Abstract
The recent focus on carbon trading has intensified interest in ‘Blue Carbon’–carbon sequestered by coastal vegetated ecosystems, particularly seagrasses. Most information on seagrass carbon storage is derived from studies of a single species, Posidonia oceanica, from the Mediterranean Sea. We surveyed 17 Australian seagrass habitats to assess the variability in their sedimentary organic carbon (Corg) stocks. The habitats encompassed 10 species, in mono-specific or mixed meadows, depositional to exposed habitats and temperate to tropical habitats. There was an 18-fold difference in the Corg stock (1.09–20.14 mg Corg cm−3 for a temperate Posidonia sinuosa and a temperate, estuarine P. australis meadow, respectively). Integrated over the top 25 cm of sediment, this equated to an areal stock of 262–4833 g Corg m−2. For some species, there was an effect of water depth on the Corg stocks, with greater stocks in deeper sites; no differences were found among sub-tidal and inter-tidal habitats. The estimated carbon storage in Australian seagrass ecosystems, taking into account inter-habitat variability, was 155 Mt. At a 2014–15 fixed carbon price of A$25.40 t−1 and an estimated market price of $35 t−1 in 2020, the Corg stock in the top 25 cm of seagrass habitats has a potential value of $AUD 3.9–5.4 bill. The estimates of annual Corg accumulation by Australian seagrasses ranged from 0.093 to 6.15 Mt, with a most probable estimate of 0.93 Mt y−1 (10.1 t. km−2 y−1). These estimates, while large, were one-third of those that would be calculated if inter-habitat variability in carbon stocks were not taken into account. We conclude that there is an urgent need for more information on the variability in seagrass carbon stock and accumulation rates, and the factors driving this variability, in order to improve global estimates of seagrass Blue Carbon storage.
Introduction
There is considerable interest in quantifying the capacity of the World’s ecosystems to trap and sequester carbon (C). The recent focus on C trading and C pricing has intensified this interest, since net C storage provides one means of valuing these ecosystems. In the early 1980s, [1] highlighted the importance of vegetated coastal habitats as carbon sinks, though since then most work on the ability of ecosystems to capture C has focused on terrestrial ecosystems. However, the publication of the ‘Blue Carbon’ report [2] has highlighted the potential of coastal marine ecosystems to sequester carbon. For example, they estimate that coastal marine systems capture up to half of the CO2 emissions from the World’s transport sector. These ecosystems include mangroves, coral reefs, saltmarshes and seagrasses, with seagrasses having a disproportionately large C storage potential relative to their global area [3].
From necessity, there has been a tendency to generalise the Corg-capture attributes of seagrasses from a very limited data set, with almost all of the estimates being based largely on the Corg content of sediments from Mediterranean Posidonia oceanica meadows (e.g. [2], [4]. However, P. oceanica is unusual in its ability to capture C. Its vertical growth dynamics and recalcitrant tissues produce a deep mat of plant debris that can extend many meters down into the sediment and persist for millennia, resulting in massive C storage, up to 40–410 kg Corg m−2 [5]–[7]. As far as is known, no other seagrass has this attribute and, in a functional form model of seagrasses, Walker et al. [8] placed P. oceanica as an outlier. Nelleman et al. [2] recognized that their assumptions were likely to have produced an upper estimate of the Blue Carbon sink, in part because of uncertainties in the C burial rates of different seagrass ecosystems.
The other 70-plus species of seagrasses [9] have a wide variation in traits relevant to C capture and storage. This includes differences in their rates of primary production, their below ground biomass, the recalcitrance of the Corg in their organs, the ability of their canopies to trap allochthonous carbon and the conditions in their sediments that drive Corg preservation (see review of factors in [10]). For most of the World’s seagrass species, and for most of their global distribution, there is little knowledge of their carbon stocks or regional cover [11], though for a wide range of seagrass species an absolute C flux to the refractory compartment was estimated ranging from 1.8 to 177.8 g C m−2 y−1 (median: 56.2 g C m−2 y−1), representing from around 5% to 65% of total plant production (median:18% [10]). Also, a review of 219 seagrass sediment data sets, encompassing 20 species, showed significant variation in organic matter content, with Corg ranging from 0.1 wt % to 11.0 wt % [12]. Recently, a first attempt to compile global Corg data [13] examined 946 distinct seagrass meadows across the globe. They estimated an average Corg in the top meter of seagrass soils at 2.5 wt % (median 1.8 wt %). Using the rough latest estimates of total area of the Earth covered by seagrass meadows (between 300,000 and 600,000 km2), they come to a conservative estimate of a global stock from 4.2 to 8.4 Pg Corg for the top meter. A preliminary regional breakdown of the areal stock is also provided showing the highest areal stocks in the Mediterranean (372.4 Mg Corg ha−1 ± 74.5, n = 29; [13]) but no details on habitat or species stock distribution can be given because of data set limitations.
In addition to the variability among seagrass species, the range of habitats in which they occur is also likely to affect their C storage potential. Seagrasses occur across a range of depositional environments, from estuaries to exposed coastal environments [14]. They occur at a range of water depths which influences their net C balances [15], [16] and the organic C preservation due to differences in sediment grain-size [17]. They also occur across latitudinal ranges and habitats with significant temperature variation that can affect sediment respiration and remineralisation rates [18]. Consequently, there are likely to be species-habitat interactions that will strongly influence the capture and retention of sedimentary Corg.
This paper presents the results of an initial survey of several species of Australian seagrasses to assess the variability in their sedimentary C stocks. While not fully comprehensive in the diversity of species examined it does, nonetheless, include about one-third of the Australian seagrass species, with estimates of Corg accumulation rates, and provides an initial contribution to broadening our understanding of the variability in the sedimentary Corg stocks of seagrass habitats. We also set out to test whether the variability in sedimentary Corg stocks among seagrasses was sufficient to warrant its inclusion in regional and global estimates of seagrass Corg storage, or whether a single global seagrass average (such as that currently based largely on P. oceanica) produces similar estimates. While our study focuses on Australian seagrasses, Australia is in a unique position of having some of the World’s largest and most diverse seagrass resources over a wide range of climates and habitat types, and encompasses much of the kind of variability found in seagrass ecosystems globally.
Methods and Materials
Ethics Statement
This research was approved by the Edith Cowan University Ethics Committee following submission of an ethics declaration. The collection of seagrass and sediment core samples undertaken for this research were approved through the issuing of collection permits by the Department of Conservation and Environment in Western Australia and the Department of Primary Industries in Queensland.
Sedimentary Carbon Characteristics
Sediment cores were extracted from 17 mono-specific or mixed-species meadows of seagrass (Table 1). The meadows sampled incorporated tropical, sub-tropical and temperate climates as well as inter-tidal and sub-tidal habitats. The sampling design was not orthogonal as not all species occurred in all habitat types; thus, some species were sampled in only one location, while others were sampled in both inter- and sub-tidal habitats or in sub-tidal habitats of different depths.
Table 1. Location of seagrass meadow sampling sites.
| Species | Zone | Location | Habitat | S | E |
| Amphibolis antarctica | subtropical | Shark Bay, WA | Sub-tidal | 7144460 | 772637 |
| Inter-tidal | 7144289 | 772989 | |||
| temperate | Geographe Bay, WA | Sub-tidal5m | 6280864 | 353582 | |
| Sub-tidal 10m | 6282348 | 342501 | |||
| C. rotundata /H.uninervis | tropical | Green Is., QLD | Sub-tidal | 8147054 | 390456 |
| C. rotundata/S. isoetifolium | tropical | Green Is., QLD | Sub-tidal | 8147003 | 390468 |
| Cymodocea serrulata | tropical | Trinity Inlet, QLD | Sub-tidal | 8132670 | 369620 |
| Halodule uninervis | tropical | Trinity Inlet, QLD | Sub-tidal | 8131418 | 373498 |
| Inter-tidal | 8131418 | 373510 | |||
| Halophila ovalis | tropical | Trinity Inlet, QLD | Sub-tidal | 8131053 | 371447 |
| Posidonia australis | subtropical | Shark Bay, WA | Sub-tidal | 7144460 | 772637 |
| Inter-tidal | 7144289 | 772989 | |||
| temperate | Waychinicup Inlet | Sub-tidal | 6137832 | 621812 | |
| Posidonia sinuosa | temperate | Geographe Bay, WA | Sub-tidal5m | 6275712 | 336571 |
| Sub-tidal 10m | 6277434 | 336006 | |||
| T. hemprichii/C. rotundata | tropical | Green Is., QLD | Sub-tidal | 8146599 | 390891 |
| Zostera muelleri | tropical | Trinity Inlet, QLD | Sub-tidal | 8131308 | 373498 |
Location of seagrass meadow sampling sites. The locations are given in UTM using WSG84 map datum and are central points of the study sites. C. rotundata = Cymodocea rotundata; T. hemprichii = Thalassia hemprichii.
At all sites except the Posidonia australis meadow at Waychinicup Inlet, PVC cores (i.d. 47 mm) were manually inserted into the sediments to a depth of 30 cm at three randomly located positions. The cores had serrated bottom edges to assist in cutting through seagrass rhizomes and were gently turned while being pushed or hammered into the sediments. The cores were retrieved, capped and returned to the boat where they were stacked vertically in a cool box until returning to the laboratory. At Waychinicup Inlet, PVC cores were inserted by manual hammering to the maximum possible depth (> 2.5 m). Sample compaction during coring was less than 25% in all cases.
In the laboratory, the sediments were extruded by inserting a plunger at the bottom of the cores and carefully drawing the PVC liner down over the plunger. The cores were sliced into 3 cm sections, at 0–3, 6–9, 12–15, 18–21 and 24–27 cm intervals. The slices were split into two sub-samples, with one sub-sampled retained for organic carbon analysis and the other for organic matter (Loss on Ignition, or LOI) and carbonate analyses. Cores from Waychinicup Inlet were sliced every cm for the first 30 cm. Analyses were performed on 1 cm sections corresponding to the depth ranges analysed for the other cores (e.g. usually the 2 cm section to correspond with the 0–3 cm section of the other cores).
Organic Content and Carbonate Content
Each sub-sample was weighed before and after drying at 50°C for 48 h to determine bulk density and porosity. The samples were then ground in a ball mill and combusted at 450°C for 4 h to determine LOI [19] and then for 2 h at 950°C to determine the carbonate content [20]. All combustions included reference samples of pure glucose and calcium carbonate to correct for incomplete combustion of Corg and carbonates.
Organic Carbon Content
The sub-sample for organic carbon analysis was dried, weighed and then dry-sieved through a 1 mm mesh to remove coarse inorganic particles. The remaining samples were then acidified with 4% HCl to remove inorganic carbon, washed in deionised water then centrifuged (3400 revolutions per minute, for 5 minutes) and the supernatant with acid residues carefully removed by pipette, avoiding resuspension. The residual samples were re-dried and then capsulated for %C and δ13C analyses using an ANCA-NT 20–20 Stable Isotope Analyser connected to an ANCA-NT Solid/Liquid Preparation Module (PDZ Europa instruments). δ13C values were reported relative to v-PDB standard. Percentage C was calculated for the bulk (pre-sieved and pre-acidified) samples.
Estimating Australian Seagrass Corg Stocks and Accumulation Rates and the Effect of Including Inter-habitat Variability on Estimates
To examine the effect of incorporating the natural variability in sedimentary organic carbon storage of seagrasses into regional estimates of seagrass Corg stocks and accumulation rate, we estimated the total sedimentary Corg stock (Cstock) of the top 25 cm of seagrass habitat in Australia as:
where, i refers to the 6 regions of Australia for which seagrass areas have been reported (Table 2), Si is the mean Corg stock of the seagrasses representative of each region and measured in this study expressed in mg m−3, Ai is the estimated area of seagrass in each region expressed in m2) (Table 2), and D is the depth of sediment layer in m (in this case, 0.25 m). The stock was integrated over 25 cm as our deepest section of sediment sampled bracketed the 24–27 cm range, and 25 cm is convenient for normalization to the top 1 m of sediment, which has been examined in other studies (e.g. Fourqurean et al. 2012). The seagrass species considered representative of each region was based by matching [22] assessment of the dominant species in each region (Table 2) with the most morphologically similar species for which we had measured Corg stocks (Table 1). Where more than one species was likely to contribute significantly to the total area of seagrass, we weighted the contribution to the C stock equally among all the species.
Table 2. Estimates of seagrass area in various region of Australia.
| State | Area(km2) | Habitat | Pre-dominantspecies | Source |
| New SouthWales | 15 | Estuarine | P, Z, H | 1 |
| 154 | Estuarine | 2 | ||
| 161 | Estuarine | 3 | ||
| Tasmania | 60 | Embayments | P, Aa, Z, H | 1 |
| 111 | NW coast | 4 | ||
| 845 | Varied | 5 | ||
| Victoria | 10 | Embayments | P, A, Z, H | 1 |
| 470 | Estuarine | 5 | ||
| SouthAustralia | >5230 | Varied | P, A, Z | 1 |
| 9620 | Varied | 6 | ||
| QLD/NT/TS | 2320 | Embayments | H, Hd, C + smallareas of | 1,7, 5, 8 |
| 6000 | Embayments | Th, E and Tc | ||
| 56473 | Varied | |||
| WesternAustralia | 2200 | Varied | P, A in the SW;≥26 species | 1 |
| 25000 | Varied | in NW, incl.H, Hd & C | ||
| Total | 92569 |
Estimates of seagrass area in various region of Australia. Bold indicate the estimates which were used in the calculations of national seagrass Corg stocks and accumulation rates.
P = Posidonia spp.;
Z = Zostera spp.;
H = Halophila spp.;
A = Amphibolis spp;
Aa = Amphibolis antarctica;
Hd = Halodule spp.;
C = Cymodocea spp.;
Th = Thalassia hemprichii;
E = Enhalus acroides;
Tc = Thalassodendron ciliatum.
Sources:
For accumulation rates, a similar approach was taken to produce a range of possible accumulation rates and to assess the effect of incorporating inter-habitat and inter-species variability in the estimates. The organic carbon accumulation rate Caccum (t Corg y−1) was determined as:
where, i refers to the 6 regions for which seagrass areas have been reported (Table 2), Si is the mean organic carbon stock (mg m−3) in the top 25 cm of the seagrasses representative of each region and that were measured in this study, Ai is the estimated area (m2) of seagrass in each region (Table 2) and R is the rate of sediment accumulation (m y−1). The stocks were the mean of all depth layers, which better represents the medium-to long-term accumulation of C than considering only the top layer.
The rate of C accumulation is highly dependent on the rate of sediment accumulation. In the absence of dating for each of the cores, we assumed a range of sedimentation rates based on published literature and unpublished dating results that we have recently obtained for other seagrass areas throughout Australia (Table 3). The published rates show a large range of sediment accumulation rates from as low as 0.15 mm y−1 to 9.9 mm y−1 in seagrass habitats and over 17 mm y−1 in tropical lagoon habitats. Therefore, we applied a range of accumulation rates. We ignored the highest reported rate as this applied to tropical lagoon environments with no evidence that these supported seagrasses. Within the remaining range (0.15 to 9.9 mm y−1) are data derived from seagrass core dating studies. The maximum sediment accumulation rate of 9.9 mm y−1 [23] was based on Pb-210 dating at one site and relates to the past 60 years. For a nearby meadow they estimated an accumulation rate of 4.7 mm y−1 for the past 60 years and for the Holocene an average of 0.44 mm y−1 (based on 14C dating). This range illustrate the wide range of accumulation rates when considering short- vs long-term periods, reflecting a combination of factors such as human impacts on sedimentation dynamics as well as diagentic process (i.e. organic matter decomposition and sediment compaction with ageing). We have a number of dated Posidonia australis sediment core profiles from Oyster Harbour, Western Australia that indicate sediment accumulation rates in the order of 1.45–2.43 mm y−1 over the past 70–80 years (unpublished data). Together, these data indicated that sediment accumulation rates in seagrass meadows are likely to be in the range of 0.15–9.9 mm y−1 but C and lead dating suggesting accumulation rates for Posidonia species in the order of 1–1.5 mm y−1 in recent times. To capture this uncertainty, we used three representative sediment accumulation rates in seagrass meadows (0.15, 1.5 and 9.9 mm y−1) to calculate Corg accumulation rates:.
Table 3. Published sediment accumulation rates (by depth) for Australian coastal marine ecosystems and P. oceanica from the Mediterranean Sea.
| Site | Habitat | Sedimentationrate (mm y−1) | Reference |
| Morton Bay | Inter-tidal | 1.3–2.7 | [56] |
| Ningaloo Reef | Fringingreef | 1.46–9.88 | [57] |
| SE Australia | Depositional | 14.2–17.3 | [58] |
| Fitzroy R., QLD | Estuary | 15 | [59] |
| Herbert R., QLD | Depositionalbay | 1.11–11.4 | [60] |
| GBR Nara Inlet | Inlet | 1 | [61] |
| Sydney | Nearshoreshelf | 2–4 | [62] |
| Port Phillip Bay | Embayment | 1.5 | [63] |
| Far North QLD | Inner shelf | 0.4–1 | [64] |
| Herbert R., QLD, | Tidalmud flats | 0.3–8.5 | [65] |
| Spencer Gulf | Seagrass | 0.15–0.25 | [66] |
| Sydney, Botany Bay | Seagrass | 4.7–9.9 | [23] |
| Mediterranean Sea | Seagrass | 0.61–4.1 | [5] |
| Mediterranean Sea | Seagrass | 1 | [42] |
| Albany, WA | Seagrass | 1.45–2.43 | Unpublisheddata |
| Total Range | 0.15–17.3 | ||
| Seagrass range | 0.15–9.9 |
NB: Mass Accumulation Rates (MAR) were not used unless sediment bulk density data were available to convert the MAR to depth accumulation rates.
Results
Seagrass Sediment Characteristics
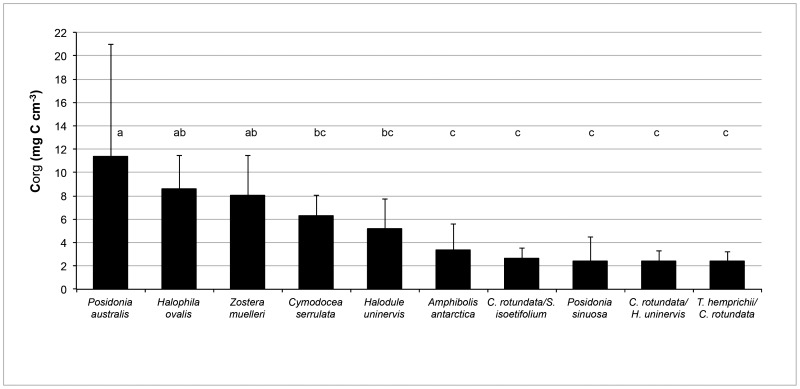
The mean organic matter content of the sediments in the Australian seagrass habitats sampled ranged from 0.67 to 9.09% DW, with a mean of 3.74 ± 3.13% (Mean ± SD; Table 4). The bulk % Corg ranged from 0.1 to 2.14% DW, with a mean of 0.64 ± 0.68%. The range across all 17 sub-habitats was 1.09 ± 0.32 mg Corg cm−3, in a shallow temperate Posidonia sinuosa habitat to 20.15 ± 10.95 mg Corg cm−3 in a temperate, estuarine P. australis habitat (Table 4). When averaging over all the sub-habitats in which a species was sampled, the mean Corg stocks in the top 25 cm of the different seagrass habitats differed significantly (Fig. 1; ANOVA d.f = 9, 269; F = 17.87; p <0.001). P australis had the highest mean Corg stock (11.42 ± 9.55 mg Corg cm−3; Fig. 1). The mean Corg stocks for Halophila ovalis, and Zostera muelleri habitats were not significantly different to those of P. australis, while a mixed meadow of T. hemprichii/C. rotundata had the lowest stock (2.38 ± 0.85 mg Corg cm−3, though this was not significantly lower than a variety of sub-tropical and temperate habitats. Averaged over all sub-habitats mixed meadows of tropical species had the lowest stocks, generally less than 2.7 mg Corg cm−3.
Table 4. Sediment characteristics of Australian seagrass habitats and Posidonia oceanica.
| Species | Climate | Habitat | N | % OM | s.d. | % C (bulk) | s.d. | mg C cm−3 | s.d. | g C m−2 | s.d. |
| Posidonia australis | all | 47 | 6.43 | 3.88 | 1.31 | 1.06 | 11.42 | 9.55 | 2741.10 | 2292.35 | |
| temperate | subtidal | 18 | 5.88 | 2.82 | 2.14 | 1.19 | 20.14 | 10.94 | 4832.88 | 2040.08 | |
| subtropical | all | 29 | 6.78 | 4.43 | 0.79 | 0.50 | 6.01 | 1.98 | 1442.75 | 474.36 | |
| intertidal | 14 | 4.31 | 1.81 | 0.55 | 0.19 | 4.92 | 0.89 | 1179.76 | 214.20 | ||
| subtidal | 15 | 9.09 | 4.94 | 1.01 | 0.60 | 7.03 | 2.18 | 1688.21 | 523.18 | ||
| Halophila ovalis | tropical | subtidal | 15 | 6.21 | 3.45 | 1.18 | 0.38 | 8.64 | 2.86 | 2072.77 | 685.41 |
| Zostera muelleri | tropical | subtidal | 15 | 4.48 | 1.30 | 1.33 | 0.83 | 8.06 | 3.38 | 1933.85 | 810.48 |
| Cymodocea serrulata | tropical | subtidal | 15 | 3.02 | 0.88 | 0.68 | 0.19 | 6.32 | 1.74 | 1516.70 | 417.02 |
| Halodule uninervis | tropical | all | 27 | 5.87 | 2.51 | 0.69 | 0.36 | 5.19 | 2.55 | 1244.96 | 610.93 |
| intertidal | 13 | 4.66 | 2.05 | 0.62 | 0.48 | 5.50 | 3.36 | 1319.62 | 805.92 | ||
| subtidal | 14 | 7.00 | 2.42 | 0.75 | 0.21 | 4.90 | 1.54 | 1175.63 | 369.02 | ||
| Amphibolis antarctica | all | 59 | 2.43 | 2.41 | 0.36 | 0.32 | 3.33 | 2.26 | 799.34 | 543.57 | |
| subtropical | intertidal | 15 | 2.47 | 1.15 | 0.25 | 0.10 | 2.80 | 1.20 | 672.28 | 288.77 | |
| subtidal | 15 | 4.16 | 3.56 | 0.54 | 0.28 | 4.84 | 1.60 | 1162.70 | 384.96 | ||
| all | 30 | 3.32 | 2.74 | 0.39 | 0.26 | 3.82 | 1.74 | 917.49 | 417.14 | ||
| temperate | subtidal 5m | 15 | 0.79 | 0.73 | 0.13 | 0.05 | 1.54 | 0.45 | 369.01 | 108.49 | |
| subtidal 10m | 14 | 2.29 | 1.90 | 0.55 | 0.45 | 4.20 | 3.29 | 1007.23 | 790.54 | ||
| all | 29 | 1.51 | 1.59 | 0.33 | 0.38 | 2.82 | 2.64 | 677.12 | 633.55 | ||
| C. rotundata/ S. isoetifolium | tropical | subtidal | 15 | 3.08 | 0.39 | 0.32 | 0.11 | 2.67 | 0.85 | 640.15 | 204.87 |
| Posidonia sinuosa | temperate | all | 43 | 1.72 | 1.70 | 0.28 | 0.31 | 2.44 | 2.01 | 585.35 | 482.62 |
| subtidal 5m | 15 | 0.67 | 0.24 | 0.10 | 0.04 | 1.09 | 0.32 | 261.93 | 75.81 | ||
| subtidal 10m | 28 | 2.26 | 1.87 | 0.39 | 0.35 | 3.16 | 2.17 | 758.61 | 519.71 | ||
| C. rotundata/H. uninervis | tropical | all | 28 | 2.55 | 2.89 | 0.28 | 0.10 | 2.43 | 0.85 | 582.33 | 203.38 |
| intertidal | 13 | 3.74 | 3.80 | 0.28 | 0.10 | 2.28 | 0.73 | 546.42 | 174.33 | ||
| subtidal | 15 | 1.51 | 1.12 | 0.28 | 0.10 | 2.56 | 0.95 | 613.45 | 226.91 | ||
| T. hemprichii/C. rotundata | tropical | intertidal | 15 | 2.94 | 2.36 | 0.30 | 0.10 | 2.38 | 0.85 | 571.82 | 204.72 |
| All Species (Australia) | 280 | 3.74 | 3.13 | 0.64 | 0.68 | 5.26 | 5.46 | 1262.05 | 1483.37 | ||
| Posidonia oceanica | 7 | 42.99 | 12.09 | 17.85 | 6.08 | 20.16 | 9.49 | 4837.42 | 2276.62 | ||
| P. oceanica/Australian Spp | 11× | 28× | 4× | 4× |
Data are means for the top 25 cm of sediment. s.d. = standard deviation. %C is for the bulk sample. Bold indicates mean of all habitats for a species; italics relates to individual habitats for a given species.
Figure 1. Organic carbon stocks (Mean ±SD) in the top 25 cm of sediment cores from different Australian seagrass meadows.
Shared letters above the bars indicate no significant difference (p>0.05) among habitats.
Several species of seagrass were sampled in more than one sub-habitat and generally showed significant variability in Corg stocks among sub-habitats. Thus, while a temperate P. australis meadow had the highest absolute stock recorded in any single sub-habitat (20.15± 10.95 mg Corg cm−3 ), a sub-tropical meadow in Shark Bay had a relatively low stock of 4.92 ± 0.89 mg Corg cm−3. Similarly, among P. sinuosa the Corg stock ranged from 1.09 ± 0.32 mg Corg cm−3, in a shallow temperate meadow, the absolute lowest value recorded in any of the sampled sub-habitats, to 3.16 ± 2.17 mg Corg cm−3 in a deeper temperate meadow.
Integrated over the depth profile of 24 cm that was sampled, the Corg content of the upper meadows ranged from 262 ± 75.8 g Corg m−2 for the temperate Posidonia sinuosa meadow to 4833 ± 2040 g Corg m−2 for temperate, estuarine P. australis meadow.(Table 4).
While there were significant differences in the total Corg stock of the different meadows, these did not consistently fall into a temperate-tropical divide; while the smallest stocks were found in a tropical species, tropical H. ovalis had the second largest stock. Post-hoc pairwise comparisons (Fig. 1) indicated that highest stocks were found in the temperate Posidonia australis and the tropical H. ovalis, Zostera muelleri and Cymodocea serrulata meadows, while the lowest stocks were found in a mixture of tropical, sub-tropical and temperate meadows (Amphibolis antarctica, P. sinuosa and mixed meadows of C. rotundata with other species).
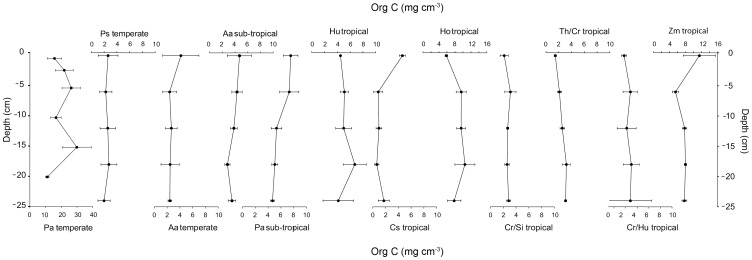
The profiles of Corg stocks through the top 25 cm of the sediment cores also showed no consistent difference among climatic zones (Fig. 2). Temperate and sub-tropical meadows showed a general pattern of declining Corg stocks with depth. While some tropical meadows showed the opposite trend (increasing Corg stocks with depth in Halophila ovalis and meadows of Cymodocea rotundata mixed with Thalassia hemprichii, Syringodium isoetifolium or Halodule uninervis), others showed the same trend as the temperate meadows, of declining stocks with depth.
Figure 2. Profiles of organic carbon stocks in the top 25 cm of cores from different Australian seagrass meadows.
All data are means ± std error.
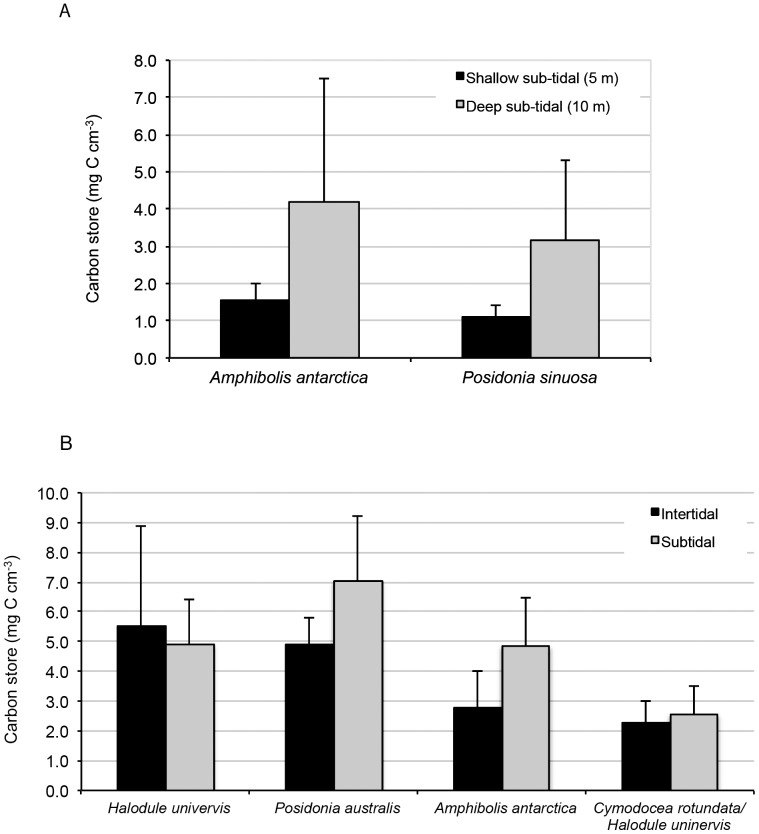
Of those species that were sampled in more than one habitat, some showed significant among-habitat differences in Corg stocks while others did not. For both Posidonia sinuosa and Amphibolis antarctica there was a significant effect of water depth on the Corg stocks (Table 5), with greater stocks in the 10 m deep sites than the 5 m deep sites (Fig. 3a). In both cases, the stock was also much more variable in the deeper sites. In contrast, for species sampled in both inter- and sub-tidal habitats, there was no habitat effect on the Corg stocks (Table 5), though there were differences in stocks among species (Fig. 3b).
Table 5. Results of statistical testing (2-way ANOVA) for significant effects of Species and Habitat (Deep v Shallow (A) or Tidal v Inter-tidal (B)) on the Corg storage in the top 25 cm of seagrass sediments.
| A) Deep v Shallow sub-tidal meadows of P. sinuosa and A. antarctica | |||||
| Effect | SS | d.f. | MS | F | p |
| Species | 9.13 | 1 | 9.13 | 2.28 | 0.135 |
| Depth | 92.98 | 1 | 92.98 | 23.25 | <0.001 |
| Spp x D | 1.45 | 1 | 1.45 | 0.36 | 0.549 |
| Error | 271.91 | 68 | 3.99 | ||
| Pairwise (Tukeys HSD) comparisons on significant effect: depth | |||||
| A. antarctica | Deep v shallow | p = 0.003 | |||
| P. sinuosa | Deep v shallow | p = 0.010 | |||
| B) Inter-tidal v Sub-tidal meadows of four different seagrass species | |||||
| Species | 252.5 | 1 | 2159.5 | 526.6 | <0.001 |
| Tidal regime | 12.1 | 1 | 12.1 | 2.9 | 0.088 |
| Spp x Tide | 27.1 | 3 | 9.0 | 2.2 | 0.090 |
| Error | 271.91 | 68 | 3.99 | ||
Figure 3. Carbon stocks in sediments of seagrass meadows occurring at different water depths: A) comparison of sub-tidal habitats (5 m vs 10 m depth); and B) comparison of inter-tidal and sub-tidal habitats.
Estimates of Australian Seagrass Sedimentary Carbon Stocks & Accumulation Rates
The estimated Corg stock of the top 25 cm of sediment in Australian seagrass habitats that took into account inter-habitat variability was in the order of 155 Mt (Table 6). The majority of this stock (54%) was in the temperate seagrass habitats, dominated by the larger, meadow-forming species of Posidonia and Amphibolis. The remaining 46% was in tropical seagrass habitats of northern Australia, which are dominated by a variety of smaller-sized seagrasses that typically have a lower sedimentary Corg stock than the larger temperate species but a larger reported areal coverage. The estimates of annual Corg accumulation for Australia ranged from 0.093 Mt when applying a sedimentation rate of 0.15 mm y−1 to 6.157 Mt at 9.9 mm y−1 (Table 7). At a sedimentation rate of 1.5 mm y−1, which, on limited dating evidence, we believe is more representative of accumulation rates in seagrass ecosystems, the annual organic carbon accumulation rate was 0.932 Mt.
Table 6. Carbon storage in the top 25 cm of Australian seagrass ecosystems and those that would be estimated by applying the carbon storage values of P. oceanica to the same area of seagrass.
| Region | Area | Habitat | Corg stock | Corg stock |
| (km2) | (mg cm−3) | (Mt) | ||
| NSW | 161 | Zm/Pa | 11.74 | 0.472 |
| TAS | 845 | Pa/Ps/Aa(Temp.) | 8.47 | 1.788 |
| VIC | 470 | Pa(subtidal)/Aa(Temp.) | 11.48 | 1.349 |
| SA | 9620 | Pa, Ps, Aa, | 8.47 | 20.361 |
| QLD/NT/TS | 56473 | Ho, Zm, Cs, Hu, Cr/Si, Cr/Hu, Th/Cr | 5.10 | 71.957 |
| WA | 25000 | Pa, Ps, Aa, | 9.53 | 59.558 |
| Total* | 92569 | 155.487 | ||
| % emissions | 104 | |||
| P. oceanica | 92569 | Po | 20.16 | 466.545 |
| % emissions | 313 |
Corg stock is the product of the area of seagrass (see Table 6) and the mean carbon storage (Table 2) of the seagrasses most likely to dominate those areas. % emission refers to the sedimentary Corg in Australian seagrass ecosystems as a % of annual CO2 carbon emissions in Australia. (Zm = Zostera muelleri; Pa = Posidonia australis; Ps = Posidonia sinuosa; Aa = Amphibolis antarctica; Ho = Halophila ovalis; Cs = Cymodocea serrulata; Hu = Halodule uninervis; Cr = Cymodocea rotundata; Si = Syringodium isoetifolium; Th = Thalassia hemprichii; Po = Posidonia oceanica).
Table 7. Estimated annual carbon accumulation rates of Australia’s seagrass habitats.
| Region | Area (km2) | Storage (km2) | Annual Corg accumulation at different sediment accumulation rates | |||
| (Mt y−1) | ||||||
| 0.15 mm y−1 | 1.5 mm y−1 | 9.9 mm y−1 | ||||
| NSW | 161 | 11.74 | <0.001 | 0.002 | 0.019 | |
| TAS | 845 | 8.47 | 0.001 | 0.011 | 0.071 | |
| VIC | 470 | 11.48 | 0.001 | 0.008 | 0.053 | |
| SA | 9620 | 8.47 | 0.012 | 0.122 | 0.806 | |
| QLD/NT/TS | 56473 | 5.10 | 0.043 | 0.432 | 2.850 | |
| WA | 25000 | 9.53 | 0.036 | 0.357 | 2.358 | |
| Total | 92569 | 0.093 | 0.932 | 6.157 | ||
| % of annual emissions | 0.063 | 0.625 | 4.129 | |||
| Mediterranean | 92569 | 20.16 | 0.280 | 2.799 | 18.476 | |
| % of annual emissions | 0.188 | 1.877 | 12.389 | |||
Discussion
Variability in C Stocks
This spatially-limited study has demonstrated a significant variability in the Corg stock in sediments beneath different seagrass habitats. Among the finding is a strong indication that a variety of biotic (species) and abiotic (habitat physic-chemical conditions) exert a strong influence on the carbons tocks of seagrass meadows, producing significant inter-habitat variability.
Posidonia australis had the highest Corg stocks, both averaged over all the sub-habitats in which it was sampled and in any individual sub-habitat sampled. This is consistent with a general expectation that larger seagrasses are likely to have larger carbon stocks due factors that affect the production, form and preservation of organic carbon. Larger seagrasses, such as P. australis, tend to have deeper, larger, more persistent rhizomes, often characterised by more refractory forms of structural carbon, more likely to be preserved in marine sediments than simpler, more labile forms of carbon [18]. The deeper canopy of larger seagrasses may also reduce near-bottom mean water velocities [24] enhancing particle trapping [25], [26] and reducing the resuspension of particles within the canopy [27], leading to higher inputs of allocthonous sedimentary organic matter inside macrophyte beds compared to unvegetated areas [25].
Despite P. australis having the largest stock of organic carbon, there was considerable variability among habitats for this species. Among the three sub-habitats sampled, there was a 4-fold range in the stocks, with the inter-tidal sub-tropical habitat having least and the sub-tidal, temperate habitat the most. This indicates a strong effect of abiotic variables on the carbon storage capacity of this species. The temperate meadow was located in Waychinicup inlet, a small, relatively sheltered coastal estuary. The combination of sheltered conditions and the inputs of allocthonous carbon from its small catchment [28] may contribute to the higher carbon stock at this site. In contrast, the sub-tropical meadow was located in very shallow water (<0.1 m at spring low tide), which would enhance hydrodynamic exposure and associated resuspension and export of sedimentary carbon matter. It is also likely that the generally warmer conditions of Shark bay where the meadow was located, and the shallowness of the site would result in higher mean temperatures that would facilitate enhanced remineralisation of sedimentary carbon [29]. Elsewhere, shallow P. oceanica meadows were observed to have higher rates of carbon remineralisation than deeper meadows [30], attributed to higher respiratory rates at the shallow sites. The effect of depth on carbon stocks is discussed further below.
Surprisingly, Halophila ovalis, which has small leaves and a very short canopy and root system relative to the other species, had the second-highest mean Corg stock, which was not significantly different to that of Posidonia australis and was greater than many larger species. The relatively high C stocks in H. ovalis meadows may be explained by their morphology and their habitat characteristics. Despite having smaller leaves than other seagrass species, Halophila decipiens increased the threshold velocity for sediment motion similar to larger seagrasses [31]. The allocation of leaf biomass and rhizomes closer to the sediment-water interface when compared to other seagrasses was hypothesized as the main physical basis for the significant sediment stabilization effects proved by H. decipiens. It has also been observed that at some water velocities denser seagrass canopies can induce ‘skimming flow’ which directs particles over the canopy and reduces the capture efficiency [32], which may explain some of the differences between H. ovalis and the larger meadows. Further, a global study of seagrass sediments found that, on average, 50% of the sedimentary Corg matter in seagrass meadows was derived from allocthonous sources [12]. In the case of Halophila, it is possible that despite its low biomass the canopy is capable of trapping a variety of C sources resulting in the relatively high carbon stocks. However, it seems more probable that this species is adapted to living in naturally depositional environments and the seagrass itself is a relatively minor contributor to the stocks. This hypothesis is supported by the stable C isotopic composition of the sediments studied for H. ovalis. While the average δ13C value of the tissues of this species has been determined to be below −14 ‰ (e.g. [12], [33]), the value for the sediments analysed for H. ovalis averaged −24.7 ‰ (data not shown), indicating a potentially strong contribution from algal production but, more likely (given the location of the H. ovalis meadows sampled for this study), from terrestrial inputs. Further studies into the sources of C would help to clarify the relative importance of seagrasses in contributing to C stocks.
The data indicate that both the species of seagrass and the abiotic habitat characteristics are important in driving variability of sedimentary Corg stocks. While the habitats we studied were characterised by one or two dominant seagrasses, we cannot conclude that these same species were dominant at the study sites over the duration of carbon accumulation to a depth of 25 cm. However, given typical sediment accumulation rates in seagrass habitats of between 1 and 10 mm y−1 (Table 3), the 25 cm deep cores may represent a period in the order of 25–250 years. For some of the smaller, disturbance-adapted species, it is likely that the seagrass composition at the sites may have changed during that time, while the larger, perennial species might be expected to have dominated the sites for most, if not all, of that time, and at least some of the variability in Corg stocks is related to the species of seagrass.
Water depth had a significant effect on Corg stocks. For Posidonia sinuosa and Amphibolis griffithiii, in Geographe Bay there were larger Corg stocks in the deeper sites than the shallow sites. The higher net productivity of meadows at shallow sites compared to deep sites [15], [34] would suggest greater carbon inputs and sedimentary organic carbon at shallow sites. However, shallower meadows may also be exposed to greater hydrodynamic forces and export of C (as wrack) may be greater than at deeper sites which, in addition, can be receiving environments for organic matter from shallower sites [30]. This hydrodynamic exposure may also result in greater exposure to oxygenated conditions for the shallower sediments [35], promoting higher respiration rates and detrital decay than in deeper sites, as observed in P. oceanica meadows [30]. Furthermore, higher sediment deposition rates at deeper sites (due to lower hydrodynamic forces) may contribute to greater vertical growth rates of seagrasses and, therefore, Corg accumulation rates and stock. Based on our results, even very similar meadows may have significantly different C storage capacities due to a combination of factors other than species composition. Water depth in particular should be considered potentially important in affecting Corg stocks in the surficial sediments and warrants further study.
Surprisingly, and in contrast to sub-tidal water depth, when a species occurred in both inter-tidal and sub-tidal habitats there were no differences in sedimentary organic carbon stocks. The effects of intermittent submergence on primary production and community respiration are complex. Inter-tidal habitats are likely to experience higher temperatures and associated respiration rates which would enhance C remineralisation [18] and lower C accumulation, particularly if tidal currents also contribute to the export of particulate matter and oxygenation of surface sediment layers. On the other hand, experimental studies have shown that some species of seagrass exhibit higher rates of photosynthesis (ETR) in air than when submerged [36]. In addition, the rates of plant respiration can be lower in inter-tidal sediments [37], and gross community metabolism is reduced during emersion periods [38], which may promote Corg preservation through reduced rates of remineralisation. A complex set of factors seems to be interacting as systems shift between intertidal and subtidal states.
As our study was not orthogonal in its design, it is not possible to draw definitive conclusions regarding the difference that climatic region may make to Corg stocks. However, as with inter-tidal and sub-tidal habitats, there was no consistent difference in the C storage of tropical, sub-tropical and temperate seagrass habitats. It is generally thought that the higher temperatures in tropical regions promote more efficient remineralisation of soil organic matter, and a similar process may well occur in shallow coastal sediments. However, while this simple models may hold for labile forms of C the refractory nature of the C substrate and a range of physico-chemical process that protect C from remineralisation may result in poor correlations between temperature and total C stocks in soils [39]. Given the high proportion of complex forms of C in seagrass rhizome material [40], [41], their relative resistance to microbial degradation [29] and the likelihood of oxygen exclusion in deeper sediments, it is likely that similar complex degradation processes relate to seagrass sedimentary Corg and that simple tropical-temperate divisions based on temperature are unlikely to be the main drivers.
The vertical profile of carbon accumulation varied among the species, with most showing the expected decrease in organic matter with depth and other showing the inverse. The decrease with depth is typical of sedimentary systems where there is little turnover of the sediment profile and carbon diagenesis results in a gradual loss of first labile and then increasingly refractory C [18]. We did not examine the cores for faunal biomass, sediment grain size or sediment dating, which may provide insights into the degree of mixing of surface sediments, but these processes may contribute to the observed differences in profiles for those cores that did not show a decline with depth. Also, it is probable that the top 25 cm is not sufficiently deep to describe the expected negative exponential profile of organic matter decomposition with aging (e.g. P. oceanica [42]).
Implications of Variability among Seagrass Habitats for Regional Estimates of Carbon Accumulation
While our data are limited in their spatial coverage and in the degree of habitat replication, the18-fold difference in the Corg storage among the different seagrass sediments is clear evidence of the significant variation among seagrass habitats. The Corg storage values presented are likely to be significant under-estimates of the true storage on an areal basis as they only apply to the top 25 cm of sediment. Many seagrass meadows have organic-rich sediment extended deeper than this, especially species of Posidonia, with mats cored to depths of over 2.5 m depth [23], [42], [43]. Furthermore, the mapping of seagrass area is incomplete for much of the country, including large regions such as the NW, and so the total area of seagrass is also likely to be an underestimate. Conservatively, it is reasonable to assume the storage is at least double that which we have estimated. If we accept our estimated stock of 155 Mt (areal stock: 1.61×109 g Corg km−2), this equates to approximately 100% of the country’s annual CO2 emissions [44] stored in the top 25 cm of sediments beneath Australian seagrass meadows, a significant portion when considering that Australia is one of the most intensive per capita greenhouse gas emitters in the World. This stock represents 7.7–15.3% of the recent global estimate for the top meter of world seagrass soils [21]. The average areal stock estimated by these authors for all seagrasses studied was of 19.42×109 g Corg km−2, about 3 times higher than that estimated in the present study for Australian seagrasses (after roughly normalizing for the top meter of sediment). This proportion increases to 4 times when comparing the Australian average against the South Australia region considered by Fourqurean et al. [21] (26.83×109 g Corg km−2), but declines to 2.3 times when comparing the average for South Australia in our study with their South Australian estimate. Again, these wide ranges and discrepancies between studies highlight the need to augment our knowledge about the natural variability of organic carbon stocks in seagrass soils.
Our estimates of the annual accumulation of Corg by Australian seagrass ecosystems was in the order of 0.09–6.15 Mt Corg. y−1 (1.01–66.5 t km−2 y−1). As explained previously, we consider a sediment accumulation rate of 1.5 mm y−1 to be more typical for seagrass ecosystems than the lower and upper estimates, and this sediment accumulation rate yields an estimated Corg accumulation rate of 0.93 Mt y−1, or 10.06 t km−2 y−1. At this rate, Australian seagrass ecosystems would be sequestering up to 0.6% of the country’s estimated annual CO2 emissions (Australian Government Geoscience Australia; www.ga.gov.au). These estimates of C accumulation by Australian seagrass ecosystems are significantly lower than previously published estimates for seagrasses but still significantly higher than those reported for most of the World’s ecosystems (Table 8). This supports earlier assertions [2], [11] that seagrasses have a high carbon sequestration potential, on a per unit area basis. The Australian Government has set the price of carbon at A$25.40 in 2014–15, after which it will be determined in the market, with estimated trading price of $35 t−1 in 2020 [45]. Using the estimated national seagrass Corg stock of 155 Mt and the above pricing, seagrass habitat would have a market value of $3.9–5.4 bill. for its carbon sequestering function alone. While he free-market trading price of carbon will vary from this predicted value, these estimates serve to emphasise the value of seagrasses and the need to conserve and restore these ecosystems.
Table 8. Estimated annual Corg stocks in soils of different ecosystems.
| Ecosystem | Soil/sediment Carbon Accumulation rate |
| (g C m−2 y−1) | |
| Tropical Forests | 2.3–2.5 |
| Temp Forests | 1.4–12 |
| Boreal Forests | 0.8–2.2 |
| Temp grassland | 2.2 |
| Temperate desert | 0.8 |
| Tundra | 0.2–0.7 |
| Posidonia oceanica | 6–175 |
| Australian seagrasses | 1.0–66.5 * |
| Soil/sediment Carbon Stock | |
| (g C m−2) | |
| Tropical montane | 6100 |
| Tropical wet | 6100 |
| Tropical moist | 5000 |
| Tropical dry | 2200 |
| Warm Temperate moist | 7600 |
| Warm temperate dry | 2600 |
| Cool temperate moist | 11600 |
| Cool temperate dry | 4900 |
| Boreal | 14900 |
| Polar | 11800 |
| Australian seagrasses | 1090 – 20140# |
| P. oceanica | 10500–40000∧ |
Data from [67] except Australian seagrasses (this study) and P. oceanica (based on the range reported [5], [7], [42], [47].
∧Value assume sediment depth of 1 m, which is a conservative estimate for the higher values which are for Posidonia ecosystems with reported organic sediment depths of >2.5 m ([23] and Pers. Obs. for Oyster Harbour and Waychinicup Inlet in SW Australia; P. oceanica references as above).
Comparison with P. oceanica-based Estimates
The mean Corg content of Australian seagrass habitats was about 4 times lower than that recorded in P. oceanica meadows from the Mediterranean Sea. However, the highest Corg content of any Australian seagrass habitat (estuarine P. australis – 4833 g Corg m−2) was almost identical to that of P. oceanica meadow in the Mediterranean Sea (4837 g Corg m−2), when normalized to the top 25 cm. The similarity of the P. australis and P. oceanica values is consistent with their similar morphology and meadow structure. Both species have large, persistent rhizomes, placing them close to each other in seagrass functional-form models [8], [14], which accumulate in the sediments producing deep, organic-rich sediment profiles. Importantly, the meadows of P. oceanica reported in the literature tend to have much deeper organic sediment profiles than P. australis (reportedly up to 8 m compared with 2.5 m for P. australis) due to the vertical growth form of the rhizome. Consequently, P. oceanica habitat will still likely contain much larger masses of sedimentary Corg than any of the Australian seagrass habitats we sampled, and probably represents a global maximum among seagrasses.
Until now, most of the global estimates of seagrass carbon storage have, necessarily, been based on indirect estimates and a few direct measurements of sedimentary Corg stocks in Posidonia oceanica meadows, plus a single study of Cymodocea nodosa (e.g. Duarte et al. 2005, Nellemann et al. 2009). The variation in Corg storage values among seagrass ecosystems reported here suggests that it is important to incorporate this variability into regional or global estimates of seagrass C stocks. The effect of this inter-habitat variation is illustrated by re-calculating the C stocks and accumulation rates for the estimated area of Australian seagrass using a uniform Corg storage value. We applied the value reported for P. oceanica (20.16 mg cm−3; derived from the data reported in Mateo et al. (1997) and Serrano et al. (2012), since this species has typically been used as the representative seagrass in global C accumulation exercises (note: while several studies report C accumulation rates for P. oceanica [3], [4], [46], they ultimately rely on earlier C stock estimates [30], [47], which are applied here.
When inter-habitat variation in seagrass organic carbon stocks was ignored and we applied only the P. oceanica carbon storage values, the stock was estimated to be about 448 Mt and the annual accumulation 0.28–18.4 Mt y−1, 3-times the estimates we made using the range of carbon storage values we measured for the Australian seagrass habitats and more than 300% of Australia’s annual CO2 emissions (compared with 100% for the estimates incorporating inter-habitat variability). These simple comparisons effectively demonstrate the need to better document inter-habitat variability in seagrass carbon storage and to incorporate this variability into regional and global estimates of seagrass carbon stocks. The significant point is not the absolute values of carbon storage, but the significant effect on the estimate of incorporating inter-habitat variability. A 3-fold discrepancy in estimates is sufficiently large to undermine confidence in Blue Carbon estimates among economic and political decision-makers. This supports Nelleman et al.’s [2] identified need to incorporate inter-habitat variability into global estimates if the uncertainties in these estimates are to be reduced.
Conclusions
There is considerable spatial variability in the Corg stock of seagrass sediments. This variability may be related to both the species of seagrass and the habitat setting in which they occur, particularly water depth. The data set presented here is limited and the errors associated with the estimates are likely to be significant, though no more so than the global estimates of seagrass C capture extrapolated from a much more limited set from the Mediterranean Sea, acknowledging that at the time those were the best available data. However, our data serve to emphasise the need for robust data sets on the carbon storage and accumulation rates in different seagrass ecosystems. There is also a pressing need to better understand the habitat features that drive this variability in C storage and accumulation rates. Assuming a uniform ability to capture and sequester carbon among the 70+ species of seagrasses will potentially lead to erroneous estimates of global C storage and improving our understanding of the variability in C stocks and accumulation rates is critical if we are to produce robust estimates of regional and global C capture and storage in seagrass ecosystems.
Acknowledgments
We thank the following people for their assistance in collecting sediment cores: Kathrin Bacher (Shark Bay Dolphin Research Project), Jim Fourqurean and the ‘Sharkies’ research team, at Monkey Mia in Shark Bay, Len McKenzie at Cairns, Kathryn McMahon and the ‘GeoBay’ research team in WA.
Funding Statement
This research was funded by a Visiting Research Fellows grant from the Faculty of Health, Engineering & Science at Edith Cowan University, project number 25139.9256.RG.06.01 (http://www.ecu.edu.au/faculties/computing-health-and-science). Part of this study was funded by the Spanish Ministry for Science and Innovation (MICINN) with the grant CTM2006-12492/MAR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Smith SV (1981) Marine macrophytes as global carbon sinks. Science 211: 838–840. [DOI] [PubMed] [Google Scholar]
- 2.Nellemann C, Corcoran E, Duarte CM, Valdés L, De Young C, et al.. (2009) Blue carbon - The role of healthy oceans in binding carbon. GRID-Arendal.
- 3.Laffoley D d’A, Grimsditch G (2009) The Management of Natural Coastal Carbon Sinks. Gland, Switzerland.
- 4. Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 1: 1–8. [Google Scholar]
- 5.Mateo MA, Romero J, Pérez M, Littler MM, Littler DS (1997) Dynamics of Millenary Organic Deposits Resulting from the Growth of the Mediterranean Seagrass Posidonia oceanica: 103–110.
- 6.Lo Iacono C, Mateo MA, Gràcia E, Guasch L, Carbonell R, et al. (2008) Very high-resolution seismo-acoustic imaging of seagrass meadows (Mediterranean Sea): Implications for carbon sink estimates. Geophysical Research Letters 35: L18601. Available: http://doi.wiley.com/10.1029/2008GL034773. Accessed 9 August 2013.
- 7.Mateo MA, Serrano O (2012) Carbon storage. In: Pergent G, Bazairi H, Bianchi CN, Boudouresque CF, Buia MC, et al.., editors. Mediterranean seagrasses: resilience and contribution to the attenuation of climate change. A short summary. Gland, Switzerland: IUCN. p. 80.
- 8.Walker D, Dennison W, Edgar G (1999) Status of Australian seagrass research and knowledge. In: Butler A, Jernakoff P, editors. Seagrasses in Australia. Collingwood, Victoria: CSIRO Publishing. 1–24.
- 9.Den Hartog C, Kuo J (2006) Taxonomy and Biogeography of Seagrasses. In: Larkum A, Orth R, Duarte C, editors. Seagrasses: Biology, ecology and conservation. Springer. 1–23.
- 10.Mateo MA, Cebrian J, Dunton K, Mutchler T (2006) Carbon Flux in Seagrass Ecosystems. In: Larkum A, Orth R, Duarte C, editors. Seagrasses: Biology, Ecology and Conservation. Netherlands: Springer-Verlag. 159–192.
- 11.Duarte CM, Marbà N, Gacia E, Fourqurean JW, Beggins J, et al. (2010) Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles 24: 1–8. Available: http://www.agu.org/pubs/crossref/2010/2010GB003793.shtml. Accessed 16 October 2012.
- 12.Kennedy H, Beggins J, Duarte CM, Fourqurean JW, Holmer M, et al. (2010) Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochemical Cycles 24: 1–9. Available: http://www.agu.org/pubs/crossref/2010/2010GB003848.shtml. Accessed 16 October 2012.
- 13. Fourqurean JW, Duarte CM, Kennedy H, Holmer M, Mateo M-A, et al. (2012) Supplementary information. Nature Geoscience 5: 1–7 doi:10.1038/NGEO1477 [Google Scholar]
- 14.Carruthers TJB, Dennison WC, Kendrick GA, Waycott M, Walker DI, et al. (2007) Seagrasses of south–west Australia: A conceptual synthesis of the world’s most diverse and extensive seagrass meadows. Journal of Experimental Marine Biology and Ecology 350: 21–45. Available: http://linkinghub.elsevier.com/retrieve/pii/S0022098107003061. Accessed 10 October 2012.
- 15.Collier C, Lavery P, Masini R, Ralph P (2007) Morphological, growth and meadow characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Marine Ecology Progress Series 337: 103–115. Available: http://www.int-res.com/abstracts/meps/v337/p103-115/. Accessed 15 Aug 2013.
- 16. Alcoverro T, Duarte C, Romero J (1995) Annual growth dynamics of Posidonia oceanica: contribution of large-scale versus local factors to seasonality. Marine Ecology Progress Series 120: 203–210. [Google Scholar]
- 17. Keil RG, Hedges JI (1993) Sorption of organic matter to mineral surfaces and the preservation of organic matter in coastal marine sediments. Chemical Geology 107: 385–388. [Google Scholar]
- 18.Burdige DJ (2007) Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chemical reviews 107: 467–485. Available: http://www.ncbi.nlm.nih.gov/pubmed/17249736. Accessed 15 Aug 2013. [DOI] [PubMed]
- 19. Kendrick GA, Lavery PS (2001) Assessing biomass, assemblage structure and productivity of algal epiphytes on seagrasses. In: Short FT, Short CA, Coles RG, editors. Global Seagrass Resarch Methods. Amsterdam: Elsevier B. V: 1–24. [Google Scholar]
- 20. Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110. [Google Scholar]
- 21. Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, et al. (2012) Seagrass ecosystems as a globally significant carbon stock. Nature Geosciences 5: 505–509. [Google Scholar]
- 22.Kirkman H (1997) Seagrasses of Australia, Australia: State of the Environment Technical Paper Series ( Estuaries and the Sea). Canberra.
- 23.Macreadie PI, Allen K, Kelaher BP, Ralph PJ, Skilbeck CG (2012) Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Global Change Biology 18: 891–901. Available: http://doi.wiley.com/10.1111/j.1365-2486.2011.02582.x. Accessed 5 October 2012.
- 24.Hansen J, Reidenbach M (2012) Wave and tidally driven flows in eelgrass beds and their effect on sediment suspension. Marine Ecology Progress Series 448: 271–287. Available: http://www.int-res.com/abstracts/meps/v448/p271-287/. Accessed 11 October 2012.
- 25.Gruber RK, Kemp WM (2010) Feedback effects in a coastal canopy-forming submersed plant bed. Limnology and Oceanography 55: 2285–2298. Available: http://www.aslo.org/lo/toc/vol_55/issue_6/2285.html. Accessed 11 October 2012.
- 26. Agawin N, Duarte C (2002) Evidence of direct particle trapping by a tropical seagrass meadow. Estuaries 25: 1205–1209. [Google Scholar]
- 27.Gacia E, Duarte CM (2001) Sediment Retention by a Mediterranean Posidonia oceanica Meadow: The Balance between Deposition and Resuspension. Estuarine, Coastal and Shelf Science 52: 505–514. Available: http://linkinghub.elsevier.com/retrieve/pii/S0272771400907534. Accessed 6 October 2012.
- 28. Phillips J, Lavery P (1997) Waychinicup estuary, Western Australia: The role of freshwater inflows on the flora and fauna. Journal of the Royal Society of Western Australia 80: 63–72. [Google Scholar]
- 29.Pedersen M, Serrano O, Mateo M, Holmer M (2011) Temperature effects on decomposition of a Posidonia oceanica mat. Aquatic Microbial Ecology 65: 169–182. Available: http://www.int-res.com/abstracts/ame/v65/n2/p169-182/. Accessed 24 February 2013.
- 30.Mateo M, Romero J (1997) Detritus dynamics in the seagrass Posidonia oceanica: elements for an ecosystem carbon and nutrient budget. Marine Ecology Progress Series 151: 43–53. Available: http://www.int-res.com/abstracts/meps/v151/p43-53/. Accessed 15 Aug 2013.
- 31. Fonseca MS (1989) Sediment stabilization by Halophila decipiens in comparison to other seagrasses. Estuarine, Coastal & Shelf Science 29: 501–507. [Google Scholar]
- 32.Wilkie L, O’Hare MT, Davidson I, Dudley B, Paterson DM (2012) Particle trapping and retention by Zostera noltii: A flume and field study. Aquatic Botany 102: 15–22. Available: http://linkinghub.elsevier.com/retrieve/pii/S0304377012000666. Accessed 9 October 2012.
- 33. Hemminga MA, Mateo MA (1996) Stable carbon isotopes in seagrasses: variability in ratios and use in ecological studies. Marine Ecology Progress Series 140: 285–298. [Google Scholar]
- 34.Collier CJ, Lavery PS, Ralph PJ, Masini RJ (2009) Shade-induced response and recovery of the seagrass Posidonia sinuosa. Journal of Experimental Marine Biology and Ecology 370: 89–103. Available: http://linkinghub.elsevier.com/retrieve/pii/S0022098108005935. Accessed 11 October 2012.
- 35. Hedges JI, Hu FS, Devol AH, Hartnett HE, Tsamakis E, et al. (1999) Sedimentary organic matter preservation: a test for selective degradation under oxic conditions. American Journal of Science 2: 529–555. [Google Scholar]
- 36.Silva J, Santos R, Calleja ML, Duarte CM (2005) Submerged versus air-exposed intertidal macrophyte productivity: from physiological to community-level assessments. Journal of Experimental Marine Biology and Ecology 317: 87–95. Available: http://linkinghub.elsevier.com/retrieve/pii/S0022098104006215. Accessed 11 October 2012.
- 37.Clavier J, Chauvaud L, Carlier A, Amice E, Van der Geest M, et al. (2011) Aerial and underwater carbon metabolism of a Zostera noltii seagrass bed in the Banc d’Arguin, Mauritania. Aquatic Botany 95: 24–30. Available: http://linkinghub.elsevier.com/retrieve/pii/S030437701100057X. Accessed 12 October 2012.
- 38.Ouisse V, Migné A, Davoult D (2011) Community-level carbon flux variability over a tidal cycle in Zostera marina and Z. noltii beds. Marine Ecology Progress Series 437: 79–87. Available: http://www.int-res.com/abstracts/meps/v437/p79-87/. Accessed 12 October 2012.
- 39.Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, et al. (2011) Temperature and soil organic matter decomposition rates - synthesis of current knowledge and a way forward. Global Change Biology 17: 3392–3404. Available: http://doi.wiley.com/10.1111/j.1365-2486.2011.02496.x. Accessed 8 October 2012.
- 40. Klap V, Hemminga M, Boon J (2000) Retention of lignin in seagrasses: angiosperms that returned to the sea. Marine Ecology Progress Series 194: 1–11. [Google Scholar]
- 41.Torbatinejad NM, Annison G, Rutherfurd-Markwick K, Sabine JR (2007) Structural constituents of the seagrass Posidonia australis. Journal of agricultural and food chemistry 55: 4021–4026. Available: http://www.ncbi.nlm.nih.gov/pubmed/17439231. Accessed 15 Aug 2013. [DOI] [PubMed]
- 42.Serrano O, Mateo MA, Renom P, Julià R (2012) Characterization of soils beneath a Posidonia oceanica meadow. Geoderma 185–186: 26–36. Available: http://linkinghub.elsevier.com/retrieve/pii/S0016706112001395. Accessed 12 October 2012.
- 43.Michot BTC, Burch JN, Arrivillaga A, Patricia S, Doyle TW, et al.. (2002) Impacts of Hurricane Mitch on Seagrass Beds and Associated Shallow Reef Communities along the Caribbean Coast of Honduras and Guatemala. Open file report OFR 03-181.
- 44.Anonymous (2012) Australian national greenhouse accounts. Quarterly Update of Australia’s national greenhouse gas inventory. March quarter 2012. Australiain Government, Department of Climate Change and Energy Efficiency.
- 45.Commonwealth of Australia Attorney-Genaral’s Department (2008) Australia’s low pollution future: The economics of climate change mitigation. Barton, ACT.
- 46.Gacia E, Duarte CM, Middelburg JJ (2002) Carbon and nutrient deposition in a Mediterranean seagrass (Posidonia oceanica) meadow. Limnology and Oceanography 47: 23–32. Available: http://www.aslo.org/lo/toc/vol_47/issue_1/0023.html. Accessed 15 Aug 2013.
- 47. Romero J, Pdrez M, Mateo MA, Sala E (1994) The belowground organs of the Mediterranean seagrass Posidonia oceanica as a biogeochemical sink. Aquatic Botany 47: 13–19. [Google Scholar]
- 48.West RJ, Thorogood C, Walford T, Williams RJ (1985) An estuarine inventory for New South Wales, Australia. Fisheries Bulletin 2. Sydney, NSW. Available: http://www.dpi.nsw.gov.au/fisheries/habitat/aquatic-habitats/estuarine. Accessed 15 Aug 2013.
- 49.Creese RG, Glasby TM, West G, Gallen C (2009) Mapping the habitats of NSW estuaries. Nelson Bay, NSW.
- 50.Donaldson P, Sharples C, Anders R (2012) The tidal characteristics and shallow-marine seagrass sedimentology of Robbins Passage and Boullanger Bay, far northwest Tasmania. A technical report to Cradle Coast Natural Resource Management. Hobart.
- 51.Green EP, Short FT (2003) World Atlas of Seagrasses. Cambridge: UNEP-WCMC.
- 52.Eddyvane KS (1999) Conserving Marine Biodiversity in South Australia - Part 2 - Identification of areas of high conservation value in South Australia. Adelaide.
- 53.Coles R, McKenzie L, Campbell S, Mellors J, Waycott M, et al.. (2004) Seagrasses in Queensland waters. Townsville, QLD.
- 54.McKenzie LJ, Collier C, Waycott M (2012) Reef rescue marine monitoring program: nearshore seagrass. Annual report fr the sampling period 1st July 2010–31st may 2011. Cairns.
- 55.Lawrence A, Baker E, Lovelock C (2012) Optimising and managing coastal carbon. Brisbane.
- 56.Morelli G, Gasparon M, Fierro D, Hu W-P, Zawadzki A (2012) Historical trends in trace metal and sediment accumulation in intertidal sediments of Moreton Bay, southeast Queensland, Australia. Chemical Geology 300–301: 152–164. Available: http://linkinghub.elsevier.com/retrieve/pii/S0009254112000514. Accessed 5 October 2012.
- 57.Twiggs EJ, Collins LB (2010) Development and demise of a fringing coral reef during Holocene environmental change, eastern Ningaloo Reef, Western Australia. Marine Geology 275: 20–36. Available: http://linkinghub.elsevier.com/retrieve/pii/S0025322710001465. Accessed 5 October 2012.
- 58. James N, Bone Y, Brown K, Cheshire A (2009) Calcareous epiphyte production in cool-water carbonate seagrass depositional environments - southern Australia. Special Publications of the international Association of Sedimentologists 41: 123–148. [Google Scholar]
- 59.Bostock HC, Brooke BP, Ryan DA, Hancock G, Pietsch T, et al. (2007) Holocene and modern sediment storage in the subtropical macrotidal Fitzroy River estuary, Southeast Queensland, Australia. Sedimentary Geology 201: 321–340. Available: http://linkinghub.elsevier.com/retrieve/pii/S0037073807002023. Accessed 5 October 2012.
- 60.Brunskill GJ, Zagorskis I, Pfitzner J (2002) Carbon Burial Rates in Sediments and a Carbon Mass Balance for the Herbert River Region of the Great Barrier Reef Continental Shelf, North Queensland, Australia. Estuarine, Coastal and Shelf Science 54: 677–700. Available: http://linkinghub.elsevier.com/retrieve/pii/S0272771401908522. Accessed 5 October 2012.
- 61. Heap AD, Dickens GR, Stewart LK (2001) Late Holocene sediment in Nara Inlet, central Great Barrier Reef platform, Australia: sediment accumulation on the middle shelf of a tropical mixed clastic/carbonate system. Marine Geology 176: 39–54 Available: http://linkinghub.elsevier.com/retrieve/pii/S0025322701001451. [Google Scholar]
- 62. Matthai C, Birch G, Jenkinson A, Heijnis H (2001) Physical resuspension and vertical mixing of sediments on a high energy continental margin (Sydney, Australia). Journal of Environmental Radioactivity 52: 67–89. [DOI] [PubMed] [Google Scholar]
- 63. Hancock G, Hunter JR (1999) Marine Freshwater Research &. Marine & Freshwater Research 50: 533–545. [Google Scholar]
- 64.Woolfe KJ, Larcombe P, Orpin AR, Purdon RG, Michaelsen P, et al. (1998) Controls upon inner-shelf sedimentation, Cape York Peninsula, in the region of 12°S. Australian Journal of Earth Sciences 45: 611–621. Available: http://www.tandfonline.com/doi/abs/10.1080/08120099808728416. Accessed 5 October 2012.
- 65. Belperio A (1983) Terrigenous sedimentation in the central Great Barrier Reef lagoon: a model from the Burdekin region Bureau of Mineral Resources. Journal of Australian Geology and Geophysics 8: 179–190. [Google Scholar]
- 66.Puttonen I, Cann JH (2006) Foraminiferal record of the postglacial (Holocene) marine transgression and subsequent regression, northern Spencer Gulf, South Australia. Australian Journal of Earth Sciences 53: 565–576. Available: http://www.tandfonline.com/doi/abs/10.1080/08120090600632433. Accessed 5 October 2012.
- 67.Batjes NH (2011) Soil organic carbon stocks under native vegetation – Revised estimates for use with the simple assessment option of the Carbon Benefits Project system. Agriculture, Ecosystems & Environment 142: 365–373. Available: http://linkinghub.elsevier.com/retrieve/pii/S0167880911001988. Accessed 17 October 2012.