Abstract
Shrinking of body size has been proposed as one of the universal responses of organisms to global climate warming. Using phytoplankton as an experimental model system has supported the negative effect of warming on body-size, but it remains controversial whether the size reduction under increasing temperatures is a direct temperature effect or an indirect effect mediated over changes in size selective grazing or enhanced nutrient limitation which should favor smaller cell-sizes. Here we present an experiment with a factorial combination of temperature and nutrient stress which shows that most of the temperature effects on phytoplankton cell size are mediated via nutrient stress. This was found both for community mean cell size and for the cell sizes of most species analyzed. At the highest level of nutrient stress, community mean cell size decreased by 46% per °C, while it decreased only by 4.7% at the lowest level of nutrient stress. Individual species showed qualitatively the same trend, but shrinkage per °C was smaller. Overall, our results support the hypothesis that temperature effects on cell size are to a great extent mediated by nutrient limitation. This effect is expected to be exacerbated under field conditions, where higher temperatures of the surface waters reduce the vertical nutrient transport.
Introduction
Shrinking of body size has been proposed as one of the universal responses of organisms to global climate warming [1], [2] and related to classic biogeographic rules [3], [4] and to the temperature-size rule (TSR) [5]. Smaller body sizes in warmer climates have been the domain of biogeographic rules since more than 1½ centuries [3], [4]. More recently, interest in the temperature response to size has been revived by Global Change research and by the “metabolic theory of ecology” [6], [7] and phytoplankton has become one of the model systems to study the size effect of warming. While most phytoplankton studies support the general trend [8], [9], [10], the mechanism remain still unresolved. A meta-analysis of monoculture studies with protists found on average a 2.5% shrinkage per °C [5], which is far less than the size trends observed in-situ and in experiments with naturally mixed plankton communities. Besides direct temperature effects, also enhanced size-selective grazing under warmer conditions [8], [9], [11], [12], [13], [14] has been suggested as proximate cause, but it is general knowledge in biological oceanography that small phytoplankton tend to dominate in warm, nutrient poor waters while large ones tend to dominate in cold, nutrient rich waters [15], [16], [17], [18]. However, identification of the causal mechanism is difficult from field data because of the global anti-correlation between temperature and nutrients in the ocean [19]. Warming of the surface waters intensifies vertical density stratification and, thereby, reduces vertical nutrient transport through the thermocline into the well illuminated surface zone.
In order to disentangle nutrient from temperature effects on phytoplankton cell size, we performed an experiment with a factorial combination of temperature and nutrient stress. We subjected mixed plankton assemblages from Kiel Bight, western Baltic Sea, to three temperature levels and three levels of nutrient limitation in a fully factorial design. The levels of nutrient limitation were manipulated by semi-continuous dilution of the cultures three times per week with fresh media and assessed by measuring the particulate matter C∶N ratio, which is the inverse of the carbon-normalized N-cell quota [20] and shows a linear relationship to the extent of nutrient limitation [21].
Materials and Methods
The field samples taken for our experiment did not involve protected species and were not taken from a protected area. No permit was needed.
The experiment was conducted for three weeks from 9th to 30th August 2012. Twenty seven Erlenmeyer flasks of 700 mL incubated in temperature (13.5, 16.5, and 19.5°C; i.e. in situ conditions and 3°C below and above) and light controlled climate cabinets served as experimental units. They were filled with Baltic Sea water (Kiel Fjord) from 1 to 3 m depth containing the natural plankton community and sieved through plankton gauze of 200 µm mesh size in order to keep out large zooplankton. Microscopic inspection of the initial plankton community indicated that microzooplankton were extremely rare. After measuring the in situ nutrient concentrations, the water was supplemented with nutrients to reach starting concentrations of concentrations of 32.7 µmolL−1 NO3, 4.47 µmolL−1 PO4 and 29 Si, µmolL−1, respectively. Part of the water was sterile filtered (0.2 µm pore size) and stored in darkness at 2°C to serve a medium for the dilutions of cultures. The strength of nutrient limitation was manipulated by semi continuous dilution three times per week on Monday, Wednesday and Friday in which 0% (N3, strongest nutrient stress), 25% (N2, medium nutrient stress), and 50% (N1, weakest nutrient stress) of the culture volume were replaced by fresh medium.
Samples were taken at the end of experiment. Samples for the elemental analysis (C, N, P) of the particulate matter were filtered onto pre-combusted Whatman GF/F filters (Whatman GmbH, Dassel, Germany). N and C were measured by gas chromatography [22] and P was measured colorimetrically after converting organic phosphorus compounds to orthophosphate [23]. Samples for counting and sizing phytoplankton >5 µm were fixed with Lugol's iodine and analyzed with the inverted microscope methodology [24]. We counted at least 100 individuals per species to achieve 95% confidence limits of ca, ±20%. Cell size measurements were taken from 20 randomly chosen individuals per species and per experimental unit and volumes were calculated after approximation to geometric proxies [25]. Phytoplankton <5 µm were counted and sized by flow cytometry. Two size metrics were used to assess the response to the experimental treatments: the cell volume of individual phytoplankton species (Vi) and the mean cell volume of the phytoplankton community (Vc) which was obtained by dividing total volume by the total cell number.
Results
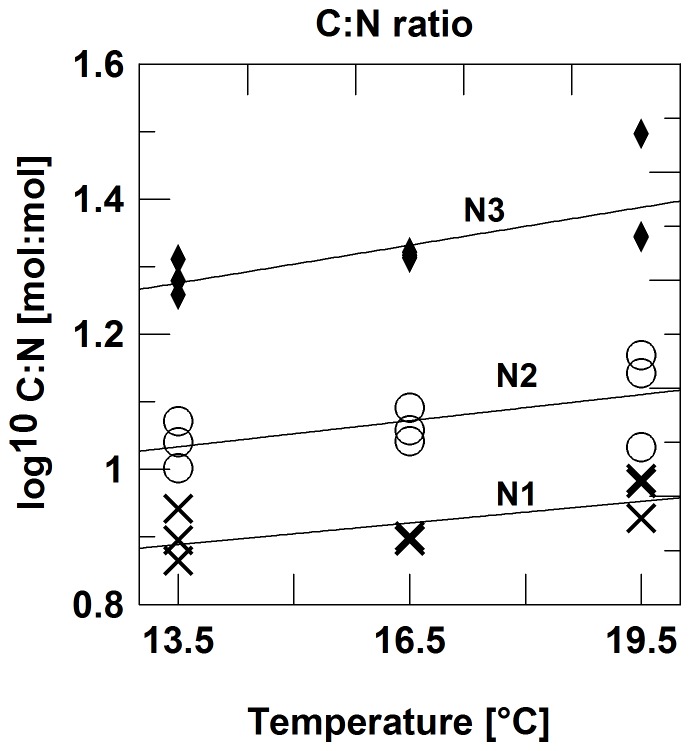
The C∶N ratios of particulate matter increased in the direction N1 to N3 and with temperature (Fig. 1). A two-factor ANOVA with log-transformed C∶N data showed a significant main effect of nutrient treatment and of temperature, but no interaction effect (Pnutr<0.0001; Ptemp = 0.0031; N = 27). The molar C∶N ratios ranged from 8.5 to 37, thus indicating weak to severe nitrogen limitation [21] while P-limitation could be excluded because of N∶P ratios <16 in all experimental units.
Figure 1. Particular matter C∶N ratios increase with tmeperature and decrease with dilution rate.
Molar C∶N ratios of particulate, organic matter in response (log10-scale) to temperature and nutrient regime; N1: 50% dilution three times per week; N2: 25% dilution three times per week; N3: no dilution.
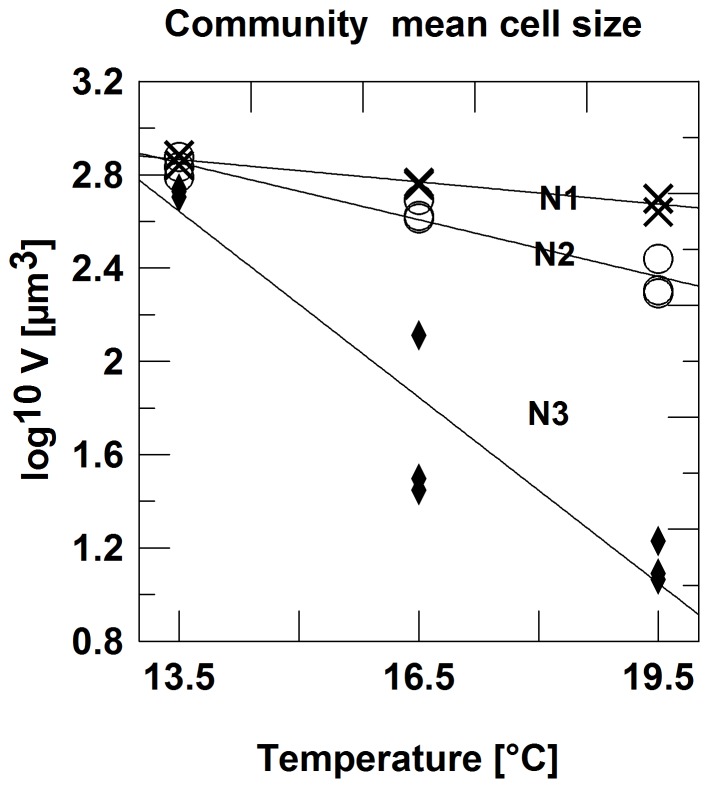
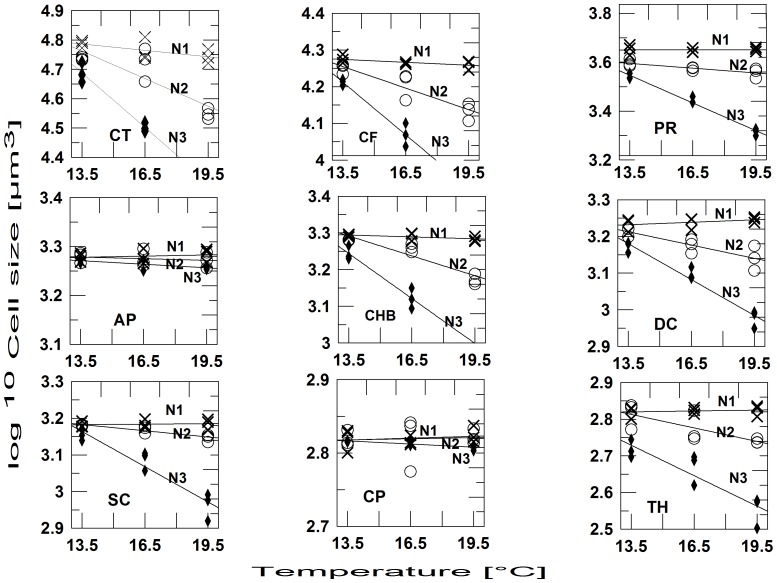
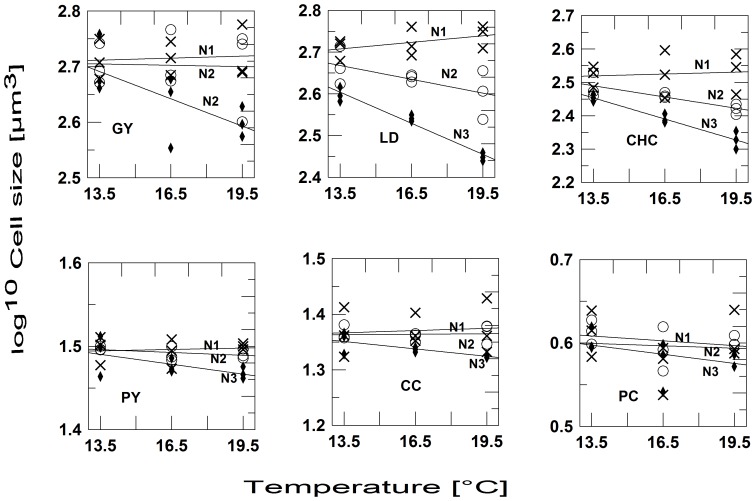
In total, we could distinguish and count 15 species. Other protists, including heterotrophic ones were too rare to be counted. The community mean cell volume (Fig. 2) and the cell volumes of the majority of the individual species (Fig. 3, Fig. 4) showed a significant negative effect of nutrient stress (13 of 15 spp.), a negative temperature effect (10 of 15 spp.) and an interaction effect (9 of 15 spp.) (Table 1). Individual regression analyses for different levels of nutrient stress showed, that the slopes of the temperature-size relationship became more negative at more stringent nutrient stress (Table 2). At the lowest nutrient stress level, no species showed a significant response to temperature. Comparing the response of Vc to the responses of Vi, it is also obvious that compositional changes, i.e. dominance shifts between differently sized species, by far outweigh intraspecific size shifts. The slopes of the Vc-temperature regression roughly conform to ca. 4.7% size reduction per °C at N1, 17.2% at N2, and 46% at N3, respectively. The most responsive individual species, the dinoflagellate Ceratium tripos, decreased by 1.7% per °C at N1 (insignificant), 6.8% at N2, and 13.3% at N3, respectively.
Figure 2. Phytoplankton community mean cell size decreases with temperature and increases with dilution rate.
Community mean cell volume in µm3 (log10-scale) in response to temperature and nutrient regime; N1: 50% dilution three times per week; N2: 25% dilution three times per week; N3: no dilution.
Figure 3. Cell sizes of phytoplankton taxa decrease with temperature and increase with dilution rate.
Cell volume in µm3 (log10-scale) in response to temperature and nutrient regime; N1: 50% dilution three times per week; N2: 25% dilution three times per week; N3: no dilution. Species codes: CT: Ceratium tripos; CF: Ceratium fusus; PR: Prorocentrum micans; AP: Amphidinium sp.; CHB: Chaetoceros brevis; DC: Dictyocha speculum; SC: Scrippsiella trochoidea; CP: Cerataulina pelagica; TH: Thalassionema nitzschioides.
Figure 4. Cell sizes of phytoplankton taxa decrease with temperature and increase with dilution rate.
(continued). Cell volume in µm3 (log10-scale) in response to temperature and nutrient regime; N1: 50% dilution three times per week; N2: 25% dilution three times per week; N3: no dilution. Species codes: GY: Gymnodinium sp.; LD: Leptocylindrus danicus; CHC: Chaetoceros curvisetus; PY: Pyramimonas sp.; CC: Cylindrotheca closterium; PC: picophytoplankton.
Table 1. ANOVA of temperature and nutrient effects.
| Species | Higher taxon | Ptemp | Pnutr | Pint | R2 |
| Community mean cell size | <0.001 | <0.001 | <0.001 | 0.92 | |
| Ceratium tripos | Dinophyta | <0.001 | <0.001 | <0.001 | 0.89 |
| Ceratium fusus | Dinophyta | <0.001 | <0.001 | <0.001 | 0.88 |
| Prorocentrum micans | Dinophyta | <0.001 | <0.001 | <0.001 | 0.92 |
| Amphidinium sp. | Dinophyta | 0.65 | 0.03 | 0.74 | 0.37 |
| Chaetoceros brevis | Bacillariophyceae | <0.001 | <0.001 | <0.001 | 0.94 |
| Dictyocha speculum | Dictychophyceae | <0.001 | <0.001 | <0.001 | 0.89 |
| Scrippsiella trochoidea | Dinophyta | <0.001 | <0.001 | 0.002 | 0.79 |
| Thalassionema nitzschioides | Bacillariophyceae | <0.001 | <0.001 | 0.001 | 0.85 |
| Cerataulina pelagica | Bacillariophyceae | 0.93 | 0.49 | 0.92 | 0.12 |
| Gymnodinium sp. | Dinophyta | 0.42 | 0.01 | 0.4 | 0.24 |
| Leptocylindrus danicus | Dinophyta | 0.002 | <0.001 | 0.002 | 0.79 |
| Chaetoceros curvisetus | Bacillariophyceae | 0.01 | <0.001 | 0.07 | 0.73 |
| Pyramimonas sp. | Prasinophycea | 0.24 | 0.02 | 0.5 | 0.48 |
| Cylindrotheca closterium | Bacillariophycea | 0.8 | 0.03 | 0.8 | 0.35 |
| Picophytoplankton | diverse taxa | 0.04 | 0.3 | 0.7 | 0.15 |
Two-factor ANOVA of temperature and nutrient level effects on log10 cell volume (µm3) of the entire phytoplankton community and of the individual species arranged by size from the largest to the smallest; N = 27, except for Ceratium tripos, Ceratium fusus, and Chaetoceros brevis which disappeared from the N3 – 19.5°C treatment combination (N = 24).
Table 2. Regression analysis of temperature-size relationships.
| Species | Nutrient level | a | b | P | R2 |
| Community mean cell size | N1 | −0.0207 | 3.0903 | 0.0009 | 0.88 |
| N2 | −0.0820 | 3.9612 | 0.0003 | 0.90 | |
| N3 | −0.2661 | 6.2376 | 0.00008 | 0.92 | |
| Ceratium tripos | N1 | −0.0073 | 4.8846 | 0.075 | 0.38 |
| N2 | −0.0306 | 5.184 | 00038 | 0.72 | |
| N3 | −0.0619 | 5.5233 | 0.001 | 0.94 | |
| Ceratium fusus | N1 | −0.0026 | 4.3100 | 0.08 | 0.35 |
| N2 | −0.0196 | 4.5197 | 0.0008 | 0.81 | |
| N3 | −0.0479 | 4.8597 | 0.0016 | 0.93 | |
| Prorocentrum micans | N1 | +0.0001 | 3.6486 | 0.950 | 0.0006 |
| N2 | −0.0065 | 3.6831 | 0.012 | 0.61 | |
| N3 | −0.0377 | 4.0556 | 0.00003 | 0.96 | |
| Amphidinium sp. | N1 | +0.0008 | 3.2672 | 0.6478 | 0.03 |
| N2 | −0.0011 | 3.2941 | 0.5736 | 0.05 | |
| N3 | −0.1124 | 3.3042 | 0.04561 | 0.46 | |
| Chaetoceros brevis | N1 | +0.0017 | 3.3174 | 0.1539 | 0.26 |
| N2 | −0.0180 | 3.5354 | 0.0003 | 0.86 | |
| N3 | −0.0408 | 3.7946 | 0.0033 | 0.90 | |
| Dicytocha speculum | N1 | +0.0022 | 3.218 | 0.2799 | 0.16 |
| N2 | −0.0121 | 3.3756 | 0.00470 | 0.70 | |
| N3 | −0.0326 | 3.6202 | 0.00001 | 0.93 | |
| Scrippsiella trochoidea | N1 | +0.0004 | 3.1765 | 0.78550 | 0.01 |
| N2 | −0.0056 | 3.2590 | 0.0019 | 0.76 | |
| N3 | −0.0320 | 3.5964 | 0.0001 | 0.90 | |
| Thalassionema nitzschioides | N1 | +0.0008 | 2.809 | 0.6558 | 0.03 |
| N2 | −0.0123 | 2.979 | 0.0195 | 0.56 | |
| N3 | −0.0277 | 3.103 | 0.0012 | 0.80 | |
| Cerataulina pelagica | N1 | +0.0008 | 2.8065 | 0.6178 | 0.04 |
| N2 | +0.0005 | 2.8110 | 0.8644 | 0.005 | |
| N3 | −0.0014 | 2.8358 | 0.0113 | 0.62 | |
| Gymnodinium sp. | N1 | +0.0012 | 2.6954 | 0.8142 | 0.09 |
| N2 | −0.0008 | 2.7155 | 0.9204 | 0.04 | |
| N3 | −0.0164 | 2.9123 | 0.00439 | 0.68 | |
| Leptocylindrus danicus | N1 | +0.0053 | 2.6367 | 0.20 | 0.22 |
| N2 | −0.0111 | 2.8173 | 0.08 | 0.37 | |
| N3 | −0.0249 | 2.9397 | 0.00006 | 0.94 | |
| Chaetoceros curvisetus | N1 | +0.0019 | 2.4940 | 0.8022 | 0.009 |
| N2 | −0.0112 | 2.6416 | 0.0077 | 0.66 | |
| N3 | −0.0211 | 2.7378 | 0.0004 | 0.92 | |
| Pyramimons sp. | N1 | +0.005 | 1.4871 | 0.779 | 0.009 |
| N2 | −0.0011 | 1.5115 | 0.2010 | 0.23 | |
| N3 | −0.0040 | 1.5436 | 0.05015 | 0.45 | |
| Cylindrotheca closterium | N1 | +0.0013 | 1.3485 | 0.8052 | 0.009 |
| N2 | +0.0003 | 1.3598 | 0.8929 | 0.003 | |
| N3 | −0.0044 | 1.4106 | 0.0239 | 0.54 | |
| Picophytoplankton | N1 | −0.0010 | 0.6125 | 0.8400 | 0.006 |
| N2 | −0.0019 | 0.6344 | 0.4637 | 0.008 | |
| N3 | −0.0037 | 0.6467 | 0.2290 | 0.19 |
Regression (Model: y = ax+b) of log10 cell volume (µm3) on temperature (°C) for the different species and nutrient levels; N = 9, except for Ceratium tripos, Ceratium fusus, and Chaetoceros brevis which disappeared from the N3 – 19.5°C treatment combination (N = 6).
Discussion
While the dominance of the nutrient effect in mediating the temperature-size effect is obvious, a remaining nutrient-independent role of temperature cannot be assessed from a direct comparison of the different treatments, because temperature itself influenced the strength of nutrient stress, as can be seen from the response of the C∶N ratios to temperature. However, if the C∶N ratio is taken as indicative of the intensity of nutrient stress [21], a nutrient-independent effect can be assessed by a multiple regression with temperature and C∶N-ratios as independent variables (Table 3). The dominance of the nutrient effect is obvious, both from the number of significant cases and from the partial correlation coefficients. The mean nutrient-independent temperature regression slope was −0.0059±0.0028 (95% CL). The slope for the community level effect indicates a 19.3% size reduction, while the most temperature sensitive species, Ceratium tripos, showed a 4.1% size reduction per °C. The mean value of species specific size reduction was 1.36% (SD = 1.16; Shapiro-Wilks test for normality: p = 0.24), while several species did not show any nutrient-independent temperature response. Overall, the range of variation overlaps with the results obtained from clonal cultures of a wide array of protists [5].
Table 3. Multiple Regression of cell volume on temperature and C∶N ratios.
| Species | a | b | %°C−1 | c | Pt | Rt | PCN | RCN | Pmodel | R2 |
| Community mean cell size | 6.55 | −0.093 | −19.3 | −2.22 | 0.0011 | −0.40 | <0.0001 | −0.69 | <0.0001 | 0.71 |
| Ceratium tripos | 5.55 | −0.018 | −4.1 | −0.49 | 0.0019 | −0.35 | <0.0001 | −0.55 | <0.0001 | 0.66 |
| Ceratium fusus | 4.8 | −0.011 | −2.5 | −0.36 | 0.005 | −0.34 | <0.0001 | −0.54 | <0.001 | 0.66 |
| Prorocentrum micans | 4.28 | −0.008 | −1.8 | −0.51 | 0.045 | −0.18 | <0.0001 | −0.86 | <0.001 | 0.81 |
| Amphidinium sp. | 3.33 | −0.001 | −0.23 | −0.04 | 0.67 | −0.07 | 0.0046 | −0.54 | 0.012 | 0.66 |
| Chaetoceros brevis | 3.76 | −0.01 | −2.3 | −0.31 | 0.0038 | −0.35 | <0.0001 | −0.54 | <0.0001 | 0.68 |
| Dictyocha sp. | 3.75 | −0.009 | −2.1 | −0.36 | 0.011 | −0.28 | <0.0001 | −0.78 | <0.0001 | 0.75 |
| Scrippsiella trochoidea | 3.62 | −0.009 | −2.1 | −0.29 | 0.017 | −0.30 | <0.0001 | −0.72 | <0.0001 | 0.66 |
| Thassionema nitzschioides | 3.38 | −0.007 | −1.6 | −0.44 | 0.024 | −0.20 | <0.0001 | −0.86 | <0.0001 | 0.83 |
| Cerataulina pelagica | 2.83 | 0.0002 | +0.05 | −0.017 | 0.85 | +0.38 | 0.24 | −0.23 | 0.54 | 0 |
| Gymnodinium sp. | 2.97 | −0.003 | −0.7 | −0.20 | 0.49 | −0.11 | 0.0006 | −0.64 | 0.0014 | 0.37 |
| Leptocylindrus danicus | 3.23 | −0.004 | −0.92 | −0.45 | 0.23 | −0.11 | <0.0001 | −0.87 | <0.0001 | 0.79 |
| Chaetoceros curvisetus | 2.93 | −0.006 | −1.4 | −0.32 | 0.071 | −0.20 | <0.0001 | −0.80 | <0.0001 | 0.71 |
| Pyramimonas sp. | 1.56 | −0.001 | −0.23 | −0.047 | 0.26 | −0.17 | 0.0029 | −0.50 | 0.0038 | 0.32 |
| Cylindrotheca closterium | 1.45 | 0.0002 | +0.05 | −0.085 | 0.92 | +0.02 | 0.003 | −0.57 | 0.0102 | 0.26 |
| Pico-phytoplankton | 0.65 | 0.002 | −0.46 | −0.017 | 0.33 | −0.20 | 0.52 | −0.13 | 0.43 | 0 |
Regression according to the model log10 V = a+b.t+c.log10(C∶N), where t is expressed in °C and V in µm3C; partial correlation coefficients (Rt, RCN), partial probabilities of error (Pt, PCN), R2 for the full model, and probability of error for the full model (Pmodel); N = 9, except for Ceratium tripos, Ceratium fusus, and Chaetoceros brevis which disappeared from the N3 – 19.5°C treatment combination (N = 6). The temperature effect is also shown as % volume reduction per °C.
An alternative explanation for the observed temperature effect could lie in the dilution effect on protistan grazers (microzooplankton) which are more strongly diluted at higher dilution rates. Since microzooplankton in general prefer smaller prey and thereby benefit the larger prey both inter- and intraspecifically. Therefore, the grazing and the nutrient effect on cell sized should have the same sign, i.e. smaller sized at lower dilution rates. However, there are good reasons to consider the contribution of the microzooplankton effect as relatively unimportant:
Microzooplankton densities were too low to count them in our phytoplankton samples, as opposed to at least 100 phytoplankton cells counted per species and sample. Therefore, grazing rates must have has little influence on the outcome of our experiment.
In our experiment, the nutrient effect on the cell size was generally stronger for the larger species which are outside the feeding spectrum of most microzooplankton species.
If the effect of microzooplankton grazing dominates the size reponse of phytoplankton, higher grazing rates at warmer temperature should lead to a positive temperature effect on cell size. This hypothesis was tested in a previous study [12] and rejected. Even under protist grazing, warming led to a shrinkage of cell size, although not as strongly as under copepod grazing.
Direct temperature effects, nutrient effects, and grazing effects as explanations for temperature dependent size trends are not mutually exclusive. However, our results strongly indicate that the direct temperature effect is much weaker than the nutrient effect. This was found both at the intra- and the interspecific level. The community effect was much stronger than the intraspecific effect, but this is no surprise, because the scope for interspecific size difference by far exceeds the scope for intraspecific ones: Size differences between species span about 9 orders of magnitude while intraspecific size changes are almost always <1 order of magnitude on a volumetric base [26]. We cannot exclude additional mechanisms such as enhanced grazing under higher temperatures. However, the effect of grazing would be less consistent, because different guild of grazer affect different parts of the phytoplankton size spectrum [27], e.g. protozoan grazer would rather suppress phytoplankton <5 or 10 µm, while copepods would rather suppress larger ones, i.e. temperature effects mediated by grazing should depend on the dominance of different grazer guilds. In a previous study [12] we found the expected stronger negative temperature effect on phytoplankton under copepod grazing, while we did not find the reversal of sign under protist grazing. This means, that a grazing independent negative temperature effect on phytoplankton must have outweighed the supposed positive effect of protist grazers. Then, we could only speculate about the possible importance of nutrient limitation, while now we have provided direct evidence for it.
Funding Statement
The authors acknowledge support by DAAD (Deutscher Akademischer Austauschdienst = German Academic Exchange Service) and Tanzania Ministry of Education and Vocational Training which supported this research by a PhD-fellowship for Kalista H. Peter. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Daufresne M, Lengefellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci USA 106: 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardner JL, Peters A, Kaarney MR, Joseph L, Heinson R (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26: 285–291. [DOI] [PubMed] [Google Scholar]
- 3. Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse [About the relationship of the thermal economy of animals to their body size]. Göttinger Studien 3: 538–545. [Google Scholar]
- 4. James FC (1970) Geographic size variation in birds and its relationship to climate. Ecology 51: 365–390. [Google Scholar]
- 5. Atkinson D, Ciotti B, Montagnes DJS (2003) Protists decrease in size linearly with temperature: ca. 2.5% C−1 . Proc Royal Soc London Ser B: Biol Sci 270: 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen AP, Brown JH, Gillooly JF (2002) Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297: 1545–1548. [DOI] [PubMed] [Google Scholar]
- 7. Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85: 1771–1789. [Google Scholar]
- 8. Sommer U, Lengfellner K (2008) Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob Change Biol 14: 1199–1208. [Google Scholar]
- 9. Sommer U, Aberle N, Lengfellner K, Lewandowska A (2012) The Baltic Sea spring phytoplankton bloom in a changing climate: an experimental approach. Mar Biol 159: 1–12. [Google Scholar]
- 10. Yvon-Durocher G, Montoya JM, Trimmer M, Woodward G (2011) Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob Change Biol 17: 1681–1694. [Google Scholar]
- 11. Sommer U, Lewandowska A (2010) Climate change and the phytoplankton spring bloom: warming and overwintering zooplankton have similar effects on phytoplankton. Glob Change Biol 17: 154–162. [Google Scholar]
- 12. Peter KH, Sommer U (2012) Phytoplankton cell size, inter- and intraspecific effects of warming and grazing. PLoS One 7: e49632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Connor MI (2009) Warming strengthens an herbivore–plant interaction. Ecology 90: 388–398 (2009). [DOI] [PubMed] [Google Scholar]
- 14. O'Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF (2009) Warming and resource availability shift food web structure and metabolism. PLoS Biology 7: e1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chisholm SW (1992) Phytoplankton size. In Primary productivity and biogeochemical cycles in the sea, eds Falkowski GP, Woodhead AD (Plenum), pp 213–237.
- 16. Kiørboe T (1993) Turbulence, phytoplankton cell size, and the structure of pelagic food webs. Adv Mar Biol 29: 1–72. [Google Scholar]
- 17. Raven J (1998) The twelfth Tansley Lecture. Small is beautiful: the picophytoplankton. Funct Ecol 12: 503–513. [Google Scholar]
- 18. Maranón E, Cermeno P, Latasa M, Tadonléké RD (2012) Temperature, resources, and phytoplankton size structure in the ocean. Limno. Oceanogr 67: 1266–1278. [Google Scholar]
- 19. Kamykowski D, Zentara SJ (1986) Predicting plant nutrient concentrations from temperature and sigma-t in the upper kilometer of the world ocean. Deep Sea Res A 33: 89–105. [Google Scholar]
- 20. Droop MR (1973) Some thoughts on nutrient limitation in algae. J Phycol 9: 264–272. [Google Scholar]
- 21. Goldman JC, McCarthy JJ, Peavey DG (1979) Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279: 210–15. [Google Scholar]
- 22. Sharp JH (1974) Improved analysis for particulate orgainic carbon and nitrogen from seawater. Limnol Oceanogr 19: 984–989. [Google Scholar]
- 23.Hansen HP, Koroleff F (2007) Determination of nutrients. In Methods of Seawater Analysis, Third Edition eds Grasshoff K, Kremling K,Ehrhard (Wiley – VCH Verlag) pp 159–228.
- 24. Utermöhl H (1958) Zur Vervollkommung der quantitativen phytoplakton Methodik. Mitt Int Ver Theor Angew Limnol 9: 263–272. [Google Scholar]
- 25. Hillebrand H, Kirschtel D, Dürselen C, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35: 402–424. [Google Scholar]
- 26.Reynolds CS (1984) The ecology of freshwater phytoplankton (Cambridge Univ Press)
- 27. Sommer U, Hansen T, Blum O, Holzner N, Vadstein O, et al. (2005) Copepod and microzooplankton grazing n mesocosms fertilised with different Si∶N ratios: no overlap between food spectra and Si∶N-influence on zooplankton trophic level. Oecologia 142: 274–283. [DOI] [PubMed] [Google Scholar]