Abstract
Context:
Although cognitive complaints are common among menopausal women, it is debatable whether there is an objective decline in cognition with menopause that exceeds what is expected with normal aging.
Objective:
The objective of the study was to determine whether reproductive senescence is associated with an age-independent decline in verbal memory.
Design and Setting:
The study was a 14-year, longitudinal, population-based cohort study of women who underwent yearly endocrine, behavioral, and cognitive assessments from pre- to postmenopause.
Participants:
Caucasian and African American premenopausal women (n = 403), who were enrolled in the Penn Ovarian Aging Study, participated in the study.
Main Outcome Measures:
Buschke Selective Reminding Test (immediate and delayed verbal recall), the digit symbol substitution task, and the symbol copy task were used to measure outcomes.
Results:
A total of 3958 assessments were conducted in this sample of 403 women. In models that were adjusted for age and important cofactors, immediate (P = .03) and delayed (P = .03) recall on the Buschke Selective Reminding Test declined from the pre- to postmenopausal stages. Further evaluation identified a significant decline (P < .002) in delayed recall early in the transition and immediate recall (P = .04) late in the transition. Race was a significant factor in performance on all tasks (all P < .0001) except the delayed verbal recall task (P = .06) in adjusted models. Endocrine measures were significantly associated with cognitive performance in unadjusted models.
Conclusions:
Certain cognitive domains are sensitive to the physiological changes of reproductive senescence independent of age. The differences in cognitive performance between African American and Caucasian women were not explained by factors examined in this study but are of important public health concern that warrants further investigation.
Whether the subjective decline in memory reported by many menopausal women can be confirmed objectively is a topic of debate (1–4). Recent cross-sectional data provide preliminary support for an age-independent effect of menopausal status on certain domains of cognition (5, 6), whereas longitudinal studies suggest that the transition to menopause dampens the learning effect typically observed with repeated testing (7). Estradiol has potent effects on brain chemistry and function underlying cognitive processes (1, 8, 9), suggesting that declines in ovarian hormone production may contribute to worsening memory above that attributed to aging alone. Referable to this point, greater endogenous estrogen exposure is associated with better cognitive performance, longer telomere length, and reduced telomerase activity among older women (10, 11).
There is growing evidence that use of estrogen and hormone therapy early in the menopause transition accentuates the potential benefits and minimizes the risks with respect to multiple estrogen-responsive tissues (12–14). Although this proverbial window of opportunity for estrogen benefit with respect to cognition, and particularly verbal memory, is supported by several studies (15, 16), a recent meta-analysis highlights the relative limitation of published studies to fully address this important clinical question (16), and randomized clinical trials have raised doubts about estrogen's neuroprotective effects (17,18).
In addition to chronological aging and reproductive status, factors that are thought to impact cognitive performance in midlife women include race, sleep quality, presence of affective disturbance, perceived stress, history of sexual abuse, body mass index (BMI), and metabolic status (3, 5, 19). Factors that may modify the effects of estrogen on cognition include subjective stress, higher cortisol and androstenedione levels, and polymorphisms in several neurotransmitter/neurotrophin-related genes (1, 20, 21). Hence, the impact of aging and hypogonadism on cognition is complex and likely to vary between individuals and across cognitive domains. We examined cognitive, endocrine, and behavioral data collected from women who participated in the 14-year Penn Ovarian Aging Study (POAS). We focused on 2 domains of cognition, verbal memory (immediate and delayed) and psychomotor processing, with verbal memory (variably defined and tested) having been previously shown to vary with estrogen status (8, 10, 22) and psychomotor processing speed, and accuracy being typically affected by aging (23, 24).
Materials and Methods
Cohort description
The population-based cohort was identified in 1996–1997 by random digit dialing to households in Philadelphia County as previously described (25) and stratified to enroll equal numbers of African American and Caucasian women (n = 436). Eligibility criteria for enrollment into the cohort included 35–47 years of age, menstrual cycles in the reference range (22–35 days) for the previous 3 months, a uterus, and at least one ovary. Exclusion criteria were current use of psychotropic or hormonal medications, including hormonal contraception and replacement therapies; pregnant or breastfeeding; serious health problems known to compromise ovarian function; and alcohol or other drug abuse within the past year. Only those 403 women in the original POAS cohort who had at least 2 assessments were considered for inclusion. The study was approved by the University of Pennsylvania Institutional Review Board, and written informed consent was obtained from participants.
Assessment periods
Data were collected during 6 assessment periods at approximately 8-month intervals through year 5 and then yearly thereafter with a 2-year gap between period 10 and period 11. Trained research interviewers administered the cognitive battery and a structured questionnaire, measured height and weight to determine BMI, and collected blood samples for hormone assays at each assessment period. Information regarding demographics, menstrual cycle dates, reproductive history, general health status and behaviors (including medications, smoking, alcohol and caffeine consumption, and history of depressive disorders), and common menopausal symptoms was obtained at each assessment.
Study variables
Demographic and health variables
Variables from the POAS study, such as age and education status, were chosen as important factors to examine in relation to cognitive change over the transition (26). BMI was chosen as a proxy for health factors such as hypertension, diabetes, and vascular disease, which are also known to adversely impact cognition (27). Education was defined as a high school diploma or less, some college/training after high school, or a college graduate. Race was examined because African American women enter the early stages of the menopause transition at a younger age than Caucasian women (28), and there are race-specific effects of certain genes involved in estrogen metabolism (29).
Cognitive assessments
Buschke Selective Reminding Test (30).
Subjects are read a list of 16 words and asked to recall as many as possible in a total of 6 trials (immediate recall) and 20 minutes after completion of the sixth trial (delayed recall). Words were substituted from a standard set so that each word list over the course of 5 years was completely different. Word lists were repeated in 5-year cycles. Performance on the Buschke was previously found to vary by age and estrogen status (30).
Digit Symbol Substitution Test (DSST) [Weschler Adult Intelligence Test III (31)].
Participants fill the spaces below rows of numbers with the corresponding symbol that is paired with a given number at the top of the page. Subjects must perform quickly and accurately, having only 90 seconds to complete as much of the 100-item task as possible. The DSST is considered to be a sensitive measure of processing speed, with memory accounting for only 5%–15% of the variance in performance (23).
Symbol copy task [Weschler Adult Intelligence Test III (31)].
Participants copy symbols (200 maximum) as quickly as possible over the course of 90 seconds. Performance on this task is thought to represent the sensorimotor processing speed component of the DSST.
Behavioral assessments
Center for Epidemiologic Studies Depression Scale (CES-D) (32).
The CES-D is a 20-item self-report inventory for assessing current depressive symptoms. Items are rated for their frequency/duration on a scale from 1 (none of the time or less than once a day) to 4 (all of the time or for 5–7 days). The standard CES-D cutoff score of 16 or greater was used to define high depressive symptoms.
Zung Self-Rating Anxiety Scale (Zung) (33).
Using a 4-point Likert scale, the 20-item Zung assesses how frequently a person experiences symptoms representing cognitive, autonomic, and motor and central nervous symptoms of anxiety. The total scores range from 20 to 80, with 20–44 being considered normal, 45–59 mild to moderate, 60–74 marked to severe, and 75–80 signifying extreme anxiety.
Perceived Stress Scale (PSS) (34).
The PSS assesses how often situations in one's life are appraised as stressful. High levels of perceived stress as measured with the PSS have been associated with markers of biological aging (35). Individuals rate on a scale of 0 (never) to 4 (very often) how frequently a situation applied to them over the previous month. The scores range from 0 to 40, with some items being reverse coded.
Hormonal assessment
Blood samples were collected between days 2 and 6 of 2 consecutive menstrual cycles or 1 month apart in nonmenstruating women during each assessment period. The samples were prepared and stored at −80°C. Assays were conducted at the Translational and Clinical Research Center (University of Pennsylvania Medical Center) in batches that included 4 visits per participant to reduce the within-subject variability due to assay conditions. Assays were performed in duplicate for all hormones, and they were repeated if the values differed by more than 15%. The interassay and intraassay coefficients of variation were calculated from the assays conducted for the 6 assessment periods, with all coefficients being less than 5%. Estradiol, FSH, and dehydroepiandrosterone sulfate (DHEAS) levels were measured by RIA using commercial kits (Coat-A-Count; Siemens Health Care Diagnostics, Malvern, Pennsylvania). For the first 10 assessments, inhibin B assays were conducted in the laboratory of Dr Patrick Sluss, PhD (Massachusetts General Hospital, Boston, Massachusetts), using an ELISA with plates coated with a monoclonal antibody specific for the α-subunit for detection. The assay was controlled in triplicate using samples with mean concentrations of 155.3, 316.3, and 919.3 pg/mL, with interassay coefficients of variation of 11.6%, 7.6%, and 9.7%, respectively. The lower limit of detection was 15 pg/mL. Thereafter inhibin B was assayed using the inhibin B ELISA kits (Beckman Coulter, Inc). The intra- and interassay coefficients of variation were 3.5%–4.6% and 6.3%–7.6%, respectively. The lower limit of detection was 7 pg/mL.
Determining menopausal status
Menopausal status was determined from the data on menstrual bleeding, recorded using daily diaries, the number of menstrual periods between assessments, and cycle length. The definitions for the menopausal status groups were based upon the Staging System for Reproductive Aging in Women (36) but added a late premenopause stage (37). At every assessment period, each participant was assigned to one of the following categories based on bleeding patterns: 1) premenopausal: regular menstrual cycles in the 22- to 35-day range; 2) late premenopause: change in cycle length of 7 days or longer in either direction from the participant's own baseline for at least 1 cycle; 3) early transition: change in cycle length of 7 days or longer in either direction from the participant's own baseline for at least 2 cycles or 60 days of amenorrhea; 4) late transition: 3–11 months of amenorrhea; and 5) postmenopausal: 12 months or more of amenorrhea without hysterectomy.
Statistical methods
For each cognitive outcome, a general linear mixed-effects regression model for repeated measures (random intercept) was used to estimate the unadjusted and adjusted associations for each study variable. Results were summarized using regression coefficients (slopes) for continuous variables and mean differences for categorical variables. Menopausal stage was considered as both to assess linear trend. The distribution of each continuous cognitive score was evaluated as well as residual plots to examine modeling assumptions. A natural logarithm transformation of hormone levels was used in all analyses to accommodate modeling assumptions and reduce the influence of skewed data.
For each outcome model, we assumed that the repeated outcomes for each woman were correlated and decreased with age over the 15 years of study participation. All models were adjusted for baseline cognitive task scores, median split low vs high baseline (assessment 1) (see Tables 3 and 4). Both subject level (race and education) and time-varying risk factors (menopausal stage, age, BMI) were considered for inclusion into the model one at a time. Covariates associated with cognition in the unadjusted analysis at P < .20 were included in the model selection process for the multivariable models. Inclusion in final multivariable models was guided by whether each variable remained statistically significant at P ≤ .05 or whether its inclusion modified other significant associations by 15% or greater. All available data for each participant were included in the repeated-measures models. Final multivariable models included baseline cognitive task scores, age, menopausal stage (ordinal), race, education, and BMI. Hormone models are adjusted for cognitive task scores, age, race, education, and BMI. Observations during pregnancy, breastfeeding, hormone use, and current psychotropic use were censored at the times of their occurrence. Hormone values measured after a participant reported hysterectomy, oophorectomy, or cancer were set to missing from reported occurrence onward. The SAS statistical software package, version 9.3 (SAS Institute), was used for all analyses. Statistical tests were 2 tailed with P ≤ .05 considered significant.
Table 3.
Impact of Menopause Stage on Cognitive Task Performance in Adjusted and Unadjusted Models
| Task | Variable | Unadjusted Estimate | Adjusted Estimate | 95% CI | P Value |
|---|---|---|---|---|---|
| BSRT: immediate recall | Menopause stage | −0.31a | −0.081 | −0.153, −0.0008 | .029 |
| Age | −0.10a | −0.083 | −0.104, −0.061 | <.0001 | |
| Race | −0.82a | −0.698 | −1.0, −0.395 | <.0001 | |
| High school or less | Referent | ||||
| Some college | 0.54b | 0.447 | 0.095, 0.08 | <.0001 | |
| College graduate | 1.17a | 1.021 | 0.660, −1.382 | <.0001 | |
| Baseline performance | 2.32a | 1.999 | 1.70, 2.29 | <.0001 | |
| BMI >30 kg/m2 | −0.42a | −0.034 | −0.228, 0.159 | .73 | |
| BSRT: delayed recall | Menopause stage | −0.36a | −0.122 | −0.231, 0.013 | .029 |
| Age | −0.12a | −0.086 | −0.120, −0.052 | <.0001 | |
| Race | −0.54c | −0.423 | −0.872, 0.026 | .065 | |
| High school or less | Referent | ||||
| Some college | 0.33 | 0.244 | −0.246, 0.733 | .329 | |
| College graduate | 0.98d | 0.897 | 0.368, 1.427 | .0009 | |
| Baseline performance | 0.68a | 0.634 | 0.557, 0.711 | <.0001 | |
| BMI >30 kg/m2 | −0.54d | −0.141 | −0.419, 0.136 | .318 | |
| Digit symbol substitution | Menopause stage | −0.51a | −0.166 | −0.584, 0.252 | .437 |
| Age | −0.16a | −0.127 | −0.250, −0.004 | .043 | |
| Race | −3.7a | −3.48 | −5.011, −1.957 | <.0001 | |
| High school or less | Referent | ||||
| Some college | 1.7c | 1.37 | −0.1114, 2.854 | .07 | |
| College graduate | 3.0b | 2.28 | 0.341, 4.221 | .021 | |
| Baseline performance | 0.72a | 0.649 | 0.570, 0728 | <.0001 | |
| BMI >30 kg/m2 | −1.0c | −0.189 | −1.088, 0.0708 | .679 | |
| Symbol copy | Menopause stage | −0.85a | −0.348 | −1.126, 0.421 | .375 |
| Age | −0.26a | −0.182 | −0.415, 0.052 | .128 | |
| Race | −7.5a | −6.479 | −9.428, −3.532 | <.0001 | |
| High school or less | Referent | ||||
| Some college | 1.1 | 0.358 | −2.815, 3.532 | .825 | |
| College graduate | 7.6d | 6.113 | 1.815, 10.410 | .005 | |
| Baseline performance | 0.77a | 0.716 | 0.628, 0.804 | <.0001 | |
| BMI >30 kg/m2 | −2.3b | −0.856 | −2.568, 0.855 | .327 |
Age refers to the age at each assessment. Menopause stage is a 5-level ordinal variable with 1 referring to premenopause to 5 referring to postmenopause; for race, the reference group is Caucasian; for education, the reference group is those with a high school diploma; for BMI, the reference group is a BMI less than 30 kg/m2.
P < .0001.
P < .01.
P < .05.
P < .001.
Table 4.
Impact of Hormones on Cognition in Adjusted and Unadjusted Models
| Task | Variable | Unadjusted Estimate | Adjusted Estimate | 95% CI | P Value |
|---|---|---|---|---|---|
| BSRT: immediate recall | |||||
| Estradiol | 0.23a | 0.01 | −0.086, 0.097 | .91 | |
| FSH | −0.34a | −0.019 | −0.108, −0.070 | .68 | |
| LH | −0.26a | 0.040 | −0.040, 0.119 | .33 | |
| Inhibin B | 0.28a | 0.049 | −0.062, 0.159 | .39 | |
| DHEAS | 2.30a | 0.022 | −0.116, 0.159 | .76 | |
| BSRT: delayed recall | |||||
| Estradiol | −0.16b | −0.109 | −0.240, −0.023 | <.10 | |
| FSH | −0.35a | 0.058 | −0.068, 0.184 | .37 | |
| LH | −0.27a | 0.093 | −0.028, 0.213 | .13 | |
| Inhibin B | 0.354a | 0.092 | −0.145, 0.169 | .88 | |
| DHEAS | 0.68a | 0.057 | −0.135, 0.249 | .56 | |
| Digit symbol substitution | |||||
| Estradiol | 0.28 | −0.065 | −0.440, 0.309 | .73 | |
| FSH | −0.47b | 0.134 | −0.273, 0.541 | .52 | |
| LH | −0.36b | 0.160 | −0.209, 0.528 | .40 | |
| Inhibin B | 0.52c | 0.498 | −0.042, 1.037 | .07 | |
| DHEAS | 0.71a | 0.936 | 0.204, 1.669 | .012c | |
| Symbol copy | |||||
| Estradiol | 1.22b | 0.791 | −0.014, 1.596 | .054 | |
| FSH | −1.06d | −0.517 | −1.419, 0.384 | .26 | |
| LH | −0.95b | −0.499 | −1.301, 0.304 | .22 | |
| Inhibin B | 0.40 | 0.308 | −0.772, 1.388 | .58 | |
| DHEAS | 0.76a | 0.642 | −0.899, 2.183 | .41 | |
Abbreviation: BSRT, Buschke Selective Reminding Task. All hormones have a significant impact on task performance in unadjusted univariate models, except for the relationship between estradiol and DSST performance and inhibin B and symbol copy task performance. Only the relationship between DHEAS and DSST remained significant in models adjusted for race, education, current age, BMI, and task performance at baseline (assessment 1).
P < 0.0001.
P < 0.01.
P < 0.05.
P < 0.001.
Results
Subject characteristics
Individuals in this sample of 403 women contributed on average 10 assessments for a total of 3958 assessments (Table 1). All participants were premenopausal at assessment 1, whereas at the 14th assessment, most subjects were postmenopausal (Table 2). Consistent with the menopause transition, mean estradiol, dehydroepiandrosterone, and inhibin B levels declined from pre- to postmenopause, whereas the opposite was observed for FSH and LH.
Table 1.
Summary of Observations and Participation in Study by Phase
| Pre- to Menopause | Late Premenopause | Early Transition | Late Transition | Postmenopause | Total | |
|---|---|---|---|---|---|---|
| Assessments, n | 970 | 613 | 1200 | 502 | 673 | 3958 |
| Women, n | 271 | 255 | 288 | 212 | 180 | 403 |
| Average number of assessments per woman | 3.6 | 2.4 | 4.2 | 2.4 | 3.7 | 9.8 |
| Minimum-maximum number of assessments | 0–13 | 0–11 | 0–12 | 0–11 | 0–11 | 0–13 |
Table 2.
Subject Characteristics
| First Assessment (n = 403) | 14th Assessment (n = 251) | |
|---|---|---|
| Age, mean (SD), y | 41.4 (3.5) | 53.6 (3.5) |
| Race | ||
| Caucasian | 206 (48.9) | 116 (46.2) |
| African American | 197 (51.1) | 135 (53.8) |
| Education, n, % | ||
| High school or Less | 93 (23.1) | 58 (23.1) |
| Some college | 139 (34.5) | 87 (34.7) |
| College graduate | 171 (42.4) | 106 (42.2) |
| CES-D ≥16, n, % | 166 (38.4) | 48 (19.1) |
| Zung Anxiety Scale, mean (SD) | 34.9 (8.1) | 29.6 (6.4) |
| PSS, mean (SD) | 21.2 (7.9) | 17.4 (8.1) |
| BMI, n, %, ≥30 | 163 (38.4) | 132 (52.8) |
| Hormones, geometric mean (95% CI) | ||
| FSH, IU/L | 7.0 (6.8, 7.3) | 85.8 (79.6, 92.0) |
| LH, IU/L | 2.8 (2.7, 3.0) | 32.6 (29.9, 35.2) |
| Estradiol, pg/mL | 36.1 (34.4, 37.9) | 25.9 (22.4, 29.3) |
| DHEAS, μg/dL | 94.0 (88.9, 99.4) | 80.0 (74.3, 85.8) |
| Inhibin B, pg/mL | 61.9 (57.6, 66.7) | ND |
| Reproductive stage, n, % | ||
| Premenopausal | 403 (100%) | 1 (0.4) |
| Late premenopause | 0 | 10 (4.0) |
| Early transition | 0 | 45 (17.9) |
| Late transition | 0 | 43 (17.1) |
| Postmenopause | 0 | 152 (60.6) |
Abbreviation: ND, not detectable.
Menopause stage and cognitive performance
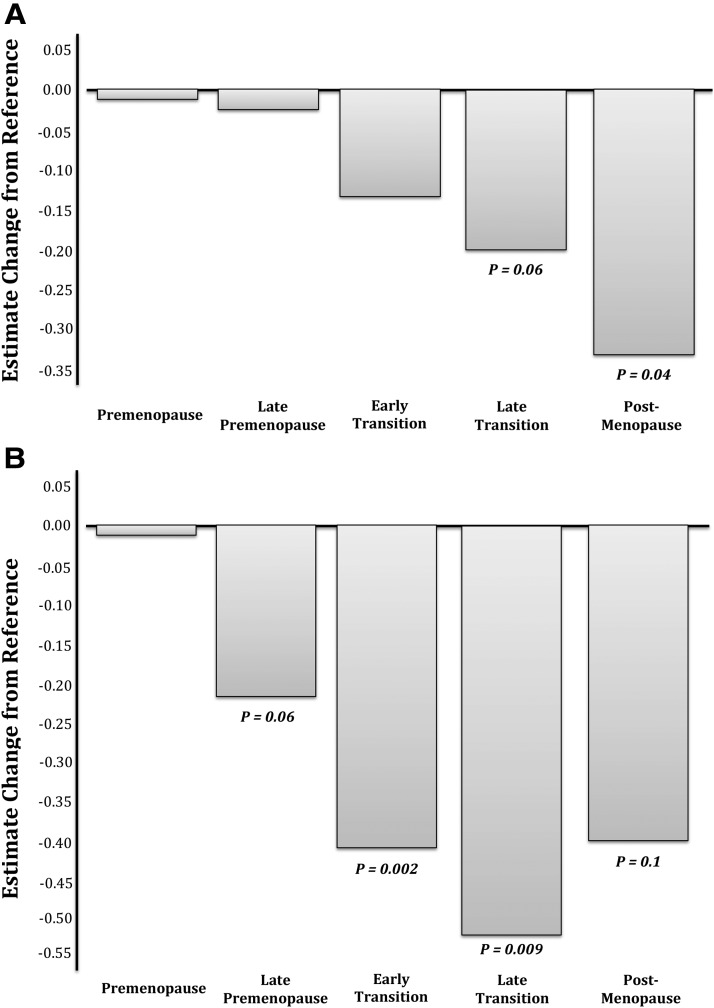
Means (SD) performance on cognitive tasks at baseline were 10.8 (2.2) and 12.5 (3.0) words for the immediate and delayed recall, respectively. Baseline performance on the DSST was 55.9 (12.0) symbol substitutions and 130.5 (25.9) symbols copied within 90 seconds. With respect to performance on the Buschke, the mean decline in immediate verbal recall was 0.31 U (words) per menopause stage [−0.31, 95% confidence interval (CI) −0.35, −0.26, P < .0001], with a similar decline of 0.36 words per stage in delayed recall (−0.36, 95% CI −0.43, 0.28, P < .0001) in the unadjusted analyses (Table 3). This main effect of menopause remained significant for both immediate (−0.08, 95% CI −0.15, −0.01, P = .03) and delayed recall (−0.12, 95% CI −0.23, 0.01, P = .03) after adjustment for chronological age as well as race, BMI, education, and baseline task performance. Post hoc analysis examining performance at each stage of menopause separately, compared with performance at assessment 1, revealed that a significant decline occurred in the postmenopause for immediate verbal recall (−0.32, 95% CI −0.62, −0.01, P = .04; Figure 1A) and even earlier, in the early transition, for the delayed verbal recall (−0.40, 95% CI −0.67, −0.14, P = .002; Figure 1B). In total, the women recalled 1.19% (7.5%) and 1.37% (9%) fewer words with the immediate and delayed-recall tasks, respectively, in the postmenopause compared with the premenopause.
Figure 1.
A, Decrement in immediate verbal memory across the menopause transition. The figure depicts the estimated change in immediate verbal recall in women at various stages of the menopause transition with performance during the premenopause as the reference. A significant decline in immediate verbal memory was observed when comparing performance on the Buschke Selective Reminding Task post- vs premenopause. B, Decrement in delayed verbal memory across the menopause transition. The estimated change in delayed verbal recall in women at various stages of the menopause transition with performance during the premenopause as the reference is depicted. A significant decline in delayed verbal memory was observed when comparing the performance on the Buschke Selective Reminding Task in the early transition and late transition vs premenopause. There was a trend for poorer performance as early as the late premenopause and as late as the postmenopause vs the premenopause. Whether the lack of significance in performance in the post- vs premenopause stages signals a leveling off of a decline in the delayed verbal recall or is a result of the relatively fewer women/assessments in the postmenopause stage is not known.
The impact of menopause stage on performance for the DSST (−0.51, 95% CI −0.75, −0.27, P < .0001) and symbol copy task (SCT; (−0.85, 95% CI −1.3, −0.37, P < .0001) was significant in the unadjusted but not adjusted models.
None of the behavioral measures (PSS, CES-D ≥ 16, or Zung scores) were significant contributors to performance on any measure after adjusting for all other variables in the model. These behavioral variables did not modify the age-independent effect of menopause stage on either verbal memory measure (data not shown).
Age and cognitive performance
In unadjusted models, age was a significant contributor to cognitive decline in both the verbal memory and psychomotor processing domains. There was a significant independent effect of age (Table 3) on immediate verbal recall (−0.10 per year, 95% CI −0.11, −0.09, P < .0001), delayed verbal recall (−0.12 per year, 95% CI −0.14, −0.09, P < .0001), DSST (−0.16 per year, 95% CI −0.23, −0.09, P < .0001), and SCT (−0.26 per year, 95% CI −0.41, −0.12, P < .001). This relationship between age and decline in cognition was present for all tasks except for the SCT in our multivariable model (Table 3).
Hormones and cognitive measures
In unadjusted models (Table 4), FSH, LH, and DHEAS were significantly associated with cognitive performance on all 4 measures across time (all P < .01), with higher levels of DHEAS and lower FSH and LH levels being associated with better performance. Higher estradiol and inhibin B levels were associated with better performance (P < .01) on all measures except the DSST and SCT, respectively. However, once these models were adjusted for age, race, education, BMI, and baseline performance, only the relationship between DHEAS and the DSST remained significant (0.71, 95% CI 0.2, 1.7, P = .01).
Demographic factors and cognitive performance
In univariate analyses, race was a significant contributor to cognitive performance on all 4 cognitive tasks: immediate verbal recall (−0.82, 95% CI −1.1, −0.51, P < .0001), delayed verbal recall (−0.52, 95% CI −0.98, −0.1, P = .02), DSST (−3.7, 95% CI −5.2, −2.2, P < .0001), and SCT (−7.5, 95% CI −10.6, −4.3, P < .0001). Race exerted a significant and independent impact on immediate verbal recall and DSST performance in models adjusted for menopause stage, age, education status, baseline performance, and BMI (Table 3). There was no race by menopause stage interaction, suggesting that menopausal stage did not differentially impact cognitive performance in African American and Caucasian women. Consistent with the literature, education status was an important contributor to verbal memory performance, with those holding a college degree performing better on the Buschke task (both immediate and delayed recall) and the DSST and SCT (all P < .01). BMI was a significant (all P < .05) contributor to performance in all 4 tasks only in the unadjusted models.
Discussion
Although there is evidence that verbal memory declines in premenopausal women in the months immediately after oophorectomy (15), this study is the first of the longitudinal studies (38–40) to demonstrate, in analyses adjusted for important covariates including chronological aging, a worsening of verbal memory performance in women during the natural menopause transition. That we observed an actual performance decline in the domains of immediate and delayed verbal recall and not simply a failure to demonstrate the previously observed practice-associated improvement in cognition (37, 39) may be due to several methodological differences between our work and that of others. Previous longitudinal studies included multiple assessments over 18 months (38), 2 years (37), or 4 years (39), limiting the investigators' ability to observe performance in the same individual over all phases of the menopause transition. In these studies verbal cognition was assessed with verbal fluency and paragraph recall tasks, which tap different aspects of verbal memory. Whereas verbal fluency depends on free recall of items in a category or beginning with a certain letter, recalling a list of words presented over several trials such as that required with the Buschke Selective Reminding Task depends on encoding as well as retrieval of new material. Paragraph recall tasks provide a context for information to be recalled. We limited practice effects by administering a different 16-word list at sequential assessments, and testing women after a relatively long interval (8–12 months). Because the average participant underwent cognitive testing almost 10 times, our finding of a decline in performance is even more impressive because one may assume that familiarity with study procedures could enhance performance. Finally, the extensive 14-year follow-up resulted in 48% of women being evaluated in 4 or 5 menopause stages, representing a total of 3958 assessments.
Age-independent effects of menopause are domain specific
These findings support our a priori principal hypothesis that verbal memory declines across the menopause transition and that this decline is independent of that expected with normal aging. Our observation that menopause stage did not contribute independently to performance on the DSST and SCT is not particularly surprising, given the well-known impact of age on mental processing speed (23) and the likelihood that menopause effects on cognition are unevenly distributed (4, 5). Because processing speed can impact multiple cognitive measures including verbal recall, our failure to detect a significant impact of menopause stage in adjusted models on the DSST and SCT performance emphasizes the primary effect of menopause stage on verbal memory performance in this study.
Our findings confirm those observed in two recent cross-sectionals studies (5, 6), which detected an age-independent impact of menopause status on verbal function in a cross-sectional sample of pre-, peri-, and postmenopausal women. Although the investigators observed an impact of menopause stage in several other cognitive domains, these were not age-independent effects. Our data are also consistent with the limited literature focusing on the cognitive effects of oophorectomy in premenopausal women showing a verbal domain-specific decline in memory in women randomized to placebo immediately after surgery but not in those randomized to estradiol treatment (15). However, until now one could not rule out that the effects of hypogonadism on verbal memory were due to the acute nature of estradiol withdrawal in young women undergoing a surgical menopause. Our findings confirm that menopause stage is an important and independent contributor to worsening verbal memory in women undergoing the natural transition to a hypogonadal state.
Hormonal contribution to cognitive performance
As with many of the cross-sectional (5, 6) and longitudinal (37–39) studies, we observed an impact of menopause-related hormonal changes on cognitive performance. However, this relationship is complicated because the only hormone to demonstrate a significant impact on cognition, specifically the DSST, in multivariable models was DHEAS. There are likely to be multiple reasons for this observation. The transition to menopause is initially characterized by periods of both hyper- and hypoestrogenism followed eventually by permanent hypogonadism. Estradiol has trophic effects on numerous neurotransmitter systems and neural structures in brain regions critical to multiple cognitive domains including both verbal memory and information processing speed and performance (1). Estradiol effect on neurotransmitters and brain regions underlying cognitive processes vary according to genotype (21) and neurotransmitter levels (8). Finally, the range of hormone levels within a given phase can vary greatly from individual to individual, and an optimal level for cognitive function has not been determined.
Adrenal DHEAS production becomes the primary source of estrogen and T in postmenopausal women and has been implicated in cognitive health in some, but not all, studies (41). There is evidence from a community sample of postmenopausal women that higher endogenous DHEAS levels are associated with better performance on measures of executive function such as concentration and working memory, although data regarding DHEAS supplementation and cognition have been disappointing (41). In our study the robust relationship between DHEAS and the DSST, and not the SCT, is interesting because the SCT is thought to represent the copy speed portion of the DSST and is responsible for the greatest variance in DSST performance (23). These data would suggest that DHEAS has a stronger protective effect on the memory or strategy learning required to perform well on the DSST.
Impact of subject characteristics on cognitive performance
A critical observation in this study is the importance of educational factors and race in cognitive performance that are not modified by age, menopause stage, behavioral measures, or hormones. Importantly, there was no interaction between race and menopause stage with respect to cognitive performance, indicating that the decline in verbal memory performance was similar between African American and Caucasian women. Although BMI, the presence of clinically meaningful depressive symptoms (CES-D scores ≥ 16), anxiety symptoms, and perceived stress were all higher in African American than Caucasian women at baseline and throughout the study, the impact of race on cognition remained significant despite adjusting for these other factors in our statistical models. We did not observe a significant impact of perceived stress, depression, or anxiety symptoms on the relationship between menopause status and cognition as modifiers in multivariable analysis. Our finding that CES-D scores were significantly lower during the post- vs premenopause is consistent with our previous report describing clinically meaningful depression during the menopause transition with a subsequent decreased risk in the postmenopausal period (25). There was a high incidence of obesity in this sample at baseline (35%), which increased to 52% by the end of the study. BMI can be used as a proxy for other adverse health outcomes such as metabolic syndrome, hypertension, and diabetes, which are known risk factors for poor cognitive aging (26). Again, when considering its relationship to cognition alone, BMI was significantly inversely correlated with cognitive performance.
Strengths and limitations
The strengths of this study include the multiple within subject assessments with 45% (180 of 403) of participants evaluated as they progressed through all 5 stages of the menopausal transition. With almost 4000 assessments, this is the largest study of its kind. Participants were well characterized with respect to behavioral and endocrine measures and overall participant retention was high and reflective of the original cohort.
Despite the many strengths of this study design, the focus on verbal memory and information processing is limited, and other aspects of memory were not assessed. There may have been important demographic, health, and behavioral factors that were not examined but contributed to our findings. Finally, none of the factors we examined provided a satisfactory explanation for the baseline racial differences in cognition that continued as women progressed from premenopause to postmenopause.
In summary, these data confirm that the natural transition to menopause exerts a negative impact on immediate and delayed verbal recall. Whether this decline can be generalized to other types of verbal memory and whether it stabilizes in the early years of the postmenopausal period are not yet known. The overall decline in verbal memory was modest, with failure to recall 1–2 of 16 words but sufficient to validate women's experience of worsening memory during the menopause transition and to quell their concern that this change represents pathological aging.
Acknowledgments
This work was supported by the following entities: the National Institute on Aging Grant R01 AG 12745 (to E.W.F.); the American Recovery and Reinvestment Act-funded Grant R01 AG030641 (to C.N.E.), the National Institute on Drug Addiction Grant K24 DA03031 (to C.N.E.); the National Institute of Mental Health and Office of Research on Women's Health (to C.N.E.); the Specialized Centers of Research on Women's Health Grant P50 MH099910; and Grant RR024134 to the Translational and Clinical Research Center, Perelman School of Medicine.
Disclosure Summary: C.N.E. has received financial (issued to the University of Pennsylvania) or product support from Shire and Novartis, respectively, for investigator-initiated research. E.W.F. reported research support (issued to the University of Pennsylvania) from Forest Laboratories, Inc, Bionovo, Inc, and Xanodyne Pharmaceuticals, Inc. M.D.S. reported honoraria for lectures from the University of Rochester and Arcadia College; travel and meeting expenses from the University of Alabama Birmingham, and The North American Menopause Society; is a consultant for Swiss Precision Diagnostics; and is a statistical editor for the American Journal of Obstetrics and Gynecology.
Footnotes
- BMI
- body mass index
- CES-D
- Center for Epidemiologic Studies Depression Scale
- CI
- confidence interval
- DHEAS
- dehydroepiandrosterone sulfate
- DSST
- Digit Symbol Substitution Test
- POAS
- Penn Ovarian Aging Study
- PSS
- Perceived Stress Scale
- SCT
- symbol copy task
- Zung
- Zung Self-Rating Anxiety Scale.
References
- 1. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in menopausal women [published online December 14, 2012]. Hum Brain Mapp. doi:10.1002/hbm.22218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause. 2011;18(5):542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2011;14(2):252–261 [DOI] [PubMed] [Google Scholar]
- 4. Weber MT, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopause transition. Menopause. 2012;19:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage [published online January 2, 2013]. Menopause. doi:10.1097/gme.0b013e31827655e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berent-Spillson A, Persad C, Love T, et al. Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinology Metab. 2012;97:E1686–E1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecolo Clin North Am. 2011;38:519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amin Z, Gueorguieva R, Cappiello A, et al. Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology. 2006;31(11):2489–2497 [DOI] [PubMed] [Google Scholar]
- 9. Amin Z, Canli T, Epperson CN. Estrogen and serotonin interaction in the modulation of mood and cognition. Rev Cogn Behav Neurosci. 2005;4(1):43–58 [DOI] [PubMed] [Google Scholar]
- 10. Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive function in later life. Psychoneuroendocrinology. 2009;34:287–298 [DOI] [PubMed] [Google Scholar]
- 11. Lin J, Kroenke CH, Epel E, et al. Greater endogenous estrogen exposure is associated with longer telemeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. North American Menopause Society The 2012 Hormone Therapy Position Statement of the North American Menopause Society. Menopause. 2012;19:257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maki PM, Dennerstein L, Clark M, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurology. 2011;69:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495 [DOI] [PubMed] [Google Scholar]
- 16. Henderson VW, Popat RA. Effects of endogenous and exogenous estrogen exposures in midlife and late-life women on episodic memory and executive functions. Neuroscience. 2011;191:129–138 [DOI] [PubMed] [Google Scholar]
- 17. Espeland MA, Rapp SR, Shumaker SA, et al. Women's Health Initiative Memory Study conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968 [DOI] [PubMed] [Google Scholar]
- 18. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probably dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958 [DOI] [PubMed] [Google Scholar]
- 19. Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wright RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Epperson CN, Bale TL. BDNF Val66Met polymorphism and brain-derived neurotrophic factor levels across the female life span: implication for the sex bias in affective disorders. Biol Psychiatry. 2012;72:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 2011;32:5286–5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical window hypothesis. Horm Behav. 2008;53(1):159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychol. 1992;79:155–170 [DOI] [PubMed] [Google Scholar]
- 24. Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol—coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19(6):759–767 [DOI] [PubMed] [Google Scholar]
- 25. Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in the transition to menopause. Arch Gen Psychiatry. 2004;61:62–70 [DOI] [PubMed] [Google Scholar]
- 26. Snitz BE, Unverzagt FW, Chang CC, et al. Effects of age, gender, education and race on two tests of language ability in community-based older adults. Int Psychogeriatr. 2009;21(6):1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerwin DR, Zhang Y, Kotchen JM, et al. The cross-sectional relationship between body mass index, waist-hip ratio, and cognitive performance in postmenopausal women enrolled in the Women's Health Initiative. J Am Geriatr Soc. 2010;58(8):1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sammel MD, Freeman EW, Liu Z, Lin H, Guo W. Factors that influence entry into stages of the menopausal transition. Menopause. 2009;16(6):1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rebbeck TR, Su HI, Sammel MD, et al. Effect of hormone metabolism genotypes on steroid hormone levels and menopausal symptoms in a prospective population-based cohort of women experiencing the menopausal transition. Menopause. 2010;17(5):1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buschke H, Fuld PA. Evaluating storage, retention and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025 [DOI] [PubMed] [Google Scholar]
- 31. Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1991 [Google Scholar]
- 32. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401 [Google Scholar]
- 33. Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379 [DOI] [PubMed] [Google Scholar]
- 34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–396 [PubMed] [Google Scholar]
- 35. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stage of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76:874–878 [DOI] [PubMed] [Google Scholar]
- 37. Gracia CR, Sammel MD, Freeman EW, Lin H, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopause transition. Menopause. 2005;12:128–135 [DOI] [PubMed] [Google Scholar]
- 38. Meyer PM, Powell LH, Wilson RS, et al. A population-based longitudinal study of cognitive functioning in the menopause transition. Neurology. 2003;61:801–806 [DOI] [PubMed] [Google Scholar]
- 39. Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopause transition in a rural community. Maturitas. 2006;53:447–453 [DOI] [PubMed] [Google Scholar]
- 40. Greendale GA, Huang MH, Wight RG. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653 [DOI] [PubMed] [Google Scholar]