Introduction
Daily maintenance therapy with an inhaled corticosteroid (ICS) can achieve optimal disease control for many patients with persistent asthma, provided that individuals adhere to their prescribed regimen. However, adherence to ICS therapy is often suboptimal with rates of use ranging from 30% - 70% of the prescribed dose.(1-5) We have recently shown that the therapeutic benefit of ICS therapy appears to have a threshold, such that asthma exacerbation rates were lower primarily among individuals taking >75% of their medication as prescribed.(6) Therefore, the threshold for achieving optimal benefit from ICS treatment appears to be considerably higher than the level of usage seen in most patients.
Unfortunately, it has often proved difficult to affect changes in patient behavior which result in improved medication adherence.(3;7) Nevertheless, studies in a variety of chronic conditions have found dosage simplification (i.e., less frequent dosing) to be associated with higher medication adherence.(8-12) These findings extend to at least one asthma clinical trial which found higher adherence rates among patients treated with once-a-day ICS dosing when compared with those treated with twice daily ICS dosing.(13) It is not unusual, however, for clinical trials to observe higher adherence rates than those found in routine practice.(14;15 The objective of the present study was to determine the real-world implications of once-daily ICS dosing as compared with multiple-daily dosing on adherence among a large, diverse patient population with asthma.
Methods
Setting and Participants
This project was approved by the Institutional Review Board of Henry Ford Hospital and is in keeping with its Health Insurance Portability and Accountability Act policy. All study individuals were members of a large integrated health system serving southeastern Michigan. By virtue of their health plan membership with pharmacy coverage, these individuals had routinely collected and electronically recorded diagnoses from clinical encounters; medication prescription information as a result of mandatory, system wide electronic prescribing; and medication fill information from pharmacies both within and outside the health system. Eligible subjects met the following criteria: age 12-56 years, ≥1 asthma-related encounter with the health system, at least two prescription fills of an ICS between March 1, 2006 and March 31, 2012 with corresponding information in the electronic prescribing database, and membership in the health plan with prescription coverage. Individuals with a prior congestive heart failure (CHF) or chronic obstructive pulmonary disease (COPD) diagnoses were excluded from the analysis.
Calculating Medication Adherence
We calculated medication adherence in a manner analogous to that which we have done previously.(2;3;16) In brief, we obtained ICS dose and frequency of use information for study subjects from the electronic prescription data. Prescription fills were identified through pharmacy claims data. We have previously shown that these data identify >99% of all ICS prescriptions filled by health plan members.(17) The National Drug Code associated with each prescription fill was used to identify the number of doses per inhaler, and when used in concert with the prescription information, we were able to calculate the days' supply for each inhaler fill. We then estimated adherence as the number of days' supply for each fill divided by the total number of days between the present fill and the subsequent ICS fill. A separate adherence estimate was calculated for each interval between fills for the duration of follow up. A final overall average measure of adherence was calculated based on the percent ICS adherence for each interval. For example, if an individual was identified in the outpatient pharmacy record as having filled an ICS with 120 actuations per inhaler prescribed at 2 puffs twice a day on a given date and the same individual refilled the same prescription 47 days later, adherence for the interval was calculated as 120 actuations ÷ (2 puffs × 2 times daily) = 30 days' supply divided by 47 days between fills which is an estimated 63.8% adherence rate for that interval between fills. If the prescribing instructions did not change, this same calculation was repeated for every interval during the observation period and then averaged for the final adherence rate. For each person, we defined the first ICS prescription in the observation period (but not necessarily that person's first ICS prescription ever) to be the index prescription. During follow-up, some individuals had a switch in their prescribed ICS dosing frequency from once daily to two or more times daily (or vice versa). For comparing adherence on both regimens, we considered the switch date to be the date of the last fill for the first observation period (i.e., for either once daily or two or more times daily dosing). Similarly, for individuals who switched to an ICS/long-acting-beta-agonist combination therapy during the observation period, follow-up ended on the date of the switch. In the matched analysis (described in greater detail below) we also considered the date that an individual switched their dosing regimen (i.e., from once daily to two or more times daily dosing or vice versa) to be the first date of observation for the second dosing regimen.
Statistical Analysis
We compared the baseline characteristics of individuals who, at the time of their index prescription, were taking their ICS medication either once daily to two or more times daily. Normally distributed continuous variables were compared using a t-test, and categorical variables were compared by a chi-square test.
Our primary outcome was patient adherence to ICS medication. We examined adherence both as a continuous measure and as a dichotomous measure (i.e., ≤75% vs. >75%). The latter was based on a recent publication of ours demonstrating a therapeutic benefit at ICS adherence levels in excess of 75%.(6) We used linear regression to assess the relationship between ICS dosing regimen (i.e., once daily vs. two or more times daily dosing) and the continuous measure of ICS adherence, and we used logistic regression to assess the relationship between ICS dosing regimen and the dichotomous measure of ICS adherence. In both types of regression models we adjusted for age, sex, race-ethnicity, number of other controller medications used (i.e., antileukotrienes, omalizumab, cromolyn sodium, or theophylline), and asthma severity at baseline. For asthma severity, we used a method described by Allen-Ramey et al,(18) which is based on oral corticosteroid (OCS) fills and short-acting beta-agonist (SABA) fills in the year prior to the index date. The most severe group had either ≥3 OCS fills OR 2 OCS fills and >6 SABA fills. The moderate-severe group had either 2 OCS fills OR >6 SABA fills OR 1 OCS fill and ≥4 SABA fills. The low severity group had no OCS fills and ≤1 SABA fill. All other combinations of SABA and OCS fills made up the low-moderate severity group. In the regression models we combined the aforementioned severity categories so as to compare the combination of low and low-moderate asthma severity (referent) with that of moderate-severe and severe asthma combined.
We examined differences in ICS adherence among various patient subgroups defined by sex, race-ethnicity (i.e. African American and white individuals), age (i.e., <18 years and ≥18 years), and asthma severity (i.e., low and low-moderate severity combined and moderate severe and severe severity combined). We used a t-test to compare mean ICS adherence between once daily ICS dosing and ≥2 times daily ICS dosing within each of the subgroups.
We also assessed for differences in adherence among those patients who had their prescribed ICS regimen changed during the observation period. We separately assessed those who started on once daily ICS medication and were switched to two or more times daily dosing, as well as those who started on two or more times daily ICS dosing and were switched to once daily use. We used a matched analysis to assess for differences in adherence under both dosing regimens, and we accounted for asthma severity at both the time of the index prescription and at the switch date.
Lastly, as a secondary analysis, we compared dosing regimens with regard to risk of a severe asthma exacerbation (i.e., a composite of burst oral corticosteroid use, asthma-related emergency department visit, and asthma-related hospitalization).(19) Cox proportional hazard regression was used to model the time to first event. Analyses were adjusted for patient age, sex, race-ethnicity, baseline asthma severity, and strength of prescribed ICS regimen (i.e., low, medium, and high). Strength of each individual's ICS regimen was based on the prescribed preparation and total daily dose as outlined in recent guidelines.(20)
Analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC).(21) A type-I error rate of 5% (P-value<0.05) was considered statistically significant.
Results
There were 88,012 individuals in the health system with ≥1 asthma diagnosis. Of those individuals, 3,845 were members of the affiliated health plan with prescription drug coverage, had no prior or concurrent diagnosis of CHF or COPD, and had ICS prescribing information available between March 1, 2006 and March 31, 2012. We excluded an additional 1,659 individuals because they were not between the ages of 12 – 56 years, and 884 individuals were excluded because they did not have at least 2 prescription fills of an ICS during the observation period. Therefore, 1,302 individuals met our inclusion requirements. Of these, 221 individuals were prescribed once-a-day ICS, and 1,081 individuals were prescribed to take their ICS medication two or more times daily. Demographic and clinical differences between these groups are shown in Table 1. Individuals on ≥2 times daily ICS dosing were more like to be female and older when compared with individuals on once daily dosing. Both groups were similar with respect to race-ethnicity, baseline asthma severity, and cumulative use of other controller medication. Based on manufacturers' dosing recommendations, it was not surprising to find that the type of ICS preparation used differed among the two groups. Those on single day dosing had a higher proportion of budesonide and mometasone use when compared with those on ≥2 times daily dosing, and those with ≥2 times daily dosing had a higher proportion of fluticasone and triamcinolone use. Total follow-up for the cohort was 2,592 person-years; 372 person-years for the 221 individuals on once-a-day ICS, and 2,220 person-years for the 1,081 individuals prescribed ICS medication taken two or more times daily.
Table 1. Characteristics of patients on once daily and two or more times daily inhaled corticosteroid use.
| Individuals on once daily ICS use (n=221) | Individuals on ≥2 times daily ICS use (n=1,081) | P-value* | |
|---|---|---|---|
| Age in years – mean ± SD | 28.2 ± 15.8 | 31.6 ± 16.0 | 0.002 |
| Female –(%) | 113 (51.1) | 656 (60.7) | 0.009 |
| Race-ethnicity –(%) | 0.120 | ||
| African American | 67 (30.3) | 406 (37.6) | |
| White | 135 (61.1) | 586 (54.2) | |
| Other/unknown | 19 (8.6) | 89 (8.2) | |
| Asthma severity –(%)† | 0.535 | ||
| Low | 78 (35.3) | 379 (35.1) | |
| Low-moderate | 98 (44.3) | 457 (42.3) | |
| Moderate-severe | 37 (17.1) | 179 (16.6) | |
| Severe | 8 (3.6) | 66 (6.1) | |
| Type of inhaled corticosteroid used –(%)‡ | 0.001 | ||
| Beclomethasone | 2 (0.9) | 16 (1.5) | |
| Budesonide | 188 (85.1) | 665 (61.5) | |
| Flunisolide | 0 (0.0) | 1 (0.1) | |
| Fluticasone | 16 (7.2) | 288 (26.6) | |
| Mometasone | 10 (4.5) | 5 (0.5) | |
| Triamcinolone | 5 (2.3) | 106 (9.8) | |
| Taking other asthma controller medication –(%)‡ | 26 (11.8) | 96 (8.9) | 0.180 |
| Type of other controller medication used –(%)‡ | |||
| Antileukotriene | 25 (11.3) | 90 (8.3) | 0.154 |
| Cromolyn sodium | 1 (0.5) | 4 (0.4) | 0.858 |
| Omalizumab | 0 (0.0) | 3 (0.3) | 0.999 |
| Theophylline | 1 (0.5) | 2 (0.2) | 0.428 |
| ICS adherence – mean ± SD | 61 ± 34 | 41 ± 30 | 0.001 |
| ICS adherence >75% – (%) | 90 (40.7) | 187 (17.3) | 0.001 |
ICS denoted inhaled corticosteroid and SD, standard deviation.
P-value for comparison of individuals on once daily ICS vs. two or more times daily use.
Baseline asthma severity based on oral corticosteroid and short-acting beta-agonist use for the year prior to the index ICS prescription.(18)
At the time of the index ICS prescription.
Adherence was significantly higher in individuals prescribed once daily ICS medication compared with those prescribed ICS ≥2 times daily (61% vs. 41%, respectively; P=0.001) (Table 1). The former group was also more likely to be >75% adherent when compared with the latter (40.7% vs. 17.3%, respectively; P=0.001).
As shown in Table 2, both the unadjusted and adjusted comparison of once-a-day dosing with ≥2 times daily dosing showed that once-a-day dosing was associated with an approximate 20% increase in adherence (i.e., 19.3% in the univariable model and 19.6% in the multivariable model). Women and African American individuals had significantly lower adherence, whereas age, asthma severity, and other controller medication use was positively associated with ICS adherence.
Table 2. Association between inhaled corticosteroid dosing (once daily vs. two or more times daily) on adherence levels and the likelihood of having a level >75%.
| Variable | Level of ICS adherence* | Likelihood of ICS adherence >75%† | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Change in level of adherence (95% CI)‡ | P-value | Change in level of adherence (95% CI)§ | P-value | OR (95% CI)‡ | P-value | aOR (95% CI)§ | P-value | |
| Once daily ICS use║ | 19.3 (19.8 – 23.7) | 0.001 | 19.6 (15.3 – 23.9) | 0.001 | 3.28 (2.41 – | 0.001 | 3.51 (2.53 – 4.85) | 0.001 |
|
| ||||||||
| Age (per 10-year interval) | 4.0 (2.9 –5.0) | 0.001 | 1.23 (1.13 – 1.35) | 0.001 | ||||
|
| ||||||||
| Female | -3.6 (-6.9 – -0.2) | 0.036 | 0.84 (0.63 – 1.13) | 0.250 | ||||
|
| ||||||||
| Race – ethnicity (referent – white) | ||||||||
| African American | -6.9 (-10.4 – - 3.5) | 0.001 | 0.61 (0.44 – 0.84) | 0.002 | ||||
| Other | 0.1 (-5.9 – 6.1) | 0.981 | 1.15 (0.72 – 1.85) | 0.552 | ||||
|
| ||||||||
| Asthma severity (referent -low/low-moderate) | 4.5 (0.7 – 8.4) | 0.022 | 1.46 (1.06 – 2.01) | 0.022 | ||||
|
| ||||||||
| Taking additional asthma controller medication | 5.6 (0.9 – 10.2) | 0.020 | 1.51 (1.05 – 2.17) | 0.025 | ||||
ICS denotes inhaled cortiosteroids; OR, odds ratio; CI, confidence interval, and aOR, adjusted odds ratio.
Linear regression model predicting adherence as a continuous outcome measure. Estimates represent the absolute change in the level of adherence (0-100 scale) per unit of change in the independent variable.
Logistic regression model predicting the likelihood of having an adherence levels >75% per unit of change in the independent variable.
Univariable analysis.
Multivariable analysis adjusted for all other variables shown.
Comparison group is individuals with two or more times daily ICS dosing.
Since we have previously shown >75% adherence to be a therapeutic threshold for improved asthma outcomes,(6) we assessed the likelihood that individuals on both dosing regimens achieved this level of adherence. As shown in Table 2, both the univarible and multivariable models suggested that once-a-day dosing was associated with a >3 fold likelihood of achieving >75% adherence as compared with ≥2 times daily dosing (odds ratio [OR] 3.28 and adjusted OR 3.51, respectively; P=0.001 for both).
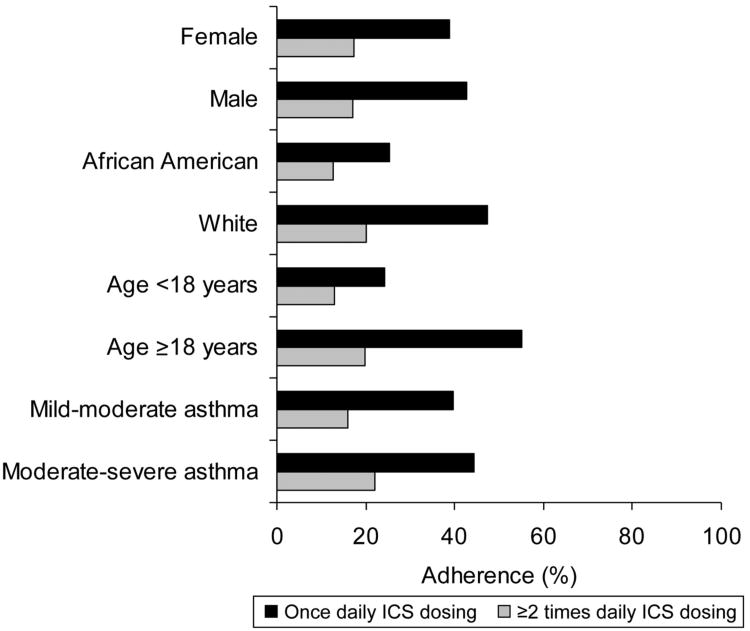
Figure 1 shows the relationship between once daily ICS dosing and ≥2 times daily ICS dosing among various subgroups defined by sex, race-ethnicity, age, and asthma severity. In all subgroups, once daily ICS dosing was associated with significantly greater adherence when compared with ≥2 times daily ICS dosing.
Figure 1.
Relationship between both once daily ICS dosing (black bars) and ≥2 times daily ICS dosing (gray bars) and ICS adherence among patient subgroups defined by sex, race-ethnicity, age, and asthma severity. Adherence between once daily and ≥2 times daily ICS dosing was statistically significant for all subgroups analyzed (P<0.01).
As a natural experiment, we also assessed changes in adherence among the subset of 106 study subjects who changed their dosing regimen during the course of observation (Table 3). Sixty-two (58.5%) of these 106 individuals started on once-a-day ICS therapy and were later prescribed ≥2 times daily therapy (group 1), and 44 (41.5%) were initially prescribed ≥2 times daily therapy and were later prescribed once-a-day therapy (group 2). Regardless of the initial dosing regimen, individuals demonstrated higher levels of adherence while on once-a-day therapy (63.7% and 74.4% for groups 1 and 2, respectively) when compared with ≥2 times daily therapy (47.8% and 48.1% for groups 1 and 2, respectively). After being switched to ≥2 times daily therapy, individuals were significantly less likely to achieve an adherence level >75% (OR 0.33; 95% confidence interval [CI] 0.13-0.82). In contrast those switched to once-a-day therapy were more likely to achieve an adherence level >75% when compared with their time on ≥2 times daily therapy (OR 6.02, 95% CI 2.52-14.38).
Table 3. Differences in adherence among individuals who switched inhaled corticosteroid dosing regimens over the course of observation (n=107).
| Initial ICS dosing regimen | Initial percent adherence – ± SD | Percent adherence after switch to alternate dosing regimen – mean ± SD* | Difference in percent adherence after switch – mean ± SD† | P-value | Likelihood for adherence >75% after switch – OR (95% CI)‡ | P-value |
|---|---|---|---|---|---|---|
| Once daily (n=62) | 63.7 ± 32.1 | 47.8 ± 30.2 | -15.9 ± 35.1 | 0.001 | 0.33 (0.13 – 0.82) | 0.018 |
| ≥2 times daily (n=44) | 48.1 ± 28.3 | 74.4 ± 30.0 | 26.3 ± 40.7 | 0.001 | 6.02 (2.52 – 14.38) | 0.001 |
ICS denotes inhaled corticosteroid; SD, standard deviation; OR, odds ratio; and CI, confidence interval.
A switch to ≥2 times daily ICS dosing for individuals initially on single day dosing, and a switch to single day dosing for individuals initially on ≥2 times daily ICS dosing.
Average of the per individual change in adherence after the switch as compared with before the switch.
Likelihood of an individual having an adherence level >75% after the dosing regimen switch as compared with before the switch.
Once-daily ICS dosing was not associated with a statistically significant difference in severe asthma exacerbations when compared with ≥2 times daily dosing (hazard ratio [HR] 0.91, 95% CI 0.73-1.14). A similar non-significant relationship was noted after adjusting for both asthma severity and the strength of the ICS dose prescribed (data not shown).
Discussion
There are few studies comparing once-daily ICS dosing with ≥2 times daily dosing in patients with asthma in a real-world setting. In the present study we found adherence to be significantly higher in patients who were prescribed once-daily ICS dosing. Moreover, we observed that individuals on once-daily ICS dosing were more than 3 times as likely to achieve >75% adherence (i.e., a threshold previously associated with therapeutic benefit)(6) when compared to individuals on an ICS dosing regimen of ≥2 times daily. We also demonstrated that mean adherence remained significantly higher for once-daily ICS dosing even among subgroups defined by sex, race-ethnicity, age, and asthma severity.
Improved adherence through a once-daily ICS medication regimen is of little benefit if clinical efficacy is compromised. Fortunately, in our present study we found a protective, albeit non-significant, association for asthma exacerbations among users of once-daily dosing when compared to multiple daily dosing. These findings are in keeping with earlier studies by our group showing an inverse relationship between ICS medication adherence and poor asthma outcomes.(2)
Our results are also supported by the findings of others. For example, using data from the United Kingdom's General Practice Research Database, Guest et al. found that patients who were switched to a once-daily ICS from a twice-daily ICS rather than another twice-daily dosing regimen experienced a significant improvement in adherence.(22) Furthermore, the authors found resources and management costs associated with asthma-related medical care to be lower among the once-daily ICS users.(22)
Two retrospective pharmacy claims analyses compared mometasone furoate (MF) (FDA approved for once-daily dosing) with fluticasone propionate (FP) and found adherence to be significantly higher with the former in both studies.(23;24) The authors attributed the higher adherence among MF users to the availability of once daily dosing, although they did not have information on how patients were instructed to take these medications. A particular strength of our study was our ability to capture actual patient prescriptions, and therefore, we could discern once daily from multiple daily dosing. Of note, nearly one-third of the individuals using MF in our study population were initially prescribed ≥2 times daily dosing. In addition, our ability to capture both prescription and filling information for our patients allowed us to estimate the effect of dosing on adherence both before and after these dosing changes.
Although clinical trials are often considered the gold standard for comparing treatments, study populations are often narrowly defined and closely monitored, making it difficult to extrapolate results to those of the real world. For example, in a randomized open-label study, asthma patients receiving MF 400μg once-daily had a mean adherence rate of 93.3% and individuals randomized to MF 200μg twice-daily had a mean adherence rate of 89.5%.(13) While these findings may suggest similar rates of adherence by dosing regimen, medication adherence in this clinical trial is markedly higher than rates observed in the current study and other population based studies of medication adherence.(25;26) Therefore, we believe that our findings present a more realistic measure of the changes in adherence that physicians can expect by switching dosing regimens.
Nevertheless, certain limitations must be considered when interpreting our study findings. First, observational studies may produce spurious results due to unknown or unaccounted for confounders. However, the consistency of our findings with the results of others suggests that our results are not unduly confounded. Another potential limitation is whether our findings can be generalized since all study individuals were members of a single health maintenance organization and all individuals had prescription drug coverage. In other words, other factors, such as medication cost, may outweigh the effects of dosing on adherence in patients who don't have prescription coverage and can't afford these medications. This limitation may become less relevant over time if national health care reform improves coverage for citizens who are currently uninsured. There is also no gold standard method by which to measure adherence. Although we have repeatedly demonstrated the predictive validity of our adherence estimates for asthma outcomes,(2;6) we cannot guarantee that all of the medication filled was used. Automated prescription fills or mail order prescription services were unlikely to have affected our estimates, as <3% of fills were from any single source (data not shown). Moreover, these services would be non-differential between once daily and ≥2 times daily ICS users as both groups had the same insurance provider. Lastly, we would expect that a dosing regimen which improves medication adherence would also improve disease outcomes. However, while once daily dosing appeared to be slightly protective for severe asthma exacerbations when compared with ≥2 times daily dosing, this difference was not statistically significant. We suspect that this was an issue of study power for this secondary outcome, as given our samples size, we estimated having 80% power at a two-tailed alpha of 0.05 to detect a HR of 1.35 between treatment groups on the composite asthma exacerbation outcome. However, we have previously estimated that a 25% difference in adherence (i.e., larger than the ∼20% adherence difference between dosing regimens) produces a 12% difference in this composite outcome.(6) Therefore, we would need a larger study to detect the likely difference in exacerbations attributable to the different dosing regimens. Future research should further investigate this outcome.
Despite these limitations, studies that quantify the risks and benefits of once-daily dosing regimens have a timely relevance to recent health care reforms. As part of its health care quality improvement initiative, the Patient Protection and Affordable Care Act mandates medication management services targeted at individuals with chronic diseases.(27) These programs are to provide multimodal interventions (i.e., information, support services, resources, and strategies) designed to enhance patient adherence. Simplifying drug dosing regimens, when feasible, has the potential to play a key role in these initiatives, so it is crucial that we understand for which pharmacotherapies once-daily dosing is a practical and effective alternative.
Collectively, this study suggests that once-daily ICS therapy provides a practicable therapeutic option for clinicians whose treatment aim is to improve adherence. Moreover, the simpler regimen did not appear to jeopardize the clinical efficacy of asthma controller therapy.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01AI079139, R01AI061774), the National Heart Lung and Blood Institute (R01HL079055), and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK064695), National Institutes of Health and the Fund for Henry Ford Hospital to Dr. Williams.
Abbreviations used
- ICS
Inhaled corticosteroid
- OCS
Oral corticosteroid
- SABA
Short-acting beta-agonist
- OR
Odds ratio
- CI
Confidence interval
- MF
Mometasone furoate
- FP
Fluticasone propionate
- HR
Hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–22. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 2.Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. The Journal of Allergy and Clinical Immunology. 2004;114(6):1288–93. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126(2):225–31. 231. doi: 10.1016/j.jaci.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors M, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–5. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 5.Bender B, Milgrom H, Rand C. Nonadherence in asthmatic patients: is there a solution to the problem? Ann Allergy Asthma Immunol. 1997;79(3):177–85. doi: 10.1016/S1081-1206(10)63001-3. [DOI] [PubMed] [Google Scholar]
- 6.Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–91. doi: 10.1016/j.jaci.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next? J Allergy Clin Immunol. 2003;112(3):489–94. doi: 10.1016/s0091-6749(03)01718-4. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD004804. CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glynn L, Fahey T. Cardiovascular medication: improving adherence. Clin Evid (Online) 2011;2011 [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150(9):1881–4. [PubMed] [Google Scholar]
- 11.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–e33. [PubMed] [Google Scholar]
- 12.Emkey RD, Ettinger M. Improving compliance and persistence with bisphosphonate therapy for osteoporosis. Am J Med. 2006;119(4 Suppl 1):S18–S24. doi: 10.1016/j.amjmed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm Med. 2010;10:1. doi: 10.1186/1471-2466-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 15.Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Unpredictability of deception in compliance with physician-prescribed bronchodilator inhaler use in a clinical trial. Chest. 2000;118(2):290–5. doi: 10.1378/chest.118.2.290. [DOI] [PubMed] [Google Scholar]
- 16.Wells K, Pladevall M, Peterson EL, Campbell J, Wang M, Lanfear DE, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med. 2008;178(12):1194–201. doi: 10.1164/rccm.200808-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–9. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Allen-Ramey FC, Bukstein D, Luskin A, Sajjan SG, Markson LE. Administrative claims analysis of asthma-related health care utilization for patients who received inhaled corticosteroids with either montelukast or salmeterol as combination therapy. J Manag Care Pharm. 2006;12(4):310–21. doi: 10.18553/jmcp.2006.12.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Gern J, et al. Asthma Outcomes: Exacerbations. J Allergy Clinc Immunol. 2012;129(3):S34–S48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 21.SAS Institute Inc. SAS/STAT Users Guide Version 9.1 ed. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- 22.Guest JF, Davie AM, Ruiz FJ, Greener MJ. Switching asthma patients to a once-daily inhaled steroid improves compliance and reduces healthcare costs. Prim Care Respir J. 2005;14(2):88–98. doi: 10.1016/j.pcrj.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navaratnam P, Friedman HS, Urdaneta E. The impact of adherence and disease control on resource use and charges in patients with mild asthma managed on inhaled corticosteroid agents. Patient Prefer Adherence. 2010;4:197–205. doi: 10.2147/ppa.s9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navaratnam P, Friedman HS, Urdaneta E. Treatment with inhaled mometasone furoate reduces short-acting beta(2) agonist claims and increases adherence compared to fluticasone propionate in asthma patients. Value Health. 2011;14(2):339–46. doi: 10.1016/j.jval.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1810–7. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 26.Bender B, Wamboldt FS, O'Connor SL, Rand C, Szefler S, Milgrom H, et al. Measurement of children's asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–21. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 27.The Patient Protection and Affordable Care Act of 2010. Pub. L. No. 111-148. 3-23-2010. 124 Stat 517.