Radioiodine therapy for thyroid disease may cause testicular damage and abnormal spermatogenesis (6). These abnormalities generally improve within 6 to 12 months, but higher dosages of radioiodine therapy (>150 mCi) may be associated with slower recovery or permanent damage to fertility. Abuse of tobacco, marijuana, or alcohol has been associated with decreased quantity or quality of spermatogenesis (7–9).
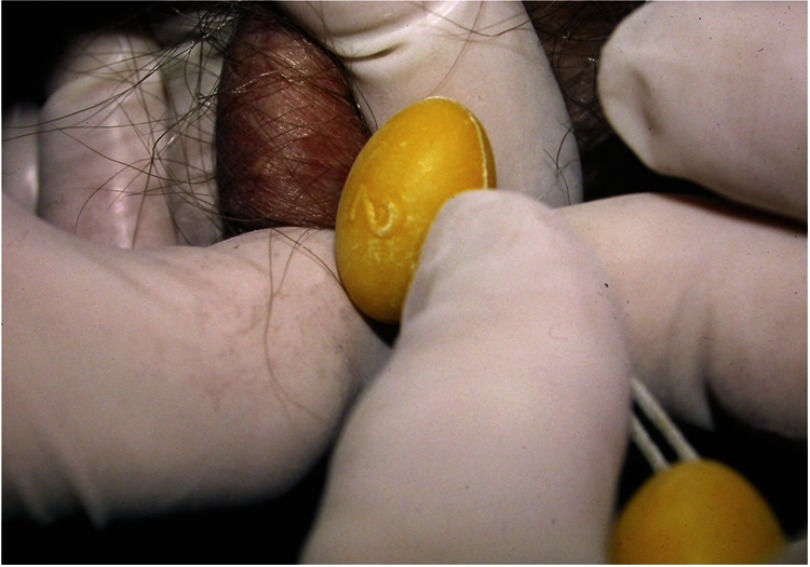
Another important cause of primary testicular dysfunction is Klinefelter syndrome. About 10–15% of infertile men with absent (azoospermia) or low sperm concentrations have Klinefelter syndrome (10, 11). Klinefelter syndrome should be suspected in any infertile man with unexplained primary hypogonadism, small testes (Figure 1), and azoospermia. Men with Klinefelter syndrome have progressive fibrosis that accelerates with the advent of puberty, and it might be desirable to surgically extract sperm as early as feasible and cryopreserve it for future attempts at conception (11, 12).
Figure 1.
Testicular examination of a man with small testes due to Klinefelter syndrome. The testis should be gently but firmly circumscribed with the fingers of 1 hand and pulled up to the scrotal skin to reduce overestimation of size due to inclusion of subtunical fluid in volumetry. The epididymis that lies posterior and extends to the superior aspect of the testis should not be included in the volumetry. Prader orchidometry with standard sizes of ovoid (2 cc ovoid shown) correlates well with ultrasound.
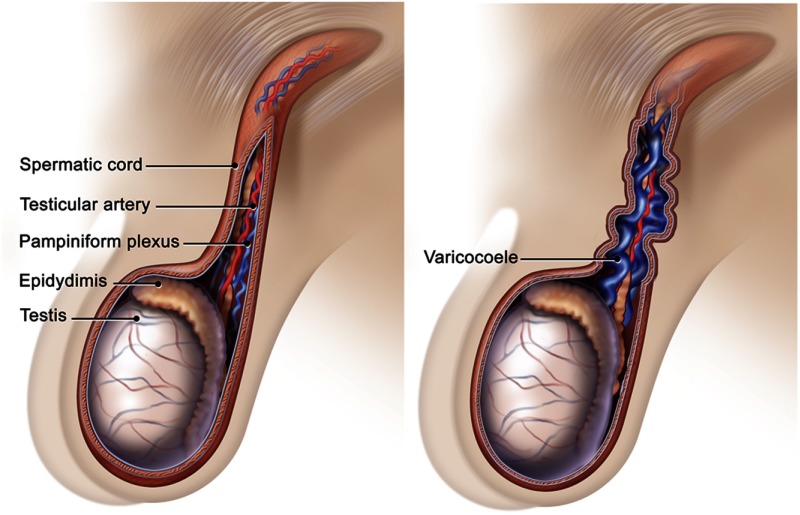
Anatomic abnormalities may cause defects in spermatogenesis. Excessive testicular heat due to undescended or partially descended testes, morbid obesity with large panni insulating the testes, or varicocele may be associated with decreased spermatogenesis. There is significant controversy whether varicoceles cause male infertility (13, 14). Large varicoceles might cause defective spermatogenesis via a variety of mechanisms including compromised testicular cooling and increased intratesticular reactive oxygen species (15). Although there are few data to support the practice, some experts continue to recommend varicocelectomy for large varicoceles in subfertile men with low sperm concentrations (16).
Endocrinopathies that are associated with reduced spermatogenesis
Hypothalamopituitary disorders are the most common cause of male subfertility due to an endocrinopathy. Because normal secretion of both FSH and LH is required for quantitatively and qualitatively normal spermatogenesis, any disease that affects hypothalamic secretion of GnRH or pituitary secretion of FSH or LH will impair spermatogenesis. Tumors and infiltrative diseases of the hypothalamus, hyperprolactinemia, hemochromatosis, Kallmann syndrome, and idiopathic hypogonadotropic hypogonadism are classic endocrine causes of male subfertility. Opioids and anabolic androgenic steroids may also cause hypogonadotropism and subfertility.
Hypothyroidism and hyperthyroidism may disrupt normal spermatogenesis, but these disorders are usually clinically obvious when thyroid dysfunction is the sole or primary cause of male subfertility (17). Adrenal disorders also may cause male subfertility via a variety of mechanisms. Cushing syndrome of any etiology may result in impaired testicular steroidogenesis and spermatogenesis. Congenital adrenal hyperplasia is often associated with male subfertility due to disruption of normal GnRH and gonadotropin secretion and hypertrophy of testicular adrenal rests. Testicular adrenal rests represent embryological remnants of nests of adrenal cells that co-migrate with the gonad during fetal development. In men with undertreated congenital adrenal hyperplasia, continuously increased ACTH secretion causes adrenal rest hypertrophy. Large adrenal rests decrease spermatogenesis and sperm transport directly (via compression of the seminiferous tubules) and indirectly (by local testicular production of corticosteroids that impair testicular sex steroidogenesis and therefore impair spermatogenesis) (18).
Obesity may also contribute to hypogonadotropism and male subfertility (19–21). Although data exist that obesity might inhibit normal testicular function directly or via insulin resistance, most of the evidence suggests that the primary mechanism of hypogonadism in obesity is the suppression of LH and FSH. Obesity-induced hypogonadotropic hypogonadism and subfertility might be due to increased aromatization of T to estradiol in the peripheral fat of obese men; estradiol is a potent inhibitor of LH secretion. Leptin resistance might contribute to obesity-induced hypogonadotropic hypogonadism. There is strong evidence from animal studies and some confirmation from a small number of human studies of leptin-deficient men that leptin is essential for normal male reproduction (22). Specifically, leptin appears to act via the kisspeptin pathway to stimulate hypothalamic release of GnRH that in turn stimulates FSH and LH secretion from the pituitary. Because obesity is commonly associated with leptin resistance, it is likely that functional leptin deficiency contributes to obesity-induced hypogonadism.
Defects in sperm transportation
Defects in sperm transportation include obstruction of the ejaculatory tract and ejaculatory dysfunction such as retrograde ejaculation or anejaculation. An important cause of ejaculatory tract obstruction is congenital bilateral absence of the vasa deferentia (CBAVD). The majority (50–80%) of men with CBAVD have mutations of the cystic fibrosis transmembrane conductance regulator (11, 16). Although nearly all men with cystic fibrosis have CBAVD, CBAVD may be the only clinical manifestation of a CFTR gene mutation. All men with CBAVD should be tested for CFTR gene mutations.
Ejaculatory dysfunction disorders are common in men with neuropathy due to systemic disease such as diabetes mellitus or central nervous system diseases including spinal cord lesions (23). Retrograde ejaculation into the bladder may cause male infertility (24). It is often due to medications (eg, α-adrenergic blockers) dysautonomia, bladder neck surgery, or any disorder that affects the neuroregulation of the bladder neck and results in failure to close the bladder neck during ejaculation. Anejaculation is usually due to neurological disorders, but it is also associated with serotonin reuptake inhibitors used for the treatment of depression (23).
Diagnostic Strategy
History and physical examination
In the evaluation of a subfertile man, the clinician should take a careful sexual and reproductive history to determine the weekly frequency of vaginal intercourse and whether the man has symptoms of hypoandrogenism (eg, decreased libido, decreased spontaneous morning erections), sexual dysfunction (erectile dysfunction or anorgasmia), pain or abnormal curvature with erections, or delayed puberty. A history of delayed puberty might indicate congenital hypogonadotropic hypogonadism. Although determining paternity by history is uncertain, it is important to query whether the man has ever impregnated a woman in the past.
The clinician should also review for symptoms of thyroid disease, corticosteroid excess, hypothalamopituitary masses (eg, headaches and visual changes), or acromegaly. In patients with long-standing diabetes mellitus and neurological disorders, gastrointestinal symptoms such as postprandial fullness or vomiting, chronic diarrhea or constipation might indicate dysautonomia and greater risk of ejaculatory dysfunction. Postcoital micturition that is cloudy might indicate retrograde ejaculation. The past medical history should be reviewed for systemic chemotherapy and scrotal or pelvic irradiation, surgery, or trauma that might cause permanent injury to spermatogenesis. Any severe systemic illness may cause a significant decrease in spermatogenesis that might improve with treatment of the systemic illness. The medication list should be reviewed for common drugs that may affect spermatogenesis including opioids, corticosteroids, 5-α reductase inhibitors, and sulfasalazine. The patient should be queried nonjudgmentally about the use or intake of tobacco products, alcohol, opioids, marijuana, and anabolic androgenic steroids.
The physical examination is useful for increasing or decreasing the probability of certain causes of male subfertility. Height, weight, body mass index, and waist-hip ratio will diagnose obesity that might contribute to subfertility. If thyroid disease is the cause of subfertility, a goiter is often present. The skin examination is useful for pigmentation changes that suggest hemochromatosis and detecting manifestations of Cushing syndrome; Cushing syndrome as a cause of male subfertility can generally be excluded on clinical grounds alone.
The most important part of the physical examination is the testicular examination (Figure 1). The clinician should examine the penis for hypospadias and fibrosis. The vasa deferentia should be palpable within the spermatic cord. The spermatic cord is a soft pliable tubular structure, and the vas deferens is firm and about the size of a small pull cord (Figure 2). Examining the man in recumbent and standing positions permits detection of varicoceles; varicoceles should shrink significantly in the recumbent position. The testes should be carefully palpated for masses; testicular cancer is more prevalent in infertile men. The testes should be measured, ideally by Prader orchidometry. Measurement with a Prader orchidometer correlates well with ultrasonography, but Prader orchidometry consistently overestimates the true volume in normal and infertile men (25, 26). Normal unilateral testicular volume is ≥ 15 cc. Smaller testicular volumes correlate with decreased spermatogenesis. Small firm testes (≤4 cc each) in a hypergonadotropic man suggest Klinefelter syndrome, whereas small testes in a hypo- or normogonadotropic man suggest congenital hypogonadotropism. In infertile men with testes < 15 cc, there generally is a direct correlation between testicular volume and successful medical treatment of fertility. A testicular volume > 6 cc generally portends a better response to treatment.
Figure 2.
Examination for the vasa deferentia and large varicoceles. The vasa extend from the base of the epididymi that lie posterior to each testis and run cephalad in the spermatic cord. The vas feels like a pull cord inside of the pliable tubular spermatic cord. Varicoceles feel like a “bag of worms” and enlarge when the patient stands.
The neurological examination should focus on determining sensation in the perineal “saddle area,” sacral reflexes (eg, the cremasteric and anal wink), and the Achilles deep tendon reflex (S1 nerve function). Evidence of significant peripheral neuropathy (decreased light touch, dry skin in extremities due to lack of sebum production) raises the likelihood of an ejaculatory disorder due to dysautonomia.
Initial diagnostic testing
The single most useful test is seminal fluid analysis. Because there is significant variation in sperm concentrations from day to day, collection of seminal fluid for analysis on at least 2 occasions is essential (3, 16). The seminal fluid samples should be collected after 2–7 days of abstinence from ejaculation and should be in accordance with international standards established by the World Health Organization (27).
Accurate measurement of an early morning serum T, FH, and LH levels should be performed on all subfertile men; equivocal results should be repeated.
Scrotal ultrasound is useful if there is a scrotal abnormality on examination, if there is a testicular mass suspected on examination, or if the scrotal examination is difficult (eg, retracted testes).
Interpretation of initial diagnostic testing and further testing
The most important findings on seminal fluid are the sperm concentration and ejaculate volume (Table 2). The sperm concentration should exceed 15 million/mL, but there is some controversy about whether sperm concentrations between 15 and 20 million are “normal” in men who have failed to conceive (27, 28). An isolated defect in sperm morphology or motility occurs rarely; these patients should be referred to a reproductive specialist. The ejaculate volume should exceed 1.5 mL; many experts use 2 or 2.5 mL as the lower limit of normal (27). If the ejaculate volume is low, then the patient should be queried about the possibility of spillage or lack of adequate duration of ejaculatory abstinence before collecting the sample. In men with sperm concentrations of zero, low ejaculate volume, low seminal fluid pH (< 7.4), and low or absent fructose levels suggest the possibility of distal ejaculatory tract obstruction near the bladder base.
Table 2.
World Health Organization Reference Values for Sperm Parametersa
| Sperm concentration | ≥15 million/mL |
| Total sperm | ≥39 million |
| Total motility (% of total) | ≥40% |
| Normal morphology (% of total) | ≥4% |
Based on the 5th percentile of recent fathers.
If the sperm concentration is zero, semen volume is low, and the serum hormones are normal, then a postejaculatory urinalysis and transrectal ultrasound should be performed to look for evidence of retrograde ejaculation or ejaculatory tract obstruction; analysis for mutations of the cystic fibrosis transmembrane conductance regulator should be performed in all men with unilateral or bilateral absence of the vasa deferentia (Figure 3). If the sperm concentration is low and the serum hormones are normal, direct testing for antisperm antibodies on the surface of sperm should be considered (16). If the sperm concentration is zero or low and serum gonadotropins are elevated (with FSH > LH levels), then the patient should be evaluated for causes of primary hypogonadism (including karyotyping). Most patients with low sperm concentrations (0–10 million/mL) and isolated elevation of serum FSH have primary spermatogenic failure. If the sperm concentration is zero or low and serum gonadotropins are low or low-normal, then the patient has hypogonadotropic hypogonadism. Evaluation for common causes of hypogonadotropism in men including hyperprolactinemia, hemochromatosis, and sellar masses should be performed.
Figure 3.
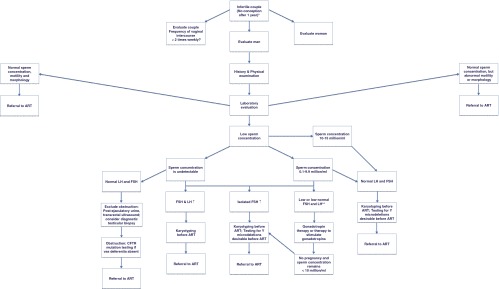
Diagnostic evaluation and management of male subfertility. The diagnostic evaluation of male subfertility begins with an evaluation of the couple. ART, ART specialist. *, Infertility is sometimes defined as no conception after 2 years of unprotected intercourse. **, Men with untreated hypogonadotropic hypogonadism typically have no sperm or very low levels (sperm concentration < 3 million/mL).
Genetic counseling and testing
Before initiating ART, it is important that patients know that low sperm concentrations may indicate that the patient has a karyotypic abnormality. The prevalence of chromosomal abnormalities is 5–6% in infertile men, and 10% of all azoospermic men have Klinefelter syndrome (11, 16). The risk of autosomal chromosomal abnormalities is also severalfold higher than the risk in fertile men. Genetic counseling and karyotype testing should be offered to all subfertile men with hypergonadotropic hypogonadism or idiopathic spermatogenic deficiency and sperm concentrations < 10 million/mL (3, 11, 16). For most couples, genetic counseling may consist of reviewing the known risks that a minority of infertile men carry a detectable genetic abnormality that might be transmitted to offspring and disclosing that most infertile men do not have a genetic abnormality that is currently detectable. Some couples might opt not to do any genetic testing including karyotype testing if the results will not alter their decision to attempt to conceive.
Testing for Y chromosome microdeletions should also be considered for all men with idiopathic spermatogenic deficiency and pretreatment sperm concentrations < 5 million/mL; some experts use a threshold of < 10 million/mL (11, 16). As mentioned above, all men with CBAVD or even unilateral absence of a vas deferens should be screened for gene mutations associated with cystic fibrosis (11, 16).
Therapeutic strategy
The therapeutic strategy is dictated by time, money, and etiology of the male subfertility. ART is expensive and emotionally draining, but it can hasten the time to conception for most causes of male subfertility. For patients with subfertility due to sexual disorders and inadequate frequency of vaginal intercourse, appropriate treatment of the underlying sexual disorder might be successful. For subfertile men with retrograde ejaculation or anejaculation, sperm harvesting (from urine or testes, respectively) followed by ICSI is often the best option. Retrograde ejaculation can also sometimes be treated medically (eg, with α-agonist therapy) or with physical stimulation (eg, electroejaculation) (23). For men with subfertility due to primary testicular defects, ART represents the best or only chance for conception, whereas men with subfertility due to hypogonadotropism might conceive after gonadotropin therapy or therapy that stimulates endogenous gonadotropin production.
Commonly available medical therapies for men with subfertility due to hypogonadotropism include gonadotropin replacement therapy, aromatase inhibitors, and selective estrogen receptor modulators (SERMs; eg, clomiphene). Aromatase inhibitors and SERMs require intact hypothalamopituitary function because they act by decreasing the inhibitory effects of estradiol on the hypothalamus and pituitary. The best data for medical therapy are based on studies of gonadotropin replacement therapy in men with infertility due to congenital hypogonadotropism. It takes approximately 9–10 weeks to complete all of the stages of spermatogenesis. In general, any medical therapy administered to stimulate spermatogenesis and increase sperm concentrations in the ejaculate will take months to years to achieve maximal effect. It is important to discuss the possible dilatory response of spermatogenesis and fertility with most medical therapies because couples will need to consider this delay when considering medical therapy to improve spermatogenesis vs immediate ART or adoption.
In men whose subfertility is due to a medically treatable cause such as an endocrinopathy that suppresses the gonadal axis and spermatogenesis, medical induction of spermatogenesis may take 6 months to 3 years before successful conception, and it ranges in cost from $60 to $500 (US dollars) monthly or more. For couples who must make haste and who can afford the cost, ART (sometimes in conjunction with medical induction of spermatogenesis) might be the best option. Finally, for all subfertile couples, adoption should be considered an option. In the United States, adoption costs range in cost from a few thousand dollars (for a foster child adoption) to $50 000 or more (in 2012 dollars) for a domestic or international infant adoption, and it might take many months until the adopted child enters the home of the adoptive parents (29). In the United States and in many other countries, the cost of adoption compares favorably with the average cost of successful ART (29).
Induction and stimulation of spermatogenesis
The most important therapeutic decision that the endocrinologist must make is whether to attempt to induce and/or stimulate spermatogenesis. Men with elevated gonadotropin levels or an isolated elevation of FSH do not benefit from gonadotropin (or other) therapy to stimulate spermatogenesis or improve fertility. When the subfertile man has hypogonadotropism and low or no spermatogenesis due to known hypothalamopituitary disease, medical therapy to stimulate spermatogenesis likely will be useful. Gonadotropin therapy is also useful to improve spermatogenesis and fertility in men with congenital or acquired isolated hypogonadotropic hypogonadism; these men will have low or low-normal serum gonadotropin, low T levels, and low sperm concentrations.
Patients with GnRH deficiency (eg, Kallmann syndrome) might be treated with replacement pulsatile GnRH therapy, but this treatment is not widely available, requires wearing a pump with a sc dispensing system, and is ineffective for patients with pituitary disease. More commonly, gonadotropin replacement therapy is generally administered as LH and FSH replacement therapy. After any exogenous androgen therapy has been discontinued, LH replacement therapy is initiated as human chorionic gonadotropin (hCG). The usual dosage of hCG is 1000–2000 IU sc 2–3 times weekly, but it is likely that lower dosages of 500–750 IU are effective (30, 31). Because some men (particularly men with pretreatment testes ≥ 8 cc) will produce sperm and become fertile with hCG alone, and because FSH therapy is expensive, hCG is often administered as monotherapy for 6 months, whereas sperm concentrations are measured monthly. If the patient can afford early initiation of FSH therapy, it could be initiated immediately and might result in more rapid increases in sperm concentrations. Men with postpubertal gonadotropin deficiency might respond to hCG monotherapy, but men with prepubertal onset of gonadotropin deficiency virtually always benefit from combination hCG plus FSH therapy. Sperm concentrations typically take at least 4–6 months to rise with hCG monotherapy, and FSH therapy is added if sperm concentrations remain below 10 million/mL and conception has not occurred. FSH may be given as recombinant human FSH (rhFSH) or human menopausal gonadotropin; the usual initial dosage is 75 IU sc every other day. The dosage of FSH may be doubled if conception has not occurred and sperm concentrations remain < 20 million/mL within 6 months of initiation of combination therapy with hCG. On average, conception occurs after 2–3 years of gonadotropin therapy and occurs when sperm concentrations are between 5 and 20 million/mL, but conception may occur at levels (<5 million/mL) well below the levels (13–20 million/mL) associated with fertility in normal men (32). If the couple desires another child within 2 years, then gonadotropin therapy should be continued until another pregnancy is achieved.
Men with baseline small testes ≤ 4 cc are more likely to respond slowly (33). There is some controversy over whether prior androgen therapy may decrease the likelihood of response to gonadotropin therapy, but the author believes that this effect is unlikely or small at worst (32).
Success of ART in treatment of male subfertility
Although the focus of this review is on the role of induction and stimulation of spermatogenesis in the treatment of male subfertility, endocrinologists must understand the success rates of ART for subfertile men with idiopathic oligozoospermia or azoospermia.
In infertile couples, 40–45% of ART procedures (including ICSI) result in pregnancy (34, 35). ICSI may be performed with sperm from the ejaculate or sperm obtained by testicular sperm extraction (TESE) or microdissection TESE (micro-TESE). The fertilized egg is then implanted into the uterus. Up to 60% of all men with nonobstructive azoospermia might have a few sperm that can be extracted with these techniques (34). Forty to 55% of men with Klinefelter syndrome may have small pockets of sperm that can be surgically extracted; rates are higher in men with mosaic Klinefelter syndrome (36).
The risks of ART include multiple gestation and possibly increased chromosomal abnormalities (35, 37). Surprisingly, small studies using ICSI with sperm of men with Klinefelter syndrome have not demonstrated an increased risk of Klinefelter syndrome in male offspring (36).
Controversies and Areas of Uncertainty
In the treatment of male infertility, there is some controversy about when to attempt to stimulate spermatogenesis with gonadotropin therapy or empiric medical therapy to increase gonadotropins (eg, aromatase inhibitor or SERM). I recommend against gonadotropin therapy or other medical therapy to subfertile men with normal serum hormone levels and idiopathic oligozoospermia, but this topic is controversial. In a recent survey of U.S. urologists (>90% of whom treated male infertility), over half of them reported that they would consider empiric medical therapy with gonadotropin therapy, aromatase inhibitors, or SERMs for men with idiopathic infertility (38). Respondents were more likely to use empiric therapy in men with low sperm concentrations and without elevated FSH levels (that would suggest primary testicular failure that would not respond to gonadotropin therapy).
Most subfertile men will not benefit from the use of gonadotropin or other empiric medical therapy. The data and clinical experience suggest that these medical therapies will be ineffective for improving spermatogenesis or fertility in men with normal, low-normal, or even slightly low serum T levels and normal gonadotropin levels. In the small minority of men with decreased spermatogenesis and infertility due to gonadotropin deficiency (eg, due to a pituitary tumor), gonadotropin therapy is likely to improve spermatogenesis. Men with gonadotropin deficiency will typically have unequivocally low (usually very low) T levels and low or low-normal gonadotropin levels.
When evaluating an infertile man for possible gonadotropin therapy, it is important for the endocrinologist to understand that the normal range of FSH levels reported by many commercial laboratories is likely to be based on a cohort that includes subfertile men. Although many commercial laboratories report an upper limit of normal of up to 12 IU/L for men, the upper limit of normal for reproductively normal men is probably closer to 8 IU/L (39). An FSH level greater than 8 IU/L should raise the suspicion of primary spermatogenic failure; gonadotropin therapy is unlikely to improve fertility.
In general, gonadotropin therapy is not useful for men with idiopathic infertility. However, some studies suggest that there might be a subset of men with normal serum T and gonadotropin levels and idiopathic infertility that might benefit from FSH therapy (40–43). Some recent studies indicate some infertile men with normal hormone levels have a polymorphism in the FSH promoter region or the FSH receptor gene that results in relatively lower (albeit normal) FSH levels or low FSH effect that results in decreased spermatogenesis and fertility. Two small pilot studies have demonstrated improved spermatogenesis with the administration of FSH to men with specific FSH or FSH receptor polymorphisms (42, 43).
The important endpoints in the treatment of male infertility are pregnancy and live birth rates. A 2007 Cochrane review concluded that there were too few randomized, controlled trials (n = 4) and too few subjects (n = 278) studied to determine whether FSH therapy to men with idiopathic infertility results in increased pregnancy rates for couples (44). It should be noted that the pregnancy rates were significantly higher in the gonadotropin treatment group (13.4%) compared to the men treated with placebo (4.4%) in the Cochrane meta-analysis.
There are no data that empirical therapy with aromatase inhibitors or SERMs are effective for treatment of infertility in men with idiopathic oligozoospermia (3). The U.S. Food and Drug Administration has not approved aromatase inhibitors or SERMS for treatment of male infertility.
My approach is to consider medical therapy with gonadotropins, aromatase inhibitors, or SERMS only in subfertile men with unequivocally low serum T levels and low to low-normal serum LH and FSH levels. Gonadotropin therapy may be offered to such men; the expense is the major limitation. Aromatase inhibitor therapy to increase serum gonadotropin levels and stimulate spermatogenesis should be offered only in selected circumstances. Obese men who have high or normal serum estradiol levels and low or low-normal serum T and gonadotropin levels might respond with increased spermatogenesis and improved fertility with an aromatase inhibitor (45). On the other hand, aromatase inhibition is likely to have deleterious effects on skeletal health, and such therapy should be limited to ≤ 1 year. Men with subfertility due to obesity-induced hypogonadotropism might also benefit from weight loss via lifestyle changes or bariatric surgery (20). It is unclear how much weight loss is required to improve fertility in these men.
Some men with infertility due to anabolic androgenic steroids continue to have persistent suppression of circulating gonadotropin levels and spermatogenesis despite discontinuation of the androgenic steroids. In these men, gonadotropin or SERM therapy might hasten the recovery of spermatogenesis and fertility (46, 47). In patients with hypogonadotropic hypogonadism due to iatrogenic Cushing syndrome or opioids that cannot be treated by weaning off the causative agent, gonadotropin therapy is the therapy most likely to be beneficial to treat subfertility. SERM or aromatase inhibition therapy might effectively improve spermatogenesis and fertility in these patients, too, by increasing pituitary gonadotropin secretion.
Returning to the Patient
This patient has a presentation consistent with adult onset idiopathic hypogonadotropic hypogonadism (48, 49). This diagnosis is uncommon and is characterized by normal puberty followed by adult onset of very low serum T levels with low or inappropriately normal gonadotropin levels. Virtually all of these men have azoospermia and infertility, but occasionally these men will have fathered a child early in adulthood. Men with adult onset idiopathic hypogonadotropic hypogonadism may have improved spermatogenesis and fertility with gonadotropin therapy. Although adult onset idiopathic hypogonadotropic hypogonadism might be more common than originally suspected, it is still uncommon. Clinicians must not thoughtlessly make this diagnosis in the common scenario of subfertile men with low-normal or slightly low T levels and normal gonadotropin levels.
To enhance the man's fertility, I would advise him to quit smoking and refrain from excessive drinking. His obesity might be contributing to his subfertility, and I would advise him to lose 5–10% of body weight via lifestyle changes. His symptoms of low libido and his reproducibly low serum T and inappropriately normal gonadotropin levels suggest hypogonadotropism. His physical examination, normal seminal fluid volume, pH and fructose levels make obstruction unlikely. By history and evaluation, the wife is likely to be ovulatory and fertile.
I would offer gonadotropin therapy, but there is some uncertainty whether gonadotropin therapy will improve his spermatogenesis. However, he has symptoms of hypogonadism, and his serum T level is very low, with an inappropriately low normal LH level. Isolated LH deficiency (without FSH deficiency) is very unusual. Although his FSH level is normal, it will suppress with the administration of LH therapy (in the form of hCG) (50). After informing the patient of the uncertainty of the diagnosis and outcome of therapy, it is reasonable to offer hCG therapy followed by rhFSH therapy if necessary.
His testicular volumes are >6 cc; his sperm concentrations might rapidly increase with hCG therapy alone. I would add rhFSH after 6 months of hCG if conception has not occurred and sperm concentration remains <10 million/mL. Because his estradiol level is low, aromatase inhibitor or SERM therapy is less likely to be effective. I would discuss the option of referral for ART without gonadotropin therapy now, but a short course of gonadotropin therapy might obviate the need for ART or improve the success rate of ART. Because his wife is 29 years old and close to entering the typical time for declining fertility, I would not treat him with gonadotropin therapy for more than 12–15 months before considering ART. If he has persistent absence of spermatogenesis despite gonadotropin therapy, the diagnosis of adult onset idiopathic hypogonadotropic hypogonadism must be reconsidered. He then must be treated as a man with idiopathic spermatogenic failure, and he should be provided genetic counseling before offering genetic testing or initiating ART.
Barriers to Ideal Practice
The barriers to ideal practice in the management of male subfertility include lack of awareness by endocrinologists about their role in identifying medically treatable causes of male subfertility. Endocrinologists must ensure that they have the skills to accurately measure testes via Prader orchidometry, assess for large varicoceles in the recumbent and standing positions, and reliably identify the presence of the vasa deferentia. In addition, endocrinologists must take the lead in their community in ensuring that laboratories have accurate assays for serum T. Finally, it is essential to understand that there is significant normal variation in serum T and seminal fluid sperm concentrations. These parameters must be assessed on at least 2–3 separate occasions.
Endocrinologists must remember to discontinue any T therapy (and counsel patients to cease or avoid any androgenic anabolic steroids), a fact that is not universally known among nonendocrinologists (38). Endocrinologists must also understand the importance of genetic counseling and testing in men with idiopathic oligozoospermia (0–10 million sperm/mL). Finally, it is essential that endocrinologists establish a collaborative relationship with experts in ART early in the management of subfertile men to ensure optimal medical therapy and timing of the use of ART.
Conclusions
Male subfertility occurs commonly in patients that endocrinologists see in their practice: men with obesity, diabetes mellitus, Klinefelter syndrome and hypogonadotropism due hyperprolactinemia, opiates, or corticosteroids. The endocrinologist plays an important role in assessing for treatable causes of impaired spermatogenesis and initiating therapy to induce or optimize spermatogenesis. Medical therapies to induce spermatogenesis may take months for maximal effect on spermatogenesis and fertility. The endocrinologist must balance this delay in maximal effect for spermatogenesis against the couples' urgency to conceive quickly (based on age and other factors) when considering when to refer for use of ART.
When medical therapy is used to stimulate spermatogenesis and enhance male fertility, the clinician must clearly communicate to the couple when the medical therapy is based on firm scientific underpinnings and when the therapy is empiric. For patients with unequivocal hypogonadotropic hypogonadism, gonadotropin therapy is very likely to improve spermatogenesis and male fertility. For men with possible hypogonadotropic hypogonadism, medical therapy should be described as empiric. The couple must be given the opportunity to understand that many of our therapies in the field of male reproduction are based not only on hormones and ART, but also on art and science.
Supplementary Material
Acknowledgments
This work has been funded by National Institutes of Health—The Eunice Kennedy Shriver National Insitute of Child Health and Human Development (NIH-NICHD) U554 HD042454-06.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- ART
- assisted reproductive technology
- CBAVD
- congenital bilateral absence of the vasa deferentia
- hCG
- human chorionic gonadotropin
- ICSI
- intracytoplasmic sperm injection
- rhFSH
- recombinant human FSH
- SERM
- selective estrogen receptor modulator
- TESE
- testicular sperm extraction.
References
- 1. Evers JL. Female subfertility. Lancet. 2002;360:151–159 [DOI] [PubMed] [Google Scholar]
- 2. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20:1144–1147 [DOI] [PubMed] [Google Scholar]
- 3. Jungwirth A, Giwercman A, Tournaye H, et al. 2012 European Association of Urology Guidelines on Male Infertility: the 2012 update. Eur Urol. 2012;62:324–332 [DOI] [PubMed] [Google Scholar]
- 4. Rowland D, McMahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. J Sex Med. 2010;7:1668–1686 [DOI] [PubMed] [Google Scholar]
- 5. Stanford JB, Dunson DB. Effects of sexual intercourse patterns in time to pregnancy studies. Am J Epidemiol. 2007;165:1088–1095 [DOI] [PubMed] [Google Scholar]
- 6. Sawka AM, Lea J, Alshehri B, et al. A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol (Oxf). 2008;68:610–617 [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123 [DOI] [PubMed] [Google Scholar]
- 8. Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci. 2011;16:498–516 [DOI] [PubMed] [Google Scholar]
- 9. Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15:31–44 [DOI] [PubMed] [Google Scholar]
- 10. Hofherr SE, Wiktor AE, Kipp BR, Dawson DB, Van Dyke DL. Clinical diagnostic testing for the cytogenetic and molecular causes of male infertility: the Mayo Clinic experience. J Assist Reprod Genet. 2011;28:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLachlan RI, O'Bryan MK. State of the art for genetic testing of infertile men. J Clin Endocrinol Metab. 2010;95:1013–1024 [DOI] [PubMed] [Google Scholar]
- 12. Radicioni AF, De Marco E, Gianfrilli D, et al. Strategies and advantages of early diagnosis in Klinefelter's syndrome. Mol Hum Reprod. 2010;16:434–440 [DOI] [PubMed] [Google Scholar]
- 13. Baazeem A, Belzile E, Ciampi A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808 [DOI] [PubMed] [Google Scholar]
- 14. Evers JH, Collins J, Clarke J. Surgery or embolisation for varicoceles in subfertile men. Cochrane Database Syst Rev. 2009;1:CD000479. [DOI] [PubMed] [Google Scholar]
- 15. Kim HH, Goldstein M. Adult varicocele. Curr Opin Urol. 2008;18:608–612 [DOI] [PubMed] [Google Scholar]
- 16. Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(5 suppl 1):S202–S209 [DOI] [PubMed] [Google Scholar]
- 17. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755 [DOI] [PubMed] [Google Scholar]
- 18. Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:209–220 [DOI] [PubMed] [Google Scholar]
- 19. Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S. Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med. 2012;172:440–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reis LO, Dias FG. Male fertility, obesity, and bariatric surgery. Reprod Sci. 2012;19:778–785 [DOI] [PubMed] [Google Scholar]
- 21. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66:103–109 [PubMed] [Google Scholar]
- 22. Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. 2011. Hum Reprod Update. 2011;17:667–683 [DOI] [PubMed] [Google Scholar]
- 23. Ohl DA, Quallich SA, Sønksen J, Brackett NL, Lynne CM. Anejaculation and retrograde ejaculation. Urol Clin North Am. 2008;35:211–220 [DOI] [PubMed] [Google Scholar]
- 24. Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril. 2012;97:306–312 [DOI] [PubMed] [Google Scholar]
- 25. Behre HM, Nashan D, Nieschlag E. Objective measurement of testicular volume by ultrasonography: evaluation of the technique and comparison with orchidometer estimates. Int J Androl. 1989;12:395–403 [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto H, Saito K, Ogawa Y, Yoshida H. Testicular volume measurements using Prader orchidometer versus ultrasonography in patients with infertility. Urology. 2007;69:158–162 [DOI] [PubMed] [Google Scholar]
- 27. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245 [DOI] [PubMed] [Google Scholar]
- 28. Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, Agarwal A. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22 [DOI] [PubMed] [Google Scholar]
- 29. Gumus G, Lee J. Alternative paths to parenthood: IVF or child adoption? Econ Inq. 2012;50:802–820 [Google Scholar]
- 30. Han TS, Bouloux PM. What is the optimal therapy for young males with hypogonadotropic hypogonadism? Clin Endocrinol (Oxf). 2010;72:731–737 [DOI] [PubMed] [Google Scholar]
- 31. Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–2602 [DOI] [PubMed] [Google Scholar]
- 32. Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–808 [DOI] [PubMed] [Google Scholar]
- 33. Warne DW, Decosterd G, Okada H, Yano Y, Koide N, Howles CM. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009;92:594–604 [DOI] [PubMed] [Google Scholar]
- 34. Palermo GD, Neri QV, Takeuchi T, Rosenwaks Z. ICSI: where we have been and where we are going. Semin Reprod Med. 2009;27:191–201 [DOI] [PubMed] [Google Scholar]
- 35. Sunderam S, Chang J, Flowers L, et al. ; Centers for Disease Control and Prevention (CDC) Assisted reproductive technology surveillance—United States, 2006. MMWR Surveill Summ. 2009;58:1–25 [PubMed] [Google Scholar]
- 36. Fullerton G, Hamilton M, Maheshwari A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum Reprod. 2010;25:588–597 [DOI] [PubMed] [Google Scholar]
- 37. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813 [DOI] [PubMed] [Google Scholar]
- 38. Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–978 [DOI] [PubMed] [Google Scholar]
- 39. Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90:5928–5936 [DOI] [PubMed] [Google Scholar]
- 40. Grigorova M, Punab M, Zilaitiene B, et al. Genetically determined dosage of follicle-stimulating hormone (FSH) affects male reproductive parameters. J Clin Endocrinol Metab. 2011;96:E1534–E1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tüttelmann F, Laan M, Grigorova M, Punab M, Sõber S, Gromoll J. Combined effects of the variants FSHB -211G>T and FSHR 2039A>G on male reproductive parameters. J Clin Endocrinol Metab. 2012;97:3639–3647 [DOI] [PubMed] [Google Scholar]
- 42. Ferlin A, Vinanzi C, Selice R, Garolla A, Frigo AC, Foresta C. Toward a pharmacogenetic approach to male infertility: polymorphism of follicle-stimulating hormone β-subunit promoter. Fertil Steril. 2011;96:1344–1349 [DOI] [PubMed] [Google Scholar]
- 43. Selice R, Garolla A, Pengo M, Caretta N, Ferlin A, Foresta C. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int J Androl. 2011;34:306–312 [DOI] [PubMed] [Google Scholar]
- 44. Attia AM, Al-Inany HG, Farquhar C, Proctor M. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2007;4:CD005071. [DOI] [PubMed] [Google Scholar]
- 45. Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Clin Pract Endocrinol Metab. 2008;4:415–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril. 2003;79(suppl 3):1659–1661 [DOI] [PubMed] [Google Scholar]
- 47. de la Torre Abril L, Ramada Benlloch F, Sánchez Ballester F, et al. Management of male sterility in patients taking anabolic steroids [in Spanish]. Arch Esp Urol. 2005;58:241–244 [PubMed] [Google Scholar]
- 48. Nachtigall LB, Boepple PA, Pralong FP, Crowley WF., Jr Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med. 1997;336:410–415 [DOI] [PubMed] [Google Scholar]
- 49. Dwyer AA, Hayes FH, Plummer L, Pitteloud N, Crowley WF., Jr The long-term clinical follow-up and natural history of men with adult-onset idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95:4235–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsumoto AM, Karpas AE, Bremner WJ. Chronic human chorionic gonadotropin administration in normal men: evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man. J Clin Endocrinol Metab. 1986;62:1184–1192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.