Abstract
Translocator protein (TSPO) present in the outer mitochondrial membrane has been suggested to be critical for cholesterol import, a rate-limiting step for steroid hormone biosynthesis. Despite the importance of steroidogenesis in regulating reproductive functions, the developmental profile of TSPO expression in the gonads and accessory sex organs has not been completely characterized. As a first step towards understanding the function of TSPO, we studied its expression in male and female murine reproductive organs. We examined testes and ovaries at embryonic days 14.5 and 18.5, and postnatal days 0, 7, 14, 21 and 56 of development. In the adult testis, TSPO was expressed in both Leydig cells and Sertoli cells. In the developing testes TSPO expression was seen in immature Sertoli cells, fetal Leydig cells and gonocytes. In the ovary, TSPO was expressed in the ovarian surface epithelium, interstitial cells granulosa cells and luteal cells. Corpora lutea of ovaries from pregnant mice showed strong expression of TSPO. In the developing ovary, TSPO expression was seen in the squamous pregranulosa cells associated with germ line cysts, together with progressively increasing expression in interstitial cells and the ovarian surface epithelium. In adult mice, the epithelia of other reproductive tissues like the epididymis, prostate, seminal vesicle, oviduct and uterus also showed distinct patterns of TSPO expression. In summary, TSPO expression in both male and female reproductive tissues was not only restricted to steroidogenic cells. Expression in Sertoli cells, ovarian surface epithelium, efferent ductal epithelium, prostatic epithelium, seminal vesiclular epithelium, uterine epithelium and oviductal epithelium suggest either previously unknown sites for de novo steroidogenesis or functions for TSPO distinct from its well-studied role in steroid hormone production.
Introduction
Translocator protein (TSPO) was first identified as a pharmacologically distinct diazepam-binding protein [1], [2], and has long been studied under its former name, peripheral-type benzodiazepine receptor (PBR) [3]. Biochemical characterization of this 18 kDa transmembrane protein showed predominant presence in the mitochondria, with specific localization to the mitochondrial outer membrane [4], [5]. Although it is highly conserved from bacteria to humans [6], the precise function of TSPO/PBR continues to remain elusive as evidence that is quite multifaceted points to several physiological and pathological roles for this protein (reviewed in [7], [8]). Experimentation on TSPO function has suggested involvement in cell proliferation [9], [10], apoptosis [11], [12], cellular respiration [13], heme synthesis [14], erythropoiesis [15], calcium flow [16], [17], cellular immunity [18], stress responses [19], photosensitization [20], malignancy [21], [22], and steroid hormone biosynthesis [23], [24]. Furthermore, its importance in vital functions was highlighted by embryonic lethality that was observed in TSPO gene deleted mice [25].
TSPO gene expression is regulated by several mechanisms in tissues and is not completely defined. Transcription factors such as specificity protein 1/specificity protein 3 (Sp1/Sp3) [26], activator protein 1 (AP1) [27], and v-ets erythroblastosis virus E26 oncogene homolog (Ets) [28], that act on the TSPO promoter have been linked to expression levels. More recently, a mechanism of regulation of TSPO expression by a natural antisense transcript called short interspersed repetitive element B2 (SINE B2) has been shown to regulate TSPO transcription [29].
Studies examining the conserved molecular structure of TSPO showed a channel-like conformation for this protein with five transmembrane alpha helices and a hydrophobic core [30]. A cholesterol recognition amino acid consensus (CRAC) has been characterized at its cytosolic carboxyl terminus suggesting cholesterol binding [31]. In the mitochondria, two specific proteins, voltage-dependent anion channel (VDAC) and the adenine nucleotide transporter (ANT) have been shown to interact with TSPO, suggesting existence of this protein as a complex [32]. This association with VDAC and ANT connected TSPO to being part of the mitochondrial permeability transition pore (MPTP), linking it to potential functions including initiation of the mitochondrial apoptosis pathway [33]. However, its interaction with VDAC and ANT was not found required for other TSPO functions like its role in steroid hormone production [34].
Specific function for TSPO in cholesterol transport required for steroidogenesis is perhaps the most characterized activity for this protein [35]. It was first identified that pharmacological agents Ro5–4864 and PK11195 that bind to TSPO modulated testosterone production in testicular Leydig cells [36], [37], and progesterone production in ovarian granulosa cells [38]. It was subsequently demonstrated that disruption of PBR/TSPO abolished steroid hormone biosynthesis in the R2C Leydig cell line [23]. Similarly, an antisense knockdown of PBR/TSPO expression in the MA-10 Leydig cell line also decreased steroid hormone biosynthesis [24].
Cholesterol transport to the inner mitochondrial membrane is essential to execute the first and rate-limiting step of the steroid hormone biosynthetic pathway [39]. In this step, mitochondrial P450 side chain cleavage enzyme (CYP11A) gains access to cholesterol at the inner mitochondrial membrane and catalyzes its conversion to pregnenolone [40]. To arrive at the inner mitochondrial membrane, cholesterol must traverse the aqueous space that lies between the outer and inner mitochondrial membranes. Involvement of the steroidogenic acute regulatory protein (StAR) in this function has been well established; mutations to the StAR gene results in lipoid congenital adrenal hyperplasia, a cholesterol transport disorder that results in impaired gonadal and adrenal steroid hormone biosynthesis [41], [42]. However, the exact steps involved in this transport, and parts played by other cooperating functional proteins remains an area of active investigation [43], [44]. Aligned with their functional similarities, there is strong accumulating evidence for a working cooperation between StAR and TSPO in steroid hormone biosynthesis [45], [46], [47].
Despite the potential importance of TSPO in regulating steroid hormone production for reproductive development and function, there have been no studies examining TSPO expression and localization exclusively focused on the reproductive system. In this study, we systematically examine the developmental expression and specific localization of TSPO in the testis, ovary and other reproductive tissues in the murine model.
Materials and Methods
Reagents
All reagents and chemicals were purchased from Sigma (St. Louis, MO), unless otherwise noted. A commercial rabbit monoclonal antibody against TSPO (Epitomics, Burlingame, CA) was used for all the localization studies and western blots. Specificity of this antibody was confirmed by blocking with the immunizing peptide (Epitomics). A mouse monoclonal antibody was used for localization of VDAC1 (Abcam, Cambridge, MA). For immunohistochemistry, a goat-anti-rabbit secondary antibody conjugated with polymerized horseradish peroxidase (pHRP) was used for diaminobenzidine (DAB)-based chemistry. Fluorescent goat anti-rabbit secondary antibody and goat anti-mouse Fab fragments were used for immunofluorescence (Jackson Immunoresearch, West Grove, PA). A micro BCA protein assay kit (Thermo Scientific, Rockford, IL) was used to quantify total protein. A mouse anti-actin polyclonal antibody (Li-Cor, Lincoln, NE) was used to estimate loading normalization in western blots. Species-specific fluorescent secondary antibodies (Li-Cor) were used to detect TSPO and actin in western blots. MA-10 Leydig cell line [48], was a generous gift from Dr. Mario Ascoli, Department of Pharmacology, The University of Iowa.
Mice
Mice (C57BL/6J) were purchased from the Jackson Laboratories (Bar Harbor, ME), and were bred for the different experiments. Timed pregnancies were setup by checking for vaginal plugs to confirm mating. Gonads were collected from fetuses at embryonic stages (E14.5 and E18.5). Gonads were also collected from postnatal pups to adult mice at different ages (P0, P7, P14, P21 and P56/adult). In adult females, oviducts and uteri were also included in the collection. In adult males, the seminal vesicles and prostates were also collected. At least four animals from each sex were collected and examined for all the different time points. All animals were maintained in accordance to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all experiments were performed with an approved protocol (# 2010-0098) from the Institutional Animal Care and Use Committee of Cornell University.
Western Blots
To evaluate the specificity of the monoclonal TSPO antibody, we examined western blots for specific detection of an 18-kDa band representing TSPO. Freshly collected MA-10 cell samples were sonicated and boiled in Laemmli sample buffer, and total protein was quantified using a bicinchoninic acid colorimetric assay. Twenty micrograms of protein was then separated by SDS-PAGE, transferred to PVDF membranes and immunoblotted for the presence of TSPO. In brief, membranes were blocked using 5% non-fat dry milk in tris buffered saline containing 0.2% Tween 20 (TBST) and incubated with rabbit anti-TSPO monoclonal antibody and control mouse anti-actin affinity purified polyclonal antibody. For testing the specificity of the TSPO antibody, a preadsorption control was performed treating the antibody with a 10-fold molar excess of the TSPO immunizing peptide before use. Detection was performed by incubation with IRDye 800 conjugated goat anti-rabbit IgG and IRDye 700 conjugated goat anti-mouse IgG followed by imaging using a laser fluorescence scanner (Li-Cor) for simultaneous detection of the two emission wavelengths.
Immunohistochemistry
Tissues were fixed with 4% formaldehyde in phosphate buffered saline for 48 hours at room temperature. Specimens were then processed, embedded in paraffin and 4 µm thin sections were prepared on glass slides. After deparrafinization and rehydration, sections were subjected to antigen retrieval using 0.01 M citrate buffer. Non-specific binding was blocked using 5% normal goat serum, then samples were incubated with anti-TSPO antibody (1∶200) in 1% BSA in PBS overnight at 4°C. For testing the specificity of the TSPO antibody, a preadsorption control was performed treating the antibody with a 10-fold molar excess of the TSPO immunizing peptide before incubation with the samples. After incubation, slides were washed in PBS and incubated with pHRP-conjugated anti-rabbit secondary antibody and processed using the DAB chemistry to visualize positive staining. To highlight morphology, slides were counterstained for a weak hematoxylin background. Images were acquired in a DM1000LED Leica microscope using an ICC50HD camera.
Immunofluorescence
Leydig cells (MA-10) grown on coverslips were fixed with 4% formaldehyde and permeabilized using 0.1% Triton X-100. Cells were then blocked using 5% normal goat serum and incubated with anti-TSPO antibody (1∶200) and anti-VDAC1 antibody (1∶500). Cells were subsequently washed with PBS and incubated with Alexa Fluor conjugated anti-mouse Fab fragments (488 nm) and anti-rabbit antibody (555 nm) and then washed again, counterstained with DAPI and mounted using an antifade reagent. Testis sections prepared as described above were also stained using anti-TSPO and anti-VDAC1 antibody using primary antibody dilutions as specified for MA-10 cells. An additional blocking step with unlabeled anti-mouse Fab fragments (13 µg/ml) was included before primary antibody incubation to prevent non-specific recognition of endogenous IgG when using the anti-mouse secondary antibody on tissues. Stained MA-10 cells and testis sections were imaged using a Zeiss LSD510 confocal microscope.
Results and Discussion
Steroid hormones play a critical role in the development and function of both male and female reproductive organs. With advances in understanding of steroid hormone biosynthesis in a cell [49], studies on the cell type specific expression of proteins in reproductive tissues have become critical to complement and reinforce any functional interpretations. In the case of proteins like TSPO that are widely expressed and known to be involved in more than one function [7], [50], specific in vivo observations can lead to not only a better comprehension, but also novel insights into physiological mechanisms. In this study, we characterize the developmental and functional expression of TSPO, to better comprehend its role in different reproductive tissues and steroidogenesis. These findings in the reproductive system also translate to functional understanding of TSPO in other organ systems and its basis for expression in specific pathologies that include ischemic stroke, neuroinflammation and certain tumors.
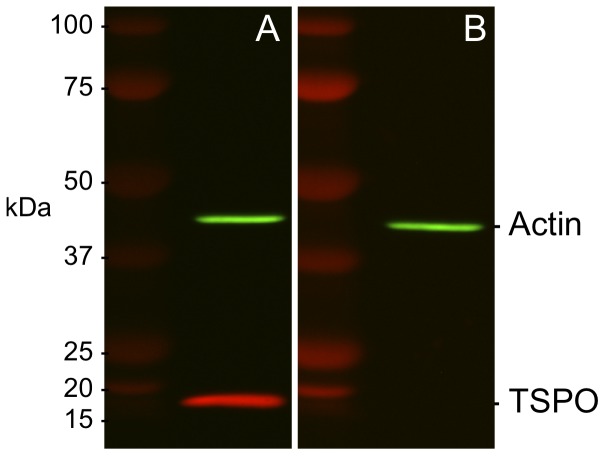
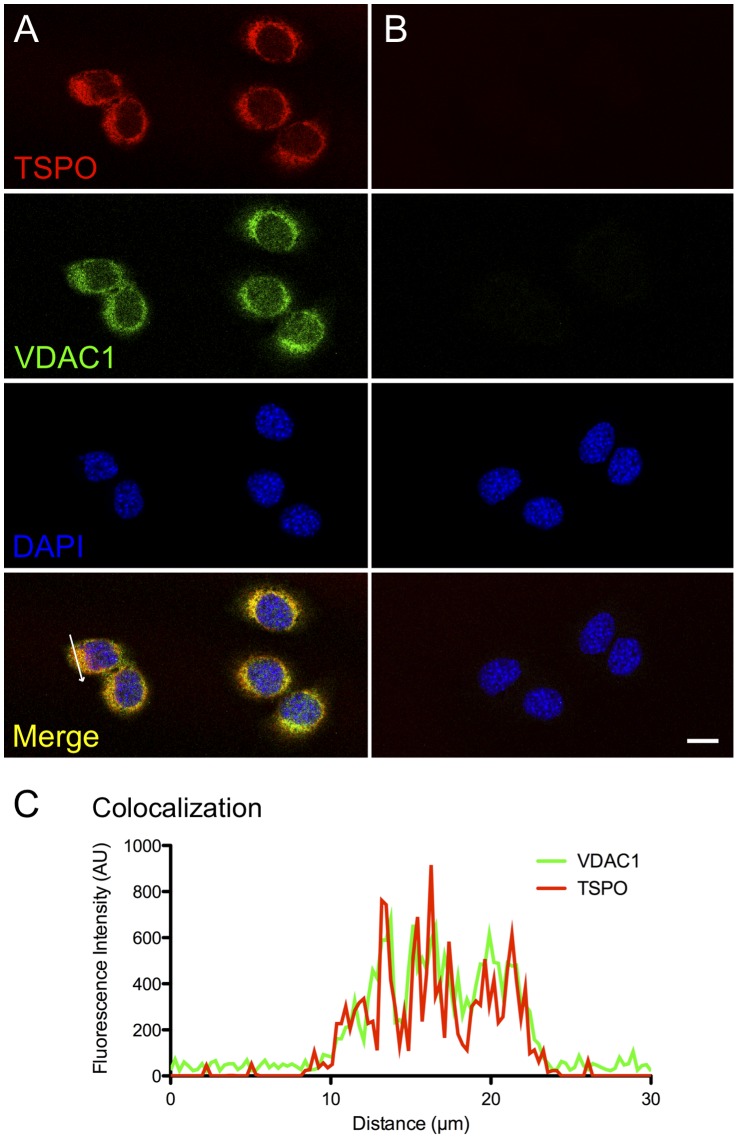
In early studies, radiolabelled lipophilic chemicals [3H]Ro5–4864 [6], or [3H]PK11195 [51] that bind TSPO have been used to roughly evaluate expression by exposing treated tissue sections to radiographic film [51]. Polyclonal anti-sera that were generated against TSPO till date have largely lacked the specificity required for immunolocalization experiments. The rabbit monoclonal TSPO primary antibody that was used for all immunolocalization experiments in this study was first validated for specific recognition of TSPO protein. In western blots from protein lysates from a Leydig cell line (MA-10), the rabbit monoclonal TSPO antibody recognized a single 18 kDa band that corresponds to the expected molecular weight defined by the murine TSPO cDNA sequence [52]; this TSPO band disappeared in the peptide-preadsorbed control showing the specificity of this antibody in recognizing TSPO (Fig. 1). Using immunofluorescence and confocal imaging, we also confirmed that TSPO fluorescence was associated with the mitochondria. TSPO fluorescence in MA-10 cells colocalized with the mitochondrial marker VDAC1 (Fig. 2). In immunohistochemistry, specific recognition of TSPO was confirmed by the absence of labeling in the peptide-preadsorbed control (Fig. 3C). Therefore, the epitope recognized by this monoclonal antibody was very specific for TSPO protein (these control experiments were repeated three times). The specificity of this reagent allowed us to perform all experiments with precision, and reliably localize TSPO in various reproductive tissues.
Figure 1. TSPO antibody is highly specific.
(A) Western blot showing specific recognition of TSPO in MA-10 cell lysates as a single 18-kDa band by the rabbit monoclonal anti-TSPO antibody that was used for all experiments in this study. (B) Peptide-preadsorbed control for the same sample does not show this band confirming the specificity of the antibody in recognizing TSPO. Beta-actin was used as a loading control for both blots as indicated.
Figure 2. TSPO expression is localized to the mitochondria.
(A) Panel shows confocal images of TSPO (red), VDAC1 (green) and nuclear counterstain (blue) in MA-10 Leydig cells. (B) Negative control panel. (C) Graph shows fluorescence intensities for TSPO and VDAC1 across the region indicated by a white arrow in Panel A. Colocalization of TSPO to the mitochondrial protein VDAC1 validates the specific localization of TSPO. Scale bar 20 µm.
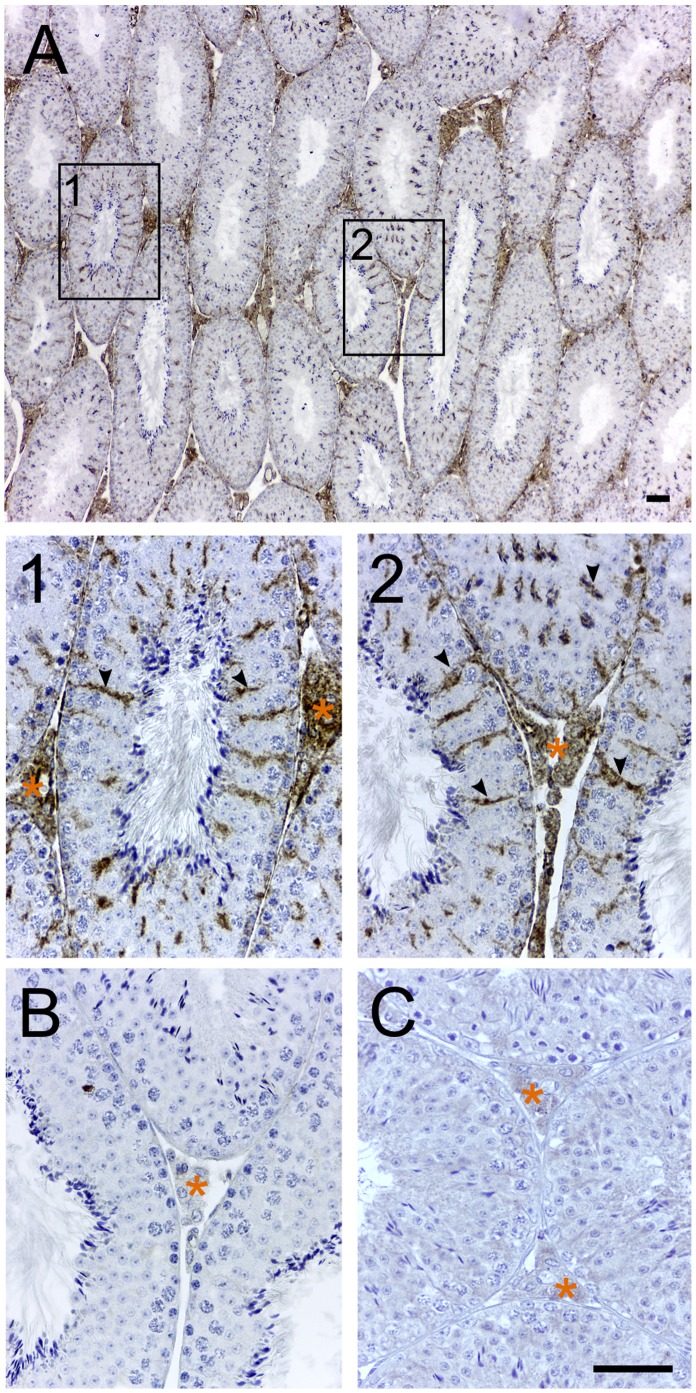
Figure 3. TSPO expression in the adult testis is localized to Leydig and Sertoli cells.
Immunohistochemical localization of TSPO in testes from an 8-week-old mouse. (A) Prominent TSPO expression was observed in the interstitial Leydig cells. Boxed regions 1 and 2 within Panel A under higher magnification showed specific TSPO expression in Sertoli cells (few indicated with arrowheads) in addition to Leydig cells (region with orange asterisk). (B) Negative control without the monoclonal primary antibody did not show any labeling. (C) Peptide-preadsorbed control did not show any labeling validating the specificity of this antibody for detecting TSPO in immunohistochemical sections. Scale bars 20 µm.
TSPO Expression in the Adult Testis
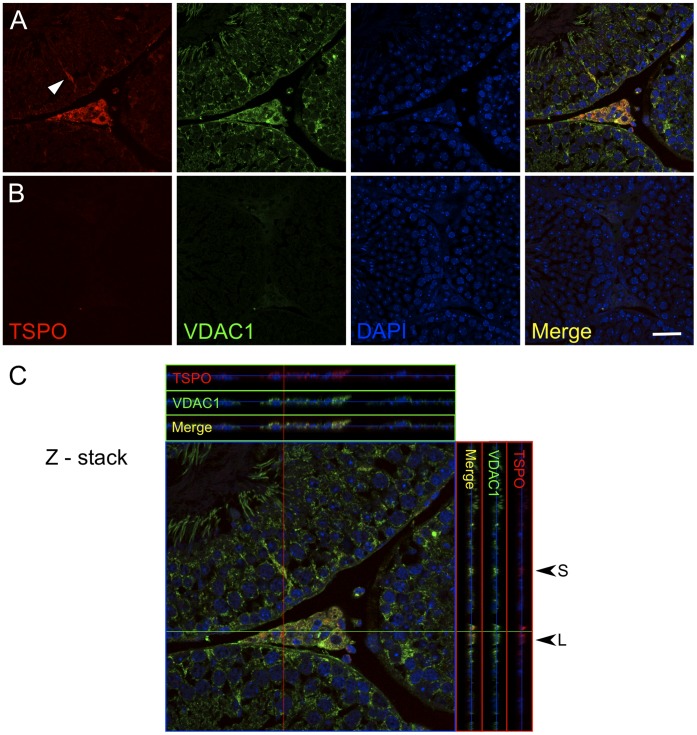
The first study to detect TSPO localization in the testis utilized [3H]Ro5–4864 as a tracer and showed high binding in interstitial regions between the seminiferous tubules [6]. In this study, we observe a specific localization of TSPO to not only the interstitial Leydig cells, but also to the Sertoli cells within the seminiferous tubules (Fig. 3). TSPO expression was not apparent in spermatogonial stem cells or in the developing germ cells at different stages of the seminiferous tubules. This observation was corroborated by immunofluorescence confocal detection in testes sections that showed TSPO and VDAC1 colocalization in Leydig and Sertoli cells (Fig. 4). TSPO expression was not evident in VDAC1-labelled mitochondria at the different stages of germ cells in the seminiferous tubules.
Figure 4. TSPO colocalizes with VDAC1 in Leydig and Sertoli cells.
(A) Panel shows confocal images of TSPO and VDAC1 localization in the testis. TSPO labeling is not apparent in germ cells but specific fluorescence is visible in Sertoli cells (arrowhead) and Leydig cells. VDAC1 localization is seen in all cellular mitochondria including developing germ cells and the midpiece of spermatozoa. TSPO colocalization with VDAC1 is seen specifically in Leydig and Sertoli cells. (B) Negative control panel. (C) A 21-image Z-stack shows colocalization of TSPO and VDAC1 across optical sections in the X (green line) and Y (red line) axes. Specific overlap is seen at regions representing Leydig cells (L) and Sertoli cells (S). Scale bar 20 µm.
Leydig cells are the steroidogenic cells in the testis that produce testosterone. Based on the known function for TSPO in cholesterol transport across the mitochondrial membranes, expression of TSPO along with StAR [53] and other steroidogenic enzymes is expected for functional Leydig cells [54]. However, in adult Sertoli cells, there is little evidence to support a role for TSPO in steroidogenesis. One can just speculate that it may perhaps be involved in cholesterol transport into the mitochondria for other modifications. Although a study examining human testis showed that StAR is expressed in Sertoli cells [55], evidence for the expression of CYP11A1 (P450 side chain cleavage) that converts cholesterol to pregnenolone in Sertoli cells remains controversial [56]. The conversion of cholesterol to pregnenolone was initially suggested to be possible within seminiferous tubules [57]; however, this observation has been refuted by several subsequent studies that have questioned Sertoli cell potential for de novo steroidogenesis [58], [59], [60]. Therefore, the function of TSPO in adult Sertoli cells remains unclear.
TSPO Expression in the Developing Testis
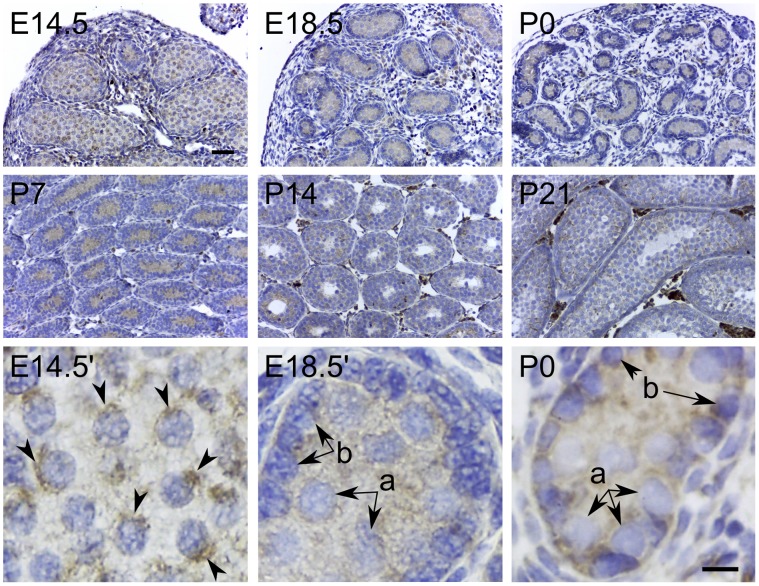
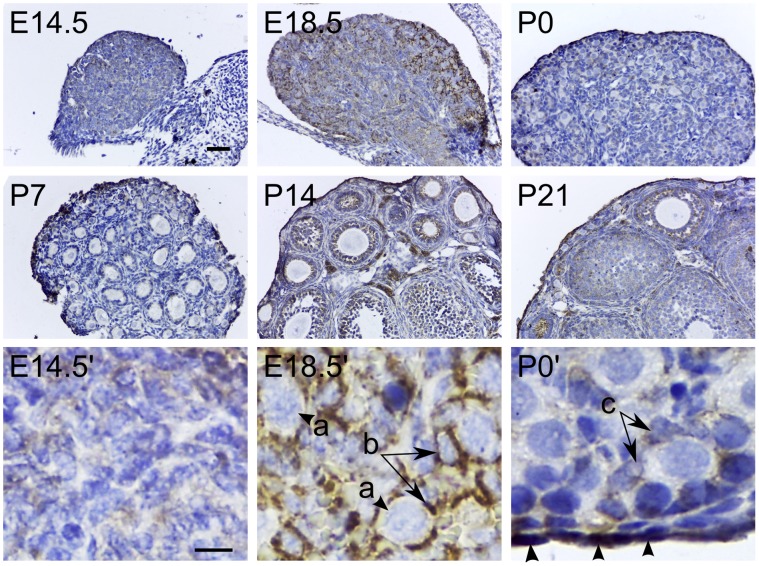
Fetal testosterone production by the testis is a critical regulator of sexual differentiation [54]. During embryonic development, it has been reported that CYP11A1 can be detected as early as E12.5 in the fetal testis [61]. Other major enzymes required for androgen biosynthesis (CYP17A1 and 3βHSD) have been detected by E13 [62], the time point immediately after sexual differentiation to the male phenotype occurs (E12). When we examined the embryonic testis, we observed weak TSPO expression within the seminiferous cords along with a few strongly positive interstitial cells at E14.5 moderately increasing in intensity to P7 testis (Fig. 5). At E14.5, TSPO expression was localized to defined juxtanuclear regions within the gonocytes. At E18.5, weak TSPO expression was seen in both the immature Sertoli cells and gonocytes. This expression in gonocytes persisted postnatally until P7. From P14 to P21, expression in the fetal proliferating Leydig cells became more prominent. The role of fetal Leydig cells in androgen production is well documented [63]. Within the tubules, TSPO expression at P21 closely resembled the pattern of expression found in the adult testis. Fetal TSPO expression in Sertoli cells could be associated with steroid hormone biosynthesis. In rats, immature Sertoli cells in the fetus are known to express CYP11A1 when cultured in vitro with follicle stimulating hormone, and can produce pregnenolone [64]. However, TSPO expression in gonocytes, and not in adult stages of germ cell development (including spermatogonial stem cells) suggest a stage-specific developmental function. A recent study examining TSPO in rat testicular germ cells reported expression in both gonocytes and adult germ cells [65]. Using a polyclonal antibody, they showed that TSPO was predominantly localized to the nucleus. In this study, we find TSPO localized to juxtanuclear regions in gonocytes (Fig. 5), but expression was not apparent in adult germ cells (Fig. 3 and Fig. 4). This dissimilarity identifies an interesting species difference between mouse and rat testicular germ cells. Similar differences in expression between mouse and rat germ cells have been reported for other genes [66].
Figure 5. Developmental expression of TSPO in the testis.
Immunohistochemical localization of TSPO in embryonic (E14.5 and E18.5), and early postnatal stages (P0, P7, P14 and P21) of the developing testis. At stages E14.5, E18.5, P0 and P7, TSPO was diffusely expressed within the seminiferous cords in both gonocytes and Sertoli cells; few strongly positive cells were also seen among the interstitial cells. At E14.5 TSPO localization in gonocytes was seen enriched in a perinuclear compartment (E14.5′ arrowheads). In higher magnification images from E18.5 and P0, TSPO localization persisted weakly in both gonocytes and proliferating Sertoli cells (E18.5′ and P0′; a - gonocytes, b – Sertoli cells). At P14 and P21, TSPO expression was approaching a pattern similar to the adult testis. However, Leydig cell expression levels at these age groups appeared higher compared to the adult (compare Fig. 2). Scale bars 20 µm.
TSPO Expression in the Epididymis
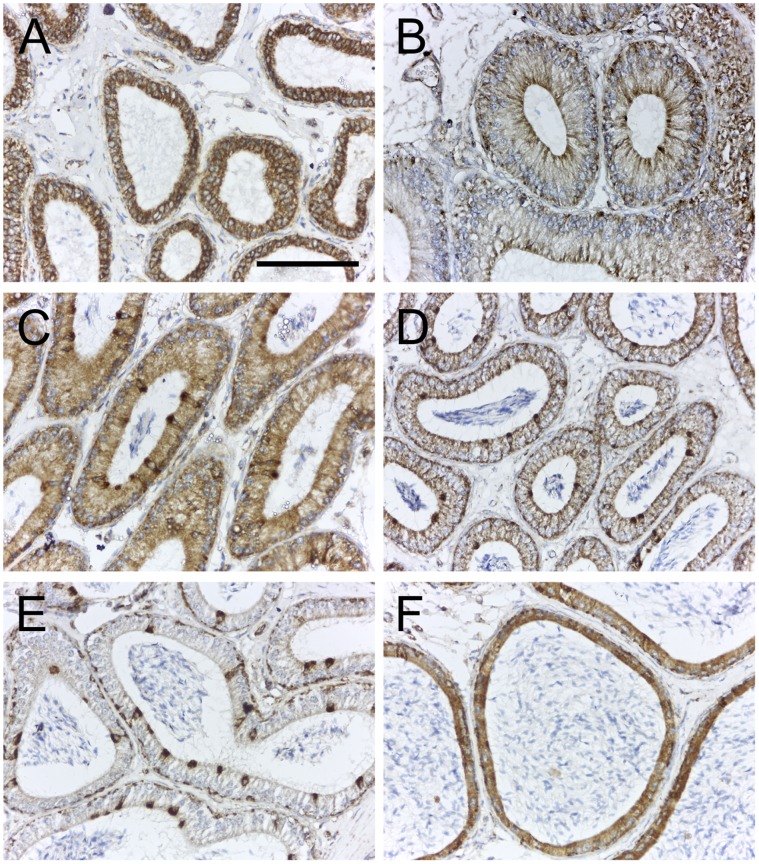
It is well established that the post-testicular maturation of spermatozoa occurs starting at the efferent ducts and extending through the length of the epididymis. However, understanding of functions mediated by the epididymal epithelium remains fairly limited [67]. In this study we find that TSPO is expressed in the epithelium of the distal efferent ducts, and in the different segments of the caput, corpus and cauda epididymis (Fig. 6). Among the different cell types forming the epididymal epithelium, TSPO expression was distinctly prominent in narrow cells, clear cells and basal cells; expression in principal cells was weaker and also varied depending on the epididymal segment – from high in segment II of the caput to completely absent in the corpus epididymis. The presence of a higher number of clear cells in the cauda versus the caput epididymis [68], could explain the difference in staining pattern seen in these regions. Clear cells are known to play a role in luminal acidification, as they express H+V-ATPase and are involved in active proton secretion [69]. This expression pattern was surprisingly similar to what was reported in the gastric mucosa where TSPO was strongly expressed in the proton-secreting parietal cells [70]. However, functional studies using pharmacological TSPO modulators did not reveal a direct relationship between TSPO and proton secretion, but rather showed an effect on chloride secretion [70]. Although not directly demonstrated in the epididymis, chloride ions in most cases accompany the protons in the acidification of luminal compartments [71]. Therefore, there could be a function for TSPO in regulating the luminal pH in the epididymis. In addition, there is evidence that steroid hormone biosynthesis occurs in the cells of the epididymal epithelium. In rams, this has been demonstrated in vitro in principal cell cultures from the epididymal epithelium [72]. In the mouse, receptors for luteinizing hormone (LH), that trigger testosterone production in Leydig cells have also been localized to principal cells of the epididymis [73]. In our observation, we did find TSPO expression in principal cells, albeit at different levels between segments, that could participate in steroidogenesis. Therefore, TSPO function could be distinct for these functional cell types in the epididymal epithelium.
Figure 6. TSPO is expressed in the epithelium of the efferent ducts and epididymis.
Immunohistochemical localization of TSPO in efferent ducts and segments of the epididymis from an 8-week-old mouse. (A) TSPO expression in the epithelium of the distal efferent ducts; all cells forming the epithelial layer showed strong expression of TSPO. (B) TSPO expression in the epithelium of segment I of the caput epididymis; expression was confined to the apical and basal regions, specific to narrow and basal cells. (C) TSPO expression in the epithelium of segment II of the caput epididymis; all cells expressed TSPO but prominently higher expression was observed in the narrow cells. (D) TSPO expression in the epithelium of segment III of the caput epididymis; expression was weaker than that observed in segment II, but the levels in narrow cells and basal cells appeared unchanged. (E) TSPO expression in epithelium of the corpus epididymis; localization was restricted exclusively to the basal cells and clear cells. (F) TSPO expression in epithelium of the cauda epididymis; almost all cells forming the epithelial layer appeared to show TSPO expression. Scale bars 20 µm.
TSPO Expression in the Male Accessory Sex Glands
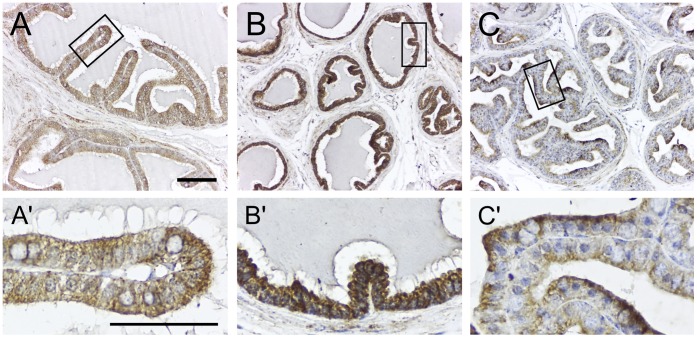
The seminal vesicle and prostate development have been largely studied as targets of androgen action in rodent models. Their secretory activity has been extensively investigated based on the composition and function of seminal fluid. We find that the seminal vesicle and prostate epithelia express TSPO (Fig. 7). In the ventral prostate, expression was higher compared to the dorsolateral prostate, in which TSPO was localized to the apical part of the cells. Based on work done in the rat, it is known that both seminal vesicle epithelia [74], and prostate epithelia [75] express LH receptor similar to testicular Leydig cells. However, there is no report of de novo steroidogenic function in these epithelia. Therefore, function of TSPO in these epithelial layers could be associated with secretory events linked to luminal pH modification [70], that may need to occur in these accessory sex glands. The paucity of data in this area also makes it plausible that de novo steroidogensis could be taking place in these accessory sex gland epithelia.
Figure 7. TSPO is expressed in the epithelium of the seminal vesicle and prostate.
Immunohistochemical localization of TSPO in the seminal vesicle and prostate from an 8-week-old mouse. (A) Epithelium of the seminal vesicle showed weak expression of TSPO that was diffuse throughout the cytoplasm. (B) Epithelium of the ventral prostate showed strong expression of TSPO also distributed evenly in the cytoplasm. (C) Epithelium of the dorsolateral prostate showed a distinct apically polarized TSPO expression. Boxed regions in panels A, B and C are magnified in panels A’, B’ and C’. Scale bars 20 µm (Low magnification panel) and 10 µm (High magnification panel).
TSPO Expression in the Adult and Pregnant Ovary
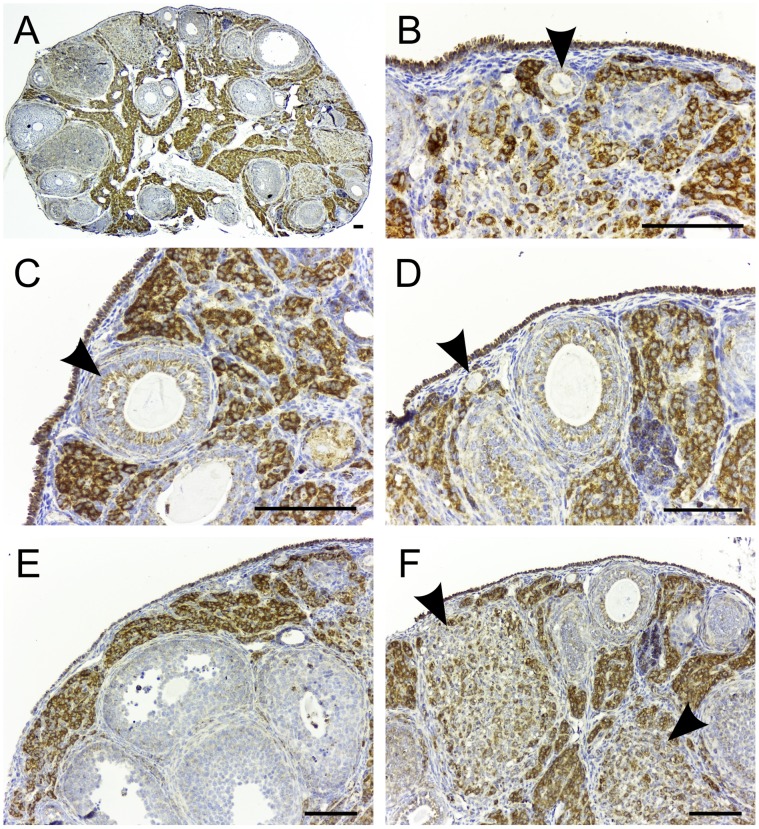
Integrated with its function, the ovary produces two major steroid hormones: estrogen during the follicular phase and progesterone during the luteal phase of the ovarian cycle. Studies using radiolabeled [3H]PK11195 showed rough localization of TSPO to the regions of interstitial cells, corpus luteum and follicles [76]. In this study, we observed high expression of TSPO in the interstitial cells of the ovary (Fig. 8). A weaker expression of TSPO was observed in the granulosa cells surrounding the developing follicles in the primordial, primary, secondary and tertiary stages. In antral follicles, TSPO expression in granulosa cells was heterogeneous. This could be due to the heterogeneity in steroidogenic potential of granulosa cells [77], that can correlate with TSPO expression. Theca cells surrounding these follicles also showed weak expression of TSPO. Based on the conserved two-cell, two-gonadotropin concept, theca and granulosa cells respond to LH and FSH to produce androstenedione and estrogen respectively [78], [79]. Ovarian interstitial cells also respond to LH and produce androstenedione [80]. Androstenedione from both theca and interstitial cells serve as a substrate for estrogen biosynthesis by the granulosa cell layers. Ovarian steroidogenesis and its regulation are well defined for these three cell types in the ovary (reviewed in [81]). Underscoring the potential cooperative role for TSPO in steroidogenic function, a pattern for StAR expression similar to our observation on TSPO has been reported for the rat ovary [82].
Figure 8. TSPO expression in the adult ovary is localized to the interstitial cells and granulosa cells.
Immunohistochemical localization of TSPO in ovaries from an adult 8-week-old mouse. (A) The staining pattern for TSPO in a section dissecting the entire ovary. There was strong expression of TSPO observed in the interstitial cells. (B) TSPO expression was also strong in the ovarian surface epithelium in addition to the interstitial cells. Granulosa cells of a primary follicle (arrowhead) also showed TSPO expression. (C) Granulosa cells of a secondary follicle (arrowhead) expressed TSPO. Few theca cells around the follicle also showed weak expression of TSPO. (D) Squamous granulosa layer of a primordial follicle (arrowhead) showed TSPO expression. (E) Granulosa cells of most antral follicles show very weak to no expression of TSPO. (F) Regressing corpora lutea (arrowheads) also contained cells that show strong expression of TSPO. Scale bars 20 µm.
Estrogen production in the ovulating follicles is terminated by the LH surge transforming their theca and granulosa cells to corpora lutea that produce progesterone. In the pregnant ovary, we found that corpora lutea express TSPO (Fig. 9), at a level higher than that seen in theca and granulosa cells of the antral follicles. TSPO expression was seen in both large and small luteal cells, both capable of making progesterone albeit at different levels [83].
Figure 9. TSPO is expressed in the active corpus luteum.
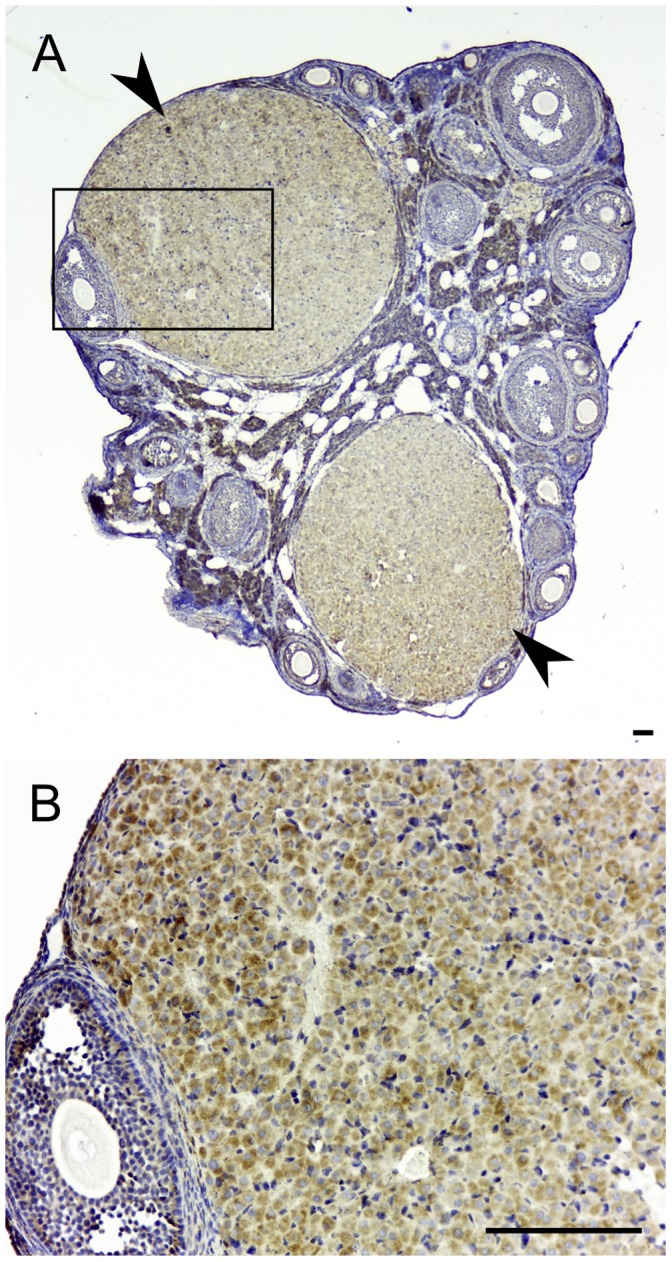
Immunohistochemical localization of TSPO in an ovary from a 14.5-day post coitus pregnant mouse. (A) The staining pattern for TSPO in a section dissecting the entire ovary. There was strong expression of TSPO in the two corpora lutea (arrowheads). (B) Higher magnification image of the boxed region in panel A showing variable levels of TSPO expression between different cells that form the corpus luteum. Both large and small luteal cells of the corpus luteum showed expression of TSPO. Scale bars 20 µm.
In addition to the steroidogenic cells, prominent TSPO expression was also distinctly present in the ovarian surface epithelium/germinal epithelium (Fig. 8). These squamous to cuboidal mesothelial cells with microvilli like projections that form the ovarian surface epithelium have been traced to form the embryonic origin of granulosa cells [84]. In pathologies, it has been suggested that these cells could be a cause for ovarian tumors [85] and they also believed to contain a population of germline stem cells for postnatal follicular renewal [86]. There is no known steroidogenic or secretory function for the germinal epithelium. Therefore, the integral activity for TSPO in this cell type remains to be investigated.
TSPO Expression in the Developing Ovary
In premeiotic germ cell development, cysts due to incomplete cytokinesis arise from division of progenitor cells that subsequently enter meiosis [87]. In the E14.5 ovary, these cysts are at prophase I of meiosis. We find that TSPO expression is weak in the E14.5 ovary and does not appear to be associated with the germ line cysts (Fig. 10). This is supported by observations at E18.5, when TSPO expression is prominent in the pre-granulosa cells associated with the germ line cysts. At birth (P0), expression of TSPO persisted in squamous granulosa cells surrounding the primordial follicles. From E18.5 to P0, there was a progressive increase in expression of TSPO in the ovarian surface epithelium that persisted through adulthood. From P7, TSPO expression was seen in clusters of interstitial cells that progressively increased in numbers through P14 and P21. Expression level at P21 closely resembled findings reported for the adult ovary. There are no reports on the developmental expression of different steroid hydroxylases and/or StAR to compare with findings on TSPO expression in this study. A report on steroidogenic factor-1 (SF-1; a nuclear receptor that regulates steroid hydroxylases) and CYP11A1 transcripts in the developing ovary showed that neither SF-1 nor CYP11A1 were expressed from E13.5 to E16.5 [61]. However, weak expression of SF-1 was detected on E18.5. This suggests that after sex differentiation of the bipotential gonad, TSPO expression preceded the expression of enzymes required for the steroid hormone biosynthesis.
Figure 10. Developmental expression of TSPO in the ovary.
Immunohistochemical localization of TSPO in embryonic (E14.5 and E18.5), and early postnatal stages (P0, P7, P14 and P21) of the developing ovary. At stages E14.5 and E18.5, TSPO was weak and appeared diffusely expressed in most cells forming the ovarian structure. Expression levels were higher at E18.5 compared to E14.5. Higher magnification images showed specific expression in the epithelial pregranulosa cells associated with the ovarian cysts (E14.5′ and E18.5′; a – progenitor in a germline cyst; b – pregranulosa cells). Compared to E18.5, TSPO expression at P0 was lower in the pregranulosa layer (P0′; c – pregranulosa cells) but there was strong expression in the germinal epithelium. Starting at P7, TSPO expression was observed in few clusters of interstitial cells that were very prominent in both numbers and expression at P14 and P21. TSPO expression pattern at P21 closely resembled that observed in the adult ovary. Scale bars 20 µm.
TSPO Expression in the Uterus and Oviduct
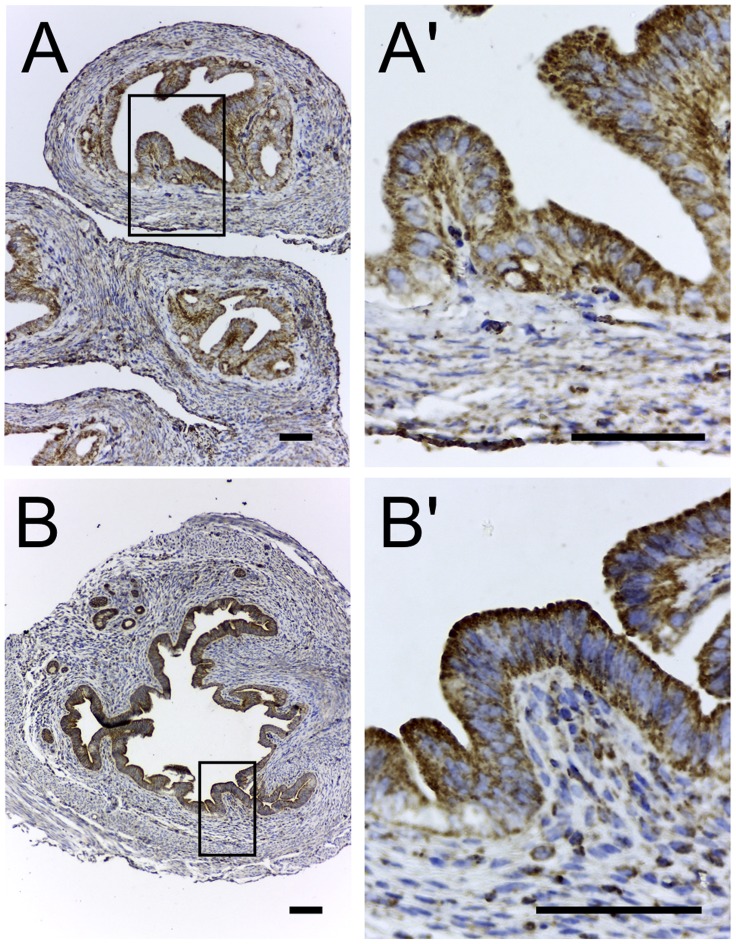
In both the non-pregnant oviduct and uterus TSPO expression was present in the epithelial layer lining the lumen, along with a few scattered cells expressing TSPO in the submucosa/stroma (Fig. 11). This expression pattern was consistent and did not appear to change with either the different stages of the estrous cycle or the different regions within the uterus and oviduct. There is evidence for de novo estrogen production by the stromal cells of the uterine endometrium [88], but not the epithelium. Moreover, there are no reports on steroidogenic activity in the oviduct. Therefore, the function for TSPO in these luminal epithelia could be associated with secretory activity similar to chloride secretion and luminal acidification as seen in the gastric mucosa [70].
Figure 11. TSPO is expressed in the epithelium of the oviduct and uterus.
Immunohistochemical localization of TSPO in the non-pregnant oviduct and uterus. (A) Epithelium of the oviduct showing strong expression of TSPO that was diffuse throughout the cytoplasm. (B) Epithelium of the uterus showing strong expression of TSPO that was distributed throughout the cytoplasm with specific enrichment towards the apical regions of the cells. Boxed regions in panels A and B are magnified in panels A’ and B’. Scale bars 20 µm.
Conclusions
TSPO is a protein that shows high sequence conservation from bacteria to mammals [89]. The well-studied function for TSPO in steroid hormone biosynthesis explains several of the physiological and pathological observations in different organ systems [90]. Equally, there are strong data suggesting multiple alternate functions for this protein [7]. Even in steroidogenic cells, it is clear that regulation of TSPO function can be complex. For example, its endogenous ligand acyl-CoA-binding protein (ACBP)/diazepam binding inhibitor was reported to induce steroidogenesis in adrenocortical cells in vitro [91]. However, mice deficient in ACBP do not have problems in steroid hormone biosynthesis [92], suggesting that multiple secondary levels of functional regulation could exist for TSPO.
At the core level of transcriptional regulation, effects of several TSPO gene upstream-binding elements (Sp1/Sp3, AP1, Ets and SINE B2) have been examined [26], [27], [28]. However, none of them completely explain the precise cell type-specific TSPO expression patterns seen in different tissues. Moreover, the basis of pathological upregulation of TSPO as seen in cancer cells remains to be established. These points suggest that regulation of this conserved gene is fairly complex and may differ based on the functional cell type.
In conclusion, this study presents several novel findings regarding TSPO localization in both the male and female reproductive system. There was clear indication of the multifunctional nature of TSPO evident from its specific expression in both steroidogenic and select non-steroidogenic cell types. These expression patterns in reproductive tissues will provide a functional reference for further studies to fully understand different TSPO functions.
Acknowledgments
The authors wish to thank Dr. Susan Quirk and Dr. Chinatsu Mukai at Cornell University for providing materials that were necessary for testing of the TSPO antibody. We also thank Dr. Mario Ascoli from The University of Iowa for sharing the MA-10 Leydig cell line used for validating the monoclonal TSPO antibody.
Funding Statement
The authors have no support or funding to report.
References
- 1. Braestrup C, Albrechtsen R, Squires RF (1977) High densities of benzodiazepine receptors in human cortical areas. Nature 269: 702–704. [DOI] [PubMed] [Google Scholar]
- 2. Braestrup C, Squires RF (1977) Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A 74: 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marangos PJ, Patel J, Boulenger JP, Clark-Rosenberg R (1982) Characterization of peripheral-type benzodiazepine binding sites in brain using [3H]Ro 5-4864. Mol Pharmacol 22: 26–32. [PubMed] [Google Scholar]
- 4. Anholt RR, Pedersen PL, De Souza EB, Snyder SH (1986) The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem 261: 576–583. [PubMed] [Google Scholar]
- 5. Antkiewicz-Michaluk L, Mukhin AG, Guidotti A, Krueger KE (1988) Purification and characterization of a protein associated with peripheral-type benzodiazepine binding sites. The Journal of biological chemistry 263: 17317–17321. [PubMed] [Google Scholar]
- 6. De Souza EB, Anholt RR, Murphy KM, Snyder SH, Kuhar MJ (1985) Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinology 116: 567–573. [DOI] [PubMed] [Google Scholar]
- 7. Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, et al. (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 51: 629–650. [PubMed] [Google Scholar]
- 8. Fan J, Lindemann P, Feuilloley MG, Papadopoulos V (2012) Structural and functional evolution of the translocator protein (18 kDa). Current molecular medicine 12: 369–386. [DOI] [PubMed] [Google Scholar]
- 9. Wang JK, Morgan JI, Spector S (1984) Benzodiazepines that bind at peripheral sites inhibit cell proliferation. Proc Natl Acad Sci U S A 81: 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carmel I, Fares FA, Leschiner S, Scherubl H, Weisinger G, et al. (1999) Peripheral-type benzodiazepine receptors in the regulation of proliferation of MCF-7 human breast carcinoma cell line. Biochem Pharmacol 58: 273–278. [DOI] [PubMed] [Google Scholar]
- 11. Maaser K, Hopfner M, Jansen A, Weisinger G, Gavish M, et al. (2001) Specific ligands of the peripheral benzodiazepine receptor induce apoptosis and cell cycle arrest in human colorectal cancer cells. Br J Cancer 85: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everett H, Barry M, Sun X, Lee SF, Frantz C, et al. (2002) The myxoma poxvirus protein, M11L, prevents apoptosis by direct interaction with the mitochondrial permeability transition pore. J Exp Med 196: 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirsch JD, Beyer CF, Malkowitz L, Beer B, Blume AJ (1989) Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol 35: 157–163. [PubMed] [Google Scholar]
- 14. Taketani S, Kohno H, Furukawa T, Tokunaga R (1995) Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J Biochem 117: 875–880. [DOI] [PubMed] [Google Scholar]
- 15. Rampon C, Bouzaffour M, Ostuni MA, Dufourcq P, Girard C, et al. (2009) Translocator protein (18 kDa) is involved in primitive erythropoiesis in zebrafish. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23: 4181–4192. [DOI] [PubMed] [Google Scholar]
- 16. Cantor EH, Kenessey A, Semenuk G, Spector S (1984) Interaction of calcium channel blockers with non-neuronal benzodiazepine binding sites. Proc Natl Acad Sci U S A 81: 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Python CP, Rossier MF, Vallotton MB, Capponi AM (1993) Peripheral-type benzodiazepines inhibit calcium channels and aldosterone production in adrenal glomerulosa cells. Endocrinology 132: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 18. Lenfant M, Haumont J, Zavala F (1986) In vivo immunomodulating activity of PK 1195, a structurally unrelated ligand for “peripheral” benzodiazepine binding sites–I. Potentiation in mice of the humoral response to sheep red blood cells. Int J Immunopharmacol 8: 825–828. [DOI] [PubMed] [Google Scholar]
- 19. Drugan RC, Basile AS, Crawley JN, Paul SM, Skolnick P (1986) Inescapable shock reduces [3H]Ro 5–4864 binding to “peripheral-type” benzodiazepine receptors in the rat. Pharmacol Biochem Behav 24: 1673–1677. [DOI] [PubMed] [Google Scholar]
- 20. Mesenholler M, Matthews EK (2000) A key role for the mitochondrial benzodiazepine receptor in cellular photosensitisation with delta-aminolaevulinic acid. Eur J Pharmacol 406: 171–180. [DOI] [PubMed] [Google Scholar]
- 21. Starosta-Rubinstein S, Ciliax BJ, Penney JB, McKeever P, Young AB (1987) Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Natl Acad Sci U S A 84: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novas ML, Medina JH, Calvo D, De Robertis E (1987) Increase of peripheral type benzodiazepine binding sites in kidney and olfactory bulb in acutely stressed rats. Eur J Pharmacol 135: 243–246. [DOI] [PubMed] [Google Scholar]
- 23. Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, et al. (1997) Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem 272: 32129–32135. [DOI] [PubMed] [Google Scholar]
- 24. Kelly-Hershkovitz E, Weizman R, Spanier I, Leschiner S, Lahav M, et al. (1998) Effects of peripheral-type benzodiazepine receptor antisense knockout on MA-10 Leydig cell proliferation and steroidogenesis. J Biol Chem 273: 5478–5483. [DOI] [PubMed] [Google Scholar]
- 25. Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, et al. (1997) Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 62: 21–28. [DOI] [PubMed] [Google Scholar]
- 26. Giatzakis C, Papadopoulos V (2004) Differential utilization of the promoter of peripheral-type benzodiazepine receptor by steroidogenic versus nonsteroidogenic cell lines and the role of Sp1 and Sp3 in the regulation of basal activity. Endocrinology 145: 1113–1123. [DOI] [PubMed] [Google Scholar]
- 27. Batarseh A, Giatzakis C, Papadopoulos V (2008) Phorbol-12-myristate 13-acetate acting through protein kinase Cepsilon induces translocator protein (18-kDa) TSPO gene expression. Biochemistry 47: 12886–12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giatzakis C, Batarseh A, Dettin L, Papadopoulos V (2007) The role of Ets transcription factors in the basal transcription of the translocator protein (18 kDa). Biochemistry 46: 4763–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan J, Papadopoulos V (2012) Transcriptional regulation of translocator protein (Tspo) via a SINE B2-mediated natural antisense transcript in MA-10 Leydig cells. Biology of reproduction 86: 147, 141–115. [DOI] [PMC free article] [PubMed]
- 30. Murail S, Robert JC, Coic YM, Neumann JM, Ostuni MA, et al. (2008) Secondary and tertiary structures of the transmembrane domains of the translocator protein TSPO determined by NMR. Stabilization of the TSPO tertiary fold upon ligand binding. Biochim Biophys Acta 1778: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V (2001) Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci U S A 98: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McEnery MW, Snowman AM, Trifiletti RR, Snyder SH (1992) Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A 89: 3170–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veenman L, Shandalov Y, Gavish M (2008) VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr 40: 199–205. [DOI] [PubMed] [Google Scholar]
- 34. Veenman L, Papadopoulos V, Gavish M (2007) Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des 13: 2385–2405. [DOI] [PubMed] [Google Scholar]
- 35. Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V (2011) Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol 336: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritta MN, Calandra RS (1989) Testicular interstitial cells as targets for peripheral benzodiazepines. Neuroendocrinology 49: 262–266. [DOI] [PubMed] [Google Scholar]
- 37. Papadopoulos V, Mukhin AG, Costa E, Krueger KE (1990) The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. The Journal of biological chemistry 265: 3772–3779. [PubMed] [Google Scholar]
- 38. Amsterdam A, Suh BS (1991) An inducible functional peripheral benzodiazepine receptor in mitochondria of steroidogenic granulosa cells. Endocrinology 129: 503–510. [DOI] [PubMed] [Google Scholar]
- 39. Stocco DM, Clark BJ (1996) Regulation of the acute production of steroids in steroidogenic cells. Endocrine reviews 17: 221–244. [DOI] [PubMed] [Google Scholar]
- 40. Black SM, Harikrishna JA, Szklarz GD, Miller WL (1994) The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proceedings of the National Academy of Sciences of the United States of America 91: 7247–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin D, Sugawara T, Strauss JF (1995) Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267: 1828–1831. [DOI] [PubMed] [Google Scholar]
- 42. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, et al. (1997) Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A 94: 11540–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller WL (2007) Mechanism of StAR’s regulation of mitochondrial cholesterol import. Mol Cell Endocrinol 265–266: 46–50. [DOI] [PubMed] [Google Scholar]
- 44. Manna PR, Dyson MT, Stocco DM (2009) Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hauet T, Liu J, Li H, Gazouli M, Culty M, et al. (2002) PBR, StAR, and PKA: partners in cholesterol transport in steroidogenic cells. Endocrine research 28: 395–401. [DOI] [PubMed] [Google Scholar]
- 46. Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, et al. (2005) Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol 19: 540–554. [DOI] [PubMed] [Google Scholar]
- 47. Liu J, Rone MB, Papadopoulos V (2006) Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. The Journal of biological chemistry 281: 38879–38893. [DOI] [PubMed] [Google Scholar]
- 48. Ascoli M (1981) Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108: 88–95. [DOI] [PubMed] [Google Scholar]
- 49. Hu J, Zhang Z, Shen WJ, Azhar S (2010) Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutrition & metabolism 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papadopoulos V (2004) In search of the function of the peripheral-type benzodiazepine receptor. Endocrine research 30: 677–684. [DOI] [PubMed] [Google Scholar]
- 51. Mercer KA, Weizman R, Gavish M (1992) Ontogenesis of peripheral benzodiazepine receptors: demonstration of selective up-regulation in rat testis as a function of maturation. Journal of receptor research 12: 413–425. [DOI] [PubMed] [Google Scholar]
- 52. Sprengel R, Werner P, Seeburg PH, Mukhin AG, Santi MR, et al. (1989) Molecular cloning and expression of cDNA encoding a peripheral-type benzodiazepine receptor. The Journal of biological chemistry 264: 20415–20421. [PubMed] [Google Scholar]
- 53. Clark BJ, Wells J, King SR, Stocco DM (1994) The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). The Journal of biological chemistry 269: 28314–28322. [PubMed] [Google Scholar]
- 54. Payne AH, Youngblood GL (1995) Regulation of expression of steroidogenic enzymes in Leydig cells. Biology of reproduction 52: 217–225. [DOI] [PubMed] [Google Scholar]
- 55. Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, et al. (1997) Localization of the steroidogenic acute regulatory protein in human tissues. The Journal of clinical endocrinology and metabolism 82: 4243–4251. [DOI] [PubMed] [Google Scholar]
- 56. Gregory CW, DePhilip RM (1998) Detection of steroidogenic acute regulatory protein (stAR) in mitochondria of cultured rat Sertoli cells incubated with follicle-stimulating hormone. Biology of reproduction 58: 470–474. [DOI] [PubMed] [Google Scholar]
- 57. Bass JJ, Bell JB, Lacy D (1973) Side-chain cleavage of (26-14 C) cholesterol by rat testicular tissues and their subcellular fractions. The Journal of endocrinology 56: 321–322. [DOI] [PubMed] [Google Scholar]
- 58. van der Vusse GJ, Kalkman ML, van der Molen HJ (1973) Endogenous production of steroids by subcellular fractions from total rat testis and from isolated interstitial tissue and seminiferous tubules. Biochimica et biophysica acta 297: 179–185. [DOI] [PubMed] [Google Scholar]
- 59. Dorrington JH, Fritz IB, Armstrong DT (1978) Steroidogenesis by granulosa and Sertoli cells. Int J Androl S2: 53–65. [Google Scholar]
- 60. Dorrington JH, Armstrong DT (1979) Effects of FSH on gonadal functions. Recent progress in hormone research 35: 301–342. [DOI] [PubMed] [Google Scholar]
- 61. Ikeda Y, Shen WH, Ingraham HA, Parker KL (1994) Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular endocrinology 8: 654–662. [DOI] [PubMed] [Google Scholar]
- 62. Greco TL, Payne AH (1994) Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3 beta-hydroxysteroid dehydrogenase, P450 17 alpha-hydroxylase/C17–20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 135: 262–268. [DOI] [PubMed] [Google Scholar]
- 63. Huhtaniemi I, Pelliniemi LJ (1992) Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med 201: 125–140. [DOI] [PubMed] [Google Scholar]
- 64. Ford SL, Reinhart AJ, Lukyanenko Y, Hutson JC, Stocco DM (1999) Pregnenolone synthesis in immature rat Sertoli cells. Molecular and cellular endocrinology 157: 87–94. [DOI] [PubMed] [Google Scholar]
- 65. Manku G, Wang Y, Thuillier R, Rhodes C, Culty M (2012) Developmental expression of the translocator protein 18 kDa (TSPO) in testicular germ cells. Current molecular medicine 12: 467–475. [PubMed] [Google Scholar]
- 66. Encinas G, Zogbi C, Stumpp T (2012) Detection of four germ cell markers in rats during testis morphogenesis: differences and similarities with mice. Cells, tissues, organs 195: 443–455. [DOI] [PubMed] [Google Scholar]
- 67. Turner TT (2011) Looking to the future of epididymal research: why this, why now? Journal of andrology 32: 705–710. [DOI] [PubMed] [Google Scholar]
- 68. Shum WW, Da Silva N, Brown D, Breton S (2009) Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. The Journal of experimental biology 212: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Breton S, Smith PJ, Lui B, Brown D (1996) Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nature medicine 2: 470–472. [DOI] [PubMed] [Google Scholar]
- 70. Ostuni MA, Marazova K, Peranzi G, Vidic B, Papadopoulos V, et al. (2004) Functional characterization and expression of PBR in rat gastric mucosa: stimulation of chloride secretion by PBR ligands. American journal of physiology Gastrointestinal and liver physiology 286: G1069–1080. [DOI] [PubMed] [Google Scholar]
- 71. Harvey WR, Wieczorek H (1997) Animal plasma membrane energization by chemiosmotic H+V-ATPases. The Journal of experimental biology 200: 203–216. [DOI] [PubMed] [Google Scholar]
- 72. Amann RP, Marengo SR, Brown DV (1987) Steroidogenesis and testosterone metabolism in cultured principal cells from the ram epididymis. Journal of andrology 8: 238–246. [DOI] [PubMed] [Google Scholar]
- 73. Adams CS, Brumlow WB (1989) Immunocytochemical detection of luteinizing hormone in epididymis of mature mouse. Histochemistry 91: 495–499. [DOI] [PubMed] [Google Scholar]
- 74. Tao YX, Lei ZM, Rao CV (1998) Seminal vesicles are novel sites of luteinizing hormone/human chorionic gonadotropin-receptor gene expression. Journal of andrology 19: 343–347. [PubMed] [Google Scholar]
- 75. Tao YX, Lei ZM, Woodworth SH, Rao CV (1995) Novel expression of luteinizing hormone/chorionic gonadotropin receptor gene in rat prostates. Molecular and cellular endocrinology 111: R9–12. [DOI] [PubMed] [Google Scholar]
- 76. Toranzo D, Tong Y, Tonon MC, Vaudry H, Pelletier G (1994) Localization of diazepam-binding inhibitor and peripheral type benzodiazepine binding sites in the rat ovary. Anat Embryol (Berl) 190: 383–388. [DOI] [PubMed] [Google Scholar]
- 77. Rao IM, Mills TM, Anderson E, Mahesh VB (1991) Heterogeneity in granulosa cells of developing rat follicles. The Anatomical record 229: 177–185. [DOI] [PubMed] [Google Scholar]
- 78. Fortune JE, Armstrong DT (1977) Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology 100: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 79. Fortune JE, Armstrong DT (1978) Hormonal control of 17 beta-estradiol biosynthesis in proestrous rat follicles: estradiol production by isolated theca versus granulosa. Endocrinology 102: 227–235. [DOI] [PubMed] [Google Scholar]
- 80. McNatty KP, Makris A, DeGrazia C, Osathanondh R, Ryan KJ (1979) The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. The Journal of clinical endocrinology and metabolism 49: 687–699. [DOI] [PubMed] [Google Scholar]
- 81. Erickson GF, Magoffin DA, Dyer CA, Hofeditz C (1985) The ovarian androgen producing cells: a review of structure/function relationships. Endocrine reviews 6: 371–399. [DOI] [PubMed] [Google Scholar]
- 82. Thompson WE, Powell J, Thomas KH, Whittaker JA (1999) Immunolocalization and expression of the steroidogenic acute regulatory protein during the transitional stages of rat follicular differentiation. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 47: 769–776. [DOI] [PubMed] [Google Scholar]
- 83. Hoyer PB, Keyes PL, Niswender GD (1986) Size distribution and hormonal responsiveness of dispersed rabbit luteal cells during pseudopregnancy. Biology of reproduction 34: 905–910. [DOI] [PubMed] [Google Scholar]
- 84. Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocrine reviews 22: 255–288. [DOI] [PubMed] [Google Scholar]
- 85. Murdoch WJ, McDonnel AC (2002) Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction 123: 743–750. [DOI] [PubMed] [Google Scholar]
- 86. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL (2004) Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428: 145–150. [DOI] [PubMed] [Google Scholar]
- 87. Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Developmental biology 234: 339–351. [DOI] [PubMed] [Google Scholar]
- 88. Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, et al. (2009) De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 106: 12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yeliseev AA, Krueger KE, Kaplan S (1997) A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proceedings of the National Academy of Sciences of the United States of America 94: 5101–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rone MB, Fan J, Papadopoulos V (2009) Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta 1791: 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Papadopoulos V, Berkovich A, Krueger KE, Costa E, Guidotti A (1991) Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology 129: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 92. Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, et al. (2011) Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. The Journal of biological chemistry 286: 3460–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]