Abstract
Over the last decade, it has become evident that 14-3-3 proteins are essential for primary cell functions. These proteins are abundant throughout the body, including the central nervous system (CNS) and interact with other proteins in both cell cycle and apoptotic pathways. Examination of cerebral spinal fluid (CSF) in humans, suggest that 14-3-3s including 14-3-3ε (YWHAE), are upregulated in several neurological diseases and loss or duplication of the YWHAE gene leads to Miller-Dieker Syndrome (MDS). The goal of this review is to examine the utility of 14-3-3s as a marker of Human Immune deficiency virus (HIV)-dependent neurodegeneration, and also as a tool to track disease progression. To that end we describe mechanisms implicating 14-3-3s in neurological diseases and summarize evidence of its interactions with HIV accessory and co-receptor proteins.
Keywords: 14-3-3, Hepatitis C virus, Neurocognition, HIV accessory proteins, gp120, Vpr, Vpu, GPR15, Nef
Dynamics of 14-3-3s
14-3-3s are proteins that regulate many cellular processes relevant to multiple pivotal points in the life cycle of a cell such as apoptosis, mutagenic signaling, and cell-cycle checkpoints (Aitken, 2006; Aitken et al, 2002; Berg et al, 2003b; Fu et al, 2000; Obsil and Obsilova, 2011; Steinacker et al, 2011; Takahashi, 2003; van Heusden, 2005; Wang and Shakes, 1996; Yaffe, 2002). 14-3-3s were first described in 1967 from bovine brains as proteins with an acidic pI and molecular masses between 29–32 kD in an attempt to identify proteins unique to the nervous system (Moore and Perez, 1967). These were later resolved to comprise nine proteins (α, β, γ, δ, ε, ζ, η, θ/τ and σ) encoded by seven distinct genes, with the α and δ isoforms being the phosphorylated forms of β and ζ genes, respectively (Aitken et al, 1995). In addition, 14-3-3s contain a number of known common modification domains, including regions for divalent cation interaction, phosphorylation, acetylation, and proteolytic cleavage, among others (Aitken, 2006; Obsil and Obsilova, 2011; Rittinger et al, 1999; Xiao et al, 1995). The 14-3-3 family is ubiquitous, with members identified in all eukaryotic species examined, including mammals, insects, nematodes, frogs, plants, and yeast (Aitken et al, 1992a; Aitken et al, 1992b; Berg et al, 2003a; Fu et al, 2000; Muslin and Xing, 2000; Takahashi, 2003; Wang and Shakes, 1996). Whether particular modifications are present in orthologs or homologs from different species and their potential functional significance is a question under active investigation.
In vertebrates, 14-3-3s are highly enriched in the cerebellum, certain cerebral areas (including the hippocampus), and motor neurons in the brainstem and spinal cord (Boston et al, 1982; VanGuilder et al, 2011; Watanabe et al, 1991). Their abundance in the brain and recent evidence of up-regulation in various neurological disorders imply that 14-3-3s may play a significant role in neuronal functions (VanGuilder et al, 2011).
14-3-3s as diagnostic tools in the CSF
14-3-3 proteins have also been detected in the cerebrospinal fluid of patients with various diseases that lead to neurodegeneration, including those with Creutzfeldt–Jakob disease (CJD), Alzheimer’s disease (AD), Multiple Sclerosis (MS), and HIV these proteins also are aggregated in Lewy bodies (LB) in those with Parkinson’s disease (PD) (Berg et al, 2003a; Ellis et al, 2007; Steinacker et al, 2011; Wakabayashi et al, 2001; Zerr and Poser, 2002). Despite this apparent correlation, the question remains as to whether 14-3-3s are truly biomarkers that can be used to track neurodegeneration. Are different isoforms specific to a particular disease? Should specific isoforms be examined for different diseases? Additionally, it is important to understand whether 14-3-3’s actually interact with the pathogens to regulate or affect in any way progression of the disease because that would likely lead to potential therapeutic interventions.
Creutzfeldt–Jakob Disease (CJD)
It has been suggested that changes in the distribution of 14-3-3s in the CNS may be linked to spongiform encephalitis (Berg et al, 2003a). Transmissible spongiform encephalopathy, or prion disease, was first described by Gerhard Creutzfeldt and Alfons Jakob in the 1920s (Creutzfeld, 1920), but recent clinical diagnostics indicate two forms, sporadic CJD and variant CJD (Zerr and Poser, 2002). Sporadic CJD occurs in patients in their seventies and is characterized by rapid dementia progressing to mortality within 6 to 14 months. In contrast, variant CJD occurs in patients from 14 to 74 years of age and typically presents slower progression (Zerr and Poser, 2002).
To look for disease-specific biomarkers, clinical investigations have focused on potential changes in the levels of various proteins in the cerebrospinal fluid (CSF) and described increases in different 14-3-3s in CJD patients (Table 1). Monitoring 14-3-3 levels in the CSF by western blot has revealed that using the anti-14-3-3β antibody, also called the pan-14-3-3 antibody, appears to be both sensitive and specific for sporadic CJD (Table 1) (Bahl et al, 2008; Baxter et al, 2002a; Bertrand et al, 2009; Brandel et al, 2000; Castellani et al, 2004; Chohan et al, 2010; Collins et al, 2010; Huang et al, 2003; Irani and Kerr, 2000; Otto et al, 2002; Peoc’h et al, 2006; Poser et al, 1999; Sanchez-Valle et al, 2002; Zerr et al, 2000a; Zerr and Poser, 2002). Furthermore, using isotype-specific antibodies, increases in the levels of γ, ζ and ε proteins have been reported in the CSF of CJD patients compared to non-CJD subjects (Table 1) (Green et al, 2001; Takahashi et al, 1999; Wiltfang et al, 1999). Distinct levels of the different 14-3-3s in the CSF appear to correlate with damage in particular areas of the brain and the rate of neurodegenerative changes (Huang et al, 2003; Zerr and Poser, 2002). Hence increased 14-3-3 levels in the CSF may result from their release upon cell death and reflect their abundance in the particular neurons affected as well their relative stability.
Table 1.
14-3-3 protein expression in the CSF of individuals with other neurodegenerative diseases
| Disease | Isoforms | Effect | References |
|---|---|---|---|
| sCJD | γ | Present | (Green et al, 2001) |
| sCJD | ε, β, γ, η | Examined 16 different antibodies; found only four isoforms present, all others not detected. | (Wiltfang et al, 1999) |
| sCJD | Pan | Present, Variant subtype should be considered when using 14-3-3 as a biomarker. | (Bahl et al, 2008; Baxter et al, 2002a; Bertrand et al, 2009; Brandel et al, 2000; Castellani et al, 2004; Chohan et al, 2010; Collins et al, 2010; Huang et al, 2003; Irani and Kerr, 2000; Otto et al, 2002; Peoc’h et al, 2006; Poser et al, 1999; Sanchez-Valle et al, 2002; Zerr et al, 2000a) |
| sCJD | ε, γ | Increased using mouse antibodies more specific then polyclonal. | (Takahashi et al, 1999) |
| CJD with PNDs | Pan | Double bands present only in patients with PNDs | (Saiz et al, 1999) |
| AD | η | Present and in those with Herpes Simplex Encephalitis. | (Wiltfang et al, 1999) |
| AD | ζ, pan | Binds to tau and co-purifies with microtubules. The ε or γ isoforms are not associated with tau. | (Hashiguchi et al, 2000) |
| AD | Pan | Not present | (Hsich et al, 1996; Tschampa et al, 2001) |
| MS | ζ, pan | Present, Dimeric and Trimeric. | (Fiorini et al, 2007) |
| MS | Pan | No change or not present. | (Bartosik-Psujek and Archelos, 2004; de Seze et al, 2002; Hsich et al, 1996) |
| HIV/AIDS | ε, γ, ζ | Increased only in patients with AIDS dementia complex or CMVE. | (Wakabayashi et al, 2001) |
| HIV | γ | Increased in those with CNS lymphoma. | (Miller et al, 2000) |
sCJD: Sporadic Creutzfeldt–Jakob disease; CMVE: Cytomegalovirus encephalitis; AD: Alzheimer’s disease; MS: Multiple Sclerosis; PNDs: Paraneoplastic neurological disorders; pan – antibody against β cross-reacts to ε, ζ, γ, η
In fact, the appearance in the CSF is hypothesized to be due to release and local loss of 14-3-3β,γ, η and ζ in areas of severe degeneration particularly in the hippocampus and thalamus shown in scrapie-infected mice (Baxter et al, 2002b; Berg et al, 2003a). However, 14-3-3 levels in the CSF may not remain elevated if the damage is not sustained. For example, in cases of herpetic encephalitis, 14-3-3s are only present in the CSF initially and decline later on.. Consequently, examining the level of 14-3-3 proteins in the CSF may not suffice for safely diagnosing such diseases, but rather provides independent support for diagnosis and characterization, in conjunction with clinical data, (Table 1) (Zerr and Poser, 2002; Zerr et al, 2000b). There is evidence of misdiagnosis using the pan-14-3-3 antibody potentially because of cross-reactivity with several other isoforms, including 14-3-3ε, ζ, γ, and η). In these cases, samples tested positive for CJD while patients were actually affected by AD or dementia with Lewy bodies (LB’s) (Table 1). Thus, we suggest that the isoform-specific antibodies are likely more appropriate for diagnostic applications (Chitravas et al, 2011; Tschampa et al, 2001). It should be pointed out however that most studies aim to use the presence or levels of 14-3-3s as a potential acute diagnostic tool, assessing their profile longitudinally as a tool to track disease progression remains largely unexplored.
Alzheimer’s disease (AD)
Increased levels of 14-3-3ζ, γ, and ε have also been reported in the CSF of AD patients (Table 1) (Hashiguchi et al, 2000; Tschampa et al, 2001; Wang et al, 1995). Interestingly, the 14-3-3ζ isoform has been suggested to affect the stability of the microtubule-associated protein Tau (Tubulin-Associated Unit) (Table 1) (Hashiguchi et al, 2000). Furthermore, association of Tau and 14-3-3ζ appears to lead to its abnormal phosphorylation via protein kinase A (PKA) and Tau hyper-phosphorylation is thought to be one of the key events in the development of AD pathology (Hashiguchi et al, 2000; Wang et al, 1995). In support of these data, 14-3-3ζ, but not 14-3-3ε and γ was found to co-purify (Hashiguchi et al, 2000). However, it should be pointed out that other studies did not find changes in the 14-3-3 levels of AD patients (Table 1) (Hsich et al, 1996; Tschampa et al, 2001) unless these patients were also infected with Herpes Simplex Encephalitis Virus (Table 1) (Wiltfang et al, 1999). These results suggest that 14-3-3s may not be appropriate as a biomarker for AD.
Multiple Sclerosis (MS)
Studies have demonstrated that elevated signal is observed with the the pan-14-3-3 and 14-3-3ζ-specific antibodies in the CSF of MS patients who present severe inflammation-induced extensive damage of the central nervous system (Table 1) (Fiorini et al, 2007; Sanchez-Valle et al, 2002). However, other studies reported absence, or at leasy no elevation of 14-3-3s in the CSF of MS patients (Table 1) (Bartosik-Psujek and Archelos, 2004; de Seze et al, 2002; Hsich et al, 1996). Again, additional broad and specific antibodies should be tested to unequivocally establish whether 14-3-3 elevation in the CSF is also characteristic of MS patients.
Human immunodeficiency virus (HIV)/Acquired immune deficiency syndrome (AIDS)
Acquired Immunodeficiency Virus (AIDS) patients may develop AIDS Dementia Complex (ADC), also known as HIV dementia, HIV-associated dementia (HAD), and HIV-associated dementia complex [HADC]). The CSF of such patients as those with Cytomegalovirus Encephalitis (CME), was reported to contain 14-3-3ε, 14-3-3γ, and 14-3-3ζ (Wakabayashi et al, 2001). However, these 14-3-3 isoforms were not present in AIDS patients who did not have neurological symptoms (Table 1). Wakabayashi et al. also found that the isoforms present in AIDS patients were different from those reported in CJD and Herpes Simplex Encephalitis, suggesting that isotype patterns in the CSF may facilitate differential diagnosis. High levels of 14-3-3ε, ζ and γ were observed in the CSF of seriously ill AIDS patients, particularly those with low CD4 levels (Table 1). They suggest that 14-3-3 proteins may have been released from destroyed neurons and making them a marker of cellular destruction (Wakabayashi et al, 2001). Both 14-3-3ζ and ε levels were found 2-fold elevated in brain sections from HIV Encephalitis (HIVE) and HIV-Associated Neurocognitive Disorders (HAND) compared to those of non-HIV controls (Gelman and Nguyen, 2010). The 14-3-3ε levels also correlated with the viral load of HIV-1 in the brain and CSF. Finally, another study examined HIV patients with lymphoma and found the 14-3-3γ isotype in their CSF in the three months preceding death (Table 1) (Miller et al, 2000). Collectively these results strongly suggest that 14-3-3 proteins are involved in changes associated with HIV infection particularly in the CNS (Gelman and Nguyen, 2010).
In Macaques infected with Simian Immunodeficiency Virus (SIV), the continued presence of 14-3-3 proteins in the CSF was tightly linked with the amount of viral replication in the CNS (Helke et al, 2005). Animals with 14-3-3 protein in the CSF harbored the highest viral loads after acute infection and the highest levels of both viral RNA and protein in the brain. Hence it was proposed that 14-3-3 protein levels may serve as a biomarker for early neuronal damage correlating to viral replication in the CNS and disease progression in individuals with HIV (Helke et al, 2005). A reasonable question which arises from all these results is: Are 14-3-3s regulating disease progression?
14-3-3s and HIV/SIV accessory and co-receptor proteins
The rate of disease progression with which HIV-1 infection leads to AIDS varies among individuals. Reasons for this variance include host susceptibility, genetics, immune function and co-infections, and the regulation and modulation of the HIV gene products (including accessory proteins). Within the brain, HIV-1 infection is associated with the degeneration due to apoptosis (Jones and Power, 2006; Shi et al, 1996) of the frontal cortex, substantia nigra, cerebellum, and striatum (Everall et al, 1993). This leads to development of HAD or HIVE (McArthur et al, 2003). Most studies of the pathogenic mechanism thus far agree that the modulation of HIV accessory and co-receptor proteins leads to neurodegeneration (Ellis et al, 2007; Iskander et al, 2004; Jones and Power, 2006; Jones et al, 2007; Kogan and Rappaport, 2011; Malim and Emerman, 2008; McArthur et al, 2003; Strazza et al, 2011; Toggas et al, 1994). These are summarized below.
Glycoprotein 120 (Gp120)
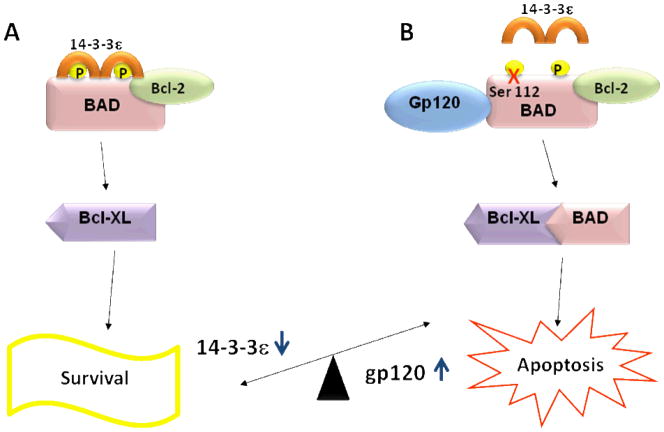
Both HIV-free and virus-infected monocyte/macrophages traverse the blood–brain barrier (BBB), infecting neighboring resident microglia, astrocytes, and other cell types (Valcour et al, 2011). The HIV-1-encoded gp120 envelope protein mediates and stimulates the entry of the virus into the host cell and induces neurotoxicity via multiple pathways, including the B-cell lymphoma-extra large (Bcl-XL)/B-cell lymphoma 2 antagonist of cell death (BAD) apoptosis pathway (Figure 1) (Bazan et al, 1998; Ellis et al, 2007; Gallo et al, 2003; Iskander et al, 2004; Lipton, 1992a; Lipton, 1992b; Ushijima et al, 1995). Understanding the players involved in this pathway may help to block the effects of gp120.
Figure 1. Proposed relation of 14-3-3ε and gp120 mediated apoptosis.
A. Binding of 14-3-3ε suppresses apoptosis in cells via phosphorylation of the pro-apoptotic Bcl-2 family protein BAD. The phosphorylation results in reduced association of BAD with Bcl-XL, thereby suppressing apoptosis. B. Gp120-dependent dephosphorylation of BAD at Serine-112 qpreventing 14-3-3ε binding and its association with the Bcl-XL in mitochondria, promotes gp120-mediated apoptosis (Yano et al, 2007).
14-3-3 proteins appear to play a role in gp120-mediated cytotoxicity in human umbilical vein endothelial cells (HUVEC) (Table 4) (Yano et al, 2007), which, like neuronal cells, have alpha- or beta-chemokine receptors, but no CD4 receptor to induce their apoptosis (Ullrich et al, 2000). The 14-3-3τ protein protects against cell death when it is associated with BAD, preventing it’s interaction with Bcl-XL. Gp120 associates with BAD, preventing the 14-3-3 protein from binding thereby allowing the BAD/Bcl-XL interaction. Suppression of BAD activity orexpression seems to be the reason cells are rescued from gp120-triggered apoptosis (Figure 1) (Yano et al, 2007). In fact, 14-3-3τ is specifically up-regulated after a 24-hour treatment with recombinant gp120 protein, while its down-regulation by RNA interference (RNAi) accelerated gp120-dependent dephosphorylation of BAD at Serine-112 and its association with the Bcl-XL in mitochondria, promoting apoptosis (Yano et al, 2007). Furthermore, in human brain microvascular endothelial cells (HBMECs), an association between gp120 and 14-3-3τ protein levels appears to regulate alpha or beta-chemokine receptors, but no CD4 receptors to induce apoptosis (Ullrich et al, 2000). In addition, an association between gp120 and 14-3-3τ protein levels appears to regulate the BBB breakdown by interfering with tight junctions between endothelial cells (Table 4) (Nakamuta et al, 2008). Furthermore, 14-3-3ε levels were inversely associated with gp120 amounts, with the lowest levels of 14-3-3ε at their highest concentrations on gp120 (Table 4, Figure 1) (Kapasi et al, 2001). These results suggest that 14-3-3 levels in the CSF may reflect either the level of HIV infection and/or neurodegeneration.
Table 4.
YWHAE/14-3-3ε genetic alterations in models of neurological impairments
| Alteration | Effect | References |
|---|---|---|
| Deletion along with TUSC5, MYO1C, CRK, PAFAH1B1 in mice | Mild to severe migration abnormalities | (Bi et al, 2009) |
| Duplication along with TUSC5, MYO1C, CRK, PAFAH1B1 in mice | Mild brain anomalies | (Bi et al, 2009) |
| Deletion along with TUSC5 in mice | Mild to severe migration abnormalities | (Bi et al, 2009) |
| Ywhae−/− mice | Defect in brain development and neuronal migration. | (Toyo-oka et al, 2003) |
| Ywhae+/− mice | Impaired working memory in radial arm maze and enhanced anxiety in plus maze. | (Ikeda et al, 2008) |
CRK: viral oncogene causes increased tyrosine-phosphorylated proteins; LIS1: encodes subunit of platelet-activating factor acetylhydrolase 1B (PAFAH1B1); Ywhae−/−: Ywhae/14-3-3ε deficient mice; siRNA - single stranded RNA
Negative factor (Nef)
Nef is a protein with a role in HIV-1 replication and pathogenesis (Foster et al, 2011; Kestler et al, 1991). Nef contributes to immune modulation of T-cells upon HIV-1 infection through its association with PKCθ (Meller et al, 1998; Smith et al, 1996). 14-3-3τ interacts directly with PKCθ resulting in inhibition of Interleukin 2 (IL-2) by preventing its translocation to the membrane in Jurkat T-cells (Table 2) (Meller et al, 1998; Meller et al, 1996). This suggests that 14-3-3τ interaction with PKCθ is necessary for normal immune function via T-cell activation (Meller et al, 1996). These results are in agreement with the notion that 14-3-3s can modulate HIV disease progression by interacting with proteins whose functions are affected by the presence of HIV accessory proteins.
Table 2.
14-3-3 protein interactions with HIV accessory and co-receptor proteins
| 14-3-3 Isoform | Cell type | Related proteins | Relationship to 14-3-3 proteins | References |
|---|---|---|---|---|
| ε | HMC | gp120 | Low-level stimulate cell proliferation and high-level inhibit of cell proliferation | (Kapasi et al, 2001) |
| τ/θ | HBMEC | gp120 | Increase expression of gp120 and blood-brain barrier permeability. | (Nakamuta et al, 2008) |
| τ/θ | HUVEC | gp120 | Binding to Bad protects it from dephosphorylation regulating gp120/CXCR4-mediated cell death. | (Yano et al, 2007) |
| τ/θ | T-cells | Nef | Binding and suppression of PKCθ–dependent IL-2 promoter activity may relate to T-cell impairments by PKCθ/Nef. | (Meller et al, 1998; Meller et al, 1996) |
| β | T-cells | Vpu1 | Binding effects translocation of K2P3 which interacts with Vpu1 releasing progeny virions from infected cells. | (Plant et al, 2005) |
| Pan | Hela, HepG2 | Vpr | Vpr leads to loss of 14-3-3/FoxO3a binding contributing to tissue-selective insulin resistance. | (Kino et al, 2005a) |
| ε/rad24 | S. pombe | Vpr | Binding with Vpr potentiates G2 cell-cycle arrest. | (Matsuda et al, 2006) |
| η, σ | HepG2, σ knockout, Hela, S. pombe | Vpr | Triple complex with Cdc25 promotes G2/M cell-cycle arrest. | (Kino et al, 2005b; Kino and Pavlakis, 2004) |
| τ/θ | T-cells | Vpr | Dissociation of 14-3-3θ to centrosomal proteins correlates to G2 cell-cycle arrest. | (Bolton et al, 2008) |
| Pan | HEK293 | GPR15 | Binding with GPR15 increased its stability and trafficking. | (Chung et al, 2009; Okamoto and Shikano, 2011) |
| ε | S. pombe, HEK293 | GPR15 | Binding motif SWTY in ε interacts with GPR15. | (Shikano et al, 2005) |
Vpu-1: HIV-1 membrane protein; K2P3 (TASK1): Potassium Channel; HBMEC: Human Brain microvascular endothelial cells; GPR15: G protein-coupled receptor 15; Vpr: Viral protein R; HCV: Hepatitis C virus; Cdc25: Cell division cycle phosphatase 25; FoxO3a: Forkhead in human rhabdomyosarcoma; S. pombe: Schizosaccharomyces pombe; HepG2: Human hepatoma; Hela: Human cervical carcinoma; SWTY: RGRSWTY; HEK293: Human embryonic kidney; PKCθ: Protein kinase C, Ca2+-independent; gp120: glycoprotein 120; BAX: Bcl-2–associated X; HMC: Human mesangial growth cells; HUVEC: Human umbilical vein endothelial cells; CXCR4: CXC chemokine receptor 4; Nef: Negative factor; pan: antibody against β cross-reacts to ε, ζ, γ, η
Viral protein U (Vpu)
The Vpu accessory protein mediates proteasomal degradation of newly synthesized CD4 receptors, leading to their down regulation (Cohen et al, 1988; Dube et al, 2010; Goff, 2007). In addition, Vpu enhances the release of newly synthesized virions by regulating Tetherin, aninterferon host restriction factor responsible for linking virons on the host cell-surface (Dube et al, 2010). The two-pore domain potassium channel (K2P) K2P3 has been shown to interact with Vpu, leading to the dissociation of the channel (Hsu et al, 2004). K2P3 also binds to 14-3-3s suppressing beta-coatomer protein (β-COP) binding and aids in the trafficking of the channel (Table 2) (Mathie et al, 2010; Plant et al, 2005). Although, no one has examined whether there is direct relationship between Vpu and 14-3-3s, the fact that these proteins both bind and regulate the same receptor suggests that 14-3-3s would have a regulatory role in Vpu function.
Viral protein R (Vpr)
Vpr is a multifunctional accessory protein that plays a role in CD4+ T-cell and macrophage viral infection (Cohen et al, 1990b; Kino and Pavlakis, 2004; Kogan and Rappaport, 2011; Zhao et al, 1994a; Zhao et al, 1994b) and the role of HIV-1 Vpr in the inhibition of normal cell growth is well known. It is suggested that the interruption of cell division by Vpr increases virus replication and induces programmed cell death. Vpr mediates cell-cycle arrest at the G2/M transition in various mammalian cells. G2 arrest provides a replication advantage for the virus because the proviral transcription level is known to be elevated during the G2 phase of the cell cycle (Belzile et al, 2007; Elder et al, 2001; Goh et al, 1998; Tyson et al, 2002). In the virions, Vpr transports the virus for integration into the host genome (Cohen et al, 1990a; Vodicka et al, 1998).
The eukaryotic cell cycle is controlled by a complex network of proteins and genes including cyclin division cycle (cdc) proteins. Cyclin-dependent protein kinases (CDKs) initiate the essential events of the cell cycle by phosphorylating specific target proteins. The phosphorylation activity of CDKs is dependent on binding to cyclins. The CDK/cyclin complexes can be down-regulated either by inhibiting the phosphorylation of the CDK subunit or by binding to inhibitory molecules (designated cyclin-dependent kinase inhibitors) (Tyson et al, 2002). G2 arrest is distinguished by low levels of cyclin B1/p34Cdc2 activity and the inhibitory phosphorylation of p34Cdc2. It has been shown that Vpr directly inhibits the in vitro activity of a phosphatase, Cdc25C, which normally activates cyclin B1-p34Cdc2 (Figure 2). Although the Vpr does not seem to bind on the catalytic site of, Cdc25C it nevertheless inactivates the phosphatase. In the absence of the Cdc25C phosphatase activity cyclin B1-p34Cdc2 remains in its inactive phosphorylated form (Goh et al, 1998; He et al, 1995).
Figure 2.
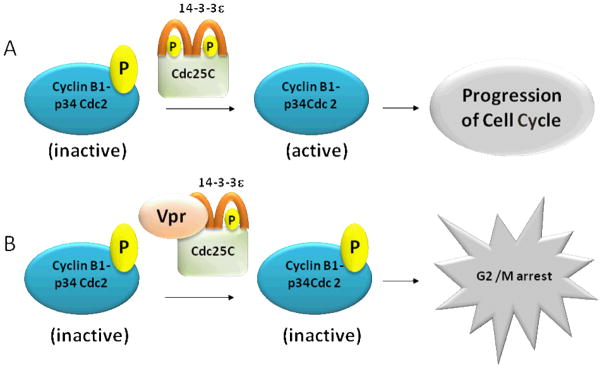
Vpr functions and molecular interactions with 14-3-3e and Cdc25C to induce cell-cycle arrest.
A. 14-3-3ε proteins bind to Cdc25C, resulting in a complex that promotes phosphatase activity. The complex removes the phosphate molecule from the inactive form of cyclin B1-p 34 Cdc2, altering it to the active form that drives the progression of the cell cycle. B. Vpr binds to the 14-3-3ε protein and Cdc25C and inactivates this complex. In the absence of the phosphatase activity of Cdc25C, cyclin B1-p34Cdc2 remains inactive, resulting in G2 arrest.
14-3-3 proteins normally regulate cell-cycle progression by modulating the activities of cyclins, including Cdc25C (He et al, 1995). DNA damage results in Cdc25C phosphorylation, which provides an active binding site for 14-3-3. Studies have shown that the C-terminal region of Vpr interacts with the C-terminal region of 14-3-3, leading to the association of 14-3-3 with Cdc25C (Figure 1) (Kino et al, 2005a; Kino et al, 2005b). The complex is not able to activate cyclin B1-p34Cdc2; therefore, the cell cycle is arrested (Figure 2) (Kino et al, 2005a; Kino et al, 2005b).
Inactivating Cdc25C is only one of the pathways utilized by Vpr to arrest the cell cycle. In addition, Vpr also plays a role in cell-cycle arrest because it binds directly onto DNA binding protein 1 and Cullin 4a-associated factor (DCAF-1), which in turn results in T-cell disruption (Kogan and Rappaport, 2011; Stewart et al, 1997; Stewart et al, 2000). Results from an S. pombemodel indicate that 14-3-3ε protein increases the levels of Wee1, a Serine/Threonine kinase (Wang et al, 2000), which contributes to Vpr-dependent G2/M cell-cycle arrest (Table 4) (Boltonet al, 2008; Matsuda et al, 2006). The 14-3-3η, and σ isotypes have also been shown to bind directly to Vpr in a complex with Cdc25, which also promotes cell-cycle arrest (Table 4) (Kinoet al, 2005b; Kino and Pavlakis, 2004). In addition, Vpr disrupts 14-3-3η and σ binding to a member of the Forkhead transcription factor (FoxO), FoxO3a, resulting in tissue-selective insulin resistance, a condition often presented by HIV-1-infected individuals (Table 4) (Kino et al, 2005a). Collectively, this evidence suggests a strong relationship between the Vpr accessory protein and 14-3-3 proteins mediating cell cycle arrest that ultimately leads to neurodegeneration.
G protein receptor 15 (GPR15)
G protein cell receptors (GPCRs) (Bernier et al, 2004) are cell surface receptors, whose role in the pathophysiology of human diseases is dependent on their density (Dunham and Hall, 2009). One GPCR that is expressed in the T-cells of both HIV-1 and SIV-infected subjects, GPR15/BOB, serves as a co-receptor for the virus (Farzan et al, 1997; Unutmaz et al, 1998). 14-3-3 proteins play a role in the trafficking of GPR15/BOB, hence controlling its cell surface density in response to phosphorylation signals (Table 2) (Chung et al, 2009; Okamoto and Shikano, 2011). Furthermore, 14-3-3ε binding substantially increases the stability of GPR15 (Table 2) (Shikano et al, 2005).
In summary, these data suggest that there is a strong relationship between HIV accessory and 14-3-3 proteins and that the latter, in addition to providing potential biomarkers for infection and disease progression, they might be also utilized in the development of therapeutic interventions.
14-3-3s and the Hepatitis C virus (HCV) core protein
Best estimates are that 20–30% of HIV-infected individuals and as high as 90% within the infected intravenous drug users, are also co-infected with the HCV. HCV infection results in liver diseases and accelerates death in those with HIV infection (Bica et al, 2001; Hernandez and Sherman, 2011). In co-infected individuals, there appears to be a link with neurocognitive impairments (Anand et al, 2010; Letendre et al, 2007) and the development of HAD (Nath et al, 2008; Valcour et al, 2011). Expression of HCV core proteins leads to the translocation of Bcl-2–associated X (Bax) protein from the cytosol to the mitochondria, where it leads to apoptosis (Aoki et al, 2000). 14-3-3ε binds to the HCV core protein and blocks Bax binding leading to caspase-dependent and independent apoptotic pathways (Lee et al, 2007). In addition, binding with HCV core protein activates Raf-1 kinase, which in turn affects hepatocyte growth regulation (Aoki et al, 2000; Nakamura et al, 2011). Interaction between 14-3-3s and FoxO1 is also important in translocation from the nucleus to the cytoplasm, which is blocked in HCV core-expressing cells (Banerjee et al, 2010). The direct interaction between 14-3-3s and HCV core proteins emphasize the importance of understanding 14-3-3s in HIV disease progression and underlines their potential as therapeutic targets in co-infected individuals.
What are the Consequences of genetic alterations to YWHAE/14-3-3ε?
YWHAE/14-3-3ε is expressed in cultured astrocytes and in the cerebral cortex, corpus callosum, frontal lobe, parietal lobe, temporal lobe, medulla oblongata, hippocampus, pons, and cerebellum (Mignon-Ravix et al, 2010). In animal models, 14-3-3ε is present homogeneously throughout brain neuropil areas and in high levels in synapses, co-localizing with synaptosomes (Baxter et al, 2002b; Bi et al, 2009; Martin et al, 1994), suggesting that 14-3-3ε may serve as a good biomarker in the CNS for degenerating synapses and by extension neurodegeneration in general.. But what happens in the brain if there are genetic alterations of YWHAE?
In humans, Miller-Dieker syndrome is caused by a deletion or duplication of genes on the 17p13 chromosome including YWHAE (Table 3) (Bi et al, 2009; Bruno et al, 2010; Cardoso et al, 2003; Hyon et al, 2011; Shimojima et al, 2011; Tenney et al, 2011). YWHAE appears to be the crucial gene, depending on which other genes are affected; whose loss leads both to neurocognitive deficits including learning disabilities, autism, epilepsy, and attention deficient hyperactivity disorder (ADHD) and to lissencephaly (Table 3) (Bi et al, 2009; Bruno et al, 2010; Cardoso et al, 2003; Hyon et al, 2011; Shimojima et al, 2011; Tenney et al, 2011). In animal models, both the duplication and the deletion of YWHAE lead to anomalous neuronal migration, which likely underlies the lissencephaly phenotypes (Table 4) (Bi et al, 2009; Spalice et al, 2009; Toyo-oka et al, 2003; Yingling et al, 2003). Heterozygous mice present reduced learning and memory and heightened anxiety (Table 4) (Ikeda et al, 2008), suggesting that YWHAE is essential for normal neuronal development and function.
Table 3.
YWHAE/14-3-3ε genetic alterations in human neurological disorders
| Genetic Alteration | Human Condition/Disease | Effect/Symptoms | References |
|---|---|---|---|
| Deletion along with TUSC5, MYO1C, CRK, LIS1 | Miller-Dieker syndrome | Severely reduced intellectual abilities, developmental delay, seizures | (Bi et al, 2009) |
| Duplication with TUSC5, MYO1C, CRK, LIS1 | Developmental delay | ADHD, autism | (Bi et al, 2009) |
| Deletion with TUSC5 | Developmental delay | Learning Difficulties | (Bi et al, 2009) |
| Microdeletion with CRK, LIS1 | Miller-Dieker syndrome with ILS | Severe brain malformations, cortical thickening | (Cardoso et al, 2003 |
| Microduplication with TUSC5 | Miller-Dieker syndrome with Autism | Autistic behavior | (Bruno et al, 2010) |
| Microduplication with LIS1 | Miller-Dieker syndrome | Moderate psychomotor retardation, speech delays, behavioral problem | (Hyon et al, 2011) |
| Deletion with CRK but not LIS1 | Miller-Dieker syndrome with Epilepsy | Generalized epilepsy, developmental delay, and non-specific white, matter changes. | (Shimojima et al, 2011; Tenney et al, 2011) |
| Polymorphisms | Schizophrenia | Frequency of SNPs different in cases vs. controls. | (Ikeda et al, 2008) |
| Polymorphisms | Schizophrenia, Bipolar Disorder | No Association | (Liu et al, 2011) |
| Polymorphism (rs34137556) | Schizophrenia | No Association | (Moens et al, 2011) |
ILS: Isolated lissencephaly; CRK: viral oncogene causes increased tyrosine-phosophorylated proteins; TUSC5: tumor suppressor candidate 5; MYO1C: Myosin-1C; LIS1: encodes subunit of platelet-activating factor acetylhydrolase 1B (PAFAH1B1); ADHD: Attention deficit hyperactivity disorder
Given the apparent importance of YWHAE in neuronal structure and function, are there polymorphisms in the gene associated with pathologies? Single nucleotide polymorphisms (SNP) in YWHAE were assayed for a possible relationship with schizophrenia, which is a complex mental disorder with a fairly high degree of heritability (Table 3) (Ikeda et al, 2008). Only one study has found SNPs associated with schizophrenia and others apparently associated with reduced risk for the condition (Table 3) (Ikeda et al, 2008). This suggests that perhaps increased YWHAE expression in humans carrying the identified SNP is protective. However, two other studies indicate no association between YWHAE SNPs in schizophrenia or bi-polar disorders (Table 3) (Liu et al, 2011; Moens et al, 2011). Another study examined YWHAE SNPs from individuals who committed suicide, and proposed that it is a potential suicide susceptibility gene (Yanagi et al, 2005). The effects of polymorphism (if any) in rodent models have not been reported yet. We propose that studies should examine if there is an association between HIV and HCV neurodegeneration and the YWHAE SNPs and whether there are other polymorphisms in other 14-3-3 isoforms related to HAND and/or other neurodegenerative disorders.
Is YWHAE/14-3-3ε a biomarker for HIV-dependent neurodegeneration?
Can 14-3-3ε protein levels be used to track disease progression? This is supported by studies indicating that 14-3-3ε is present in the CSF in those with HIVE and/or HAD (Gelman and Nguyen, 2010). 14-3-3ε does interact with Vpr, modulating G(2)/M cell-cycle arrest via Cdc25C phosphorylation-dependent association (Figure 2) (Matsuda et al, 2006). Also, it directly interacts with GPR15 HIV accessory protein to modulate receptor stability (Shikano et al, 2005). In addition, gp120 levels, which regulate cell cycle and apoptosis, are inversely related to those of 14-3-3ε (Figure 2) (Kapasi et al, 2001). In human 293T cells, cleavage of 14-3-3ε releases BAD, facilitating its translocation and subsequent interaction with Bcl to promote cell death (Fong et al, 2010; Won et al, 2003). 14-3-3ε also interacts with the core protein of HCV a commonly co-infecting virus in HIV patients an interaction which regulates apoptosis. There is also evidence that in normal cells, 14-3-3ε is necessary for maintaining neuronal integrity by promoting both survival and neuronal regeneration (Berg et al, 2003a; Datta et al, 2000). Therefore, the collective evidence clearly indicates that 14-3-3ε is involved in multiple processes implicated in HIV pathogenesis and disease progression.
Conclusion
14-3-3s are present in the CSF of those with HIV. Many of the isoforms, including YWHAE/14-3-3ε either directly or indirectly interact and modulate HIV-related proteins involved in BBB trafficking, stability of receptors, apoptosis, and cell-cycle arrest. Taking that into consideration, we propose that 14-3-3ε would be an appropriate biomarker for HIV-related neurodegeneration and that, additionally, it may also offer a target for therapeutic intervention.
We propose that the presence of 14-3-3 proteins in the CSF of HIV seropositive patients is likely the consequence of apoptotic or necrotic lysis of neurons and their release in the CSF of HIV-infected patients. In humans, CSF volume is about 150 ml and the rate of CSF production is about 550 ml/day, indicating that 14-3-3 proteins in CSF are turned over about 3.7 times per day (Thomson and Bertram, 2001; Wakabayashi et al, 2001). Hence, 14-3-3 proteins in CSF might be a biomarker reflecting the state of neuronal destruction and neurodegeneration. However, further studies looking at the prognostic significance of specific antibodies against 14-3-3 isoforms are required.
Acknowledgments
The study was funded by National Center for Research Resources (NCRR) grant 1U54RR026139-01A1 (awarded to the University of Puerto Rico-Medical Science Campus (UPR-MSC)). This publication (journal article, etc.) was supported by a grant from the Johns Hopkins NIMH Center for Novel Therapeutics of HIV-associated Cognitive Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of The Johns Hopkins University or any grantor providing funds to its NIMH Center for Novel Therapeutics of HIV-associated Cognitive Disorders with special thanks to Dr. Avindra Nath and Dr. Valerie Wojna. The study was partially supported by National Institute of Neurological Disorders and Stroke (NINDS) grants S11NS46278 and U54NS43011 (SNRP). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIMH, or NINDS. We acknowledge the support of Tirtsa Porrata-Doria and the Molecular Biology Core Lab of the Ponce School of Medicine and Health Sciences (Grant RR003050). Special thanks go to Robert Ritchie of the RCMI/Ponce School of Medicine and Health Sciences Publications Office (G12 RR003050) for editing services.
Abbreviations
- ACD
AIDS dementia complex
- AD
Alzheimer’s disease
- ADHD
Attention deficient hyperactivity disorder
- AIDS
Acquired Immunodeficiency Virus (AIDS) dementia complex (ADC)
- BAD
B-cell lymphoma 2 antagonist of cell death
- BAX
Bcl-2–associated X
- Bax
Bcl-2–associated X
- BBB
Blood–brain barrier
- C. elegan
Caenorhabditis elegans
- Cdc25
Cell division cycle phosphatase 25
- CDKs
Cyclin-dependent protein kinases
- CJD
Creutzfeldt–Jakob disease
- CME
Cytomegalovirus encephalitis
- CNS
Central nervous system
- CRK
Viral oncogene causes increased tyrosine-phosphorylated proteins
- CSF
Cerebral spinal fluid
- CXCR4
CXC chemokine receptor 4
- DCAF-1
DNA binding protein 1 and Cullin 4a-associated factor
- FoxO
Forkhead transcription factor
- Gp120
Glycoprotein 120
- GPR15
G protein receptor 15
- GPRs
G protein cell receptors
- HAD
HIV-associated dementia
- HADC
HIV-associated dementia complex
- HAND
HIV-Associated Neurocognitive Disorders
- HBMEC
Human Brain microvascular endothelial cells
- HBMECs
Human brain microvascular endothelial cells
- HCV
Hepatitis C virus
- HCV
Hepatitis C virus
- HEK293
Human embryonic kidney
- Hela
Human cervical carcinoma
- HepG2
Human hepatoma
- HIV
Human Immune deficiency virus
- HIVE
HIV encephalitis
- HMC
Human mesangial growth cells
- HUVEC
Human umbilical vein endothelial cells
- IL
Interleukin
- ILK
Isolated lissencephaly
- K2P
Potassium channel
- LB
Lewy bodies
- LISI
Encodes subunit of platelet-activating factor acetylhydrolase 1B (PAFAH1B1)
- MDS
Miller-Dieker Syndrome
- MS
Multiple Sclerosis (MS)
- MYO1C
Myosin-1C
- Nef
Negative factor
- PKA
Protein kinase A
- PKC
Protein kinase C
- Raf
Proto-oncogene serine/threonine-protein kinase
- RNAi
RNA interference
- S. pombe
Schizosaccharomyces pombe
- siRNA
Single stranded RNA
- SIV
Simian immunodeficiency virus
- TAU
Tubulin-Associated Unit
- TUSC5
Tumor suppressor candidate 5
- Vpr
Viral protein R
- Vpu
Viral protein U
- Ywhae−/−
Ywhae/14-3-3ε-deficient mice
- YWHEA
14-3-3ε (human gene)
Footnotes
Competing interest
The authors have no conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
References
- Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Aitken A, Amess B, Howell S, Jones D, Martin H, Patel Y, Robinson K, Toker A. The role of specific isoforms of 14-3-3 protein in regulating protein kinase activity in the brain. Biochem Soc Trans. 1992a;20:607–611. doi: 10.1042/bst0200607. [DOI] [PubMed] [Google Scholar]
- Aitken A, Baxter H, Dubois T, Clokie S, Mackie S, Mitchell K, Peden A, Zemlickova E. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans. 2002;30:351–360. doi: 10.1042/bst0300351. [DOI] [PubMed] [Google Scholar]
- Aitken A, Collinge DB, van Heusden BP, Isobe T, Roseboom PH, Rosenfeld G, Soll J. 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci. 1992b;17:498–501. doi: 10.1016/0968-0004(92)90339-b. [DOI] [PubMed] [Google Scholar]
- Aitken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological function and domain structure. Biochem Soc Trans. 1995;23:605–611. doi: 10.1042/bst0230605. [DOI] [PubMed] [Google Scholar]
- Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav. 2010;14:1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Hayashi J, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J Virol. 2000;74:1736–1741. doi: 10.1128/jvi.74.4.1736-1741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl JM, Heegaard NH, Falkenhorst G, Laursen H, Hogenhaven H, Molbak K, Jespersgaard C, Hougs L, Waldemar G, Johannsen P, Christiansen M. The diagnostic efficiency of biomarkers in sporadic Creutzfeldt-Jakob disease compared to Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Meyer K, Mazumdar B, Ray RB, Ray R. Hepatitis C virus differentially modulates activation of forkhead transcription factors and insulin-induced metabolic gene expression. J Virol. 2010;84:5936–5946. doi: 10.1128/JVI.02344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Archelos JJ. Tau protein and 14-3-3 are elevated in the cerebrospinal fluid of patients with multiple sclerosis and correlate with intrathecal synthesis of IgG. J Neurol. 2004;251:414–420. doi: 10.1007/s00415-004-0336-0. [DOI] [PubMed] [Google Scholar]
- Baxter HC, Fraser JR, Liu WG, Forster JL, Clokie S, Steinacker P, Otto M, Bahn E, Wiltfang J, Aitken A. Specific 14-3-3 isoform detection and immunolocalization in prion diseases. Biochem Soc Trans. 2002a;30:387–391. doi: 10.1042/bst0300387. [DOI] [PubMed] [Google Scholar]
- Baxter HC, Liu WG, Forster JL, Aitken A, Fraser JR. Immunolocalisation of 14-3-3 isoforms in normal and scrapie-infected murine brain. Neuroscience. 2002b;109:5–14. doi: 10.1016/s0306-4522(01)00492-4. [DOI] [PubMed] [Google Scholar]
- Bazan HA, Alkhatib G, Broder CC, Berger EA. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 2007;3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003a;4:752–762. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- Berg D, Riess O, Bornemann A. Specification of 14-3-3 proteins in Lewy bodies. Ann Neurol. 2003b;54:135. doi: 10.1002/ana.10621. [DOI] [PubMed] [Google Scholar]
- Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bertrand A, Brandel JP, Grignon Y, Sazdovitch V, Seilhean D, Faucheux B, Privat N, Brault JL, Vital A, Uro-Coste E, Pluot M, Chapon F, Maurage CA, Letournel F, Vespignani H, Place G, Degos CF, Peoc’h K, Haik S, Hauw JJ. Wernicke encephalopathy and Creutzfeldt-Jakob disease. J Neurol. 2009;256:904–909. doi: 10.1007/s00415-009-5038-1. [DOI] [PubMed] [Google Scholar]
- Bi W, Sapir T, Shchelochkov OA, Zhang F, Withers MA, Hunter JV, Levy T, Shinder V, Peiffer DA, Gunderson KL, Nezarati MM, Shotts VA, Amato SS, Savage SK, Harris DJ, Day-Salvatore DL, Horner M, Lu XY, Sahoo T, Yanagawa Y, Beaudet AL, Cheung SW, Martinez S, Lupski JR, Reiner O. Increased LIS1 expression affects human and mouse brain development. Nat Genet. 2009;41:168–177. doi: 10.1038/ng.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- Bolton DL, Barnitz RA, Sakai K, Lenardo MJ. 14-3-3 theta binding to cell cycle regulatory factors is enhanced by HIV-1 Vpr. Biol Direct. 2008;3:17. doi: 10.1186/1745-6150-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston PF, Jackson P, Thompson RJ. Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem. 1982;38:1475–1482. doi: 10.1111/j.1471-4159.1982.tb07928.x. [DOI] [PubMed] [Google Scholar]
- Brandel JP, Delasnerie-Laupretre N, Laplanche JL, Hauw JJ, Alperovitch A. Diagnosis of Creutzfeldt-Jakob disease: effect of clinical criteria on incidence estimates. Neurology. 2000;54:1095–1099. doi: 10.1212/wnl.54.5.1095. [DOI] [PubMed] [Google Scholar]
- Bruno DL, Anderlid BM, Lindstrand A, van Ravenswaaij-Arts C, Ganesamoorthy D, Lundin J, Martin CL, Douglas J, Nowak C, Adam MP, Kooy RF, Van der Aa N, Reyniers E, Vandeweyer G, Stolte-Dijkstra I, Dijkhuizen T, Yeung A, Delatycki M, Borgstrom B, Thelin L, Cardoso C, van Bon B, Pfundt R, de Vries BB, Wallin A, Amor DJ, James PA, Slater HR, Schoumans J. Further molecular and clinical delineation of co-locating 17p13.3 microdeletions and microduplications that show distinctive phenotypes. J Med Genet. 2010;47:299–311. doi: 10.1136/jmg.2009.069906. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Leventer RJ, Ward HL, Toyo-Oka K, Chung J, Gross A, Martin CL, Allanson J, Pilz DT, Olney AH, Mutchinick OM, Hirotsune S, Wynshaw-Boris A, Dobyns WB, Ledbetter DH. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13.3. Am J Hum Genet. 2003;72:918–930. doi: 10.1086/374320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Colucci M, Xie Z, Zou W, Li C, Parchi P, Capellari S, Pastore M, Rahbar MH, Chen SG, Gambetti P. Sensitivity of 14-3-3 protein test varies in subtypes of sporadic Creutzfeldt-Jakob disease. Neurology. 2004;63:436–442. doi: 10.1212/01.wnl.0000135153.96325.3b. [DOI] [PubMed] [Google Scholar]
- Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, Leigh RJ, Cohen ML. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol. 2011;70:437–444. doi: 10.1002/ana.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan G, Pennington C, Mackenzie JM, Andrews M, Everington D, Will RG, Knight RS, Green AJ. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry. 2010;81:1243–1248. doi: 10.1136/jnnp.2009.197962. [DOI] [PubMed] [Google Scholar]
- Chung JJ, Okamoto Y, Coblitz B, Li M, Qiu Y, Shikano S. PI3K/Akt signalling-mediated protein surface expression sensed by 14-3-3 interacting motif. FEBS J. 2009;276:5547–5558. doi: 10.1111/j.1742-4658.2009.07241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990a;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990b;3:11–18. [PubMed] [Google Scholar]
- Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988;334:532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- Collins SJ, McGlade A, Boyd A, Masters CL, Klug GM. 14-3-3 protein detection and sporadic CJD: the status quo serves well while awaiting progress. J Neurol Neurosurg Psychiatry. 2010;81:1181. doi: 10.1136/jnnp.2010.219691. [DOI] [PubMed] [Google Scholar]
- Creutzfeld HG. Uber eine egenartige herdformige erkrankung des zentralnervensystems. Neurol Psychiat. 1920;57:1–18. [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- de Seze J, Peoc’h K, Ferriby D, Stojkovic T, Laplanche JL, Vermersch P. 14-3-3 Protein in the cerebrospinal fluid of patients with acute transverse myelitis and multiple sclerosis. J Neurol. 2002;249:626–627. doi: 10.1007/s004150200074. [DOI] [PubMed] [Google Scholar]
- Dube M, Bego MG, Paquay C, Cohen EA. Modulation of HIV-1-host interaction: role of the Vpu accessory protein. Retrovirology. 2010;7:114. doi: 10.1186/1742-4690-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JH, Hall RA. Enhancement of the surface expression of G protein-coupled receptors. Trends Biotechnol. 2009;27:541–545. doi: 10.1016/j.tibtech.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder RT, Yu M, Chen M, Zhu X, Yanagida M, Zhao Y. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology. 2001;287:359–370. doi: 10.1006/viro.2001.1007. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal number and volume alterations in the neocortex of HIV infected individuals. J Neurol Neurosurg Psychiatry. 1993;56:481–486. doi: 10.1136/jnnp.56.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini M, Zanusso G, Benedetti MD, Righetti PG, Monaco S. Cerebrospinal fluid biomarkers in clinically isolated syndromes and multiple sclerosis. Proteomics Clin Appl. 2007;1:963–971. doi: 10.1002/prca.200700091. [DOI] [PubMed] [Google Scholar]
- Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180–186. doi: 10.1007/s12035-010-8103-y. [DOI] [PubMed] [Google Scholar]
- Foster JL, Denial SJ, Temple BR, Garcia JV. Mechanisms of HIV-1 Nef function and intracellular signaling. J Neuroimmune Pharmacol. 2011;6:230–246. doi: 10.1007/s11481-011-9262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SP. Host factors exploited by retroviruses. Nat Rev Microbiol. 2007;5:253–263. doi: 10.1038/nrmicro1541. [DOI] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod MA, Ironside JW, Will RG, Knight RS. Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;70:744–748. doi: 10.1136/jnnp.70.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Sobue K, Paudel HK. 14-3-3zeta is an effector of tau protein phosphorylation. J Biol Chem. 2000;275:25247–25254. doi: 10.1074/jbc.M003738200. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helke KL, Queen SE, Tarwater PM, Turchan-Cholewo J, Nath A, Zink MC, Irani DN, Mankowski JL. 14-3-3 protein in CSF: an early predictor of SIV CNS disease. J Neuropathol Exp Neurol. 2005;64:202–208. doi: 10.1093/jnen/64.3.202. [DOI] [PubMed] [Google Scholar]
- Hernandez MD, Sherman KE. HIV/hepatitis C coinfection natural history and disease progression. Curr Opin HIV AIDS. 2011;6:478–482. doi: 10.1097/COH.0b013e32834bd365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med. 1996;335:924–930. doi: 10.1056/NEJM199609263351303. [DOI] [PubMed] [Google Scholar]
- Hsu K, Seharaseyon J, Dong P, Bour S, Marban E. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol Cell. 2004;14:259–267. doi: 10.1016/s1097-2765(04)00183-2. [DOI] [PubMed] [Google Scholar]
- Huang N, Marie SK, Livramento JA, Chammas R, Nitrini R. 14-3-3 protein in the CSF of patients with rapidly progressive dementia. Neurology. 2003;61:354–357. doi: 10.1212/01.wnl.0000078890.89473.ed. [DOI] [PubMed] [Google Scholar]
- Hyon C, Marlin S, Chantot-Bastaraud S, Mabboux P, Beaujard MP, Al Ageeli E, Vazquez MP, Picard A, Siffroi JP, Portnoi MF. A new 17p13.3 microduplication including the PAFAH1B1 and YWHAE genes resulting from an unbalanced X;17 translocation. Eur J Med Genet. 2011;54:287–291. doi: 10.1016/j.ejmg.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hikita T, Taya S, Uraguchi-Asaki J, Toyo-oka K, Wynshaw-Boris A, Ujike H, Inada T, Takao K, Miyakawa T, Ozaki N, Kaibuchi K, Iwata N. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- Irani DN, Kerr DA. 14-3-3 protein in the cerebrospinal fluid of patients with acute transverse myelitis. Lancet. 2000;355:901. doi: 10.1016/S0140-6736(99)04745-5. [DOI] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–3711. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi AA, Fan S, Singhal PC. Role of 14-3-3epsilon, c-Myc/Max, and Akt phosphorylation in HIV-1 gp 120-induced mesangial cell proliferation. Am J Physiol Renal Physiol. 2001;280:F333–342. doi: 10.1152/ajprenal.2001.280.2.F333. [DOI] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes. 2005a;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Valentin A, Tsopanomihalou M, Ilyina-Gragerova G, Erwin-Cohen R, Chrousos GP, Pavlakis GN. Vpr protein of human immunodeficiency virus type 1 binds to 14-3-3 proteins and facilitates complex formation with Cdc25C: implications for cell cycle arrest. J Virol. 2005b;79:2780–2787. doi: 10.1128/JVI.79.5.2780-2787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Pavlakis GN. Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1. DNA Cell Biol. 2004;23:193–205. doi: 10.1089/104454904773819789. [DOI] [PubMed] [Google Scholar]
- Kogan M, Rappaport J. HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology. 2011;8:25. doi: 10.1186/1742-4690-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Park SO, Joe CO, Kim YS. Interaction of HCV core protein with 14-3-3epsilon protein releases Bax to activate apoptosis. Biochem Biophys Res Commun. 2007;352:756–762. doi: 10.1016/j.bbrc.2006.11.098. [DOI] [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992a;15:75–79. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992b;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou G, Ji W, Li J, Li T, Wang T, Li Y, Zeng Z, Hu Z, Zheng L, Ji J, Wang Y, Wei Z, Feng G, He L, Shi Y. No association of the YWHAE gene with schizophrenia, major depressive disorder or bipolar disorder in the Han Chinese population. Behav Genet. 2011;41:557–564. doi: 10.1007/s10519-010-9426-1. [DOI] [PubMed] [Google Scholar]
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Martin H, Rostas J, Patel Y, Aitken A. Subcellular localisation of 14-3-3 isoforms in rat brain using specific antibodies. J Neurochem. 1994;63:2259–2265. doi: 10.1046/j.1471-4159.1994.63062259.x. [DOI] [PubMed] [Google Scholar]
- Mathie A, Rees KA, El Hachmane MF, Veale EL. Trafficking of neuronal two pore domain potassium channels. Curr Neuropharmacol. 2010;8:276–286. doi: 10.2174/157015910792246146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Tanaka H, Yamazaki S, Suzuki J, Tanaka K, Yamada T, Masuda M. HIV-1 Vpr induces G2 cell cycle arrest in fission yeast associated with Rad24/14-3-3-dependent, Chk1/Cds1-independent Wee1 upregulation. Microbes Infect. 2006;8:2736–2744. doi: 10.1016/j.micinf.2006.08.003. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Meller N, Altman A, Isakov N. New perspectives on PKCtheta, a member of the novel subfamily of protein kinase C. Stem Cells. 1998;16:178–192. doi: 10.1002/stem.160178. [DOI] [PubMed] [Google Scholar]
- Meller N, Liu YC, Collins TL, Bonnefoy-Berard N, Baier G, Isakov N, Altman A. Direct interaction between protein kinase C theta (PKC theta) and 14-3-3 tau in T cells: 14-3-3 overexpression results in inhibition of PKC theta translocation and function. Mol Cell Biol. 1996;16:5782–5791. doi: 10.1128/mcb.16.10.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon-Ravix C, Cacciagli P, El-Waly B, Moncla A, Milh M, Girard N, Chabrol B, Philip N, Villard L. Deletion of YWHAE in a patient with periventricular heterotopias and pronounced corpus callosum hypoplasia. J Med Genet. 2010;47:132–136. doi: 10.1136/jmg.2009.069112. [DOI] [PubMed] [Google Scholar]
- Miller RF, Green AJ, Giovannoni G, Thompson EJ. Detection of 14-3-3 brain protein in cerebrospinal fluid of HIV infected patients. Sex Transm Infect. 2000;76:408. doi: 10.1136/sti.76.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens LN, De Rijk P, Reumers J, Van den Bossche MJ, Glassee W, De Zutter S, Lenaerts AS, Nordin A, Nilsson LG, Medina Castello I, Norrback KF, Goossens D, Van Steen K, Adolfsson R, Del-Favero J. Sequencing of DISC1 pathway genes reveals increased burden of rare missense variants in schizophrenia patients from a northern Swedish population. PLoS One. 2011;6:e23450. doi: 10.1371/journal.pone.0023450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BW, Perez VJ. Physiological and biochemical aspects of nervous integration. Prentice-Hall; New York, NY: 1967. [Google Scholar]
- Muslin AJ, Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12:703–709. doi: 10.1016/s0898-6568(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Aoki H, Hino O, Moriyama M. HCV core protein promotes heparin binding EGF-like growth factor expression and activates Akt. Hepatol Res. 2011;41:455–462. doi: 10.1111/j.1872-034X.2011.00792.x. [DOI] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Shikano S. Phosphorylation-dependent C-terminal binding of 14-3-3 proteins promotes cell surface expression of HIV co-receptor GPR15. J Biol Chem. 2011;286:7171–7181. doi: 10.1074/jbc.M110.199695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M, Wiltfang J, Cepek L, Neumann M, Mollenhauer B, Steinacker P, Ciesielczyk B, Schulz-Schaeffer W, Kretzschmar HA, Poser S. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2002;58:192–197. doi: 10.1212/wnl.58.2.192. [DOI] [PubMed] [Google Scholar]
- Peoc’h K, Delasnerie-Laupretre N, Beaudry P, Laplanche JL. Diagnostic value of CSF 14-3-3 detection in sporadic CJD diagnosis according to the age of the patient. Eur J Neurol. 2006;13:427–428. doi: 10.1111/j.1468-1331.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- Plant LD, Rajan S, Goldstein SA. K2P channels and their protein partners. Curr Opin Neurobiol. 2005;15:326–333. doi: 10.1016/j.conb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Poser S, Mollenhauer B, Kraubeta A, Zerr I, Steinhoff BJ, Schroeter A, Finkenstaedt M, Schulz-Schaeffer WJ, Kretzschmar HA, Felgenhauer K. How to improve the clinical diagnosis of Creutzfeldt-Jakob disease. Brain. 1999;122 (Pt 12):2345–2351. doi: 10.1093/brain/122.12.2345. [DOI] [PubMed] [Google Scholar]
- Rittinger K, Budman J, Xu J, Volinia S, Cantley LC, Smerdon SJ, Gamblin SJ, Yaffe MB. Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol Cell. 1999;4:153–166. doi: 10.1016/s1097-2765(00)80363-9. [DOI] [PubMed] [Google Scholar]
- Saiz A, Graus F, Dalmau J, Pifarre A, Marin C, Tolosa E. Detection of 14-3-3 brain protein in the cerebrospinal fluid of patients with paraneoplastic neurological disorders. Ann Neurol. 1999;46:774–777. [PubMed] [Google Scholar]
- Sanchez-Valle R, Saiz A, Graus F. 14-3-3 Protein isoforms and atypical patterns of the 14-3-3 assay in the diagnosis of Creutzfeldt-Jakob disease. Neurosci Lett. 2002;320:69–72. doi: 10.1016/s0304-3940(02)00045-9. [DOI] [PubMed] [Google Scholar]
- Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano S, Coblitz B, Sun H, Li M. Genetic isolation of transport signals directing cell surface expression. Nat Cell Biol. 2005;7:985–992. doi: 10.1038/ncb1297. [DOI] [PubMed] [Google Scholar]
- Shimojima K, Sugiura C, Takahashi H, Ikegami M, Takahashi Y, Ohno K, Matsuo M, Saito K, Yamamoto T. Genomic copy number variations at 17p13.3 and epileptogenesis. Epilepsy Res. 2011;89:303–309. doi: 10.1016/j.eplepsyres.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Smith BL, Krushelnycky BW, Mochly-Rosen D, Berg P. The HIV nef protein associates with protein kinase C theta. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- Spalice A, Parisi P, Nicita F, Pizzardi G, Del Balzo F, Iannetti P. Neuronal migration disorders: clinical, neuroradiologic and genetics aspects. Acta Paediatr. 2009;98:421–433. doi: 10.1111/j.1651-2227.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Steinacker P, Aitken A, Otto M. 14-3-3 proteins in neurodegeneration. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Jowett JB, Chen IS. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Iwata T, Kitagawa Y, Takahashi RH, Sato Y, Wakabayashi H, Takashima M, Kido H, Nagashima K, Kenney K, Gibbs CJ, Jr, Kurata T. Increased levels of epsilon and gamma isoforms of 14-3-3 proteins in cerebrospinal fluid in patients with Creutzfeldt-Jakob disease. Clin Diagn Lab Immunol. 1999;6:983–985. doi: 10.1128/cdli.6.6.983-985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y. The 14-3-3 proteins: gene, gene expression, and function. Neurochem Res. 2003;28:1265–1273. doi: 10.1023/a:1024296932670. [DOI] [PubMed] [Google Scholar]
- Tenney JR, Hopkin RJ, Schapiro MB. Deletion of 14-3-3{varepsilon} and CRK: a clinical syndrome with macrocephaly, developmental delay, and generalized epilepsy. J Child Neurol. 2011;26:223–227. doi: 10.1177/0883073810379638. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Jr, Bertram H. Laboratory diagnosis of central nervous system infections. Infect Dis Clin North Am. 2001;15:1047–1071. doi: 10.1016/s0891-5520(05)70186-0. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, Ward HL, Ayala R, Tsai LH, Dobyns W, Ledbetter D, Hirotsune S, Wynshaw-Boris A. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- Tschampa HJ, Neumann M, Zerr I, Henkel K, Schroter A, Schulz-Schaeffer WJ, Steinhoff BJ, Kretzschmar HA, Poser S. Patients with Alzheimer’s disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;71:33–39. doi: 10.1136/jnnp.71.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JJ, Csikasz-Nagy A, Novak B. The dynamics of cell cycle regulation. Bioessays. 2002;24:1095–1109. doi: 10.1002/bies.10191. [DOI] [PubMed] [Google Scholar]
- Ullrich CK, Groopman JE, Ganju RK. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood. 2000;96:1438–1442. [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Littman DR. G protein-coupled receptors in HIV and SIV entry: new perspectives on lentivirus-host interactions and on the utility of animal models. Semin Immunol. 1998;10:225–236. doi: 10.1006/smim.1998.0134. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Nishio O, Klocking R, Perovic S, Muller WE. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor-mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–1359. doi: 10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden GP. 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life. 2005;57:623–629. doi: 10.1080/15216540500252666. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H, Yano M, Tachikawa N, Oka S, Maeda M, Kido H. Increased concentrations of 14-3-3 epsilon, gamma and zeta isoforms in cerebrospinal fluid of AIDS patients with neuronal destruction. Clin Chim Acta. 2001;312:97–105. doi: 10.1016/s0009-8981(01)00595-2. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- Wang W, Shakes DC. Molecular evolution of the 14-3-3 protein family. J Mol Evol. 1996;43:384–398. doi: 10.1007/BF02339012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jacobs C, Hook KE, Duan H, Booher RN, Sun Y. Binding of 14-3-3beta to the carboxyl terminus of Wee1 increases Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 2000;11:211–219. [PubMed] [Google Scholar]
- Watanabe M, Isobe T, Okuyama T, Ichimura T, Kuwano R, Takahashi Y, Kondo H. Molecular cloning of cDNA to rat 14-3-3 eta chain polypeptide and the neuronal expression of the mRNA in the central nervous system. Brain Res Mol Brain Res. 1991;10:151–158. doi: 10.1016/0169-328x(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Wiltfang J, Otto M, Baxter HC, Bodemer M, Steinacker P, Bahn E, Zerr I, Kornhuber J, Kretzschmar HA, Poser S, Ruther E, Aitken A. Isoform pattern of 14-3-3 proteins in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J Neurochem. 1999;73:2485–2490. doi: 10.1046/j.1471-4159.1999.0732485.x. [DOI] [PubMed] [Google Scholar]
- Won J, Kim DY, La M, Kim D, Meadows GG, Joe CO. Cleavage of 14-3-3 protein by caspase-3 facilitates bad interaction with Bcl-x(L) during apoptosis. J Biol Chem. 2003;278:19347–19351. doi: 10.1074/jbc.M213098200. [DOI] [PubMed] [Google Scholar]
- Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, Gamblin SJ. Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature. 1995;376:188–191. doi: 10.1038/376188a0. [DOI] [PubMed] [Google Scholar]
- Yaffe MB. How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513:53–57. doi: 10.1016/s0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- Yanagi M, Shirakawa O, Kitamura N, Okamura K, Sakurai K, Nishiguchi N, Hashimoto T, Nushida H, Ueno Y, Kanbe D, Kawamura M, Araki K, Nawa H, Maeda K. Association of 14-3-3 epsilon gene haplotype with completed suicide in Japanese. J Hum Genet. 2005;50:210–216. doi: 10.1007/s10038-005-0241-0. [DOI] [PubMed] [Google Scholar]
- Yano M, Nakamuta S, Shiota M, Endo H, Kido H. Gatekeeper role of 14-3-3tau protein in HIV-1 gp120-mediated apoptosis of human endothelial cells by inactivation of Bad. AIDS. 2007;21:911–920. doi: 10.1097/QAD.0b013e32810539f3. [DOI] [PubMed] [Google Scholar]
- Yingling J, Toyo-Oka K, Wynshaw-Boris A. Miller-Dieker syndrome: analysis of a human contiguous gene syndrome in the mouse. Am J Hum Genet. 2003;73:475–488. doi: 10.1086/378096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro Cuesta J, Knight RS, Bernheimer H, Cardone F, Delasnerie-Laupretre N, Cuadrado Corrales N, Ladogana A, Bodemer M, Fletcher A, Awan T, Ruiz Bremon A, Budka H, Laplanche JL, Will RG, Poser S. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology. 2000a;55:811–815. doi: 10.1212/wnl.55.6.811. [DOI] [PubMed] [Google Scholar]
- Zerr I, Poser S. Clinical diagnosis and differential diagnosis of CJD and vCJD. With special emphasis on laboratory tests. APMIS. 2002;110:88–98. doi: 10.1034/j.1600-0463.2002.100111.x. [DOI] [PubMed] [Google Scholar]
- Zerr I, Schulz-Schaeffer WJ, Giese A, Bodemer M, Schroter A, Henkel K, Tschampa HJ, Windl O, Pfahlberg A, Steinhoff BJ, Gefeller O, Kretzschmar HA, Poser S. Current clinical diagnosis in Creutzfeldt-Jakob disease: identification of uncommon variants. Ann Neurol. 2000b;48:323–329. [PubMed] [Google Scholar]
- Zhao LJ, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994a;269:15577–15582. [PubMed] [Google Scholar]
- Zhao LJ, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J Biol Chem. 1994b;269:32131–32137. [PubMed] [Google Scholar]