Abstract
Context:
Endometrium in polycystic ovary syndrome (PCOS) presents altered gene expression indicating progesterone resistance and predisposing to reduced endometrial receptivity and endometrial cancer.
Objective:
We hypothesized that an altered endocrine/metabolic environment in PCOS may result in an endometrial “disease phenotype” affecting the gene expression of different endometrial cell populations, including stem cells and their differentiated progeny.
Design and Setting:
This was a prospective study conducted at an academic medical center.
Patients and Main Outcome Measures:
Proliferative-phase endometrium was obtained from 6 overweight/obese PCOS (National Institutes of Health criteria) and 6 overweight/obese controls. Microarray analysis was performed on fluorescence-activated cell sorting-isolated endometrial epithelial cells (eEPs), endothelial cells, stromal fibroblasts (eSFs), and mesenchymal stem cells (eMSCs). Gene expression data were validated using microfluidic quantitative RT-PCR and immunohistochemistry.
Results:
The comparison between eEPPCOS and eEPCtrl showed dysregulation of inflammatory genes and genes with oncogenic potential (CCL2, IL-6, ORM1, TNAIFP6, SFRP4, SPARC). eSFPCOS and eSFCtrl showed up-regulation of inflammatory genes (C4A/B, CCL2, ICAM1, TNFAIP3). Similarly, in eMSCPCOS vs eMSCCtrl, the most up-regulated genes were related to inflammation and cancer (IL-8, ICAM1, SPRR3, LCN2). Immunohistochemistry scoring showed increased expression of CCL2 in eEPPCOS and eSFPCOS compared with eEPCtrl and eSFCtrl and IL-6 in eEPPCOS compared with eEPCtrl.
Conclusions:
Isolated endometrial cell populations in women with PCOS showed altered gene expression revealing inflammation and prooncogenic changes, independent of body mass index, especially in eEPPCOS and eMSCPCOS, compared with controls. The study reveals an endometrial disease phenotype in women with PCOS with potential negative effects on endometrial function and long-term health.
Polycystic ovary syndrome (PCOS), characterized by oligo/anovulation, hyperandogenism, and polycystic-appearing ovaries, is the most common endocrine disorder among reproductive-age women and is a leading cause of female infertility (1). Although anovulation and impaired oocyte maturation are the main causes of decreased fecundity in PCOS, several abnormalities in PCOS endometrium have been reported, including aberrant steroid hormone action with high sex steroid receptor and coactivator expression, low expression of αvβ3 integrin, abnormal immune cell trafficking, and resistance to progesterone (P4) (2–5). These changes likely contribute to reduced endometrial receptivity, subfertility, and poor pregnancy outcome in women with PCOS (6–9).
Endometrial cell populations include epithelial (eEP), endothelial (eEN), and vascular smooth muscle cells, stromal fibroblasts (eSFs), and resident and transient immune cell populations. Many of these cells respond to ovarian-derived steroid hormones with follicular phase estradiol (E2) driving endometrial cellular proliferation that is curtailed by corpus luteum P4 production in the secretory phase. In anovulatory disorders such as in PCOS, an E2-dominant environment prevails because ovulation and P4 production are infrequent or completely absent. This results in the increased risks of endometrial hyperplasia and endometrial cancer in PCOS women (10, 11). It can be postulated that an endometrial disease phenotype is promoted by anovulation and aggravated by hyperandrogenism and metabolic and inflammatory changes related to obesity, insulin resistance, and accompanying hyperinsulinemia, all common in PCOS women (12, 13).
Endometrial mesenchymal stem cells (eMSCs), presumptive progenitors of eSFs, reside in the perivascular space in human endometrium, and likely contribute to endometrial cyclic regeneration and lineage-specific differentiation (14, 15). Adult stem cells exist in a niche that maintains their stemness or signals their differentiation and can be affected by changes in their microenvironment (eg, inflammation, hypoxia) that may lead to abnormal/dysfunctional lineage progeny (16). Because inflammation and metabolic and endocrine abnormalities prevail in women with PCOS, the purpose of the current study was to determine whether the gene expression profile of specific endometrial cell populations, including eMSCs, in PCOS endometrium can give insights into the origin of endometrial abnormalities and subfertility common in PCOS women.
Materials and Methods
Study subjects and tissues
Tissue samples were obtained through the National Institutes of Health/University of California, San Francisco (UCSF), Human Endometrial Tissue and DNA Bank in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants in the UCSF Center for Reproductive Health, and the study was approved by the UCSF Committee on Human Research. The clinical summary of the study participants is shown in Table 1. Eleven proliferative-phase endometrial biopsies (Pipelle; Cooper Surgical) and one curettage specimen were collected from overweight [body mass index (BMI) ≥ 27 kg/m2 < 29.9 kg/m2; n = 1] and obese (BMI ≥ 30 kg/m2; n = 5) women with PCOS [age 30.5 ± 2.1 y, BMI 34.13 ± 2.2 kg/m2, National Institutes of Health criteria (17)] and overweight (n = 2) and obese (n = 4) control women (age 36.50 ± 1.70 y, BMI 35.73 ± 3.96 kg/m2). All PCOS subjects had normal 17-hydroxyprogesterone, prolactin, and thyroid hormone levels. Control samples were obtained from healthy volunteer and women undergoing benign gynecological surgery. All controls reported menstrual cycles with regular intervals (25–35 d) and no clinical evidence of having PCOS. Neither PCOS nor control subjects were exposed to hormonal medications for at least 2 months prior to tissue sampling and were confirmed not pregnant.
Table 1.
Clinical Characteristics of the Study Subjects
| ID | Group | Agea | BMIa | IR | PCOS | Diagnosis | Procedure | Medication |
|---|---|---|---|---|---|---|---|---|
| PC01b | PCOS | 28 | 26.89 | No | OA, HA, hirsutism, PCO | PCOS | Pipelle | None |
| PC02b | PCOS | 29 | 41.15 | Yes | A, HA, hirsutism, PCO | PCOS | Pipelle | Metformin, esomeprazole, sertraline |
| PC03b | PCOS | 36 | 33.00 | No | OA, HA, PCO | Ovarian cyst, PCOS | Right salpingo-oophorectomy, pipelle | Albuterol, phenylpropanolamine |
| PC04c | PCOS | 25 | 30.37 | No | OA, HA, acne, PCO | PCOS | Pipelle | None |
| PC05c | PCOS | 38 | 39.76 | Yes | OA, HA, hirsutism, PCO | PCOS | Pipelle | None |
| PC06d | PCOS | 27 | 33.60 | Yes | OA, HA, hirsutism, PCO | PCOS | Pipelle | Metformin |
| C01b | Ctrl | 36 | 43.48 | No | Control | Fibroids, adenomyosis, menorrhagia | Hysterectomy, pipelle | Iron |
| C02b | Ctrl | 37 | 32.36 | No | Control | Undesired fertility | Tubal ligation, pipelle | Nifedipine |
| C03c | Ctrl | 29 | 27.93 | No | Control | Volunteer | Pipelle | None |
| C04c | Ctrl | 41 | 51.48 | No | Control | Fibroids, adenomyosis, polyp, menorrhagia, ovarian cyst | Hysterectomy, bilateral salpingectomy, curettage | Iron |
| C05d | Ctrl | 36 | 26.87 | No | Control | Undesired fertility, stress urinary incontinence | Tubal ligation, urethral sling, pipelle | Simvastatin |
| C06c | Ctrl | 40 | 32.27 | No | Control | Undesired fertility | Tubal ligation, pipelle | None |
Abbreviations: A, amenorrhea; Ctrl, control; HA, biochemical hyperandrogenism; IR, insulin resistance (2 h oral glucose tolerance test); OA, oligoamenorrhea; PCO, polycystic ovaries.
No statistical difference between the study groups according to t test (age: P = .732; BMI: P = .054).
Sample used for array analysis, Q-RT-PCR, and IHC.
Sample used for array analysis and Q-RT-PCR.
Sample used for IHC.
Tissue processing and fluorescence-activated cell sorting (FACS) of endometrial cell populations
Tissue biopsies were divided into two fresh tissue samples processed separately for FACS and for histological examination in formalin-fixed, paraffin-embedded tissue. Tissue processing for viable cell isolation and FACS analysis were performed as previously described (15). Briefly, enzymatically dissociated endometrial cells were incubated in blocking buffer [PBS with 40% human serum and 1% BSA] for 30 minutes and then labeled with the following fluorochrome-conjugated antibodies (BD Biosciences) in PBS containing 10% human serum and 1% BSA: cluster of differentiation (CD)-45 (phycoerythrin-Cy7 anti-CD45) at 1:20 dilution to label contaminating leukocytes for their removal; epithelial cell adhesion molecule (EPCAM; allophycocyanin anti-EPCAM) at 1:20 dilution to label eEP; cluster of differentiation 146 [CD146 or melanoma cell adhesion molecule (MCAM), CD146, fluorescein isothiocyanate anti-MCAM] at 1:5 dilution to label eEN/perivascular cells; β-type platelet-derived growth factor receptor (PDGFRB; phycoerythrin anti-PDGFRB) at 1:5 dilution to label eSFs. eMSCs were sorted using double labeling for CD146 and PDGFRB antibodies, respectively, both at 1:5 dilutions. The cell suspension was sorted using a FACS Aria II with FACS Diva software (BD Biosciences). The FACS-sorted cell pellets were stored at −80°C until RNA extraction.
RNA and cDNA preparation for microarray analysis and quantitative real-time PCR (Q-RT-PCR)
Total RNA was isolated form FACS-sorted cell populations and purified using the Arcturus PicoPure RNA isolation kit (Applied Biosystems, Life Technologies Corporation) following the manufacturer's instructions. An additional deoxyribonuclease treatment was performed using the ribonuclease-free deoxyribonuclease set (Qiagen). Reverse transcription and amplification of isolated RNA into cDNA was performed using NuGEN WT-Ovation Exon FFPE System V2 (NuGen). The integrity of resultant cDNA was assessed using the Agilent 2100 bioanalyzer (Agilent Technologies), and individual samples meeting yield and quality standards were further processed and hybridized to Affymetrix Human Gene 1.0 ST arrays (Affymetrix), probing 21 014 genes. Arrays were scanned according to the protocol described in the wild-type sense target labeling assay manual from Affymetrix (version 4; FS450_0007).
Validation of microarray data by fluidigm-based Q-RT-PCR
Eighty-one genes were chosen from the differentially expressed genes (≥2.0 fold changes [FCs]) to validate lineage-specific gene profiles and cell type-specific differences between the study groups. A total of 29 cDNA samples from FACS-sorted endometrial cell populations and 6 internal controls were analyzed in duplicate by Q-RT-PCR using the Fluidigm 96.96 dynamic array with integrated fluidic circuits and the BioMark HD system (Fluidigm) as previously described (15). The updated Fluidigm protocol 37, with updated modifications to volume/concentration of reagents and timing of reactions for preamplification and Biomark quantitative PCR, was used for all procedures. Briefly, cDNA was preamplified to generate a pool of target genes in 5-μL reactions using Taq-Man Pre-Amp master mix (Applied Biosystems), 200 ng cDNA, and 500 nM for each primer pair. Samples were then exonuclease treated (Exonuclease I; New England BioLabs). Using previously generated optimal dilution curves, samples were diluted 1:5 in a Tris-EDTA dilution buffer (TEKnova). Q-RT-PCR was performed using SsoFast Evagreen supermix with low ROX binding dye (Biotium Inc) with a final primer concentration of 5 μM. Data were processed by user-detected threshold settings and linear baseline correction using Biomark real-time PCR Analysis Software (version 3.0.4). Melt curves were assessed using the melting temperature threshold.
The comparative cycle threshold (Ct) method was used to obtain relative expression for each grouping comparison, in which the amount of target was normalized to the hypoxanthine phosphoribosyltransferase 1 represented by ΔCt (15). Expression was normalized to an internal calibrator for sorted cells ΔΔCt and total FC calculated by 2−ΔΔCt (ABI. http://hcgs.unh.edu/protocol/realtime/userbulletin2.pdf). If one or more samples in eEPPCOS/Ctrl, eENPCOS/Ctrl, eSFPCOS/Ctrl, or eMSCPCOS/Ctrl groups failed to produce a ΔCt value leaving less than 3 samples for the analysis, the FC calculation was not conducted [not calculated (NC), Tables 2 and 3].
Table 2.
Microfluidic Q-RT-PCR Validation of Differentially Expressed Genes Between Different Endometrial Cell Populations
| Select genes | Microarraya | Q-RT-PCRb | P Valuec |
|---|---|---|---|
| eMSC vs eEP (FC) | |||
| eMSC-EP | |||
| MMP2 | 24.21 | 18.47 | .009 |
| MCAM | 11.41 | 103.93 | <.001 |
| ITGB1 | −6.14 | −72.68 | .028 |
| DEFB4 | −6.39 | −28.30 | .045 |
| MUC1 | −6.40 | −71.06 | .001 |
| MUC16 | −6.96 | −51.37 | .014 |
| LAMC2 | −15.61 | −45.24 | .001 |
| CDH1 | −16.34 | −29.40 | .034 |
| EPCAM | −16.94 | −218.26 | .010 |
| MMP7 | −20.74 | 34.03 | .021 |
| MMP26 | −24.21 | −61.87 | .005 |
| eMSC vs eEN (FC) | |||
| PDGFRB | 18.64 | 285.28 | .005 |
| COL3A1 | 11.79 | NC | NC |
| MMP16 | 7.40 | 81.19 | .028 |
| MCAM | 1.70 | 1.00 | .982 |
| FLT1 | −5.92 | −17.09 | .001 |
| CDH5 | −11.09 | −36.21 | .013 |
| CD34 | −11.11 | −28.65 | .021 |
| VWF | −18.52 | −53.15 | .007 |
| PECAM1 | −19.03 | −52.99 | .006 |
| SELE | −21.44 | −59.51 | .029 |
| EMCN | −22.55 | −60.02 | .001 |
| MMRN1 | −28.11 | 98.98 | .020 |
| eMSC vs eSF (FC) | |||
| RGS5 | 39.86 | 203.99 | .001 |
| ANGPT2 | 25.62 | 99.01 | <.001 |
| SLC38A11 | 17.57 | 189.63 | <.001 |
| CDH6 | 16.53 | 175.16 | .015 |
| MCAM | 10.22 | 177.96 | <.001 |
| JAG1 | 7.54 | 62.89 | <.001 |
| PDGFRB | 6.41 | 9.96 | .007 |
| HEY2 | 4.84 | 34.73 | .034 |
| NOTCH3 | 2.50 | 162.62 | .045 |
| NOTCH1 | 2.31 | 94.15 | .053 |
| PDGFRA | −9.46 | −25.18 | .033 |
Abbreviations: EP, endometrial epithelium; EN, endometrial endothelium/perivascular cells.
Differentially expressed genes (ANOVA; P < .05); cutoff for fold change set as 2.0.
Comparative Ct method, FC.
Independent-samples t test (PASW) comparing the fold change between different cell types in quantitative PCR (P < .05, bolded).
Table 3.
Microfluidic Q-RT-PCR Validation of Select Differentially Expressed Genes Between Overweight/Obese Women With PCOS and Overweight/Obese Controls
| Gene | Microarraya | Q-RT-PCRb | P Valuec |
|---|---|---|---|
| eEP | |||
| LGR5 | 3.87 | 1.80 | .427 |
| ORM1 | 3.15 | NC | NC |
| ORM2 | 2.88 | 5.75 | .384 |
| LICAM | 2.64 | NC | NC |
| SEMA3E | 2.48 | 1.33 | .765 |
| UBD | 2.38 | 4.34 | .436 |
| IL6 | 2.35 | 6.64 | .182d |
| SERPINE2 | 2.25 | 1.63 | .504 |
| CCL2 | 2.14 | NC | NC |
| TNFAIP6 | 2.01 | NC | NC |
| SPP1 | −2.28 | NC | NC |
| TGFB1 | −2.48 | NC | NC |
| CLDN4 | −2.81 | −11.30 | .042 |
| IGF1 | −3.24 | −86.99 | .026 |
| OLFM4 | −3.46 | −5.49 | .263 |
| ENDRA | −4.37 | −269.42 | .083 |
| DCN | −5.57 | −163.30 | .057 |
| SPARCL1 | −5.10 | −19.68 | .021 |
| SPARC | −6.73 | −108.15 | .028 |
| SFRP4 | −9.53 | −33.18 | .028 |
| eEN | |||
| MAL2 | 3.14 | NC | NC |
| TTN | 2.29 | 18.58 | .068 |
| APOD | 2.16 | 4.03 | .003 |
| TGFB2 | 2.16 | 1.99 | .386 |
| INSR | −2.02 | −1.58 | .360 |
| IL6 | −2.05 | NC | NC |
| RHEB | −2.06 | −1.28 | .791 |
| AKTIP | −2.15 | −11.80 | .140 |
| SLC16A6 | −4.65 | NC | NC |
| eSF | |||
| LRP2 | 3.62 | 1.65 | .456 |
| CCL2 | 3.31 | 9.63 | .008 |
| ICAM 1 | 2.87 | NC | NC |
| TNFAIP3 | 2.51 | 9.65 | .003 |
| RHOJ | 2.49 | 18.79 | .003 |
| VLDLR | 2.43 | 1.38 | .269 |
| C4A/B | 2.20 | 34.41 | .002 |
| HHIP | −2.21 | −3.97 | .016 |
| RSPO3 | −2.35 | −3.21 | .174 |
| ADAMTS5 | −2.56 | −3.16 | .102 |
| ANLN | −2.69 | −3.09 | .089 |
| KIF23 | −3.15 | −4.44 | .038 |
| FGF7 | −3.74 | NC | NC |
| eMSC | |||
| SPRR3 | 6.65 | NC | NC |
| LCN2 | 5.06 | 100.14 | .020 |
| CEACAM7 | 3.62 | NC | NC |
| MAL | 2.26 | NC | NC |
| ICAM1 | 2.00 | NC | NC |
| IL8 | 2.24 | 3.98 | .039 |
| SMAD2 | −2.31 | −1.45 | .078 |
| OR51E2 | −2.37 | −7.71 | .055 |
| SERPINI1 | −2.48 | −1.44 | .639 |
| TAGLN | −2.65 | −2.67 | .306 |
| ACTG2 | −3.12 | −4.56 | .085 |
Differentially expressed genes (ANOVA; P < .05); cutoff for FC set as 2.0.
Comparative Ct method.
Independent-samples t test (PASW), comparing the FC in each cell type between the study groups in quantitative PCR (P < .05, bolded).
After excluding an outlier (FC = 76.72, P = .044).
Immunohistochemistry
Chemokine (C-C motif) ligand 2 (CCL2) and IL-6 proteins were localized in paraffin-embedded human endometrial tissue sections using the IMPRESS Universal Polymer detection kit (Vector Laboratories) and rabbit polyclonal antibodies for CCL2 (ab9669; Abcam) at 1:100 and IL-6 (ab6672; Abcam) at 1:600. Normal rabbit IgG was used as a negative control. Sections were deparaffinized in xylene, rehydrated through graded ethanols, and washed with PBS. Antigen retrieval was performed at 100°C in trisodium citrate (10 mM, pH 6) for 20 minutes followed by quenching endogenous peroxidase (3% H2O2 in methanol) for 10 minutes and blocking at 22°C for 30 minutes in Ready-to-Use (R.T.U.) horse serum (Vectastain universal quick kit, R.T.U.; Vector Laboratories). Primary antibodies were incubated at 4°C overnight and secondary antibody (ImmPRESS universal antibody polymer detection kit; Vector Laboratories) was added for 30 minutes at 22°C. The immunoreactive antigen was visualized using 3,3′-diaminobenzidine (DAB peroxidase substrate kit; Vector Laboratories) and counterstained with hematoxylin blue (Vector Laboratories). Slides were viewed on a Leica DM 5000 microscope equipped with a Leica 350DX camera (Leica Microsystems, Inc), and the staining in each section was evaluated independently by several observers. Additional protein validation was limited by tissue availability.
To quantify and statistically analyze the intensity of immunoreactive cells, a modified version of the H-score system, the Quick Score, was used (18). Here the intensity and the proportion of nuclear and cytoplasmic brown staining (for CCL2 and IL-6) throughout each image were termed category A and were assigned scores from 1 to 6 (1, 0%–4%; 2, 5%–19%; 3, 20%–39%; 4, 40%–59%; 5, 60%–79%; 6, 80%–100%). The whole image was scanned at ×200 magnification to gauge the general level of intensity throughout. The average intensity, corresponding to each of the categories, labeled negative, weak, intermediate, and strong staining, was given a score from 0 to 3, respectively, and termed category B. A × B was used as a multiplicative Quick Score, and thus, the maximum score achievable is 18. Blinded scorers (n = 4) were instructed to judge images taken for each sample (control vs PCOS), and these scores were subsequently used for statistical analysis.
Statistics
Microarray data analysis was performed using GeneSpring as previously described (15). Briefly, the intensity values of the probe sets in the GeneChip operating software (Affymetrix) were imported into GeneSpring version 11.02 software (Agilent Technologies) normalized and log2 transformed using the robust multiarray analysis as the background correction algorithm for ST array technology. Pairwise comparisons of differentially expressed genes (P < .05, ≥ 2.0 FC) between different cell types or individual cell types between different study populations were performed using ANOVA with Tukey post hoc analysis with Benjamini-Hochberg multiple-testing correction for false discovery rate. Unbiased principal component analysis (PCA) algorithm was applied to all samples, using all 21 014 genes on the chip to identify similar expression patterns and visualize underlying cluster structures in 3-dimensional space. Hierarchical clustering (HC) analysis was conducted using differentially expressed genes with 2.0-fold or greater change difference from all samples and among all experimental conditions. The clustering algorithm used Euclidean distance measure with centroid linkage rule to identify samples with similar patterns of gene expression. Where Genespring was not the primary statistical analysis program (age and BMI comparisons between the study groups, FC differences in ΔCt, Quick Score), an independent-samples t test (with logarithmic transformation to ensure normal distribution of variables as needed) and a nonparametric test were used to determine statistical significance, and the analyses were conducted using PASW Statistics 18 software (IBM).
Results
Principal component analysis and hierarchical clustering of FACS-isolated endometrial cell populations
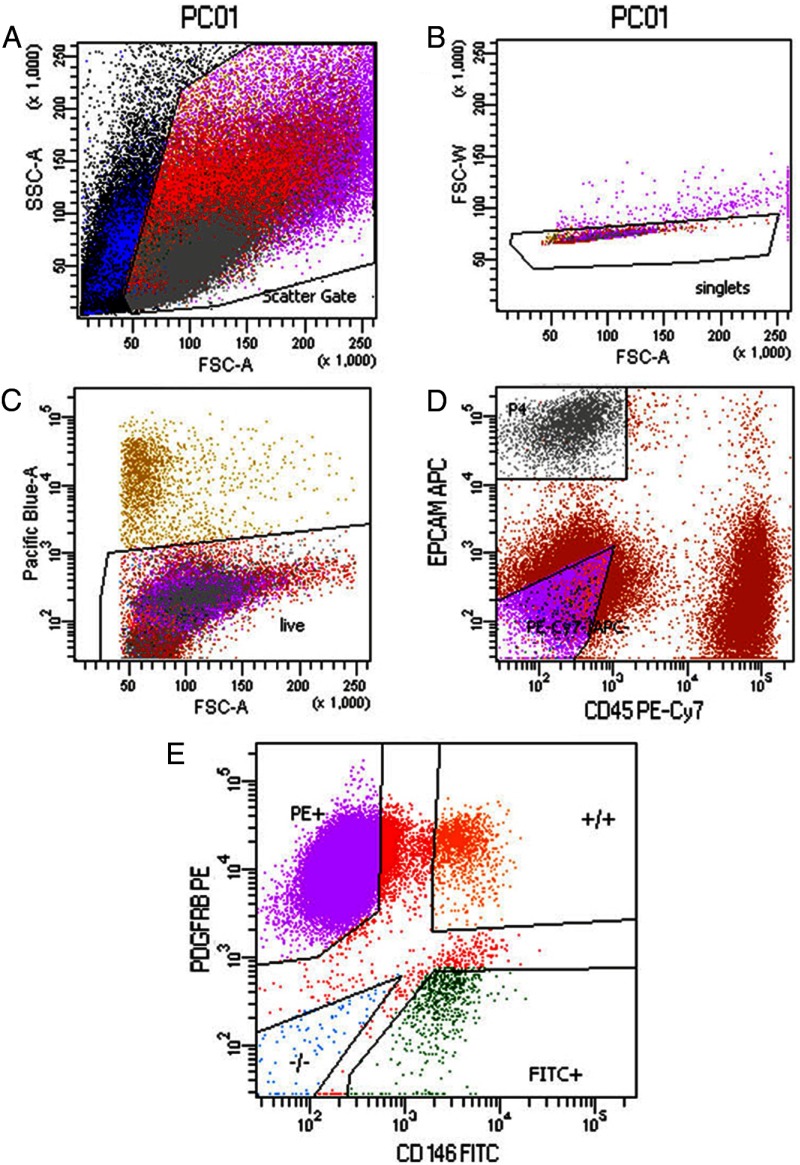
Five different cell populations were identified using FACS according to fluorochrome antibody labeling: CD45+ (leukocytes), EPCAM+ (eEPs), CD146+/PDGFRB− (eENs), CD146−/PDGFRB+ (eSFs), and CD146+/PDGFRB+ (eMSCs) (Figure 1). CD45+ cell populations were sorted out and not collected for the present study. Analysis of microarray data from the four isolated cell types showed that they clustered separately in 3-dimensional space by PCA primarily according to cell type (Figure 2A). Cells derived from the hysterectomy specimen clustered by cell type and did not cluster together by PCA or hierarchical clustering, although gene expression can be altered by, for example, uterine fibroids (19) or endometrial polyps (20). Furthermore, the control samples clustered tightly together (Figure 2A), suggesting they have similar gene expression.
Figure 1.
Isolation of the different cell types by FACS from human endometrium. A–C, FACS analysis of live single-cell fraction. D, EPCAM+ epithelial cells (eEPa) and CD45+ lymphocytes isolated from the cell population. E, Cells sorted into 3 populations according to the label for MCAM (CD146, eENs), PDGFRB (eSFs), or MCAM (CD146) and PDGFRB (eMSCs). SSC, side scatter; FSC, forward scatter; APC, allophycocyanin, FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 2.
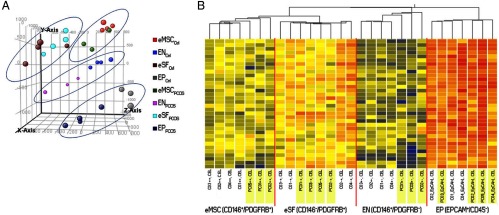
A, Endometrial cell populations of overweight/obese women with PCOS and overweight/obese controls clustered in PCA by cell type and disease. B, Hierarchical clustering analysis of the differentially expressed genes (≥2 FC, P < .05) between eMSCs, eSFs, eENs, and eEPs in overweight/obese women with PCOS (yellow) and overweight/obese controls (white).
HC showed a fairly close initial tree-way branching segregating epithelial (eEP), endothelial (eEN), and the other mesenchymal (eSF, eMSC) cell types, and a second branching separating the two nonendothelial (eSF, eMSC) mesenchymal cell types (Figure 2B). Within cell type, clustering showed clear segregation of control vs PCOS samples for eENs and eMSCs but not within eEPs and eSFs (Figure 2B).
Differential expression analysis of FACS-isolated endometrial cell populations
The transcriptomes of isolated cells types were analyzed by comparing the gene expression of the mesenchymal stem cell population with the individual profiles of other cell types (Supplemental Tables 1, A–C, Journals Online web site at http://jcem.endojournals.org). Some of these differentially expressed genes were chosen for further validation with microfluidic Q-RT-PCR (Table 2).
The endometrial epithelial cells highly expressed genes characteristic for the epithelial lineage, including epithelial specific integrins, matrix metalloproteinases, defensins, tight junction proteins, mucinsm and lamnins compared with the eMSC population (Supplemental Table 1A). Comparison of eMSCs vs eENs and vs eSFs revealed the eEN and eSF populations expressing genes related to endothelial and fibroblast functions, respectively (Supplemental Tables 1, B and C). The eMSC population clustered close to the eSF population in PCA and HC (Figure 2) and had a gene expression profile characteristic of adult endometrial mesenchymal stem/progenitor cells (Supplemental Table 1C) (15). Cell type-specific gene expression was validated by Q-RT-PCR (Table 2).
Endometrial cell type-specific differential gene expression between PCOS women and controls
No statistical differences among participants were observed in age (P = .734) or BMI (P = .054) between the 2 study groups (Table 1). To assess cell type-specific differences in gene expression of each cell type between women with PCOS and controls, 4 major comparisons were performed: epithelial (eEPPCOS vs eEPCtrl), endoethelial (eENPCOS vs eENCtrl), fibroblast (eSFPCOS vs eSFCtrl,), and mesenchymal stem cell(eMSCPCOS vs eMSCCtrl). The corresponding lists of differentially expressed genes (P < .05, ≥ 2.0 FC, Supplemental Tables 2, A–D) comprised 216 eEP, 168 eEN, 113 eSF, and 69 eMSC differentially expressed genes in PCOS vs controls. Select differentially expressed genes were also analyzed by microfluidic Q-RT-PCR, which largely validated the microarray approach (Table 3).
The comparison eEPPCOS vs eEPCtrl revealed increased expression of inflammation-related genes, eg, CCL2, IL-6, orosomucoid 1 (ORM1), TNF, and α-induced protein 6 (TNFAIP6). In addition, several genes implicated in oncogenesis showed increased expression: cell adhesion molecule with homology to L1CAM (CHL1) or decreased expression: claudin 4 (CLDN4), secreted protein, acidic secreted frizzled-related protein 4 (SFRP4), and secreted protein acidic and rich in cysteine (SPARC, osteonectin). Interestingly, the expression of insulin growth factor 1 (IGF-1) was decreased in PCOS endometrium compared with controls. Of note in eENPCOS vs eENCtrl, there was up-regulation of apolipoprotein D (APOD). Similar to eEPPCOS, eSFPCOS showed increased expression of inflammatory genes vs eSFCtrl, eg, complement component 4A and 4B (C4A/B), CCL2, intercellular adhesion molecule 1 (ICAM1), and TNAIFP3, whereas the expression of genes related to cell growth and proliferation [fibroblast growth factor 7 (FGF7); Hedgehog-interacting protein (HHIP); kinesin-like protein (KIF23)] were decreased. Among the most highly differentially expressed genes between eMSCPCOS and eMSCCtrl were genes related to inflammatory processes (IL-8 and ICAM1) and cancer [proline rich protein 3 (SPRR3) and lipocalin 2 (LCN2)].
Immunohistochemistry for IL-6 and CCL2
Consistent with the microarray and Q-RT-PCR data, proliferative-phase endometrium sections from overweight/obese women with PCOS showed significantly (P < .05) increased immunostaining for CCL2 in glandular epithelium (GE) and luminal epithelium (LE) in the Quick Score system, compared with control epithelium (Figure 3A). In addition, the Quick Score for CCL2 expression in the stroma (ST) of PCOS endometrium was significantly increased (P < .05), compared with the ST of control endometrium (Figure 3A). For IL-6, the immunostaining for PCOS LE and GE was significantly increased (Quick Score, P < .05) compared with control epithelium (Figure 3B).
Figure 3.
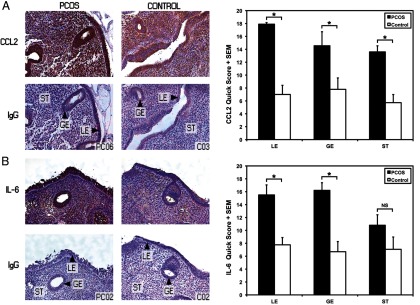
Immunohistochemical localization of CCL2 (monocyte chemoattractant protein-1) and IL-6 protein in human endometrium. Representative paraffin-embedded endometrial sections in anovulatory overweight/obese women with PCOS (n = 4) and overweight/obese controls (n = 4) are shown. A, CCL2 was highly expressed in LE and GE PCOS epithelium and ST compared with the control tissue. The Quick Score showed increased immunostaining for LE, GE, and ST in PCOS endometrium (*, P < .05). B, IL-6 showed increased expression in LE and GE in the PCOS endometrium compared with control (Quick Score; LE and GE, P < .05) (nonimmune rabbit IgG, negative control; ×200 magnification).
Discussion
This unique study investigating FACS-isolated endometrial cell populations in women with PCOS revealed an endometrial disease phenotype in PCOS involving aberrant expression of inflammation and cancer-related genes. We postulate that this phenotype is promoted by oligo/anovulation and possibly hyperandrogenism and metabolic disturbances in PCOS, beyond contributions of solely the obese state. Furthermore, the present findings give insight into endometrial function and pathologies of subfertility, poor pregnancy outcomes, and endometrial cancer observed in PCOS women (9, 11, 13).
Up-regulation of proinflammatory genes in PCOS endometrium and reproductive function
Cytokines and infiltrating leukocytes participate in endometrial cyclic changes, modulating endometrial structural and functional components required for embryo attachment (21) CCL2 (also known as monocyte chemoattractant protein-1, MCP-1), a potent chemoattractant/chemokine, which induces monocytes to leave the bloodstream and enter tissues to become resident macrophages during tissue repair (22), was increased in eEPPCOS and eSFPCOS in anovulatory PCOS women, compared with controls. CCL2 protein increases in cycling endometrial eEP during the window of implantation (WOI), suggesting a role in embryo attachment (23, 24). The observed increase in CCL2 in eEPPCOS in the absence of P4 suggests an aberrancy, which could theoretically lead to enhanced macrophage influx in the endometrium of women with PCOS and warrants further investigation (25). The data regarding eSF CCL2 expression is interesting because some studies report decreased/absent secretion of CCL2 during WOI and in vitro E2 and P4 administration decreases eSF CCL2 secretion (23, 26). The increased expression of CCL2 could result in negative consequences for the endometrium in women with PCOS, especially if the altered secretion pattern prevails during the secretory phase.
IL-6, increased in eEPPCOS, is a multifunctional cytokine with a wide range of biological activities inducing cytokine production and recruitment of macrophages and megakaryocytes and cell growth (27). Similar to CCL2, IL-6 is secreted by endometrial epithelium and is also expressed in endometrial stromal cells (28, 29). IL-6 is present during the WOI and has an important role coordinating placental morphogenesis and trophoblast invasion (29, 30). IL-6-deficient mice have reduced fertility and fewer implantation sites, whereas, in vitro IL-6 exposure decreases embryo attachment and growth (29, 31). Women experiencing recurrent miscarriage have decreased endometrial IL-6 production, whereas elevated IL-6 levels in plasma and cervical mucus are associated with unexplained infertility (29, 32, 33). PCOS endometrium demonstrates progesterone resistance and impaired decidualization (3) by unclear mechanisms, and the role of IL-6 in this abnormality awaits further clarification. Although we are not aware of previous studies on IL-6 expression in PCOS endometrium, other experimental approaches suggest an aberrant immune response in PCOS endometrium in the secretory phase (3, 4). That inflammation is pronounced in overweight/obese PCOS (proliferative phase) endometrium compared with overweight/obese controls suggests it is independent of high BMI. An increased or otherwise altered inflammatory network as part of the endometrial disease phenotype could result in compromised endometrial cellular differentiation and endometrial receptivity, impaired embryonic implantation, and subsequent poor pregnancy outcomes in women with PCOS.
Proinflammatory changes may promote cancer in PCOS endometrium
In addition to normal reproductive processes, cytokines and a proinflammatory milieu are involved in tumorigenesis, including in the endometrium (12). Up-regulated inflammatory genes in eEPPCOS and/or eSFPCOS may contribute to the increased risk of endometrial cancer in women with PCOS. CCL2 increases the expression of survivin, an apoptosis inhibitor, and stimulates tumor cell proliferation, migration, and invasiveness by activating the phosphatidylinositol 3-kinase/Akt and MAPK/ERK1/2 signaling pathways that are also activated in endometrial cancer (34, 35). IL-6 has also been linked to several cancers, including endometrial adenocarcinoma, by promoting a proinflammatory environment, metastasis, and growth, presumably through signal transducer and activator of transcription-3 (STAT3) activation (36, 37). Metformin and statin treatments decrease the inflammatory response in endometrial stromal cells in vitro (38, 39). The fact that 2 PCOS women were on metformin [samples used in microarray and in immunohistochemistry (IHC)] and 1 control woman was using simvastatin (sample used in IHC) could have blunted some of the differences in inflammatory gene expression between the study groups.
Prooncogenic gene expression of PCOS endometrial cells
In addition to normal endometrial function, increased WNT/β-catenin signaling is involved in endometrial hyperplasia and cancer (40). Down-regulation of SFRP4 in PCOS endometrium indicates abnormal proliferative phase development in PCOS epithelium (41). Because SFRP4 serves as WNT signaling antagonist, EPPCOS compared with EPCtrl is of note because SFRP4 inhibits endometrial cancer cell proliferation through inhibition of WNT7A signaling in vitro (42). Furthermore, SFRP4 expression correlates with poor outcome in ovarian cancer, supporting its role as a tumor suppressor gene (43). SPARC, which has tumor suppressor properties, was also down-regulated in eEPPCOS vs eEPCtrl. SPARC functions as a key regulator of matrix proteinase-associated tissue remodeling; however, its function in endometrial epithelium is not well understood (44). Previous studies have implicated its involvement with TGFB signaling both restricting growth and proliferation and also presenting with immunomodulatory properties, attenuating the mitogenic and proinvasive effects of CCL2 in ovarian cancer cells (45). Interestingly, CLD4 and CHL1, which are aberrantly expressed in several cancers, including endometrial cancer, were decreased in eEPPCOS (46, 47). The fact that genes regulating cellular growth and proliferation (IGF1, FGF7, HHIP, KIF23) were down-regulated in EPPCOS and eSFPCOS compared with controls suggests that the changes in PCOS endometrium may occur prior to or even without excessive proliferation. Altogether the data support that a proinflammatory and procancerous milieu promotes an endometrial disease phenotype in PCOS, which likely contributes to the predisposition to endometrial cancer in PCOS women in the long term.
Altered genotype in eMSCs of the PCOS endometrium
Mesenchymal stem cells participate in wound healing relevant to endometrial regeneration responding to changes in their microenvironment (48). Herein we found 69 differentially expressed genes in eMSCPCOS vs eMSCCtrl with up-regulation of the proinflammatory chemokine IL-8 and adhesion molecule ICAM1. Mesenchymal stem cells secrete IL-8, which has a paracrine function in the stem cell niche (49) and is linked to an abnormal microenvironment related to several malignancies (50). In PCOS endometrium IL-8 may promote a proinflammatory milieu with ICAM1 and an abnormal footprint in downstream progeny, having a potential effect on lineage cell development and differentiation, resulting in altered endometrial function. SPRR3, whose function is not well defined, and oncogene LCN2, which is a potent inflammatory mediator (51), were the most up-regulated genes in eMSCPCOS vs. eMSCctrl. Increased expression of both genes has been observed in several malignancies including high LCN2 expression in endometrial cancer (52).
Given that the transcriptome of eMSC was altered in PCOS endometrium together with other cell types suggests that the endocrine and metabolic PCOS environment may contribute to differences in gene expression between the study groups. Moreover, several studies have shown that proper maintenance and regulation of the stem cell microenvironment is crucial to sustain normal stem cell development (53). It should be noted that the eMSCctrl were from cycling endometrium, whereas eMSCPCOS were from anovulatory women. It is unknown at this time whether eMSCs differ in the cycling vs noncycling endometrium, although a limited study revealed no menstrual cycle phase dependence of the eMSC transcriptome (15), and turnover of eMSC in quiescent endometrium is not known. Whether the eMSC transcriptome found herein is due to the anovulatory state or to metabolic disturbances or hyperandrogenemia in PCOS remains to be determined. Altogether the altered eMSCPCOS genotype is a novel finding and may give new insights into endometrial abnormalities related to PCOS.
Summary and conclusions
The pronounced proinflammatory cytokine expression in PCOS endometrium was one of the most striking findings herein because the different endometrial cell populations presented with increased expression of several cytokine and immune response-related genes relevant to subfertility, endometrial receptivity, miscarriage, and endometrial cancer. Changes in several cancer-related genes were observed in eEPPCOS, consistent with the increase risk of endometrial carcinoma in PCOS and interestingly also in eMSCPCOS, suggesting a possible role of this cell population in endometrial carcinogenesis in PCOS. Furthermore, that different endometrial cell types, including eMSCs, presented with altered inflammation and cancer-related gene expression independent of BMI supports the hypothesis of an endometrial disease phenotype related to PCOS. These findings underscore the importance of assessing cell-specific changes within the endometrium and pave the way for future studies with a larger sample size and in vitro experiments to validate and capitalize on the current observations to elucidate the impact of persistent abnormal endocrine, metabolic, and inflammatory environments on stem cell populations in tissues more broadly as well as on endometrial health and function in women with PCOS.
Supplementary Material
Acknowledgments
We acknowledge John Tamaresis, PhD, and Risto Bloigu, PhD, for statistical advice; Amy Hamilton and Florence Ng for technical support with the Fluidigm analysis and the Biomark system; Ny Jieng for assistance with sample collection: Kim Chi Vo and Brittni Johnson for tissue collection and processing, and the University of California, San Francisco National Institutes of Health Human Endometrial Tissue and DNA Bank for tissue acquisition. We also acknowledge Tara Rombaldo for technical support with FACS analysis and Linda Ta and Yanxia Hao (Genomics Core at the Gladstone Institute) for their technical skills regarding microarray processing.
This work was supported by the Sigrid Juselius Foundation, the Academy of Finland, the Finnish Medical Foundation, the Orion-Farmos Research Foundation, the Maud Kuistila Foundation (to T.T.P.), the Ruth L. Kirschstein National Research Service Award 1F32HD074423-01 (to J.C.C.), and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54HD 055764-06 Specialized Cooperative Centers Program in Reproduction and Infertility Research (to LCG).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CCL2
- chemokine (C-C motif) ligand 2
- CD
- cluster of differentiation
- Ct
- cycle threshold
- E2
- estradiol
- eEN
- endometrial endothelium
- eEP
- endometrial epithelium
- eMSC
- endometrial mesenchymal stem cell
- EPCAM
- epithelial cell adhesion molecule
- eSF
- endometrial stromal fibroblast
- FACS
- fluorescence-activated cell sorting
- FC
- fold change
- GE
- glandular epithelium
- HC
- hierarchical clustering
- IHC
- immunohistochemistry
- LE
- luminal epithelium
- MCAM
- melanoma cell adhesion molecule
- NC
- not calculated
- P4
- progesterone
- PCA
- principal component analysis
- PCOS
- polycystic ovary syndrome
- PDGFRB
- β-type platelet-derived growth factor receptor
- Q-RT-PCR
- quantitative real-time PCR
- ST
- stroma
- UCSF
- University of California, San Francisco
- WOI
- window of implantation.
References
- 1. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25 [DOI] [PubMed] [Google Scholar]
- 2. Villavicencio A, Bacallao K, Avellaira C, Gabler F, Fuentes A, Vega M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol Oncol. 2006;103(1):307–314 [DOI] [PubMed] [Google Scholar]
- 3. Savaris RF, Groll JM, Young SL, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96(6):1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matteo M, Serviddio G, Massenzio F, et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil Steril. 2010;94(6):2222–7, 2227.e1–3 [DOI] [PubMed] [Google Scholar]
- 5. Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66(2):297–304 [DOI] [PubMed] [Google Scholar]
- 6. Sagle M, Bishop K, Ridley N, Alexander FM, et al. Recurrent early miscarriage and polycystic ovaries. BMJ. 1988;297(6655):1027–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tulppala M, Stenman UH, Cacciatore B, Ylikorkala O. Polycystic ovaries and levels of gonadotrophins and androgens in recurrent miscarriage: prospective study in 50 women. Br J Obstet Gynaecol. 1993;100(4):348–352 [DOI] [PubMed] [Google Scholar]
- 8. Koivunen R, Pouta A, Franks S, et al. Northern Finland Birth Cohort 1966 Study. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 study. Hum Reprod. 2008;23(9):2134–2139 [DOI] [PubMed] [Google Scholar]
- 9. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673–683 [DOI] [PubMed] [Google Scholar]
- 10. Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb). 2000;3(2):101–105 [DOI] [PubMed] [Google Scholar]
- 11. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. 2010. 21(12):2303–2308 [DOI] [PubMed] [Google Scholar]
- 12. Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2840–2847 [DOI] [PubMed] [Google Scholar]
- 13. Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27(5):1327–1331 [DOI] [PubMed] [Google Scholar]
- 14. Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–2911 [DOI] [PubMed] [Google Scholar]
- 15. Spitzer TL, Rojas A, Zelenko Z, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86(2):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9(1):11–21 [DOI] [PubMed] [Google Scholar]
- 17. Zawadski J. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, ed. Polycystic Ovary Syndrome. Boston: Blackwell Scientific: 1992:377–384 [Google Scholar]
- 18. Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity. Fertil Steril. 2011;95(8):2690–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11(6):613–630 [DOI] [PubMed] [Google Scholar]
- 22. Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14(3):149–154 [DOI] [PubMed] [Google Scholar]
- 23. Caballero-Campo P, Dominguez F, Coloma J, et al. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol Hum Reprod. 2002;8(4):375–384 [DOI] [PubMed] [Google Scholar]
- 24. Ulukus M, Ulukus EC, Tavmergen Goker EN, Tavmergen E, Zheng W, Arici A. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil Steril. 2009;91(3):687–693 [DOI] [PubMed] [Google Scholar]
- 25. Meter RA, Wira CR, Fahey JV. Secretion of monocyte chemotactic protein-1 by human uterine epithelium directs monocyte migration in culture. Fertil Steril. 2005;84(1):191–201 [DOI] [PubMed] [Google Scholar]
- 26. Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by estrogen and progesterone. Biol Reprod. 1999;61(1):85–90 [DOI] [PubMed] [Google Scholar]
- 27. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(suppl 3):S233–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim KJ, Odukoya OA, Ajjan RA, Li TC, Weetman AP, Cooke ID. Profile of cytokine mRNA expression in peri-implantation human endometrium. Mol Hum Reprod. 1998;4(1):77–81 [DOI] [PubMed] [Google Scholar]
- 29. Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95(1–2):1–14 [DOI] [PubMed] [Google Scholar]
- 30. Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30(4):320–328 [DOI] [PubMed] [Google Scholar]
- 31. Jacobs AL, Sehgal PB, Julian J, Carson DD. Secretion and hormonal regulation of interleukin-6 production by mouse uterine stromal and polarized epithelial cells cultured in vitro. Endocrinology. 1992;131(3):1037–1046 [DOI] [PubMed] [Google Scholar]
- 32. Naz RK, Butler A. Interleukins-6 and -8 levels in sera and cervical mucus of fertile, idiopathic infertile, and immunoinfertile women: implication in infertility. Am J Reprod Immunol. 1996;35(6):534–540 [DOI] [PubMed] [Google Scholar]
- 33. Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Reprod Immunol. 2009;62(4):261–267 [DOI] [PubMed] [Google Scholar]
- 34. Roca H, Varsos ZS, Mizutani K, Pienta KJ. CCL2, survivin and autophagy: new links with implications in human cancer. Autophagy. 2008;4(7):969–971 [DOI] [PubMed] [Google Scholar]
- 35. Li MQ, Li HP, Meng YH, et al. Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating akt and MAPK/ERK1/2 signal pathway. Fertil Steril. 2012;97(4):919–929 [DOI] [PubMed] [Google Scholar]
- 36. Dossus L, Rinaldi S, Becker S, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17(4):1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril. 2010;94(5):1639–46.e1 [DOI] [PubMed] [Google Scholar]
- 39. Takemura Y, Osuga Y, Yoshino O, et al. Metformin suppresses interleukin (IL)-1β-induced IL-8 production, aromatase activation, and proliferation of endometriotic stromal cells. J Clin Endocrinol Metab. 2007;92(8):3213–3218 [DOI] [PubMed] [Google Scholar]
- 40. Liao X, Siu MK, Au CW, et al. Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through β-catenin. Mod Pathol. 2009;22(6):839–847 [DOI] [PubMed] [Google Scholar]
- 41. Petracco RG, Kong A, Grechukhina O, Krikun G, Taylor HS. Global gene expression profiling of proliferative phase endometrium reveals distinct functional subdivisions. Reprod Sci. 2012;19(10):1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–1028 [DOI] [PubMed] [Google Scholar]
- 43. Jacob F, Ukegjini K, Nixdorf S, et al. Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients. PLoS One. 2012;7(2):e31885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagaraju GP, Sharma D. Anti-cancer role of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev. 2011;37(7):559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Said NA, Elmarakby AA, Imig JD, Fulton DJ, Motamed K. SPARC ameliorates ovarian cancer-associated inflammation. Neoplasia. 2008;10(10):1092–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Senchenko VN, Krasnov GS, Dmitriev AA, et al. Differential expression of CHL1 gene during development of major human cancers. PLoS One. 2011;6(3):e15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pan XY, Wang B, Che YC, Weng ZP, Dai HY, Peng W. Expression of claudin-3 and claudin-4 in normal, hyperplastic, and malignant endometrial tissue. Int J Gynecol Cancer. 2007;17(1):233–241 [DOI] [PubMed] [Google Scholar]
- 48. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396 [DOI] [PubMed] [Google Scholar]
- 49. Barbet R, Peiffer I, Hatzfeld A, Charbord P, Hatzfeld JA. Comparison of gene expression in human embryonic stem cells, hESC-derived mesenchymal stem cells and human mesenchymal stem cells. Stem Cells Int. 2011;2011:368192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hwang WL, Yang MH, Tsai ML, et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141(1):279–91, 291.e1–5 [DOI] [PubMed] [Google Scholar]
- 51. Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol. 2003;171(12):6630–6639 [DOI] [PubMed] [Google Scholar]
- 52. Mannelqvist M, Stefansson IM, Wik E, et al. Lipocalin 2 expression is associated with aggressive features of endometrial cancer. BMC Cancer. 2012;12(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche—there goes the neighborhood? Int J Cancer. 2011;129(10):2315–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.