Abstract
The incidence of pediatric non-alcoholic fatty liver disease has increased dramatically, and growing evidence indicates that the pathophysiology may be unique from the adult form, suggesting a role for early-life events. Recent radiologic techniques have now demonstrated that maternal obesity contributes to hepatic fat storage in newborn infants. In this review, we will explore how maternal obesity and a hyperlipidemic environment can initiate liver histopathogenesis in utero, including steatosis, mitochondrial dysfunction, oxidative stress, and inflammatory priming. Thus, early exposure to excess lipids may represent the 'first hit' for the fetal liver, placing it on a trajectory towards future metabolic disease.
Keywords: NAFLD, Nonalcoholic steatohepatitis, Pediatric NASH, Maternal Obesity, Fetal Programming, Mitochondrial Dysfunction, Lipotoxicity, Sirtuins
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now the most common cause of adult chronic liver disease in the United States [1]. The occurrence of NAFLD in children has also increased, from the first case report in 1983 to a current prevalence of 8 to 11 percent in adolescents [2–5]. The tripling of childhood obesity in the past thirty years is likely a significant contributor to this recent epidemic [6, 7], with rates of NAFLD in obese adolescents reported to be as high as 38 to 83 percent [8, 9]. NAFLD describes a spectrum of pathology from isolated hepatic steatosis to more severe necro-inflammatory non-alcoholic steatohepatitis (NASH). The natural history of NASH in pediatrics is unknown, but in adults approximately one third of patients with NASH will have progressive inflammation and fibrosis, ending in cirrhosis within 5 to 10 years [10]. A small retrospective study in 66 children with NAFLD over a 20 year observational period found a 13.8 fold increased risk of dying or requiring liver transplant from decompensating liver failure [11].
Pediatric NAFLD is a histologic diagnosis with greater than 5 percent steatosis in the absence of other specific cause, and NASH is the more severe form with steatosis, inflammation, and variable degrees of fibrosis [12]. For unclear reasons, pediatric NASH is characteristically different from the adult form with geographically unique portal inflammation and fibrosis [13, 14]. The pathogenesis of NAFLD and progression to NASH is likely multifactorial and has been described by a “two-hit” or more recently a “multiple-hit” hypothesis [15, 16]. Hepatic lipid accumulation, commonly regarded as the first hit, results in the characteristic lipid droplets seen in hepatic steatosis, which is thought to be a protective mechanism to minimize lipotoxicity [17, 18]. Secondary events from lipid likely involve a combination of factors including mitochondrial dysfunction, oxidative stress, pro-inflammatory cytokine production, and endotoxemia from the gastrointestinal microbiota [16, 19, 20]. Such events are associated with activation of Kupffer cells and hepatic stellate cells, leading to further inflammation, apoptosis, and the development of fibrosis and, eventually, cirrhosis.
While obesity is the most common risk factor for NAFLD, sedentary lifestyle, increased waist circumference, high fructose consumption, decreased polyunsaturated fatty acid intake, insulin resistance, and metabolic syndrome are also associated with increased disease risk and/or severity [21–26]. Additionally, gene polymorphisms associated with lipid metabolism, inflammatory cytokines, fibrotic mediators and oxidative stress also appear to play a role in both susceptibility and severity of pathology [27]. However, the dramatic increase in pediatric NAFLD over the past 30 years and high prevalence of obesity in mothers suggests that environmental factors may be igniting the shift.
There is now growing evidence that exposures prior to birth, such as maternal obesity, may contribute to an individual’s risk of developing metabolic diseases such as NAFLD. In this review, we will explore how exposure to excess maternal lipids in utero can promote hepatic steatosis, mitochondrial dysfunction, oxidative stress, and inflammation – the characteristic features of NAFLD progression. Further, we will discuss how these processes can sensitize the infant to postnatal inflammatory and metabolic challenges, thus driving the growing epidemic of pediatric NAFLD, and providing evidence that perinatal programming from lipid excess may, in fact, be the new first hit.
Fetal Liver Development and Early Life Exposures
The fetal liver development begins at week four of gestation with the generation of the hepatic bud from the ventral endoderm [28, 29]. Hepatic cellular specification occurs even earlier, as shown by serum albumin mRNA expression in mice [30]. Gross hepatic morphogenesis is completed by the end of the first trimester, however, more refined cellular determination continues throughout gestation. The hepatic bud is populated by bi-potential hepatoblasts, which differentiate into mature hepatocytes or cholangiocytes that can be further refined to achieve unique cellular phenotypes [31]. As reviewed elsewhere, a vast array of genes and their transcriptional regulators are involved in the development of hepatocyte metabolic processes, including gluconeogenesis, glycogenolysis, lipid oxidation, and de novo lipogenesis, however the majority of genes underlying these processes are normally not highly expressed until after birth [31, 32]. Adding to the complexity, the liver is the primary location of hematopoietic development from week six to twenty-one of gestation, and hematopoietic stem cells account for 60 percent of total liver mass during peak hematopoiesis followed by regression to the fetal bone marrow by term [33]. Thus, the developing fetal liver is constantly in flux over most of gestation, with large changes in cell determination and population, as well as more refined changes in cellular metabolic phenotypes.
The Developmental Origins of Disease Hypothesis posited by Dr. David Barker argues that exposure to an adverse environment during critical windows of cellular plasticity results in increased risk of later life disease [34]. During these critical periods, changes in the local environment can impact the “programming” of gene expression pathways, with consequent long-term changes in organ function and/or growth [35]. Multiple observational and experimental studies in humans and animals have demonstrated that fetal exposure to stressors such as maternal malnutrition and environmental toxins results in the programming of later life disease [36]. Importantly, the crux of the Barker Hypothesis focuses on how in utero under-nutrition programs a “thrifty” phenotype, resulting in metabolic mismatch to a postnatal obesogenic environment [37, 38]. However, the opposite scenario of maternal hyper-nutrition and obesity has also been shown to have programming effects on offspring. As we shall see, this is likely not due to nutrient mismatch, but rather nutrient overload and resulting maladaptation of metabolic pathways, thus promoting disease upon further nutrient challenge. Whether and how these changes are passed on to subsequent generations through epigenetic changes is currently the subject of much debate, and will be reviewed elsewhere.
Maternal Obesity and Lipid Overload During Fetal Liver Development
It is well established that maternal diabetes increases pregnancy-related maternal and fetal complications. However, both human epidemiological evidence and animal studies indicate that maternal obesity, independent of diabetes, contributes to adverse metabolic outcomes in children, including insulin resistance, obesity, and metabolic syndrome [39–41]. Therefore, it is not surprising that maternal obesity may also be an important risk factor for pediatric NAFLD.
Because a definitive diagnosis for NAFLD requires a liver biopsy, neonatal studies for NAFLD are limited in humans due to its invasive nature. In animal models, however, maternal obesity clearly shows an association with early onset NAFLD, even prior to birth [42–44]. We previously demonstrated in non-human primates that maternal obesity and a high fat diet (HFD) during gestation promotes fetal hepatic steatosis and lipotoxicity in the early third trimester [45]. Further, this steatotic phenotype persisted into the juvenile period, suggesting persistent hepatic programming. Recently, two innovative human studies utilized magnetic resonance technology as a non-invasive means to screen for steatosis in newborn infants. Brumbaugh et al. found a 68 percent increase in intrahepatocellular lipid content in newborns born to pregnancies complicated by maternal obesity and insulin resistance [46], and Modi et al. reported an 8.6 percent increase in intrahepatocellular lipid content for each one point increase in maternal BMI [47]. Both groups found a direct correlation with maternal BMI and neonatal fatty liver and not with maternal weight gain. Importantly, neonatal liver fat did not correlate with newborn adiposity, suggesting that the drivers for hepatic fat storage and subcutaneous fat may be different and that factors associated with maternal obesity, such as excess serum lipids, could be associated with newborn fatty liver.
Lipid accretion is critical for normal fetal development; however, excess lipids can be cytotoxic and induce metabolic dysfunction. In adults, lipotoxicity is caused by increases in intracellular lipids, resulting in increased levels of ceramides, diacylglycerols and reactive oxygen species (ROS), subsequent activation of cellular stress and inflammation pathways, and consequent cell death. In normal human pregnancy, placental lipid flux is minimal during the first and second trimester and exponentially increases in later gestation with increased maternal lipolysis and placental lipid transport, which coincides with fetal adipose tissue development [48, 49]. A driving force in fetal lipid accretion relates to physiologic insulin resistance in the mother during the second half of gestation, when it is common to see a two to three fold increase in maternal serum triglycerides [50]. This increased substrate load, ultimately, creates a concentration gradient driving lipid flux to the fetus. Pregnancies complicated by maternal obesity, however, are associated with pregravid insulin resistance, which, when combined with the metabolic stress of pregnancy, can lead to an early catabolic switch [51], resulting in exaggerated serum lipid levels and increased placental lipid transport [52, 53]. Excess fetal lipid exposure in early or mid-gestation may therefore utilize the liver and other developing organs as ectopic sites of lipid deposition in the absence of adipose tissue.
The adult liver is a main regulator of lipid homeostasis, and as such it carries out multiple metabolic processes, including de novo lipogenesis, fatty acid esterification, lipoprotein processing and export, and β-oxidation for energy. Increases in hepatic lipid influx and synthesis relative to utilization and export can lead to cellular lipotoxicity, and is fundamental in the pathophysiology of adult NAFLD [54]. In adults, this imbalance occurs from the development of hepatic insulin resistance, but whether this is the case in the developing fetus is still unknown. De novo lipogenesis is limited in the fetus [55], and while mature hepatocytes routinely process fatty acids for ATP synthesis via β-oxidation, lipid oxidation in utero is limited, with glucose instead being the primary metabolic fuel [56]. However, fetal hepatic β-oxidation must occur at some basal level, because pregnancies complicated by fetal fatty acid oxidation defects lead to toxic accumulation of intermediate metabolites and maternal liver disease [57]. Nevertheless, excess lipid influx into the fetal liver, particularly prior to fetal adipose tissue development, may result in a lipid accumulation, which, as we shall discuss, could promote metabolic dysfunction, cellular stress, and inflammation in an organ not yet competent in handling such a substrate overload.
Potential Mechanisms for the Developmental Programming of NAFLD in the Fetal Liver
Mitochondrial Dysfunction and Oxidative Stress
As in adults, children with NAFLD can progress to cirrhosis and end stage liver disease [11]. Mitochondrial dysfunction is frequently observed in adult NAFLD and NASH. Mitochondria are the major endpoint in fatty acid oxidation; however, in excess, fatty acids can promote their dysfunction. Markers of reduced hepatic mitochondrial oxidative capacity have been reported in rodent weanling and adult offspring of HFD mothers, including decreased mitochondrial electron transport complex (ETC) I,II/III, and IV activity, and reduced carnitine palmitoyltransferase 1 (CPT-1) expression, resulting in reduced basal hepatic fatty acid oxidation and a blunting in oxidative response to post-weaning HFD challenge [58, 59]. Under normal physiologic conditions, CPT-1 expression and ETC complex activity are induced by increases in cellular lipid influx; however in NAFLD, pathological reduction in these proteins’ function and expression has been implicated as a mechanism of impaired fatty acid oxidation [60]. Importantly, in adult NAFLD, this is tied to an unchecked increase in de novo lipogenesis and the negative feedback of malonyl-CoA on CPT-1. However, whether this holds true for the fetal liver is still unclear.
Fatty acid oxidation contributes to normal free radical leak from mitochondria, which can lead to cell damage if not balanced by antioxidant enzymes such as glutathione peroxidase (GPX) and superoxide dismutase (SOD). Lipid influx beyond mitochondrial oxidative capacity can result in accumulation of partially-oxidized lipid products and generate additional ROS that can overwhelm cellular defenses leading to oxidative stress and accumulation of free radical injury [61]. In experimental models, ROS-induced mitochondrial DNA damage was linked to decreased mitochondria number and increased steatosis [62]. Ultimately, this mitochondrial damage creates a vicious cycle of reduced oxidative capacity and increased oxidative stress and is thought to be one of the tenets underlying the development of NASH.
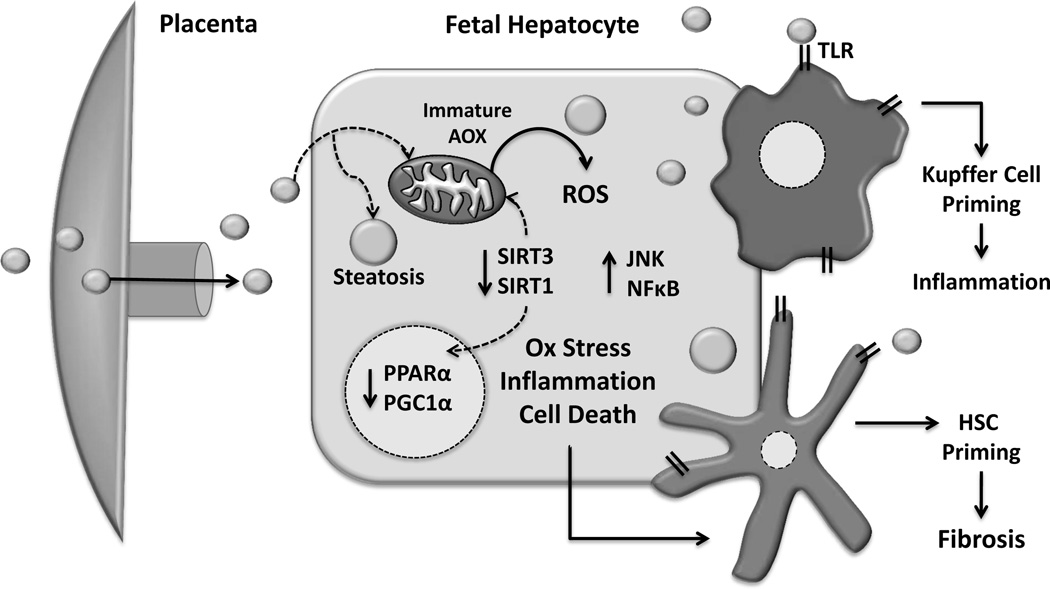
In utero exposure to excess lipids may potentiate both mitochondrial dysfunction and resulting oxidative damage (Figure 1). Our findings in the non-human primate clearly demonstrated hepatic oxidative stress as early as the third trimester concomitant with the previously discussed steatosis [45]. This is reinforced by data in rodent models, showing maternal over-nutrition resulted in activation of hepatic oxidative stress pathways in neonatal pups, which persisted into adolescence, even after weaning on to a control diet [63]. The reduced oxidative capacity of the fetal liver, coupled with an immaturity in antioxidant defense systems [64], may promote the accumulation of excess ROS and partially oxidized lipid intermediates under conditions of excess lipid influx. Supporting this are the findings in a mouse model of maternal HFD combined with an antioxidant supplementation cocktail during gestation and lactation showed improved redox homeostasis and oxidative stress in the fetus, in addition to normalizing metabolic parameters and total body adiposity at 2 months of age [65]. Further, there is evidence that maternal HFD may induce mitochondrial damage even earlier in mouse embryonic development, including in the oocyte prior to fertilization [66]. Thus, mitochondrial dysfunction and consequent oxidative damage of the fetal liver may be a primary mechanism for reduced hepatic oxidative capacity in later life.
Figure 1. In utero lipid excess and hepatocyte maladaptive changes.
Increased lipid flux to the developing fetal liver establishes a lipotoxic environment characterized by oxidative stress, increased inflammation, cell death, and consequent dysregulation in oxidative metabolism genes. Additionally, Kupffer cell and hepatic stellate cell (HSC) pro-inflammatory priming by hepatocellular stress as well as direct stimulation by circulating lipids may also increase susceptibility to later disease. Antioxidant enzymes (AOX), Reactive Oxidative Species (ROS), Sirtuin 1 and 3 (SIRT1 and SIRT3), Toll-like receptors (TLR).
Sirtuins and the PPARα/PGC1α Pathway
Impairments in mitochondrial metabolism can often become a chicken-or-egg scenario, with reduced mitochondrial function leading to metabolic dysregulation, and dysregulation further impairing mitochondrial function. Fatty acid oxidation is controlled by a number of inputs. Among these are the sirtuins- a family of NAD+ dependent deacetylases that act as nutrient sensors, and have a role in modifying both genes and proteins via post-translational protein activity [67]. Sirtuin-3 (SIRT3), specifically, is a key regulator of mitochondrial function and antioxidant capacity [68], and SIRT3 knockout results in reduced ETC function and increased production of free radicals from lipid excess [69]. In a HFD mouse model of NAFLD, we showed decreased hepatic SIRT3 activity and increased oxidative damage of mitochondrial proteins [70]. Further, rat weanlings from HFD mothers were shown to have decreased hepatic SIRT3 expression along with impaired SIRT3 activation [59], suggesting an early role for SIRT3 in impaired nutrient sensing and mitochondrial dysfunction.
SIRT1, similar to SIRT3, is a key cellular deactelyase important in post-translation regulation of various proteins and genes involved in glucose and lipid homeostasis. Importantly, SIRT1 positively regulates peroxisome proliferator-activated receptor alpha (PPARα) transcription of target proteins involved in fatty acid oxidation. Further, reduced SIRT1 expression and activity decreases PPARα transactivation, and increases HFD-susceptibility to NAFLD, as seen in SIRT1 knockout mice [71]. Interestingly, fetal livers of non-human primates exposed to a maternal high fat diet had decreased SIRT1 expression, protein, and activity in the presence of heightened oxidative stress [45, 72]. While lipids and their derivatives are the natural ligands for PPARα, resulting in increased PPARα transcriptional activity to promote fatty acid oxidation, this activation appears to be impaired in patients with NAFLD [60, 73].
While the mechanisms of sirtuin regulation remain to be fully elucidated, increased sirtuin expression is closely linked to periods of caloric restriction [68, 69]. Further, sirtuins have been shown to be upregulated by peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α), the co-activator of PPARα, and a master regulator of mitochondrial biogenesis [74]. In liver biopsies obtained from adult NAFLD patients, Sookoian and colleagues found increased methylation of specific PGC1α promoter regions resulted in gene silencing that significantly correlated with histologic diagnosis [75]. Further, in newborn cord blood, the same PGC1α epigenetic modifications correlated with pregravid maternal BMI [76]. This was supported in a mouse model of maternal HFD, where they observed reduced PGC1α expression in the liver of adult male offspring [77].
While reduced SIRT1 and SIRT3 expression and/or activity are likely linked to decreased mitochondrial function and oxidative capacity, consequent mitochondrial dysfunction and elevated ROS may in turn further impair SIRT1 and SIRT3 function and expression. There is early evidence in rodent models that ROS activation of cellular stress kinases, particularly c-Jun N-terminal Kinase-1 (JNK1) promotes SIRT1 degradation leading to hepatic steatosis [78]. Further, the lipid peroxidation product 4-hydroxynonenol (4-HNE), which is increased under conditions of oxidative stress, has been shown to reduce SIRT3 activity by direct protein modification [79]. Thus, early mitochondrial dysfunction and oxidative stress due to fetal exposure to excess lipids may result in reduced sirtuin activity and expression, contributing to impairments in overall hepatic oxidative capacity and its downstream consequences.
Priming of Hepatic Inflammation and Inflammation-Associated Insulin Resistance
Inflammation is another major component in the pathophysiology of NAFLD, as it relates to development of insulin resistance and disease progression to NASH. Obesity, and particularly maternal obesity, increases markers of chronic low-grade systemic inflammation in the mother as well as in cord blood samples from the fetus [80–82]. Whether this increase can lead to fetal insulin resistance, as measured by increased cord blood insulin and glucose levels, is not yet known [83, 84]. Importantly, the relative contribution of the mother, the placenta, and the fetus to observed increases in cord blood pro-inflammatory cytokines is an open question. Numerous animal models of maternal obesity have demonstrated inflammatory changes in adult offspring liver, corresponding with the progression of NAFLD, including increased hepatic levels of TNFα and IL1β [85], altered hepatic insulin signaling proteins, increased hepatic fibrogenesis [42], and enhanced Kupffer cell density [86]. However, given that all these changes occurred with the concomitant development of offspring obesity, the relative impact of pro-inflammatory changes in utero is still unclear.
The Kupffer cell is the tissue macrophage of the liver, and its activation may be critical for the development of insulin resistance and NASH [87]. Kupffer cell activation is regulated by a number of factors, including the binding of cell surface Toll-Like Receptors (TLRs). TLRs are pattern recognition receptors that sense pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) and bacterial DNA, but can also be activated by saturated fatty acids [88]. TLRs promote inflammation by stimulating local cytokine production via NF-κB activation in the presence of antigen. They can also promote insulin resistance via JNK and IκB kinase pathways. Additionally, lipotoxicity within hepatocytes themselves can promote the activation of stress kinases, inflammation and consequent insulin resistance as well [89].
Evidence for early activation of Kupffer cells within the fetal liver is currently lacking, however our findings in the non-human primate suggest that maternal HFD can ‘prime’ their pro-inflammatory response, such that Kupffer cells derived from juvenile offspring on a healthy diet have an exacerbated response when exposed to lipid or LPS to any observed development of obesity [90]. Macrophages, including Kupffer cells, are known to up-regulate TLR4 expression in response to pro-inflammatory stimuli, thus priming them for further response to antigen [91, 92]. Increased TLR4 expression, likewise, has also been implicated in the promotion fibrogenesis via hepatic stellate cell (HSC) activation [93]. Of importance, TLR4 knockout mice were protected from HFD-induced insulin resistance and obesity [94]. In utero exposure to the increased placentally-derived pro-inflammatory cytokines and lipids observed in our non-human primate model [81], as well as local hepatic pro-inflammatory stimuli, could promote such a priming and thus place the fetal liver on a path toward postnatal metabolic and inflammatory dysfunction, resulting in progression through NAFLD to NASH with continued metabolic challenge.
Many animal studies have indicated increased de novo lipogenesis in obese adult offspring from high-fat fed mothers [58, 65, 95]. While the early stimulus for enhanced de novo lipogenesis leading to the pathologic development of NAFLD remains unclear, programmed changes in inflammation and consequent hepatic insulin resistance may shift fuel utilization toward steatosis [60]. Insulin resistance results in elevated hepatic glucose uptake, which is shuttled into the fatty acid synthetic pathway as acetyl-CoA, providing substrate for de novo lipogenesis enzymes acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). Sterol regulatory element-binding protein 1c (SREBP-1c) regulates the expression of these genes, and is traditionally activated by insulin. Importantly, SREBP-1c appears to remain insulin sensitive, even in the face of hepatic glucose insulin resistance, as seen in inflammatory NAFLD [60, 96, 97]. Thus, in the pediatric liver, programming of inflammation-related insulin resistance could result in continued triglyceride synthesis in the presence of hyperinsulinism as well as unchecked gluconeogenesis. All of which could be further exacerbated by a heightened pro-inflammatory response to repeated hypercaloric and particularly high-fat dietary challenge.
The Importance of the Neonatal Environment: Early Challenges and Defenses
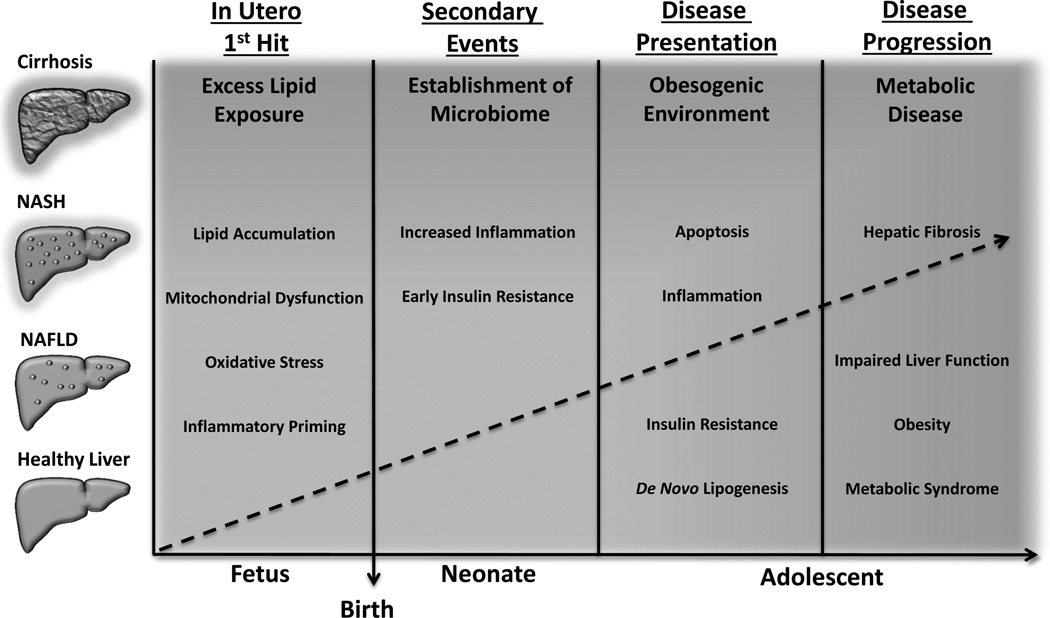
Developmental programming can extend beyond the intrauterine environment to include important postnatal exposures. In fact, in utero exposure to maternal obesity may impair fetal liver development such that it is less capable of counteracting continued postnatal challenges, resulting in susceptibility to NAFLD disease progression. Of particular concern during early neonatal life is the development of innate immune responses to environmental exposures, as exemplified by the establishment of the infant microbiome (Figure 2).
Figure 2. A revised pediatric NAFLD paradigm: In utero challenges establish the new “first hit”.
Maternal obesity, insulin resistance, and a high-fat diet lead to excess fetal hepatic lipid exposure. This results in cytotoxic lipid accumulation and maladaptive changes in oxidative metabolism and pro-inflammatory pathways, leading accelerated disease progression in a future obesogenic environment.
Babies are born essentially sterile, and acquire their initial microbiome through direct contact with maternal enteric flora, and subsequently through breast milk and skin contact during lactation [98]. In humans, mode of maternal delivery, method of feeding, and early antibiotic exposure can all impact the neonatal microbiome, and significantly increase risk for later obesity [99–102]. Endotoxins released from the gut microbiota are elevated in obesity, which is termed “metabolic endotoxemia” [103]. This is also true in pregnancy, where obese mothers were found to have serum endotoxin levels twice that of lean mothers at term, indicating subclinical endotoxemia [104]. Further, elevated endotoxin levels have been observed in children with NALFD, and appear to be critical to disease progression [105, 106]. Defining how a mother’s physiology influences her intestinal bacteria, and how such changes might impact the development of the infant’s microbiome, may provide better understanding of the early origins of pediatric NAFLD, obesity, and chronic metabolic disease.
Mechanisms behind microbiota influence on development of NAFLD continue to be investigated, however, alterations in nutrient absorption and chronic systemic inflammation from intestinal microbes are important factors [107–110]. Because the liver receives the majority of its blood supply from the portal vein, hepatocytes are often the first line defense for intestinal-derived antigens [107]. In rodents, HFD combined with chronic LPS infusion resulted in elevated endotoxin levels, increased inflammatory cytokines, hepatic steatosis, and insulin resistance [103]. Ultimately, chronic hepatic inflammation and insulin resistance appear to be related to gastrointestinal microbes, and the direct correlation between common neonatal exposures and the risk for NAFLD is waiting to be discovered.
Conclusions and Future Directions
The true pathogenesis of pediatric is likely multifactorial; adverse in utero events, however, likely play a role. Exposure to excess maternal lipids and resulting hepatic steatosis may promote fetal hepatic mitochondrial dysfunction, oxidative damage, and inflammation, leading to increased susceptibility to postnatal insults required for the progression from NAFLD to NASH. Of particular importance is whether these changes are accelerated in the context of a postnatal obesogenic environment, including high-fat, hypercaloric diets, sedentary lifestyle, and metabolic endotoxemia; and whether they are reversible if infants are raised in a more health-conscious environment. Following the adult NAFLD paradigm, it is likely that liver cells with reduced oxidative capacity and increased pro-inflammatory priming would not only contribute to liver disease progression but also whole-body hyperlipidemia, insulin resistance, and consequent metabolic syndrome.
Further studies are needed in both humans and animal models to better characterize the role of events prior to birth in the programming of metabolic disease as well as identify potential therapeutic interventions. Improving maternal metabolic adaptations associated with pregravid obesity, including insulin resistance and low-grade inflammation, may be a desirable therapeutic target during pregnancy. Postnatally, improved pediatric screening methods are needed to better identify at-risk populations, and at an earlier age, such that immediate action can be taken to minimize disease progression.
In conclusion, pediatric NAFLD is accelerating in incidence, and while this corresponds with the overall rise in pediatric obesity, there may also be a liver-specific programming mechanism in utero promoting this devastating trend. New evidence supports the idea that maternal obesity may initiate the pathogenesis of NAFLD, even before birth. Consequently, the first hit in the development of pediatric NAFLD may, in fact, be in utero.
Acknowledgments
Acknowledgments/Financial Sources of Support:
NIH/NICHD T32 HD007186 (MSS)
NIH/CTSI TL1-RR-025778 (MJRH)
AHA-11PRE502001 (MJRH)
NIH-R24-DK090964 (JEF)
NIH-RO1-DK077630 (JEF)
NIH-RO1-DK078645 (JEF)
NIH-R29-DK088324 (JEF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have nothing to disclose.
References
- 1.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Moran JR, Ghishan FK, Halter SA, et al. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374–377. [PubMed] [Google Scholar]
- 3.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegand S, Keller KM, Robl M, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond) 2010;34:1468–1474. doi: 10.1038/ijo.2010.106. [DOI] [PubMed] [Google Scholar]
- 5.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barshop NJ, Francis CS, Schwimmer JB, et al. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health. 2009;3:271–281. doi: 10.2217/phe.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 9.Xanthakos S, Miles L, Bucuvalas J, et al. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226–232. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 10.Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Marcellini M, Devito R, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 15.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 16.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 17.Thaler H. Relation of steatosis to cirrhosis. Clin Gastroenterol. 1975;4:273–280. [PubMed] [Google Scholar]
- 18.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 21.Mager DR, Patterson C, So S, et al. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. 2010;64:628–635. doi: 10.1038/ejcn.2010.35. [DOI] [PubMed] [Google Scholar]
- 22.Fishbein MH, Mogren C, Gleason T, et al. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83–88. [PubMed] [Google Scholar]
- 23.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JB, Deutsch R, Rauch JB, et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 25.Manco M, Bedogni G, Marcellini M, et al. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut. 2008;57:1283–1287. doi: 10.1136/gut.2007.142919. [DOI] [PubMed] [Google Scholar]
- 26.Manco M, Marcellini M, Devito R, et al. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 27.Day CP. Genetic and environmental susceptibility to non-alcoholic fatty liver disease. Dig Dis. 2010;28:255–260. doi: 10.1159/000282098. [DOI] [PubMed] [Google Scholar]
- 28.Godlewski G, Gaubert-Cristol R, Rouy S, et al. Liver development in the rat and in man during the embryonic period (Carnegie stages 11–23) Microsc Res Tech. 1997;39:314–327. doi: 10.1002/(SICI)1097-0029(19971115)39:4<314::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 30.Gualdi R, Bossard P, Zheng M, et al. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- 31.Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Xanthopoulos KG, Mirkovitch J. Gene regulation in rodent hepatocytes during development, differentiation and disease. Eur J Biochem. 1993;216:353–360. doi: 10.1111/j.1432-1033.1993.tb18152.x. [DOI] [PubMed] [Google Scholar]
- 33.Dame C, Juul SE. The switch from fetal to adult erythropoiesis. Clin Perinatol. 2000;27:507–526. doi: 10.1016/s0095-5108(05)70036-1. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 35.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–55. [PubMed] [Google Scholar]
- 36.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 37.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJ, Hales CN, Fall CH, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 39.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115(Suppl 1):S6–S10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 40.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 41.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oben JA, Mouralidarane A, Samuelsson AM, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 43.George LA, Uthlaut AB, Long NM, et al. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol. 2010;8:75. doi: 10.1186/1477-7827-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan M, Katewa SD, Palaniyappan A, et al. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 45.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brumbaugh DE, Tearse P, Cree-Green M, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.11.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modi N, Murgasova D, Ruager-Martin R, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70:287–291. doi: 10.1203/PDR.0b013e318225f9b1. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein IM, Goran MI, Amini SB, et al. Differential growth of fetal tissues during the second half of pregnancy. Am J Obstet Gynecol. 1997;176:28–32. doi: 10.1016/s0002-9378(97)80006-3. [DOI] [PubMed] [Google Scholar]
- 49.Widdowson EM. Growth and composition of the fetus and newborn. In: Assali NS, editor. The Biology of Gestation. New York: Academic Press; 1968. pp. 1–49. [Google Scholar]
- 50.Salameh WA, Mastrogiannis DS. Maternal hyperlipidemia in pregnancy. Clin Obstet Gynecol. 1994;37:66–77. doi: 10.1097/00003081-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 52.Dube E, Gravel A, Martin C, et al. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87:1–11. doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 53.Zhu MJ, Ma Y, Long NM, et al. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1224–R1231. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:351–359. doi: 10.1055/s-0028-1091979. [DOI] [PubMed] [Google Scholar]
- 55.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 56.Battaglia FC, Meschia G. Principal substrates of fetal metabolism. Physiol Rev. 1978;58:499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- 57.Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107:115–120. doi: 10.1097/01.AOG.0000191297.47183.bd. [DOI] [PubMed] [Google Scholar]
- 58.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 59.Borengasser SJ, Lau F, Kang P, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6:e24068. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohjima M, Enjoji M, Higuchi N, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–358. [PubMed] [Google Scholar]
- 61.Wei Y, Rector RS, Thyfault JP, et al. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demeilliers C, Maisonneuve C, Grodet A, et al. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology. 2002;123:1278–1290. doi: 10.1053/gast.2002.35952. [DOI] [PubMed] [Google Scholar]
- 63.Bayol SA, Simbi BH, Fowkes RC, et al. A maternal "junk food" diet in pregnancy and lactation promotes nonalcoholic fatty liver disease in rat offspring. Endocrinology. 2010;151:1451–1461. doi: 10.1210/en.2009-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato EF, Nakagawa E, Hiramoto K, et al. Oxidative stress promotes the regression of fetal liver hemopoiesis. Biochemistry (Mosc) 2004;69:18–22. doi: 10.1023/b:biry.0000016346.61403.24. [DOI] [PubMed] [Google Scholar]
- 65.Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of western diet-fed rats. Diabetes. 2010;59:3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luzzo KM, Wang Q, Purcell SH, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newsom SA, Boyle KE, Friedman JE. Sirtuin 3: A major control point for obesity-related metabolic diseases? Drug Discovery Today. 2013 doi: 10.1016/j.ddmec.2013.04.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu X, Brown K, Hirschey MD, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Bao J, Scott I, Lu Z, et al. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49:1230–1237. doi: 10.1016/j.freeradbiomed.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kendrick AA, Choudhury M, Rahman SM, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433:505–514. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purushotham A, Schug TT, Xu Q, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suter MA, Chen A, Burdine MS, et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. Faseb J. 2012;26:5106–5114. doi: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeon JE, Choi KM, Baik SH, et al. Reduced expression of peroxisome proliferator-activated receptor-alpha may have an important role in the development of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2004;19:799–804. doi: 10.1111/j.1440-1746.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- 74.Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sookoian S, Rosselli MS, Gemma C, et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology. 2010;52:1992–2000. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 76.Gemma C, Sookoian S, Alvarinas J, et al. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity (Silver Spring) 2009;17:1032–1039. doi: 10.1038/oby.2008.605. [DOI] [PubMed] [Google Scholar]
- 77.Burgueno AL, Cabrerizo R, Gonzales Mansilla N, et al. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J Nutr Biochem. 2013;24:6–13. doi: 10.1016/j.jnutbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Gao Z, Zhang J, Kheterpal I, et al. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem. 2011;286:22227–22234. doi: 10.1074/jbc.M111.228874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fritz KS, Galligan JJ, Smathers RL, et al. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24:651–662. doi: 10.1021/tx100355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmatz M, Madan J, Marino T, et al. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–446. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- 81.Grant WF, Gillingham MB, Batra AK, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One. 2011;6:e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 83.Catalano PM, Presley L, Minium J, et al. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashino NG, Saito KN, Souza FD, et al. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012;23:341–348. doi: 10.1016/j.jnutbio.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 86.Mouralidarane A, Soeda J, Visconti-Pugmire C, et al. Maternal obesity programs offspring non-alcoholic fatty liver disease via innate immune dysfunction in mice. Hepatology. 2013 doi: 10.1002/hep.26248. In Press. [DOI] [PubMed] [Google Scholar]
- 87.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 90.Thorn SR, Bacquero KC, Aikens RM, et al. Maternal high fat diet sensitivity programs juvenile hepatic lipid accumulation and kupffer cell inflammation in non-human primates in the absence of obesity. Proc Natl Acad Sci USA. 2013 In Review. [Google Scholar]
- 91.Romics L, Jr, Dolganiuc A, Kodys K, et al. Selective priming to toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555–564. doi: 10.1002/hep.20350. [DOI] [PubMed] [Google Scholar]
- 92.De Nardo D, De Nardo CM, Nguyen T, et al. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- 93.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Huang J, Li JS, et al. Accumulation of endoplasmic reticulum stress and lipogenesis in the liver through generational effects of high fat diets. J Hepatol. 2012;56:900–907. doi: 10.1016/j.jhep.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto M, Han S, Kitamura T, et al. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perfield JW, 2nd, Ortinau LC, Pickering RT, et al. Altered hepatic lipid metabolism contributes to nonalcoholic fatty liver disease in leptin-deficient ob/ob mice. J Obes. 2013;2013:296537. doi: 10.1155/2013/296537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palmer C, Bik EM, DiGiulio DB, et al. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 100.Kalliomaki M, Collado MC, Salminen S, et al. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 101.Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ajslev TA, Andersen CS, Gamborg M, et al. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 103.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 104.Basu S, Haghiac M, Surace P, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19:476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alisi A, Manco M, Devito R, et al. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2010;50:645–649. doi: 10.1097/MPG.0b013e3181c7bdf1. [DOI] [PubMed] [Google Scholar]
- 107.Aron-Wisnewsky J, Gaborit B, Dutour A, et al. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013 doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 108.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 109.Zambell KL, Fitch MD, Fleming SE. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. J Nutr. 2003;133:3509–3515. doi: 10.1093/jn/133.11.3509. [DOI] [PubMed] [Google Scholar]
- 110.Frasinariu OE, Ceccarelli S, Alisi A, et al. Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: An input for novel therapies. Dig Liver Dis. 2012 doi: 10.1016/j.dld.2012.11.010. [DOI] [PubMed] [Google Scholar]