Abstract
Background: Traditional animal toxicity tests can be time and resource intensive, thereby limiting the number of chemicals that can be comprehensively tested for potential hazards to humans and/or to the environment.
Objective: We compared several types of data to demonstrate how alternative models can be used to inform both human and ecological risk assessment.
Methods: We reviewed and compared data derived from high throughput in vitro assays to fish reproductive tests for seven chemicals. We investigated whether human-focused assays can be predictive of chemical hazards in the environment. We examined how conserved pathways enable the use of nonmammalian models, such as fathead minnow, zebrafish, and Xenopus laevis, to understand modes of action and to screen for chemical risks to humans.
Results: We examined how dose-dependent responses of zebrafish embryos exposed to flusilazole can be extrapolated, using pathway point of departure data and reverse toxicokinetics, to obtain human oral dose hazard values that are similar to published mammalian chronic toxicity values for the chemical. We also examined how development/safety data for human health can be used to help assess potential risks of pharmaceuticals to nontarget species in the environment.
Discussion: Using several examples, we demonstrate that pathway-based analysis of chemical effects provides new opportunities to use alternative models (nonmammalian species, in vitro tests) to support decision making while reducing animal use and associated costs.
Conclusions: These analyses and examples demonstrate how alternative models can be used to reduce cost and animal use while being protective of both human and ecological health.
Citation: Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, Johnson MS, Tietge JE, Villeneuve DL. 2013. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ Health Perspect 121:1002–1010; http://dx.doi.org/10.1289/ehp.1306638
Introduction
The use of traditional animal models and assays to assess the potential human and ecological hazards and risks posed by tens of thousands of chemicals that are currently being evaluated both in Europe and the United States would be prohibitively costly and time consuming, and vastly increase the number of testing animals needed (Rovida and Hartung 2009). As a result, toxicology has shifted from standard empirical testing to a pathway-based vision relying on in vitro systems and predictive models [National Research Council (NRC) 2007]. Although the challenges differ, a pathway-based vision is equally applicable to ecotoxicology (Villeneuve and Garcia-Reyero 2010).
As regulatory toxicology increases its reliance on predictive approaches, the historical distinction between human and ecological toxicology is increasingly blurred. These disciplines should no longer be defined by the animal models they employ. Greater emphasis should be placed on understanding chemical perturbation(s) of pathways at key junctures, including activation or inactivation of specific receptors, enzymes, or transport proteins (molecular initiating events) that in many instances are conserved across species.
Our increasing knowledge of pathway conservation facilitates the use of nontraditional species as toxicological models. Extrapolation across species, and selection of testing organisms, can be improved by focusing on the similarity (or lack thereof) of biological pathways among species, as opposed to direct comparisons of apical responses alone via species sensitivity distributions. Fundamental pathways underlying development (Adamska et al. 2007; Martindale 2005; Vallee et al. 2008), reproduction (Ankley and Johnson 2004), and stress response (Simmons et al. 2009) are highly conserved in metazoans. Nonmammalian models such as zebrafish have been found to possess orthologs for 62% of all human genes (Howe et al. 2013). Species as phylogenetically remote as Drosophila possess pathways important in human disease and development such as, for example, the lateral inhibition pathway involved in lung cancer and sleep regulation (Chen et al. 1997; Foltenyi et al. 2007). Numerous studies have identified conserved pathways for diseases in nonmammalian vertebrates and invertebrates, further supporting the use of alternative models for toxicity testing (Embry et al. 2010; Hill 2005). Although caution is still needed in extrapolation, the similarities between humans and nontraditional species provide great potential for improving efficiencies in hazard assessments.
Embryos offer alternatives to in vivo testing with adult animals, but embryos may not have a complete organ system (e.g., reproductive) or metabolic capacity (Embry et al. 2010). Nevertheless, transcriptional analysis of zebrafish embryos exposed to ethinylestradiol or genistein have detected alterations to genes and pathways involved in estradiol response, steroid biosynthesis, and neurodevelopment, demonstrating that the developing embryo has potential in screening for endocrine-disrupting chemicals that affect reproduction (Schiller et al. 2013; Vosges et al. 2010). Zebrafish embryos can also be predictive of in vivo chemical effects in both adult fish and rats, depending on the pathway involved. Knöbel et al. (2012) found that chemical toxicity to zebrafish embryos was predictive of acute toxicity in adult fish, with the possible exception of chemicals requiring metabolic activation. Enough pathway conservation is present in 24 hr post-fertilized zebrafish embryos that the toxicity of 60 chemicals was well correlated to toxicity in rats (Ali et al. 2011). This correlation was also dependent on the chemical class examined where carboxylic acids, glycosides, and alkaloids were more toxic to zebrafish, whereas alcohols were more toxic to rats than zebrafish. Zebrafish embryos also have complete pathways for thyroid hormone synthesis (Thienpont et al. 2011), heart development and more (Hill 2005). While it remains to be shown that embryo tests are fully predictive of effects in other species, evidence to date supports the view that fish embryos can be protective of both adult alternative species and mammals when used in a pathway context.
Here we postulate that, using a pathway-based hazard assessment approach, data from multiple species and non-animal alternative models are equally valuable for both ecological and human health hazard assessment. Using the adverse outcome pathway (AOP) framework (Ankley et al. 2010), we provide examples of how data from human-focused assays can be useful in identifying key initiators and predicting effects in nonmammalian species. Likewise, we describe how alternative models can be predictive of effects of human health concern (e.g., endocrine disruption) and link chemicals to toxicity pathways, or modes of action, in both mammals and ecological species. Finally, we demonstrate how dose-dependent effects in alternative models can be translated using a pathway-based measure to chemical hazard levels that are similar to those generated using mammalian species in chronic tests. These examples highlight the scientifically credible foundation that supports the predictive application and/or extrapolation of pathway-based toxicological data across species.
Use of Alternative Species and in Vitro Assays in an AOP Framework
A mechanistic understanding of the effects of pathway perturbation is required to accurately relate chemical impacts across species. Adverse outcome pathways provide a framework that organizes mechanistic and/or predictive relationships between initial chemical–biological interactions (i.e., molecular initiating events, or MIEs), pathways, and adverse phenotypic outcomes relevant to hazard assessment. Using an AOP framework allows the use of alternative models by informing extrapolation of chemical impacts across species.
Extrapolation across species may occur at several different levels once an AOP, or even elements of one, has been defined. The simplest is at the molecular initiating event level where sequences or structures of proteins can be compared. More complex extrapolations occur at a pathway level, from molecular initiating event to adverse outcome including the sequence of events and the dose or threshold concentrations required to activate these events. Pathways can be explored either as discrete pathways or as networks using cross-species comparative genomics. [For a brief review, see Burgess-Herbert and Euling (2011).] Ultimately, the most realistic approach is to translate effects through systems-level models where dynamic events are incorporated, such as chemical concentrations, homeostasis, effects over time, and species-specific parameters related to absorption, distribution, metabolism, and excretion.
Extrapolation at the molecular initiating event level facilitates prediction of potential adverse effects. Cross-species comparisons focused on conserved events permit anchoring an AOP in a nonmammalian species to those relevant to rodents or humans through the identification of common biological machinery, under the assumption that evolutionarily conserved proteins may have conserved functions. Identification of a protein ortholog for a known chemical molecular target can be used to infer possible effects, especially where an AOP exists. Numerous human drug targets are conserved across ecologically relevant vertebrate and nonvertebrate species (Gunnarsson et al. 2008; Huggett et al. 2003; Kostich and Lazorchak 2008). Assigning functions via sequence similarity should be done with caution because genome duplication in species such as zebrafish has resulted in multiple orthologs for 15% of human genes (Howe et al. 2013). Duplicated genes are generally free to evolve and acquire new functions, which can confuse functional attribution by sequence similarity.
Recent concerns regarding the potential of pharmaceuticals to harm nontarget aquatic organisms illustrate how extrapolation of human-focused data can be used to infer potential ecological effects for prioritizing pharmaceutical chemicals/classes for hazard assessment purposes (Boxall et al. 2012; Schreiber et al. 2011; Tarazona et al. 2007). For example, although the estrogen receptor (ER) is well conserved across vertebrate species (Baker 2011), a functional ortholog has not been found in invertebrate species (Baker et al. 2008; Thomson et al. 2009). Therefore, chemicals that bind to the ER should affect vertebrates more than invertebrates, regardless of dose—a prediction confirmed in comparative studies (Goto and Hiromi 2003; Jukosky et al. 2008; Santos et al. 2007).
Using human-focused molecular initiating event assays to predict higher-level effects across species. Most, if not all high throughput screening (HTS) programs designed to assess the toxicity or biological effects of chemicals are centered on molecular initiating events known to be relevant to human health (Collins et al. 2008). Where these events are conserved across species, the HTS data could be relevant to ecological hazard assessment (Kavlock et al. 2012). This is demonstrated by examination of 309 chemicals tested in the U.S. Environmental Protection Agency (EPA) ToxCast™ phase I project (Knudsen et al. 2011) and of 40 chemicals for which fathead minnow 21-day short-term reproduction assay data have been reported in the peer-reviewed literature. Both HTS and fathead minnow reproduction data exist for nine chemicals [atrazine, bisphenol A (BPA), fenarimol, fipronil, methoxychlor, prochloraz, propiconazole, prometon, and vinclozolin; Table 1]. In general, significant responses in HTS assays for each of the nine chemicals (Knudsen et al. 2011; see Supplemental Material, Figures S1–S9) were predictive of the response in the fathead minnow (Table 1) when the HTS assays were relevant to established AOPs involving fish reproduction (Ankley et al. 2010).
Table 1.
Extrapolation of MIEs predicts adverse outcomes in other species: Comparison of human in vitro screening of nine chemicals for potential MIEs related reproductive toxicity (ToxCastTM MIE) to in vivo effects of the same chemicals on reproductive end points in female fathead minnows.
| ToxCast | In vivo, female fathead minnows | |||
|---|---|---|---|---|
| MIE linked to reproductive toxicity in fisha | Plasma E2 | Plasma Vtg | Cumulative fecundity | |
| Abbreviations: E2, estradiol; MIE, molecular initiating event; sig ↓, significantly decreased; Vtg, vitellogenin.aIndicates whether activity was observed in one or more ToxCast assays (Knudsen et al. 2011) corresponding to molecular initiating events previously associated with reproductive toxicity in fish (Ankley et al. 2010). Specific MIEs of interest included androgen receptor (AR) agonism, estrogen receptor (ER) antagonism, and inhibition of steroidogenic enzyme activities, particularly aromatase (CYP19A1). bAll effects are after 21 days of continuous exposure. See Ankley et al. (2005). cFrom a 96-hr range finding study, all other data are from a 21-day exposure (Skolness et al. 2013). dMeasured after 96 hr continuous exposure (Villeneuve et al. 2012). eMartinović et al. 2008. fBencic et al. 2013. gVilleneuve et al. 2006. hAnkley et al. 2001. iTillitt et al. 2010. jBringolf et al. 2004. | ||||
| Prochlorazb | Inhibition of CYP19A1 and AR binding | Sig ↓, 0.3 mg/L | Sig ↓, 0.1 mg/L | Sig ↓, 0.1 mg/L |
| Propiconazolec | Inhibition of CYP19A1 | Sig ↓, 0.5 mg/L | Sig ↓, 0.5 mg/L | Sig ↓, 0.5 mg/L |
| Bisphenol Ad | Interacted with ER and also with the AR at higher concentrations | Sig ↓, 10 μg/L | Sig ↓, 10 μg/L | |
| Fenarimolb | None | Sig ↓, 1.0 mg/L | Sig ↓, 1.0 mg/L | Sig ↓, 1.0 mg/L |
| Vinclozoline | None | No effect | Sig ↓, 0.4 mg/L | Sig ↓, 0.1 mg/L |
| Fipronilf | None | No effect (≤ 1 mg/L) | No effect (≤ 1 mg/L) | No effect (≤ 1 mg/L) |
| Prometong | None | No effect (≤ 5 μg/L) | No effect (≤ 5 μg/L) | No effect (≤ 5 μg/L) |
| Methoxychlorh | None | No effect (≤ 5 μg/L) | No effect (≤ 5 μg/L) | Sig ↓, 5 μg/L |
| Atrazinei,j | None | No effect (≤ 50 μg/L)j | No effect (≤ 50 μg/L)j | Sig ↓, 0.5 μg/Li |
| No effect (≤ 50 μg/L) | ||||
Reproductive effects of BPA in female fish were consistent with the ER interactions identified in HTS assays, (Table 1; see also Supplemental Material, Figure S1). Likewise, the two chemicals identified as aromatase (CYP191A) inhibitors (prochloraz and propiconazole; see Supplemental Material, Figures S2 and S3) caused reproductive and endocrine effects in the fathead minnow (Table 1) consistent with aromatase inhibition. This was true even when multiple molecular targets, including many with lower AC50 (half-maximal activity concentration) values than those related to reproduction, were affected (see Supplemental Material, Figures S1–S9), indicating that the most sensitive assay may not be driving the toxicity outcome. Interestingly, the three chemicals that had either no impact (fipronil and prometon; Table 1) or inconsistent impacts on reproduction (atrazine; Table 1) had the least activity in HTS assays (see Supplemental Material, Figures S4–S6). No ER activity was detected in HTS with methoxychlor, a chemical for which metabolites of the parent chemical are thought to be largely responsible for its endocrine activities (Bulger et al. 1978; see also Supplemental Material, Figure S7). However, an interaction with the androgen receptor (AR) was one of the few activities detected for vinclozolin, another chemical with endocrine-active metabolites (Kelce et al. 1994; see Supplemental Material, Figure S8). For only one of the nine chemicals (fenarimol) was there an apparent disagreement between the HTS results and fish reproduction results that was not readily explainable. In vivo results suggested ER antagonism as a mode of action (Ankley et al. 2005) (Table 1). However, HTS results did not identify interactions with the ER as a likely event (see Supplemental Material, Figure S9). The reason for the discrepancy remains unclear but raises the possibility that the hypothesized in vivo mode of action may be inaccurate.
Clearly, human- or mammal-focused HTS assays targeting conserved molecular initiating events can be useful in predicting potential higher-level effects in other species. The failure to identify a relevant molecular initiating event for methoxychlor in HTS assays demonstrates the current limitations of in vitro screening in predicting in vivo activities of chemicals that are metabolically activated. Nonetheless, currently available data supports the application of mammalian molecular screening data to assessing chemical hazards for a range of nonmammalian species, provided that relevant toxicity pathways are reasonably conserved and understood. Data on the binding kinetics of chemicals to receptors from multiple species, in addition to toxicokinetic and toxicodynamic information, will be necessary to begin quantitatively predicting effects across species using HTS data.
Pathway conservation also enables assessment of toxicity in alternative species based on mammalian AOP models. Where orthologous pathways occur, pathway-based data can often be extended well beyond the phylogenetic group from which it was derived. For example, hexahydro-1,3,5-trinitro-1,3,5-trizine (RDX) causes seizures and other indicators of neurotoxicity in species as varied as human, rat, quail, and earthworm (Garcia-Reyero et al. 2011) (Figure 1). Williams et al. (2010) screened for RDX binding to different neurological receptors and found that RDX binds to the picrotoxin binding site in the chloride channel of the γ-aminobutyric acid A (GABAA) receptor, an interaction associated with the onset of seizures in rats. The picrotoxin binding site in the GABAA receptor is highly conserved from humans to earthworms, indicating that binding the GABAA receptor is a likely molecular initiating event in multiple species (Garcia-Reyero et al. 2011) (Figure 1). Given the conserved role of GABAergic signaling, results of assays assessing impacts on this pathway are likely to be informative of potential effects across a similarly broad range of species and can provide a reasonable basis for extrapolating mammalian health data to other species.
Figure 1.
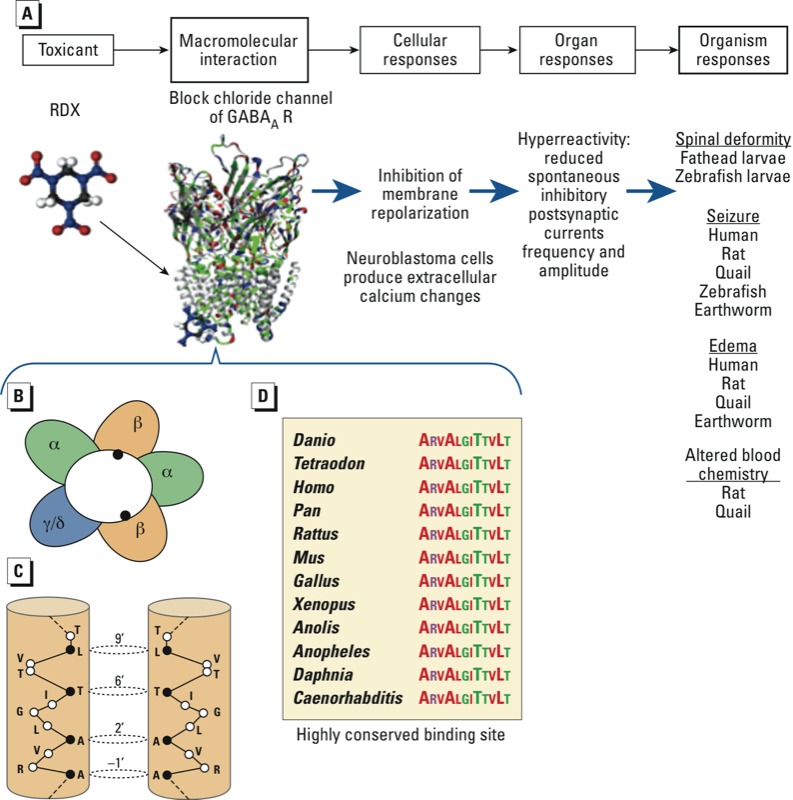
Cross-species similarity of GABAA receptor (GABAAR) and RDX toxicity in an AOP framework. (A) Schematic of pathway. (B) Schematic view of a GABAAR heteropentamer with the channel in the center formed of two copies of an α subunit (α1–α6), two copies of a β subunit (β1, β2, or β3), and a third subunit of γ or δ protein (adapted from Olsen 2006). (C) Cytoplasmic end of the transmembrane channel for two β-3 subunits, indicated by closed circles in (B) in GABAAR, showing the residues that form the binding site for picrotoxin (PTX) and RDX [closed circles in (C): residues numbered 1’–23’ from the N-terminal bottom; open circles represent residues that are not part of the binding site]. (D) Sequence alignment of the PTX/RDX binding site for several species: Danio rerio, Tetraodon nigroviridis, Homo sapiens, Pan troglodytes, Rattus norvegicus, Mus musculus, Gallus gallus, Xenopus tropicalis, Anolis carolinensis, Anopheles gambiae, Daphnia pulex, and Caenorhabditis elegans.
Pathway conservation enables prediction of potential endocrine activity across species. The hypothalamus–pituitary–gonad (HPG) axis is one example of a system highly conserved across vertebrates (Norris 2007). Conservation of molecular initiating events across species should enable nonmammalian vertebrate models to be used in determining the potential for chemicals to interact with the HPG axis in any vertebrate species (including humans), despite the fact that actual apical responses associated with endocrine disruption may differ across species.
Direct consideration of this hypothesis can be done using data from the U.S. EPA Endocrine Disruptor Screening Program (EDSP), which uses multiple in vivo tests with rats and fish as screening tests (Tier 1) to identify substances with the potential to interact with the estrogen, androgen, or thyroid hormone system which are then characterized in depth using Tier 2 in vivo assays (Marty et al. 2011). The rat tests include a) the 3-day uterotrophic assay, which detects ER agonists through increases in uterine weight; b) the 10-day Hershberger assay, which detects AR agonists or antagonists through changes in weights of several androgen-responsive tissues; and c) two pubertal assays with males and females, 20–30 days in length, which detect impacts on ER- and AR-mediated responses, including sex steroid synthesis. The EDSP Tier 1 fish test is a 21-day fathead minnow reproduction assay featuring a range of biochemical, histological, and apical end points that capture chemical effects on multiple HPG pathways, including activation and antagonism of ERs and ARsas well as inhibition of sex steroid synthesis (Ankley et al. 2001). Because fecundity is a general indicator of reproduction, the fish assay can detect a broader range of chemical effects including those acting via non-endocrine mechanisms.
Ankley and Gray (2013) examined results from method validation studies with 12 model endocrine-active chemicals, which act via several different molecular initiating events, that were tested both in the fathead minnow and one or more of the rat EDSP assays; they confirmed that effects on a highly conserved toxicological pathway in one species are predictive of effects in the other species (Table 2). For example, both species were responsive to ethinylestradiol and methoxychlor, which are known to activate ER-mediated pathways, although estrogenic activity in fish was manifested as induction of vitellogenin and in rats as changes to relative weights of estrogen-responsive tissues (uterotrophic and female pubertal assays). Responses of the fish and female rat EDSP tests to a third weak ER agonist (BPA) were more variable, demonstrating a lesser effect in the rats that was likely due to first-pass hepatic metabolism associated with oral dosing and highlighting the fact that metabolism and route of exposure need to be considered in cross-species extrapolation, in addition to pathway conservation.
Table 2.
Conservation of endocrine-active chemicals effects in rats and fish (fathead minnow).
| Pathway | Chemical | Assay | ||||
|---|---|---|---|---|---|---|
| Rat uterotrophic | Rat Hershberger | Rat pubertal female | Rat pubertal male | Fathead minnow | ||
| Abbreviations: NT, not tested. +, positive result. –, negative result. An overview of comparative responses of the various U.S. EPA Endocrine Disruptor Screening Program Tier 1 in vivo assays to chemicals representing different pathways within the vertebrate HPG axis. Modified from Ankley and Grey (2013). | ||||||
| Estrogen agonist | 17α‑Ethynylestradiol | + | NT | + | NT | + |
| Methoxychlor | + | – | + | –/+ | + | |
| Bisphenol A | + | – | – | NT | + | |
| Androgen agonist | Methyltestosterone | + | + | NT | + | + |
| 17β-Trenbolone | NT | + | NT | NT | + | |
| Androgen antagonist | Flutamide | NT | + | NT | + | + |
| Vinclozolin | NT | + | NT | + | + | |
| p, p´-DDE | – | + | NT | + | + | |
| Steroidogenesis | Ketoconazole | NT | NT | + | + | + |
| Fadrozole | NT | NT | + | NT | + | |
| Fenarimol | NT | NT | – | NT | + | |
| Prochloraz | + | NT | NT | + | + | |
Similar results were seen in rodent and fish assays for AR agonists and antagonists [methyltestosterone, 17β-trenbolone, 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene (p,p´-DDE), flutamide, and vinclozolin; Table 2]. That is, although the apical end points that were affected differed, changes in the end points in the two species reflected the conserved molecular initiating event. Chemicals inhibiting sex steroid synthesis (ketoconazole, fadrozole, and prochloraz) were also consistently detected in both the fish assay and one or more rat assays (Table 2). A fourth sex steroid synthesis inhibitor (fenarimol) was detected in the fish assay but not with in vivo rat assays (Table 2). Although not detected with in vivo tests, fenarimol has been identified as a sex steroid synthesis inhibitor using the mammal-based H295R in vitro assay (Villeneuve et al. 2007). In the context of the 12 model chemicals examined, the fathead minnow assays were essentially predictive of pathway-specific responses in the four rodent assays. The Tier 1 tests were focused on sensitively detecting the potential to cause effects, rather than accurate quantification of hazard thresholds. Therefore, the ability of nonmammalian models to quantitatively predict hazard effects in mammals requires further examination and development. This analysis demonstrates that apical effects can be predicted across species if conserved molecular initiating event and pathways exist and, as such, supports hazard screening and assessment for HPG-active chemicals applicable both to mammalian and nonmammalian (vertebrate) species.
Pathway conservation enables effective resource use in prediction of thyroid and developmental effects on both mammalian and nonmammalian species. Pathways associated with the hypothalamic–pituitary–thyroidal (HPT) axis are common and important features of embryonic development and metabolic control in all vertebrate species (Paris and Laudet 2008). Early development in vertebrates is typically characterized by transient elevations of thyroid hormone (TH) that elicit species-specific physiological and morphogenetic responses with lasting developmental consequences as seen in the metamorphosis of free-swimming tadpoles into juveniles (Dodd 1976; Leloup and Buscaglia 1977), reorganization of the flatfish body (Power et al. 2001), and a TH-dependent shift in physiology in salmonids during migration (Dickhoff et al. 1978). Further, vertebrate post-embryonic neurodevelopment is TH-dependent in that deviations from normal TH concentrations can result in neurological defects and deficits (Zoeller and Rovet 2004). As a result, chemical disruption of TH-dependent pathways in vertebrates can have significant adverse impacts (Zoeller 2007).
Disruption of TH activity can result from several established events (Crofton 2008; Figure 2). Of these, thyroid peroxidase and sodium iodide symporter are key proteins in the metabolic pathway of TH. Both thyroid peroxidase and sodium iodide symporters from numerous species are inhibited by the same chemicals resulting in readily predictable reductions in circulating TH, though downstream effects may be species specific. Other molecular initiating events affecting TH activity (i.e., enhanced phase II metabolism of THs via glucuronosyl- or sulfo-transferases, enhanced cellular transport of thyroid hormone, deiodinase inhibition, and interference with thyroid receptor function) are found primarily in peripheral tissues where chemical effects and subsequent consequences are highly variable among species, as is the effectiveness of cross-species extrapolation using these mechanisms. For example, these other events can lead to thyroid hypertrophy, hyperplasia, and thyroid follicular tumors in rats, which are not relevant to the mode of action of concern for humans (Capen 1997; Hill et al. 1998) (Figure 2, pathway 1). Conversely, in frogs, these same events can lead to decreased serum TH, decreased tissue TH, decreased tissue mRNA and protein synthesis, and disruption of development [which is relevant to the mode of action of concern for humans (Degitz et al. 2005) (Figure 2, pathway 2)].
Figure 2.
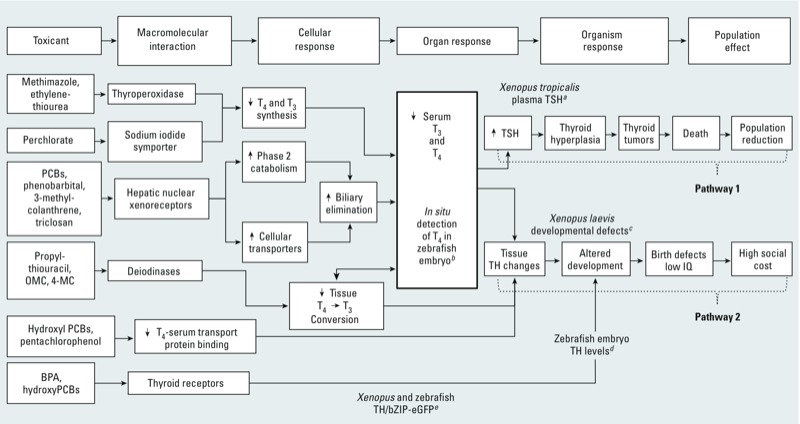
Major AOPs for thyroid disruption with example toxicants and alternative models applicable to both human and ecological hazard assessment. The thick black outlined box indicates the critical event of serum level concentrations of thyroid hormones. Pathway 1: rat pathway leading to tumors via thyroid hyperplasia. Pathway 2: principle pathway of concern affecting humans. Abbreviations: IQ, intelligence quotient; 4-MC, 4-methylbenzylidene camphor; OMC, octyl methoxycinnamate; T3, triiodothyronine; T4, thyroxine; TR, thyroid receptor. Figure modified from Crofton (2008). aQuantification of plasma TSH levels in Xenopus tropicalis (Korte et al 2011). bDirect quantification of intrafollicular concentrations of T4 in zebrafish embryos (Thienpont et al. 2011). cDetection of developmental defects with X. laevis metamorphosis assay (Degitz et al. 2005 Organisation for Economic Co-operation and Development 2004). dDetection of developmental defects using zebrafish embryos. eReporter gene (eGFP) detection of TR activity (Fini et al. 2007).
Rodent studies have long been a standard way to assess the impact of chemicals on thyroid function (Capen 1997). Alternative species are useful in evaluating the risks, both to humans and other vertebrates, associated with chemicals that perturb the HPT axis. The African clawed frog, Xenopus laevis, is a well-documented developmental and physiological model used to assess thyroid disruption (Degitz et al. 2005; Miyata and Ose 2012; Pickford 2010). A comparison of amphibian and mammalian models for detecting thyroid disruption found good concordance in that both detected the majority of 32 environmental chemicals (Pickford 2010). An advantage of the assays using X. laevis, when compared with mammals, is that effects on thyroid disruption can be observed via distinct, relatively specific changes in development (i.e., metamorphosis) as opposed to the more subtle changes in humans and other mammals, such as behavioral and neural developmental effects requiring extensive resources to assess (Grim et al. 2009; Nieuwkoop and Faber 1994). An additional advantage of the amphibian model is that there is no serum protein sink/buffer to bind TH as is found in rodents to complicate the toxicokinetics of TH responses.
Like other vertebrates, X. laevis responds to low levels of TH by release of thyroid stimulating hormone (TSH) from the pituitary to upregulate TH synthesis and release by the thyroid gland through a negative feedback loop. Adverse outcomes occur when the degree of inhibition of TH synthesis and release exceeds the ability of the feedback loop to maintain TH levels. As a result, serum TH levels are a reliable predictor of HPT axis disruption across species. Methods to measure circulating TSH in X. laevis have been recently developed and successfully applied in a toxicological context (Korte et al. 2011; Tietge et al. 2012). Zebrafish embryos have also been used to directly quantify thyroid disruption effects through immunodetection of TH levels in intrafollicular cells (Thienpont et al. 2011). Receptor function can be examined in vivo through use of transgenic X. laevis thyroid receptor-activated promoter reporter systems (Terrien et al. 2011). These alternative species models clearly provide data relevant to both human and ecological risk assessment when analyzed in a pathway context, yet allowing refinement for follow-on studies if needed (Figure 2).
Use of alternative species as models to define and refine adverse effects of chemicals. Alternative species provide models that can be manipulated to assess effects on subtle outcomes such as motor and sensory behaviors and cognitive function (Levin and Tanguay 2011; MacPhail et al. 2009), validate in vivo predictions and provide useful in vivo data for human health hazard assessment. Alternative models are especially useful in understanding the specific mechanisms and pathways through which chemicals cause toxicity to mammals, thereby reducing and refining the use of mammalian models. For example, thalidomide has been identified as a prenatal developmental toxicant and vascular disrupting compound based on potential molecular initiating events identified through HTS results (Kleinstreuer et al. 2011; Sipes et al. 2011). Although thalidomide is a well known developmental toxicant and teratogen in humans, in vitro studies in zebrafish embryos have helped define that it causes limb malformation and other developmental defects through the thalidomide-binding protein cereblon, a protein important in limb outgrowth, and the fibroblast growth factor, Fgf8, which disrupts vascular development (D’Amato et al. 1994; Ito et al. 2010; Yabu et al. 2005).
Perfluorooctane sulfonate (PFOS), a breakdown product of perfluorinated surfactants, has also been identified as a developmental toxicant and vascular disrupting compound via HTS. Effects seen in alternative models are consistent with this prediction and also predictive of effects observed in mammals, including humans. PFOS has been shown to impair cardiac development in marine medaka embryos (Huang et al. 2011); cause altered cardiac function, behavior, and developmental toxicity in zebrafish embryos (Huang et al. 2010); and alter lipid metabolism in salmon larvae (Arukwe et al. 2013). In mammals, PFOS has been shown to cause developmental, reproductive, neuroendocrine, and hepatic steatosis effects (Austin et al. 2003; Bijland et al. 2011; Thibodeaux et al. 2003). PFOS has also been linked to altered cholesterol levels in human epidemiological studies (Eriksen et al. 2013). In addition, demonstration of thyroid disruption by environmentally relevant levels of PFOS in X. laevis (Cheng et al. 2011) confirms early observations that PFOS may impact thyroid function in humans (Organisation for Economic Co-operation and Development 2002). Used in a pathway context, nonmammalian vertebrates and embryo tests can clearly show similar effects to chemicals as found in mammals.
Extrapolation of dose–response relationships between species for hazard assessment. Alternative models offer significant advantages to answering questions requiring a well-characterized constant, controlled exposure to test chemicals where low-dose and compensatory effects are important considerations. For example, Ankley et al. (2009) conducted several intensive studies to characterize dose/time–dependent changes in the fathead minnow HPG function relative to direct effects, compensation, and recovery to collect robust data sets with a) temporally intensive sampling of several hundred animals over a short period, and b) use of controlled, aqueous exposures for highly consistent and predictable dosimetry (Ankley et al. 2009). The aqueous exposures used in fish tests provide constant exposure levels not found in the oral and dermal exposure methods commonly used in mammalian studies that result in fluctuating internal doses rather than a truly constant exposure. So, although relevant to real-world exposures in human health, oral and dermal exposure routes complicate understanding system dynamics because of the complex interplay between biology (e.g., signaling, feedback) and varying chemical intensity.
Used in a pathway context, nonmammalian vertebrates and embryo tests can show effects similar to those found in mammals; however, the concentrations needed to cause an effect and the mechanisms of compensation may be different. Both commonalities and differences underlying response effects can be extrapolated between species using transcriptomics and proteomics to identify genes and signaling pathways in one species that can then be mapped to functional pathways that are conserved across species (Bauer-Mehren et al. 2009). Concentrations required to activate pathways and pathway data can be linked to adverse effects either by known functional linkages (key signaling cascades, metabolism, or experimental demonstration) or de novo linkages (data driven approaches such as network analysis or functional genomics with gene knockout and rescue experiments). Mapping of concentration-responsive genes to pathways would allow use of pathway and concentration data in more traditional risk frameworks that use no observable effect level (NOEL), no observed adverse effect level (NOAEL), or benchmark concentration methods to calculate a point of departure of a biomarker or phenotype related to an adverse effect in an exposed animal group from that of a control animal group. Because regulatory agencies use such a point of departure, modified by uncertainty factors, to set safe levels for chemical exposures (Gaylor and Aylward 2004), dose–response effects could potentially be translated into values more amenable to current risk or hazard frameworks.
Choosing a key event in a pathway as a point of departure is currently difficult (Miller et al. 2009; Woodruff et al. 2008). An alternative is to use a pathway-based point of departure derived from the lowest concentration at which a functionally enriched, co-regulated cluster of transcripts is significantly different from controls. Ideally this pathway would be significantly linked to an adverse effect so that pathways related to compensation or secondary effects are not mistaken for toxicity. However, even changes proceeding from, or indirectly related to, adverse effects may be indicators of sensitivity to a chemical and therefore useful in hazard assessment and prioritization (Thomas et al. 2011). For example, treatment of zebrafish embryos from 0 to 24 hr after fertilization with flusilazole, a known developmental toxicant, caused a concentration-dependent response in morphological effects (Hermsen et al. 2012). Developmental delays, pericardial edema, and malformations of head and heart were observed at concentrations > 28 µM, whereas 2.8 µM flusilazole was the lowest concentration where functionally enriched, co-regulated clusters were observed. Retinol metabolism, a pathway highly conserved across vertebrates, was the most significant function changed at 2.8 µM. Because deregulation of this pathway is linked to developmental skeletal deformities and the mechanism is conserved in both zebrafish and mammals (Laue et al. 2011), a pathway-based NOEL of 1.35 µM and a lowest observable effect level of 2.8 µM flusilazole could be reasonably derived from the point at which the retinol metabolism pathway significantly departed from the control. Use of key events upstream as conservative points of departure has been widely used in mammalian toxicology (Hill et al. 1998; NRC 2005).
A disadvantage to extrapolation based on experimental data is that it is limited to the range of concentrations or doses used. As an alternative, benchmark dose or concentration modeling, which estimates the point at which chemically treated groups diverge from a control group by regression modeling of response curves, can be used to identify low-concentration effects below those concentrations empirically tested (Crump 1995). This approach has been applied to toxicogenomic- and HTS-based pathway data to derive quantitative hazard values from short-term in vivo rat exposures and human-focused in vitro assays (Burgoon and Zacharewski 2008; Judson et al. 2011; Thomas et al. 2011). When applied to toxicogenomic data from flusilazole-exposed zebrafish embryos, benchmark concentration modeling identified retinol metabolism as the most sensitive pathway, with a pathway-based benchmark concentration no effect level much lower than the pathway-based NOEL and closer to observed values in adult fish and values predicted by ToxCast™ analyses (Table 3). This data could potentially be used as a threshold level of sensitivity or as a no observable pathway effect level that could be extrapolated across species using species-specific toxicokinetic and toxicodynamic modeling.
Table 3.
Dose– and concentration–response values can be compared across species and in vitro models using pathway-based measures and reverse toxicokinetics to derive a common hazard value for prioritization.
| Animal model | Dose/concentration reference point | Toxicity value | Human lower oral equivalent (mg/kg/day) |
|---|---|---|---|
| No observable effect levels (NOEL), biological pathway altering dose (BPAD), and pathway-based bench mark concentration lower level (pathway BMCL) values from flusilazole exposures in animal and in vitro models were converted to human oral 95% lower bound dose equivalents using human reverse toxicokinetics (Wetmore et al. 2012). The pathway BMCL for zebrafish was derived from gene expression data (Thomas et al. 2011).aU.S. EPA 2007. bHermsen et al. 2012. cWetmore et al. 2012. dEuropean Commission 2007. eFood and Agricultural Organization of the United Nations 2008. | |||
| Dog | Chronic oral, NOEL | 0.20 mg/kg/daya | 0.002a |
| Zebrafish embryo | 24 hr, pathway NOEL | 1.35 μMb | |
| Zebrafish embryo | 24 hr, pathway BMCL | 0.310 μM | 0.037 |
| ToxCast™ in vitro | Most sensitive assay, BPAD | 0.023 μM | 0.003c |
| Fathead minnow | 252-day flow through, NOEL | 0.073 μMd | 0.009 |
| Rainbow trout | 96-hr acute toxicity, NOEL | 0.010 μMe | 0.001 |
Determination of species-specific dose and kinetic parameters. Toxicokinetic and toxicodynamic modeling is an important step in extrapolating from pathway- or effects-based concentration–response values to whole-animal chemical hazards. The absorption, distribution, metabolism, and elimination of chemicals have been studied extensively in humans and, increasingly, in alternative models. Huggett et al. (2003) predicted pharmacological responses in fish utilizing human therapeutic plasma concentrations [measured maximum concentration (Cmax)] normalized to predicted steady-state fish plasma concentrations to determine whether further toxicity testing may be warranted. In another study, Berninger and Brooks (2010) found that the ratio of acutely toxic drug dose to therapeutic drug dose in mammals has the potential to be predictive of chronic responses to pharmaceuticals in fish. Other methods, such as the well-stirred liver homogenate model, have been useful in predicting hepatic clearance measures of a chemical in trout as a means to estimate metabolism and bioaccumulation (Han et al. 2007). A logical next step will be to determine whether readily available human toxicokinetic–toxicodynamic information can be predictive of the action of a chemical in select aquatic species, or vice versa.
Species-specific toxicokinetic–toxicodynamic modeling, pathway-based point of departures, and modeling of uncertainty and population variability can be combined to translate dose responses in one species to NOEL-like dose values for another species. Judson et al. (2011) used the concept of biological pathway–altering concentration as a measure of the lowest chemical concentration at which an in vitro assay in a pathway is significantly changed. Once defined, the biological pathway–altering concentration was then extrapolated using reverse toxicokinetic modeling to estimate the external dose required to achieve the internal dose that is equal to the biological pathway–altering concentration, ultimately yielding a human dose equivalent that is required to cause toxicity. A reverse toxicokinetics model, suitable for chemicals that are mainly eliminated through metabolism and renal excretion, can be used to obtain a concentration-to-dose scaling factor in micromoles per milligram per kilogram body weight per day (Wetmore et al. 2012). The advantage of this approach is that simple assays exist for the rate of disappearance of parent chemical via hepatic metabolism and the fraction of chemical bound (or conversely unbound) to plasma proteins and allows one to tailor these parameters to specific species, including sensitive ecological species or humans (Han et al. 2007; Pacifici and Viani 1992).
Quantitatively, extrapolation across species can be illustrated by applying pathway-based benchmark concentration modeling in combination with the human reverse toxicokinetic modeling of Wetmore et al. (2012) to the flusilazole concentration-responsive retinol metabolism pathway of zebrafish embryos, described above. When the pathway-based benchmark concentration lower limit for flusilazole and zebrafish embryos is extrapolated using the human dose scaling factor determined for flusilazole (Wetmore et al. 2012), an oral dose equivalent required to cause an effect in humans is derived. In the case of flusilazole, values extrapolated from zebrafish embryos are within an order of magnitude of no effect level values derived from mammalian data and high throughput chemical hazard assessment values for prioritization derived from ToxCast™ assays (Table 3). Reverse toxicokinetics can also be used to extrapolate no effect levels from acute and chronic adult fish tests yielding values similar to those from in vitro assays and mammalian data (Table 3). These observations are consistent with a high conservation of flusilazole’s mechanism of action, including concentration sensitivity, among vertebrate species, such that effects seen in one species can be predictive of effects on another perhaps even to the extent of quantitatively identifying chemical hazard levels.
Conclusions
As we move from largely empirical approaches in chemical safety assessment toward a more predictive paradigm focusing on perturbation of well-conserved pathways and processes, toxicologists should have greater flexibility in the selection of model organisms for testing. As part of this review, we have cited a body of literature documenting the effective use of nonmammalian vertebrates and invertebrates for studying mechanisms and pathway perturbations relevant to human disease. Using the AOP context, we provide evidence for the suitability and practicality of such models for applications such as neurotoxicology and for identifying endocrine-active chemicals, identifying AOPs, and extrapolating concentration–response effects to mammals. In turn, we illustrate how rich sources of human-oriented effects data can be efficiently employed to address pertinent ecological risk challenges such as prioritizing pharmaceutical contaminants in terms of potential effects in nontarget species. For both types of hazard assessments, data can be efficiently employed to address pertinent ecological risk challenges, such as prioritizing data from “alternative species.”
The use of alternative species and pathway-based approaches for hazard assessment is still evolving; therefore, caution should be used when applying these approaches so that ample scientific evidence is presented to support extrapolations and other conclusions. In some cases such as pathway-based benchmark concentrations, we do not yet understand the relationship between the chemically most sensitive pathway and a specific toxicological outcome. In other cases, rapidly evolving technologies such as next generation sequencing or biological network analysis do not have firmly established, reliable, and accurate analysis methods or standards. Although each approach and alternative model is likely to have limitations, the availability of numerous options at different levels of biological organization should compensate and enable more accurate assessment while using fewer animals.
In summary, the movement of toxicology and hazard assessment toward a pathway-based paradigm opens numerous opportunities in applying nontraditional approaches for hazard screening and understanding the risks of chemical exposures. Alternative species can provide valuable and relevant information more rapidly, and at lower cost, than traditional species used for human chemical risk assessment. In a pathway centric world, all species can provide information to protect other species, each with different advantages in use, sensitivity, and accessibility. Given this perspective, the distinctions between the disciplines of human health and ecotoxicology are blurring and heading towards a more unified and integrated application of toxicological data.
Supplemental Material
Acknowledgments
We thank M. Embry for valuable comments in reviewing this manuscript.
Footnotes
This work was funded partly by the U.S. Army Environmental Quality Research Program (including BAA 11-4838).
Permission for publishing this information has been granted by the Chief of Engineers. The paper also has been approved for publication by the U.S. Environmental Protection Agency. However, mention of trade names or products does not indicate endorsement by the federal government.
The authors declare they have no actual or potential competing financial interests.
References
- Adamska M, Degnan SM, Green KM, Adamski M, Craigie A, Larroux C, et al. 2007Wnt and TGF-β expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS One e 1031; 10.1371/journal.pone.0001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Mil HGJV, Richardson MK.2011Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS One 6e21076; 10.1371/journal.pone.0021076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Bencic DC, Breen MS, Collette TW, Conolly RB, Denslow ND, et al. 2009Endocrine disrupting chemicals in fish: developing exposure indicators and predictive models of effects based on mechanism of action. Aquat Toxicol 92168–178.; 10.1016/j.aquatox.2009.01.013 [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. 2010Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29730–741.; 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- Ankley GT, Gray LE.2013Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environ Toxicol Chem 321084–1087.; 10.1002/etc2151 [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Durhan EJ, Makynen EA, Butterworth BC, Kahl MD, et al. 2005Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas). Toxicol Sci 86300–308.; 10.1093/toxsci/kfi202 [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem. 2001;20:1276–1290. [PubMed] [Google Scholar]
- Ankley GT, Johnson RD. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004;45:469–483. doi: 10.1093/ilar.45.4.469. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Cangialosi MV, Letcher RJ, Rocha E, Mortensen AS.2013Changes in morphometry and association between whole-body fatty acids and steroid hormone profiles in relation to bioaccumulation patterns in salmon larvae exposed to perfluorooctane sulfonic or perfluorooctane carboxylic acids. Aquatic Toxicol 131219–230.; 10.1016/j.aquatox.2012.12.026 [DOI] [PubMed] [Google Scholar]
- Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SMJ. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–1489. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME.2011Insights from the structure of estrogen receptor into the evolution of estrogens: Implications for endocrine disruption. Biochemical Pharmacol 821–8.; 10.1016/j.bcp.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Baker ME, Ruggeri B, Sprague LJ, Eckhardt-Ludka C, Lapira J, Wick I, et al. 2008Analysis of endocrine disruption in southern California coastal fish using an aquatic multispecies microarray. Environ Health Perspect 117223–230.; 10.1289/ehp.11627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer-Mehren A, Furlong LI, Sanz F.2009Pathway databases and tools for their exploitation: benefits, current limitations and challenges. Mol Syst Biol 5290; 10.1038/msb.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencic DC, Villeneuve DL, Biales AD, Blake L, Durhan EJ, Jensen KM, et al. 2013Effects of the insecticide fipronil on reproductive endocrinology in the fathead minnow. Environ Toxicol Chem; 10.1002/etc.2254[Online 27 April 2013] [DOI] [PubMed] [Google Scholar]
- Berninger JP, Brooks BW.2010Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicol Lett 19369–78.; 10.1016/j.toxlet.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Bijland S, Rensen PCN, Pieterman EJ, Maas ACE, van der Hoorn JW, van Erk MJ, et al. 2011Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol Sci 123290–303.; 10.1093/toxsci/kfr142 [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, et al. 2012Pharmaceuticals and personal care products in the environment: What are the big questions? Environ Health Perspect 1201221–1229.; 10.1289/ehp.1104477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringolf RB, Belden JB, Summerfelt RC. Effects of atrazine on fathead minnow in a short-term reproduction assay. Environ Toxicol Chem. 2004;23:1019–1025. doi: 10.1897/03-180. [DOI] [PubMed] [Google Scholar]
- Bulger WH, Muccitelli RM, Kupfer D.1978Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol 272417–2423.; 10.1016/0006-2952(78)90354-4 [DOI] [PubMed] [Google Scholar]
- Burgess-Herbert SL, Euling SY. Use of comparative genomics approaches to characterize interspecies differences in response to environmental chemicals: challenges, opportunities, and research needs. Toxicol Appl Pharmacol. 2011 doi: 10.1016/j.taap.2011.11.011. [Online 28 November 2011] [DOI] [PubMed] [Google Scholar]
- Burgoon LD, Zacharewski TR.2008Automated quantitative dose-response modeling and point of departure determination for large toxicogenomic and high-throughput screening data sets. Toxicol Sci 104412–418.; 10.1093/toxsci/kfn083 [DOI] [PubMed] [Google Scholar]
- Capen CC.1997Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol Pathol 2539–48.; 10.1177/019262339702500109 [DOI] [PubMed] [Google Scholar]
- Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci USA. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cui Y, Chen HM, Xie WP.2011Thyroid disruption effects of environmental levels perfluorooctane sulfonates (PFOS) in Xenopus laevis. Ecotoxicology 202069–2078.; 10.1007/s10646-011-0749-3 [DOI] [PubMed] [Google Scholar]
- Collins FS, Gray GM, Bucher JR.2008Transforming environmental health protection. Science 319906–907.; 10.1126/science.1154619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM.2008Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl 31209–23.; 10.1111/j.1365-2605.2007.00857.x [DOI] [PubMed] [Google Scholar]
- Crump KS.1995Calculation of benchmark doses from continuous data. Risk Anal 1579–89.; 10.1111/j.1539-6924.1995.tb00095.x [DOI] [Google Scholar]
- D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degitz SJ, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Tietge JE.2005Progress towards development of an amphibian-based thyroid screening assay using Xenopus laevis. Organismal and thyroidal responses to the model compounds 6-propylthiouracil, methimazole, and thyroxine. Toxicol Sci 87353–364.; 10.1093/toxsci/kfi246 [DOI] [PubMed] [Google Scholar]
- Dickhoff WW, Folmar LC, Gorbman A.1978Changes in plasma thyroxine during smoltification of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrin 36229–232.; 10.1016/0016-6480(78)90027-8 [DOI] [PubMed] [Google Scholar]
- Dodd MHI. New York:Academic Press. 1976. The biology of metamorphosis. I. In Physiology of Amphibia (Lofts B, ed) pp. 467–599. [Google Scholar]
- Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE, et al. 2010The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquatic Toxicol 97279–87.; 10.1016/j.aquatox.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, et al. 2013Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One 8e56969; 10.1371/journal.pone.0056969.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. Review Report for the Active Substance Flusilazole Finalized in the Standing Committee on Plant Health at its Meeting on 3 March 2006 in View of the Inclusion of Flusilazole in Annex I of Directive 91/414/EEC. Health and Consumer Protection Directorate-General of the European Commission, Brussels, Belgium. 2007. Available: http://ec.europa.eu/food/plant/protection/evaluation/existactive/list_flusilazole.pdf [accessed 10 July 2013]
- Fini JB, Le Mével S, Turque N, Palmier K, Zalko D, Cravedi JP, et al. An in vivo multiwell-based fluorescent screen for monitoring vertebrate thyroid hormone disruption. Environ Sci Technol. 2007;41:5908–5914. doi: 10.1021/es0704129. [DOI] [PubMed] [Google Scholar]
- Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the United Nations. FAO Specifications and Evaluations for Agricultural Pesticides: Flusilazole, bis(4-fluorophenyl)(methyl)(1H-1,2,4-triazol-1-ylmethyl)silane. New York:United Nations. 2008. Available: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Flusilazole08.pdf [accessed 10 July 2013]
- Garcia-Reyero N, Habib T, Pirooznia M, Gust KA, Gong P, Warner C, et al. 2011Conserved toxic responses across divergent phylogenetic lineages: a meta-analysis of the neurotoxic effects of RDX among multiple species using toxicogenomics. Ecotoxicology 20580–594.; 10.1007/s10646-011-0623-3 [DOI] [PubMed] [Google Scholar]
- Gaylor DW, Aylward LL.2004An evaluation of benchmark dose methodology for non-cancer continuous-data health effects in animals due to exposures to dioxin (TCDD). Regul Toxicol Pharmacol 409–17.; 10.1016/j.yrtph.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Goto T, Hiromi J.2003Toxicity of 17α-ethynylestradiol and norethindrone, constituents of an oral contraceptive pill to the swimming and reproduction of cladoceran Daphnia magna, with special reference to their synergetic effect. Mar Pollut Bull 47139–142.; 10.1016/S0025-326X(03)00052-3 [DOI] [PubMed] [Google Scholar]
- Grim KC, Wolfe M, Braunbeck T, Iguchi T, Ohta Y, Tooi O, et al. 2009Thyroid histopathology assessments for the amphibian metamorphosis assay to detect thyroid-active substances. Toxicol Pathol 37415–424.; 10.1177/0192623309335063 [DOI] [PubMed] [Google Scholar]
- Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DGJ.2008Evolutionary Conservation of Human Drug Targets in Organisms used for Environmental Risk Assessments. Environ Sci Technol 425807–5813.; 10.1021/es8005173 [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Mingoia RT, Yang CH.2007Determination of xenobiotic intrinsic clearance in freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat and its application in bioaccumulation assessment. Environ Sci Technol 413269–3276.; 10.1021/es0626279 [DOI] [PubMed] [Google Scholar]
- Hermsen SAB, Pronk TE, van den Brandhof EJ, van der Ven LTM, Piersma AH.2012Concentration-response analysis of differential gene expression in the zebrafish embryotoxicity test following flusilazole exposure. Toxicol Sci 127303–312.; 10.1093/toxsci/kfs092 [DOI] [PubMed] [Google Scholar]
- Hill AJ.2005Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 866–19.; 10.1093/toxsci/kfi110 [DOI] [PubMed] [Google Scholar]
- Hill RN, Crisp TM, Hurley PM, Rosenthal SL, Singh DV. Risk assessment of thyroid follicular cell tumors. Environ Health Perspect. 1998;106:447–457. doi: 10.1289/ehp.98106447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. 2013The zebrafish reference genome sequence and its relationship to the human genome. Nature 496498–503.; 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, et al. 2010Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat Toxicol 98139–147.; 10.1016/j.aquatox.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Fang C, Wu X, Fan J, Dong S.2011Perfluorooctane sulfonate impairs the cardiac development of a marine medaka (Oryzias melastigma). Aquat Toxicol 10571–77.; 10.1016/j.aquatox.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Huggett DB, Cook JC, Ericson JF, Williams RT.2003A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum Ecol Risk Assess Int J 91789–1799.; 10.1080/714044797 [DOI] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. 2010Identification of a primary target of thalidomide teratogenicity. Science 3271345–1350.; 10.1126/science.1177319 [DOI] [PubMed] [Google Scholar]
- Judson RS, Kavlock RJ, Setzer RW, Cohen Hubal EA, Martin MT, Knudsen TB, et al. 2011Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem Res Toxicol 24451–462.; 10.1021/tx100428e [DOI] [PubMed] [Google Scholar]
- Jukosky JA, Watzin MC, Leiter JC.2008Elevated concentrations of ethinylestradiol, 17β-estradiol, and medroxyprogesterone have little effect on reproduction and survival of Ceriodaphnia dubia. Bull Environ Contam Toxicol 81230–235.; 10.1007/s00128-008-9462-1 [DOI] [PubMed] [Google Scholar]
- Kavlock R, Chandler K, Houck K, Hunter S, Judson R, Kleinstreuer N, et al. 2012Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chem Res Toxicol 251287–302.; 10.1021/tx3000939 [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr1994Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol 126276–285.; 10.1006/taap.1994.1117 [DOI] [PubMed] [Google Scholar]
- Kleinstreuer NC, Judson RS, Reif DM, Sipes NS, Singh AV, Chandler KJ, et al. 2011Environmental impact on vascular development predicted by high-throughput screening. Environ Health Perspect 1191596–1603.; 10.1289/ehp.1103412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöbel M, Busser FJM, Rico Rico A, Kramer NI, Hermens JLM, Hafner C, et al. 2012Predicting adult fish acute lethality with the zebrafish embryo: relevance of test duration, endpoints, compound properties, and exposure concentration analysis. Environ Sci Technol 469690–9700.; 10.1021/es301729q [DOI] [PubMed] [Google Scholar]
- Knudsen TB, Houck KA, Sipes NS, Singh AV, Judson RS, Martin MT, et al. 2011Activity profiles of 309 ToxCast™ chemicals evaluated across 292 biochemical targets. J Toxicol 2821–15.; 10.1016/j.tox.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Korte JJ, Sternberg RM, Serrano JA, Thoemke KR, Moen SM, Lillegard KE, et al. 2011Thyroid-stimulating hormone (TSH): measurement of intracellular, secreted, and circulating hormone in Xenopus laevis and Xenopus tropicalis. Gen Comp Endocrinol 171319–325.; 10.1016/j.ygcen.2011.02.017 [DOI] [PubMed] [Google Scholar]
- Kostich MS, Lazorchak JM.2008Risks to aquatic organisms posed by human pharmaceutical use. Sci Total Environ 389329–339.; 10.1016/j.scitotenv.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Laue K, Pogoda HM, Daniel PB, van Haeringen A, Alanay Y, von Ameln S, et al. 2011Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am J Hum Genet 89595–606.; 10.1016/j.ajhg.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine, hormone de la métamorphose des amphibiens. CR Acad Sci Paris. 1977;284:2261–2263. [in French] [Google Scholar]
- Levin ED, Tanguay RL.2011Introduction to zebrafish: current discoveries and emerging technologies for neurobehavioral toxicology and teratology. Neurotoxicol Teratol 33607; 10.1016/j.ntt.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S.2009Locomotion in larval zebrafish: influence of time of day, lighting and ethanol. Neurotoxicology 3052–58.; 10.1016/j.neuro.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Martindale MQ.2005The evolution of metazoan axial properties. Nat Rev Genet 6917–927.; 10.1038/nrg1725 [DOI] [PubMed] [Google Scholar]
- Martinović D, Blake LS, Durhan EJ, Greene KJ, Kahl MD, Jensen KM, et al. 2008Reproductive toxicity of vinclozolin in the fathead minnow: confirming an anti-androgenic mode of action. Environ Toxicol Chem 27478–488.; 10.1897/07-206R.1 [DOI] [PubMed] [Google Scholar]
- Marty MS, Carney EW, Rowlands JC.2011Endocrine disruption: historical perspectives and its impact on the future of toxicology testing. Toxicol Sci 120S93–S108.; 10.1093/toxsci/kfq329 [DOI] [PubMed] [Google Scholar]
- Miller MD, Crofton KM, Rice DC, Zoeller RT.2009Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect 1171033–1041.; 10.1289/ehp.0800247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Ose K.2012Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J Toxicol Pathol 2511–9.; 10.1293/tox.25.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. New York:Garland Science. 1994. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis (Faber J, ed). 3rd ed. [Google Scholar]
- Norris DO. Burlington, MA: Elsevier Academic Press; 2007. Vertebrate Endocrinology. [Google Scholar]
- NRC (National Research Council). Health Implications of Perchlorate Ingestion. Committee to Assess the Health Implications of Perchlorate Ingestion, National Research Council, Washington, DC:National Academies Press. 2005. Available: http://www.nap.edu/openbook.php?isbn=0309095689 [accessed 10 July 2013]
- NRC (National Research Council). Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC:National Academies Press. 2007. Available: http://www.nap.edu/openbook.php?record_id=11970 [accessed 10 July 2013]
- Olsen RW.2006Picrotoxin-like channel blockers of GABAA receptors. Proc Natl Acad Sci USA 103166081–6082.; 10.1073/pnas.0601121103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development. Co-Operation on Existing Chemicals. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts. ENV/JM/RD(2002)17/FINAL. 2002. Available: http://www.oecd.org/dataoecd/23/18/2382880.pdf [accessed 10 July 2013]
- Organisation for Economic Co-operation and Development. Detailed Review Paper on Amphibian Metamorphosis Assay for the Detection of Thyroid Active Substances. ENV/JM/MONO(2004)17. OCED Series on Testing and Assessment, No. 46. 2004. Available: http://www.epa.gov/endo/pubs/attachment_e_oecd_amphibian_drp.pdf [accessed 10 July 2013]
- Pacifici GM, Viani A. Methods of determining plasma and tissue binding of drugs. Pharmacokinetic consequences. Clin Pharmacokinet. 1992;23:449–468. doi: 10.2165/00003088-199223060-00005. [DOI] [PubMed] [Google Scholar]
- Paris MM, Laudet VV.2008The history of a developmental stage: metamorphosis in chordates. Genesis 46657–672.; 10.1002/dvg.20443 [DOI] [PubMed] [Google Scholar]
- Pickford DB.2010Screening chemicals for thyroid-disrupting activity: a critical comparison of mammalian and amphibian models. Crit Rev Toxicol 40845–892. . 10.3109/10408444.2010.494250 [DOI] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, et al. 2001Thyroid hormones in growth and development of fish. Comp Biochem Physiol C Toxicol Pharmacol 130447–459.; 10.1016/S1532-0456(01)00271-X [DOI] [PubMed] [Google Scholar]
- Rovida C, Hartung T. Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals—a report by the Transatlantic Think Tank for Toxicology (t4). ALTEX. 2009;26:187–208. [PubMed] [Google Scholar]
- Santos EM, Paull GC, Van Look KJW, Workman VL, Holt WV, van Aerle R, et al. 2007Gonadal transcriptome responses and physiological consequences of exposure to oestrogen in breeding zebrafish (Danio rerio). Aquat Toxicol 83134–142.; 10.1016/j.aquatox.2007.03.019 [DOI] [PubMed] [Google Scholar]
- Schiller V, Wichmann A, Kriehuber R, Muth-Köhne E, Giesy JP, Hecker M, et al. 2013Studying the effects of genistein on gene expression of fish embryos as an alternative testing approach for endocrine disruption. Comp Biochem Physiol C Toxicol Pharmacol 15741–53.; 10.1016/j.cbpc.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Schreiber R, Gündel U, Franz S, Küster A, Rechenberg B, Altenburger R.2011Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regulatory Toxicol Pharmacol 61261–275.; 10.1016/j.yrtph.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Simmons SO, Fan CY, Ramabhadran R.2009Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol Sci 111202–225.; 10.1093/toxsci/kfp140 [DOI] [PubMed] [Google Scholar]
- Sipes NS, Martin MT, Reif DM, Kleinstreuer NC, Judson RS, Singh AV, et al. 2011Predictive models of prenatal developmental toxicity from toxcast high-throughput screening data. Toxicol Sci 124109–127.; 10.1093/toxsci/kfr220 [DOI] [PubMed] [Google Scholar]
- Skolness SY, Blanksma C, Cavallin J, Churchill JJ, Durhan EJ, Jensen KM, et al. 2013Propiconazole inhibits steroidogenesis and reproduction in the fathead minnow (Pimephales promelas). Toxicol Sci 132284–297.; 10.1093/toxsci/kft010 [DOI] [PubMed] [Google Scholar]
- Tarazona JV, Escher B, Giltrow E, Sumpter JP, Knacker T.2007Targeting the environmental risk assessment of pharmaceuticals: facts and fantasies. Integr Environ Assess Manag 6supp603–613.; 10.1897/IEAM_2009-052.1 [DOI] [PubMed] [Google Scholar]
- Terrien X, Fini JB, Demeneix BA, Schramm KW, Prunet P.2011Generation of fluorescent zebrafish to study endocrine disruption and potential crosstalk between thyroid hormone and corticosteroids. Aquat Toxicol 10513–20.; 10.1016/j.aquatox.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, et al. 2003Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol Sci 74369–381.; 10.1093/toxsci/kfg121 [DOI] [PubMed] [Google Scholar]
- Thienpont B, Tingaud-Sequeira A, Prats E, Barata C, Babin PJ, Raldúa D.2011Zebrafish eleutheroembryos provide a suitable vertebrate model for screening chemicals that impair thyroid hormone synthesis. Environ Sci Technol 457525–7532.; 10.1021/es202248h [DOI] [PubMed] [Google Scholar]
- Thomas RS, Clewell HJ, Allen BC, Wesselkamper SC, Wang NCY, Lambert JC, et al. 2011Application of transcriptional benchmark dose values in quantitative cancer and noncancer risk assessment. Toxicol Sci 120194–205.; 10.1093/toxsci/kfq355 [DOI] [PubMed] [Google Scholar]
- Thomson SA, Baldwin WS, Wang YH, Kwon G, Leblanc GA.2009Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genomics 10500; 10.1186/1471-2164-10-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietge JE, Degitz SJ, Haselman JT, Butterworth BC, Korte JJ, Koian PA, et al. 2012Inhibition of the thyroid hormone pathway in Xenopus laevis by 2-mercaptobenzothiazole. Aquat Toxicol 126C128–136. . 10.1016/j.aquatox.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Tillitt DE, Papoulias DM, Whyte JJ, Richter CA.2010Atrazine reduces reproduction in fathead minnow (Pimephales promelas). Aquat Toxicol 99149–159.; 10.1016/j.aquatox.2010.04.011 [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). Flusilazole: Pesticide Tolerance. Final rule. Fed Reg. 2007;72:49654–49660. [Google Scholar]
- Vallee M, Aiba K, Piao Y, Palin MF, Ko MSH, Sirard MA.2008Comparative analysis of oocyte transcript profiles reveals a high degree of conservation among species. Reproduction 135439–448.; 10.1530/REP-07-0342 [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Ankley GT, Makynen EA, Blake LS, Greene KJ, Higley EB, et al. 2007Comparison of fathead minnow ovary explant and H295R cell-based steroidogenesis assays for identifying endocrine-active chemicals. Ecotoxicol Environ Saf 6820–32.; 10.1016/j.ecoenv.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Garcia-Reyero N.2010Vision & strategy: Predictive ecotoxicology in the 21st century. Environ Toxicol Chem 301–8.; 10.1002/etc.396 [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Garcia-Reyero N, Escalon BL, Jensen KM, Cavallin JE, Makynen EA, et al. 2012Ecotoxicogenomics to support ecological risk assessment: a case study with bisphenol A in fish. Environ Sci Technol 4651–59.; 10.1021/es201150a [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Murphy MB, Kahl MD, Jensen KM, Butterworth BC, Makynen EA, et al. Evaluation of the methoxytriazine herbicide prometon using a short-term fathead minnow reproduction test and a suite of in vitro bioassays. Environ Toxicol Chem. 2006;25:2143–2153. doi: 10.1897/05-604r.1. [DOI] [PubMed] [Google Scholar]
- Vosges M, Le Page Y, Chung B-C, Combarnous Y, Porcher JM, Kah O, et al. 201017α-ethinylestradiol disrupts the ontogeny of the forebrain GnRH system and the expression of brain aromatase during early development of zebrafish. Aquat Toxicol 99479–491.; 10.1016/j.aquatox.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, et al. 2012Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 125157–174.; 10.1093/toxsci/kfr254 [DOI] [PubMed] [Google Scholar]
- Williams LR, Aroniadou-Anderjaska V, Qashu F, Finne H, Pidoplichko V, Bannon DI, et al. 2010RDX binds to the GABAA receptor–convulsant site and blocks GABAa receptor–mediated currents in the amygdala: a mechanism for RDX-induced seizures. Environ Health Perspect 119357–363.; 10.1289/ehp.1002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zeise L, Axelrad DA, Guyton KZ, Janssen S, Miller M, et al. 2008Meeting report: moving upstream—evaluating adverse upstream end points for improved risk assessment and decision-making. Environ Health Perspect 1161568–1575.; 10.1289/ehp.11516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu T, Tomimoto H, Taguchi Y, Yamaoka S, Igarashi Y, Okazaki T.2005Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood 106125–134.; 10.1182/blood-2004-09-3679 [DOI] [PubMed] [Google Scholar]
- Zoeller RT.2007Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17811–817.; 10.1089/thy.2007.0107 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J.2004Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol 16809–818.; 10.1111/j.1365-2826.2004.01243.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


