Abstract
Background
Nigeria has a significant burden of lymphatic filariasis (LF) caused by the parasite Wuchereria bancrofti. A major concern to the expansion of the LF elimination programme is the risk of serious adverse events (SAEs) associated with the use of ivermectin in areas co-endemic with Loa filariasis. To better understand this, as well as other factors that may impact on LF elimination, we used Micro-stratification Overlap Mapping (MOM) to highlight the distribution and potential impact of multiple disease interventions that geographically coincide in LF endemic areas and which will impact on LF and vice versa.
Methodology/Principal findings
LF data from the literature and Federal Ministry of Health (FMoH) were collated into a database. LF prevalence distributions; predicted prevalence of loiasis; ongoing onchocerciasis community-directed treatment with ivermectin (CDTi); and long-lasting insecticidal mosquito net (LLIN) distributions for malaria were incorporated into overlay maps using geographical information system (GIS) software. LF was prevalent across most regions of the country. The mean prevalence determined by circulating filarial antigen (CFA) was 14.0% (n = 134 locations), and by microfilaria (Mf) was 8.2% (n = 162 locations). Overall, LF endemic areas geographically coincided with CDTi priority areas, however, LLIN coverage was generally low (<50%) in areas where LF prevalence was high or co-endemic with L. loa.
Conclusions/Significance
The extensive database and series of maps produced in this study provide an important overview for the LF Programme and will assist to maximize existing interventions, ensuring cost effective use of resources as the programme scales up. Such information is a prerequisite for the LF programme, and will allow for other factors to be included into planning, as well as monitoring and evaluation activities given the broad spectrum impact of the drugs used.
Author Summary
Nigeria is estimated to have the highest burden of lymphatic filariasis (LF), a disease also known as elephantiasis, which is transmitted by mosquitoes and caused by the parasite Wuchereria bancrofti. The National LF Elimination Programme is planning to scale up the elimination programme through mass drug administration of ivermectin and albendazole. However, a major constraint to this expansion is the risk of serious adverse events (SAEs) associated with the use of ivermectin in areas co-endemic with Loa, the causative agent of tropical eye worm (loiasis). To better understand this and other factors that may impact on LF elimination, we collated and mapped all available LF data, and highlighted the overlaps with predicted loiasis prevalence distributions, onchocerciasis ivermectin treatment areas, and bed net distributions for malaria. This study provides a baseline overview for the LF Programme and will help to maximize existing disease interventions, ensuring cost effective use of resources as the programme scales up.
Introduction
Lymphatic filariasis (LF) is one of the most debilitating neglected tropical diseases (NTD) in the world [1]. It is caused by the parasitic worms Wuchereria bancrofti, Brugia malayi and B. timori and is transmitted by Anopheles, Culex, Aedes, Ochlerotatus and Mansoni mosquitoes [1]. Wuchereria bancrofti is transmitted throughout the tropics in Africa, Asia, the Pacific and the Americas while B. malayi and B. timori are found in east and south Asia. The disease is endemic in 73 countries with an estimated 120 million people infected and 40 million people with clinical manifestations including lymphoedema (elephantiasis) of the limbs and urogenital disorders, especially hydrocele in men [2] [3]. In Africa, 34 countries are endemic, and Nigeria is believed to bear the highest burden of LF, with an estimated 80 to 120 million people at risk [3]–[5].
The Global Programme to Eliminate LF (GPELF) was launched in 2000 with the goal of eliminating LF as a public health problem by 2020 [1]. The principal elimination strategy is to interrupt transmission using Mass Drug Administration (MDA) with the combinations of albendazole plus ivermectin or albendazole plus diethylcarbamazine (DEC) administered once a year for at least five consecutive years. [1]–[3]. Overall, significant progress has been made, however, the scale up of programmatic activities has been slow in Africa, especially in countries with logistical challenges, conflict, instability and fragile infrastructures [6]. The wide and overlapping distribution of the filarial parasite Loa in Africa [7] is also a major impediment due to the risk of severe adverse events (SAEs) in co-infected individuals when treated with ivermectin [8] [9].
These constraints pose significant problems for the national LF programmes and GPELF with the potential to severely hinder the 2020 goal of LF elimination globally. To begin to address these complexities, a number of specific objectives and strategies have been developed. First, the GPELF strategic plan aims to achieve full geographical coverage with MDA by 2016, targeting the countries with the highest burden, including Nigeria [1]. Second, the use of integrated vector management (IVM) [10] is advocated in malaria co-endemic areas where both diseases are transmitted by Anopheles mosquitoes [11]. Finally, a provisional strategy for interrupting LF transmission in loiasis endemic countries recently developed recommends albendazole (400 mg) twice yearly in combination with vector control in all co-endemic areas [12]. Finally, mapping LF and L. loa at the lowest possible administrative unit is also considered important to identify small areas that can be treated for LF using the most appropriate regimes to reduce the risk of SAEs, which is considered to be highest when L. loa microfilaremia (mf) prevalence is ≥20%.
The coordinated effort of global disease control programmes is becoming increasingly important as many operate in the same countries and distribute interventions that have multiple benefits [13]–[16]. GPELF is likely to benefit from the activities of the Global Malaria Programme, including the recent scale up of insecticide treated/long-lasting insecticidal mosquito nets (ITNs/LLINs) and indoor residual spraying (IRS) [11]. These interventions have also been shown to impact LF transmission in a range of ecological settings [17], thus more synergy between the programmes in Africa could optimize resources and increase the impact on both diseases [15]–[18]. In countries such as Nigeria where malaria and LF are co-endemic and both transmitted by Anopheles mosquitoes [19] [20], the use of ITNs has shown to be effective at reducing LF transmission in L. loa co-endemic areas [21]. ITNs have also been successfully integrated with MDA activities in Central Nigeria with report of an increase in ITN ownership and retention [22] [23]. However, to take advantage of these programmatic links, more data on LF vectors is critical as there are many gaps in our knowledge as highlighted in the Anopheles database recently compiled for Nigeria [19].
Integrating activities and combining resources across the various NTD programmes will also have many advantages [13] [24]. For example, the African Programme for Onchocerciasis Control (APOC) has developed a sustainable community-directed treatment with ivermectin (CDTi) for the parasitic disease caused by the filarial worm Onchocerca volvulus, [25]–[27]. The CDTi approach has been successful in reaching millions of people across high transmission areas of onchocerciasis in Africa, and has also been used to distribute other health interventions including LF treatment and bed nets for malaria control [26]. Moreover, the maps of CDTi priority areas highlight the potential geographical overlap of onchocerciasis with LF, and it is likely that the wide and frequent use of ivermectin has reduced transmission in co-endemic areas [28]–[31]. However, the extent of this impact is yet to be determined at a large scale and needs to be quantified so that benefits from this and future NTD control programmes can be better understood and fully exploited [5] [32]–[34].
These issues are particularly relevant for Nigeria, given the large population at risk of W. bancrofti infection [3]–[5]. The National Lymphatic Filariasis Elimination Programme (NLFEP) is yet to complete LF mapping [3] [35] and will need significant financial and technical support to scale up MDA activities across this large, populous country. The aim of this paper, therefore, is to use the Micro-stratification Overlap Mapping (MOM) approach [24] to review and synthesize the current knowledge of the distribution of W. bancrofti in Nigeria, and factors that will impact on the control and elimination of LF such as loiasis co-endemicity, onchocerciasis control programmes, and malaria bed net distributions. This information is a prerequisite for effective planning and will help to optimize the future LF MDA implementation strategy to ensure safety, maximum cost effectiveness as well as impact.
Methods
Study location
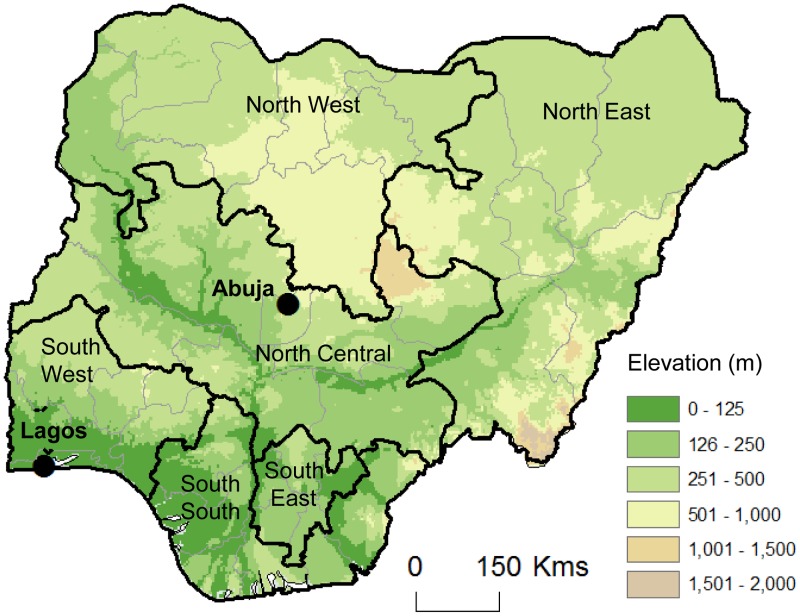
Nigeria is a Federal Republic comprising 36 States and its Federal Capital Territory, Abuja [35] [36]. The states are grouped into six geopolitical zones, the North Central (NC), North East (NE), North West (NW), South West (SW), South East (SE) and South (SS). Nigeria covers an area of approximately 923,768 sq. km, and has a large low plateau intersected by two major rivers, the Niger and Benue, in the central region of the country (Figure 1). It shares borders with Benin in the west, Chad and Cameroon in the east, and Niger in the north. Its coast in the south lies on the Gulf of Guinea on the Atlantic Ocean and Lagos, the former capital, is an important port city. Nigeria is Africa's most populous country with the total population estimated to be 160 million in 2012, with approximately 50% living in urban areas.
Figure 1. Map of Nigeria and its geopolitical zones.
North Central - Benue, FCT, Kogi, Kwara, Nasarawa, Niger, Plateau. North East - Adamawa, Bauchi, Borno, Gombe, Taraba, Yobe. North West - Kaduna, Katsina, Kano, Kebbi, Sokoto, Jigawa,, Zamfara. South East - Abia, Anambra, Ebonyi, Enugu, Imo. South - Akwa-Ibom, Bayelsa, Cross-River, Delta, Edo, Rivers. South West - Ekiti, Lagos, Osun, Ondo, Ogun, Oyo. Note: Elevation data based on ETOPO2 global 2-minute gridded resolution from National Oceanic and Atmospheric Administration (NOAA) available from ESRI Redland, CA.
LF prevalence data
To review and synthesize the current knowledge of the human distribution of LF in Nigeria, a systematic search for data in peer-reviewed published literature and national reports was carried out. The search was conducted using PubMed, JSTOR, Google, SCOPUS and other online scientific and historical databases. References were also obtained from the references listed within articles, and then from the references within those articles.
Studies and reports with data on the prevalence of i) LF infection as circulating filarial antigen (CFA) from using immunochromatographic tests (ICTs), antibodies by ELISAs, and microfilaria (Mf) from blood slides, and ii) disease cases (hydrocele, lymphodema) [2] [37] were identified and collated into a database. Information on the location/collection site (village, local government area (LGA) and State), and time period (month, year), was also collected for mapping and descriptive analyses. Specific information on whether MDA for LF had been administered prior to the LF prevalence measure was recorded and considered in the analysis. The range of methods used to detect LF in the different studies was recorded, as well as information on the mosquito species, which was cross-checked with the Nigerian Anopheles database [19].
The locations of the community or collection site were geo-referenced using the latitude and longitude coordinates obtained from references directly or by cross-checking the names with data from the GEOnet Names Server, Directory of Cities and Towns in the World databases [38] [39]. The coordinates of the midpoint of the LGA was used as a proxy for the locations that could not be allocated exact latitude and longitude coordinates. This is considered to be a limitation of the review and restricts any accurate detailed mapping. It is also acknowledged that LF prevalence distribution has a degree of bias as the data are based on the locations selected by the investigators in the original study, and does not take sampling methodologies between studies into account, which may affect the outcome.
In addition, selected data from the Federal Ministry of Health (FMoH) collected during LF mapping activities were collated and included in the database. The LF data available for this study were based on Mf prevalence rates collected in selected LGA sentinel sites during baseline surveys in 31 LGAs across 18 States of Nigeria. The WHO standard protocol was used to collect blood samples at night and examined for the presence of Mf. The coordinates of the midpoint of each LGA was used to map the LF prevalence. A national LF endemicity map by LGA was also available from the FMoH, which provided an overall CFA prevalence based on ICT survey in each State carried out between 2000 and 2010. Specific LGA data is not publicly available and not included in this database, however, the State-level information on the number of LGAs surveyed, prevalence range and year of survey is available in the recently published Master Plan for NTDs [35].
All the relevant information was entered into an Excel worksheet and data analysis was performed using Stata software (version 12, StataCorp, Texas, USA). All data were mapped using the geographical information systems (GIS) software ArcGIS 10.0 (ESRI, Redlands, CA) to produce maps of LF prevalence distributions, and to examine the geographical overlaps with loiasis-endemic areas, and the different intervention distributions.
Loiasis co-endemicity
To examine the potential extent of LF and L. loa co-endemicity, the recent map of the predicted loiasis prevalence produced from a Rapid Assessment Procedure of Loiasis (RAPLOA) based on eye worm history carried out between 2004 and 2010 across Africa, including Nigeria [7], was imported into ArcGIS. Three levels of predicted loiasis prevalence were digitised (i.e. outlined, shaded) based on the defined distribution boundaries, which included low <20%, medium 20–40% and high >40% prevalence areas; the latter is equivalent to mf prevalence of >20%. The different levels of loiasis prevalence and the overlap with LF prevalence distributions were highlighted to help identify potential low risk (i.e. loiasis <20%) and medium to high risk (i.e loiasis >20%) SAE areas.
Interventions overlap maps
Onchocerciasis control programmes
To examine the distribution of ivermectin and its association with LF and loiasis endemicity, the CDTi map produced from REMO surveys carried out in 2004 and 2005 [31] was imported into ArcGIS. The CDTi priority (i.e. ivermectin treatment) areas were digitized, and the overlap with LF and loiasis distributions highlighted. It is acknowledged that the CDTI priority areas do not identify the specific location, frequency and duration of ivermectin distribution, but provide a broader overview of potential treatment areas. Detailed data on treatment areas are not publicly available.
Malaria bed net distributions
To examine the potential impact of vector control in co-endemic areas, data on LLIN coverage based on the percentage of households with at least one LLIN for each geopolitical zone was obtained from the Malaria Indicator Survey (MIS) carried out in 2010. The LLIN coverage rates were based on data collected from 7200 households in 12 states across all geopolitical zones [40]. Coverage rates by each zone were mapped based on three levels, which included <25%, 25–50% >50%. The different levels of LLIN coverage and overlap with LF and L. loa co-endemic areas were highlighted to help identify areas that could potentially benefit from this intervention.
Results
LF data summary
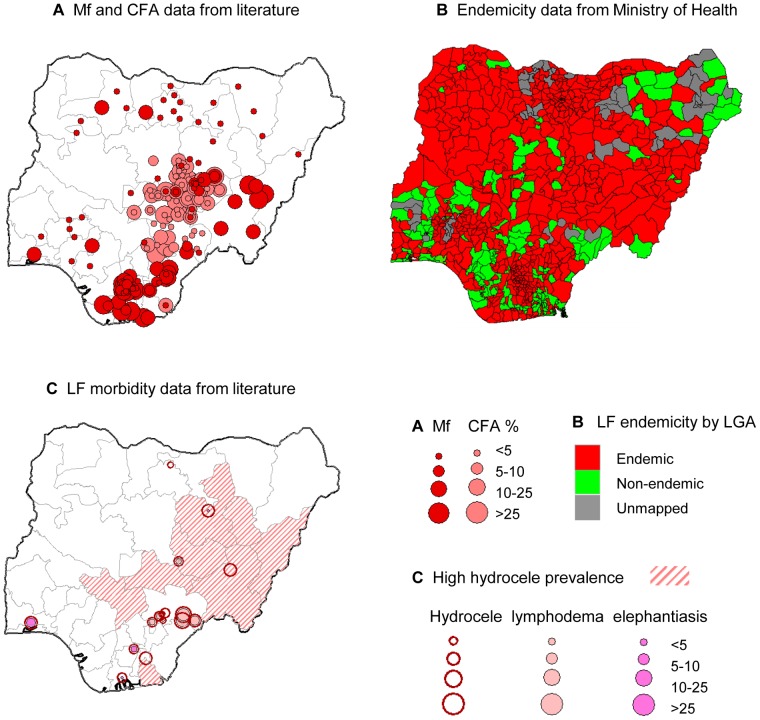
In total, 41 studies [41]–[81] from 68 published and unpublished filariasis studies identified in the literature were found to have examined the prevalence, clinical manifestations and entomological aspects of LF in Nigeria (Table S1). The studies excluded from the review had reported data on L. loa and/onchocerciasis only. The majority of LF studies (n = 30) were conducted post 2000 [41]–[69], nine studies were conducted between 1980 and 2000 [70]–[78], and three studies were conducted pre 1980 [79]–[81]. The studies indicate that LF is present in 19 States across all six geopolitical zones of the country (Figure 2a). The majority of studies were from the NC geopolitical zone, with the most comprehensive studies carried out in Plateau and Nassarawa States [49] [53] [54] [63] [66].
Figure 2. LF prevalence data, endemicity status and disease data.
a. CFA and Mf data. b. LF endemicity. c. Disease data. Note: Data source for CFA, MF prevalence (2a) and disease (2c) data available in Table S1. LF endemicity map (2c) developed by FMoH.
The FMoH sentinel site Mf prevalence data were carried out more widely in 31 LGAs across 18 States. All information was added to the database (Table S1) and mapped with the other specific Mf data described above (Figure 2a). The FMoH national endemicity map indicated that out of the 774 LGAs in Nigeria, 541 were classified as endemic, 164 were classified as non-endemic and 69 remained to be mapped (Figure 2b). The related state-level data are found in Nigeria Master Plan for NTDs [35].
The range of methods used to detect the presence of LF in Nigeria included serological methods (using ICTs or ELISA), parasitological methods (blood films for Mf) and physical examination for clinical manifestations (lymphodema, hydrocele), and were used either alone or in combination. Studies carried out before the 1980s only used parasitological examination of blood films, whereas post 2000, a combination of serology and parasitological methods were most widely used (Table S1).
LF prevalence distribution
In total there were 258 individual data points from 152 unique locations where the prevalence of W. bancrofti was measured by CFA or Mf. The average CFA and Mf rates by State are summarized in Table 1 and 2. The overall mean CFA prevalence rate across the country was 14.0% (n = 134; range 0% to 66.0%), and the overall mean Mf prevalence rate was 8.2% (n = 162; 0 to 47.4%). The CFA and Mf prevalence rates by geopolitical zones indicate that the highest rates occur in the NC, NE, NW and SS zones. The highest CFA prevalence rate recorded was 66% recorded at Ogi-Utonkon, Ado LGA, Benue State, NC [64], while the highest Mf rate was 47.4% recorded at Zing LGA, Taraba State, NE [55] (Table S1).
Table 1. Summary of CFA prevalence by state.
| Zone and State | No. of sites | No. of persons tested | Mean (%) | 95% CI (lower) | 95% CI (Upper) |
| North Central | |||||
| Benue | 23 | 2189 | 12.9 | 11.5 | 14.4 |
| Nassarawa | 46 | 19026 | 11.4 | 11.0 | 11.9 |
| Plateau | 61 | 27325 | 16.5 | 16.1 | 16.9 |
| Plateau, Nassarawa | 1 | 4120 | 22.5 | 21.2 | 23.8 |
| North West | |||||
| Kaduna | 1 | 341 | 10.0 | 7.0 | 13.7 |
| South | |||||
| Bayelsa | 1 | 1803 | 11.3 | 9.9 | 12.9 |
| Cross River | 1 | 222 | 17.0 | 12.4 | 2.7 |
| Total | 134 | 55026 | 14.0 | 13.7 | 14.3 |
Note: All data included i.e. number of MDA rounds not taken into account.
Table 2. Summary of Mf prevalence by state.
| Zone and State | No. of sites | No. of persons tested | Mean (%) | 95% CI (lower) | 95% CI (Upper) |
| North Central | |||||
| Benue | 3 | 1903 | 7.0 | 5.9 | 8.2 |
| FCT* | 1 | - | 0.0 | - | - |
| Kogi* | 1 | - | 0.0 | - | - |
| Kwara* | 1 | - | 0.0 | - | - |
| Kwara, Kogi, Plateau | 1 | 172 | 13.9 | 9.1 | 20.0 |
| Nassarawa | 21 | 4431 | 1.1 | 0.8 | 1.5 |
| Niger* | 1 | - | 3.8 | - | - |
| Plateau | 30 | 6322 | 3.4 | 3.0 | 3.9 |
| North East | |||||
| Adamawa* | 1 | - | 1.2 | - | - |
| Bauchi | 4 | 4114 | 1.2 | 0.8 | 1.5 |
| Taraba | 7 | 3966 | 23.5 | 22.2 | 24.9 |
| Yobe* | 3 | - | 0.0 | - | - |
| North West | |||||
| Jigawa* | 3 | - | 0.3 | - | - |
| Kaduna* | 1 | - | 0.3 | - | - |
| Kano | 6 | 180 | 1.0 | 0.1 | 4.0 |
| Katsina | 1 | 257 | 22.6 | 17.6 | 28.2 |
| Kebbi* | 1 | - | 0.0 | - | - |
| Zamfara* | 3 | - | 4.6 | - | - |
| South East | |||||
| Anambra* | 1 | - | 18.8 | - | - |
| Ebonyi | 1 | 1243 | 16.9 | 14.9 | 19.1 |
| Imo | 39 | 9131 | 12.8 | 12.1 | 13.5 |
| Imo/Anambra | 5 | 500 | 16.0 | 12.9 | 19.5 |
| South South | |||||
| Akwa Ibom* | 1 | - | 17.6 | - | - |
| Bayelsa | 2 | 2583 | 18.0 | 16.5 | 19.5 |
| Cross River | 11 | 1903 | 10.2 | 8.9 | 11.6 |
| Edo* | 1 | - | 2.2 | - | - |
| Rivers | 5 | 2837 | 24.2 | 22.6 | 25.8 |
| South West | |||||
| Ekiti* | 1 | - | 1.2 | - | - |
| Ogun | 1 | 317 | 17.0 | 13.1 | 21.6 |
| Ondo* | 3 | - | 5.6 | - | - |
| Osun* | 1 | - | 1.8 | - | - |
| Oyo | 1 | 915 | 3.4 | 2.3 | 4.8 |
| Total | 162 | 40775 | 8.2 | 7.9 | 8.5 |
Note: All data included i.e. number of MDA rounds not taken into account.
Value for number of persons tested not available.
Confidence intervals calculated in Stata software (version 12, StataCorp, Texas, USA).
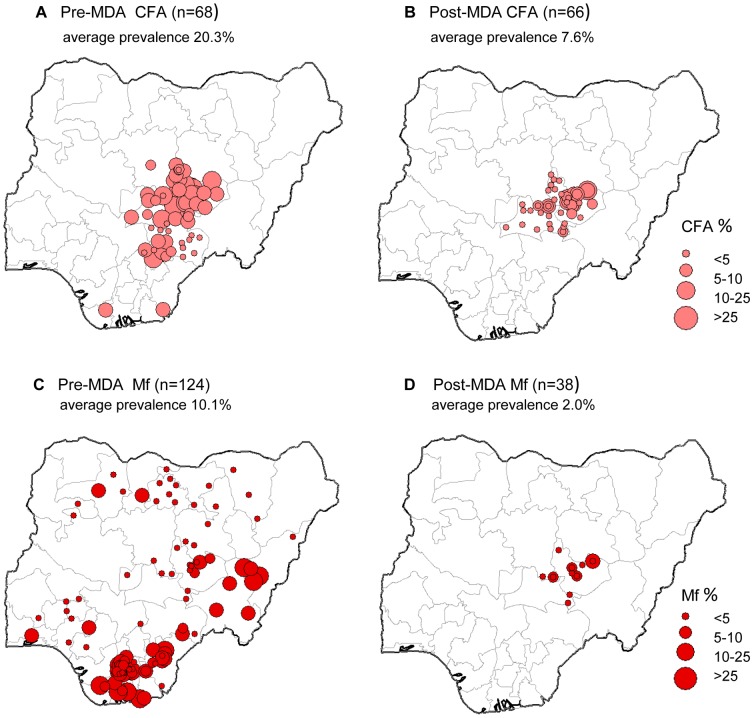
Overall, there were marked differences in W. bancrofti prevalence at sites that had not received MDA i.e. pre-MDA, compared to those sites that had received MDA i.e. post-MDA. The average CFA prevalence in pre-MDA sites, was 20.3% (n = 68; range 0% to 66.0%), which was approximately 3 times higher than the average CFA prevalence in post-MDA sites, 7.6% (n = 66, range 0.2% to 31.5%) (Figure 3a,b). The average Mf prevalence in pre-MDA sites was 10.1% (n = 124; range 0% to 47.4%), which was approximately 5 times higher than the average Mf prevalence in post-MDA sites, 2.0% (n = 66, range 0% to 12.1%) (Figure 3c,d). The distribution of post-MDA sites occur in the two States of Plateau and Nassarawa, and are the result of an extensive MDA programme delivering the combination of ivermectin and albendazole as detailed in the study publications [49] [53] [54] [63]. Baseline LF mapping was conducted in 1999 and 2000 in 30 LGAs of the two States, and MDA launched in 2000, and monitored from 2000 to 2009. Details are contained in the specific reference [63] (Table S1).
Figure 3. LF prevalence data pre-MDA and post-MDA.
a. Pre-MDA CFA (n = 68). b. Post-MDA CFA (n = 66). c. Pre-MDA Mf (n = 124). d. Post-MDA Mf (n = 38). Note: Data source for CFA, MF prevalence available in Table S1.
Clinical manifestations
The most extensive study on clinical manifestations was conducted by Nwoke et al. [82], who used hydrocele as a clinical marker to estimate LF prevalence. A rapid epidemiological mapping survey (REM-LF) was conducted across 25 States and 536 villages in Nigeria. Details of the specific study sites are not available, however, the survey found that hydrocele was absent in 339 (63.3%) villages, and present in 197 (36.8%) villages, which were found to have different levels of hydrocele severity. Hydrocele was absent in Jigawa and Kano (NW), and Ogun (SW) States. Very few hydrocele cases (1–3%) were found in northern Borno (NE), Kaduna and Zamfara (NW), Edo (SS), Imo (SE),and in Ekiti, Ondo, Osun, Oyo (SW) States. The highest hydrocele rates were found in the NE States of Adamawa, Bauchi, Gombe, Taraba and southern Borno, in the NC states of Kogi, Plateau, Nassarawa, and in the northern part of Akwa Ibom State in the SS (Figure 2c) [82].
The clinical signs that were reported included limb lymphodema, hydrocele. chyluria and elephantiasis, and were from a few specific areas of the country [45]–[49] [59]–[62] [65] [68]–[71] [74]–[75] [77]. For hydrocele, there were 22 sites with prevalence data ranging from 0.1% to 50%, while for lymphodema, 12 sites recorded prevalence rates ranging from 1% to 49%. The prevalence of limb elephantiasis was also recorded in 5 sites, which ranged from 1.7% to 11.8%. The distribution of these study sites is shown in Figure 2c, together with the high hydrocele prevalence states described by Nwoke et al. [82], and highlight the geographical concordance with CFA and Mf distributions which occur in the central south and eastern regions of the country (Figure 2a).
LF and loiasis co-endemicity
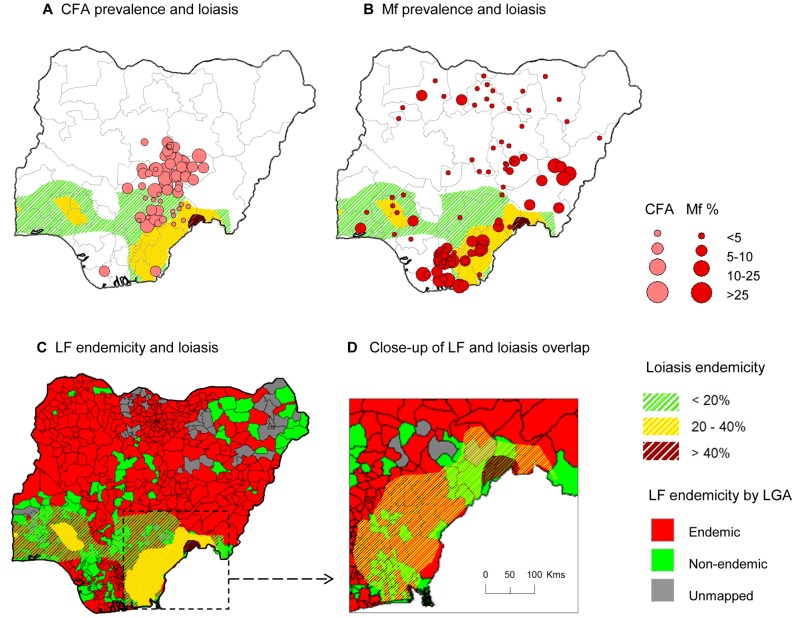
LF prevalence was examined in relation to the L. loa distribution in Nigeria defined by the RAPLOA surveys reporting eye worm history, which were carried out in 381 villages between 2002 and 2010 [7]. Loiasis was found predominately in the southern region of the country, with the highest risk in east along the border with Cameroon, which had a localized area >40% in the States of Taraba and Benue (Figure 4). Overall, there was minimal geographical overlap with the number of LF prevalence sites determined by CFA. The majority of sites with medium to high LF prevalence rates >25%, were found in low loiasis prevalence areas (<20%) where the risk of SAEs are considered to be low (Figure 4a). Similarly, there was minimal geographical overlap with the number of LF prevalence sites determined by Mf, however, more sites with medium to high LF prevalence rates >25% were found in medium loiasis prevalence areas (20–40%) where the risk of SAEs is potentially high (Figure 4b). The overlap with the LF endemicity map available from the FMoH shows a combination of endemic and non-endemic LGAs in the low (<20%) to medium (20–40%) loiasis prevalence areas (Figure 4c). Only LF non-endemic LGAs were found in the high risk loiasis area (>40%), which is highlighted in the close up of the map in Figure 4d.
Figure 4. LF prevalence overlapping loiasis areas.
a. CFA prevalence and loiasis. b. Mf prevalence and loiasis. c. LF endemicity and loiasis. d. Close up of LF and loiasis overlap. Note: Loiaisis endemicity based on eye worm history map determined from RAPLOA surveys published by Zouré et al. 2011 [7]. Three levels of loiasis shaded green <20%, yellow 20–40% and dark brown >40% highlight the extent of geographical overlap between CFA (4a) and MF (4b) prevalence data points available in Table S1. Figure 4c shows loiasis overlap with LF endemicity map developed by the FMoH, and the medium to high risk loiasis areas (yellow and dark brown shading) of the south eastern region is shown close-up in 4d. The small localized high risk loiasis area (dark brown) geographically coincides with areas classified as LF non-endemic (solid green).
Intervention overlap maps
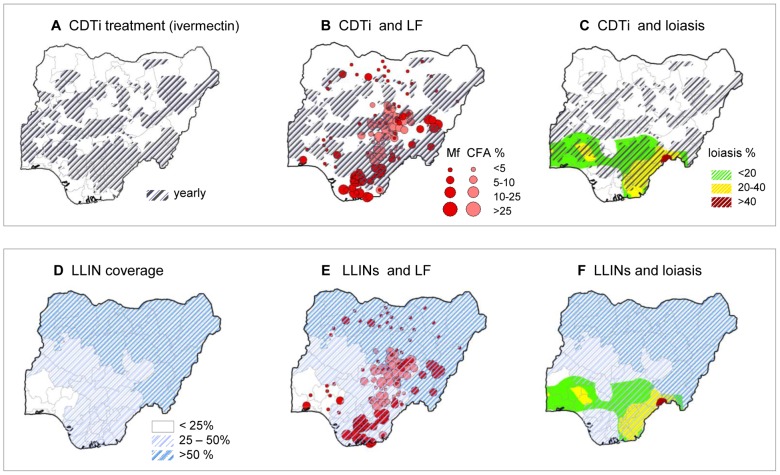
The onchocerciasis CDTi priority areas [31] are shown in Figure 5a, and illustrate that large areas across the central region of the country are being targeted with ivermectin treatment. Since 1992, ivermectin has been distributed annually to 80% of the total population at risk, estimated at ∼38,331,140 people in 430 endemic LGAs [35]. The LF endemic areas to potentially benefit from CDTi priority areas are extensive and include large areas of NC, NW and NE zones of the country. The LF programme could readily add albendazole to the ivermectin being distributed in these areas (Figure 5b). The potential risks associated with ivermectin treatment for O. volvulus are related to potential SAEs in areas where W. bancrofti and L. loa are co-endemic in the southern region of the county, especially in the States of Benue, Cross River, Ebonyi, Enugu, Osun, Ekiti and regions of Edo, Ondo and Ogun States (Figure 5c).
Figure 5. LF prevalence and intervention distribution overlap.
a. CDTi treatment (ivermectin). b. CDTi and LF. c. CDTI and loaisis. d. LLIN coverage. e. LLINs and LF. f. LLINs and loiasis. Note: Data source for CDTi (5a) based on WHO-APOC Country profile – Nigeria [31] (shaded grey) and for LLIN coverage (5d) based on Malaria Indicator Survey [40] (shaded blue) to highlight the geographical overlap with CFA and Mf data prevalence points from Table S1(5b and 5e) and loiasis map by Zouré et al. 2011 [7] (5c and 5f) respectively.
The distribution of LLIN coverage across the six different geopolitical zones is shown in Figure 5d. The highest LLIN coverage occurred in the NE (61.8%) and NW (58.2%) zones, followed by the SS (43.5%), SE (32.1%), NC (32.1%) and SW (20.3%) zones respectively. This shows that the highest LLIN coverage occurred in the northern region of the country where LF does not appear to be highly endemic (Figure 5e). The lowest LLIN coverage occurred across the southern region of the country, which coincides with areas where both LF and loiasis are considered endemic and co-endemic (Figure 5f).
Discussion
The database and series of maps produced in this study provide critical information to the Nigerian National NTD Programme. The LF Programme, specifically, will benefit as the implementation of MDA will be more effective in the coming years as it scales up to reach national coverage with support from the government and international stakeholders. This database represents the largest geo-referenced source of data on W. bancrofti publicly available for any country in Africa, and provides a basis on which to add future programmatic and research data collected from routine activities and systematic surveys in Nigeria over time. Importantly, it highlights the few selected studies and large data gaps on the burden of disease, which is an increasingly important area for GPELF and the international community given the significant social stigma and economic consequences of this disabling disease [2]. This database also complements the extensive Anopheles database recently developed by Okorie et al. [19], which comprises all available information on the main vectors of malaria and LF in Nigeria. The combination of the two databases will help to prioritise programmatic activities by appropriately targeting high LF prevalence areas, and by identifying areas with data gaps where more information is needed.
The LF prevalence data and maps clearly show the widespread endemicity across the country, and the need to implement MDA on a large scale with high levels of coverage. This will require significant support as currently only 18,591,932 (17.5%) of the targeted population are receiving MDA with ivermectin and albendazole, with varying levels of coverage [35]. The impact of MDA on LF transmission in most of the States and LGAs is yet to be determined as this intervention has only started to expand. However, detailed studies carried out in the States of Plateau and Nassarawa have highlighted the effectiveness of this intervention and the importance of documenting impact and progress through systematic monitoring and evaluation [53] [63]. These results suggest that Nigeria could significantly reduce W. bancrofti transmission in the next few years with a collective effort and commitment through increased human and financial resources from both national and external donors and stakeholders. For example, the concerted efforts geared towards the control of onchocerciasis by APOC have made a huge impact on the population by markedly reducing the prevalence and morbidity associated with the disease [83].
The LF programme also needs to take advantage of the interventions already being distributed by other programmes that could enhance its efforts towards elimination. This study highlights that large areas of Nigeria are CDTi priority areas and have received multiple rounds of ivermectin for the control of O. volvulus over the past decade [31] [34]. It is likely that ivermectin has already impacted on LF as shown elsewhere in West Africa [28] [29], and possibly interrupted transmission in low prevalence areas, especially those that have also received LLINs for malaria control. More detailed studies examining the impact of ivermectin on LF in Nigeria are important to fully appreciate its role in reducing prevalence levels. The CDTi priority areas also provide an opportunity for the LF programme to add albendazole to the current ivermectin regimes, which will save time and lead to considerable cost savings given the shared use of human resources and infrastructure. Further, the collation and examination of intervention data at State and LGA level using the MOM approach [24] will help produce finer scale maps informing the regional and local programmes of the risk and benefits, and actions that need to be taken. It will also help determine if other NTDs, such as the soil-transmitted helminths (STH), need to be considered.
To date only mebendazole has been used for STH in Nigeria, however, albendazole is being introduced in addition to medendazole in 2013 by the FMoH and is also distributed during child health weeks, thus providing some indirect treatment in LF endemic areas [35]. Integrating activities with the STH programme will be essential as the expansion of the LF Programme will also provide treatment for STH and support for the new STH programme. Importantly, if albendazole is introduced and scaled up for LF in the loiasis endemic regions the impact on both diseases may be significant. Taking these factors into account is important in a large, ecologically diverse and populous country like Nigeria, where W. bancrofti is co-endemic with other diseases and varies considerably in the different geopolitical zones and ecological settings [20] [32]–[35]. This variability requires that different intervention strategies should be developed and implemented consonant with the most up to date information and guided by WHO guidelines.
Nigeria, unlike several other L. loa endemic countries, has not reported any SAEs associated with ivermectin treatment for O. volvulus, which is shown to cover more than 90% of the L. loa area [35]. The reason for this is unclear but may be related to the different levels of endemicity and the strategies that have successfully been used to address the risk [31]. It may also be related to the extent of geographical overlap in high risk L. loa areas, which appears to be less than countries in Central Africa such as Cameroon and the Democratic Republic of Congo, where SAEs have had a major negative impact on NTD programmes [7]–[9]. In Nigeria, LF and L. loa appear to be co-endemic at low to medium prevalences (<40%) and only in the southern region of country. This information will help focus the distribution of the current WHO recommended alternative strategy of twice yearly treatment albendazole and distribution of ITN/LLINs [12]. Coordination with STH programmes will be essential, especially as new funding becomes available and programmes expand into co-endemic areas [31] [32]. In the future new alternative drugs and regimes such as the doxycycline an anti Wolbachia ' macrofilaricide or adult sterilising agent [84]–[86], may become available for co-endemic area and fine scale mapping will help to identify those populations most appropriate to target.
Strengthening linkages with the National Malaria Control Programme will be crucial, especially as there has been significant scale up of bed net distribution through a new campaign since previously reported ITN coverage in 2005 [87] and the LLIN coverage in 2010 reported here [88]. In mid-2012, nearly 50 million LLINs were reported to have been distributed across the country representing 73% of the total number expected for universal coverage distribution. [89], [90]. This dramatic increase in vector control has major implications for LF as bed nets have shown to impact on W. bancrofti transmission, especially in Anopheles-transmitted areas [17] [21] [91] [92]. Moreover, entomological data from a longitudinal study conducted in South East Nigeria has showed that full coverage with LLINs can interrupt LF transmission even in the absence of MDA [21]. More information on Anopheles vectors and their susceptibility to insecticides being used in vector control is urgently needed [19]. The role of xenomonitoring should also be considered as it may be more reliable and cost effective in the long term, especially in Nigeria where there is a wide range of existing entomological capacity and expertise that could be specifically developed and strengthened to help monitor the elimination of the disease.
Conclusion
This MOM work builds on the recent study carried out in Democratic Republic of Congo [24], which first used the new overlap mapping approach to collate and map all available country data on W. bancrofti, examine the extent of L. loa co-endemicity and determine the risk and benefits of different intervention strategies. Collectively the two studies address two important countries in Africa with respect to the elimination of LF, as they have the highest burdens of disease, collectively accounting for more than 170 million people at risk. Furthermore, their LF Programmes are yet to scale up to reach full national MDA coverage taking into account the co-endemic areas of L. loa, which may require alternative treatment strategies in selected areas, and coordination with other NTD elimination and malaria control programmes. We advocate that the MOM approach should be used more widely over time and space, and at different geographical scales to better monitor and understand the impact of single and multiple interventions, and to assess progress towards elimination of LF and other diseases. Such an approach is also necessary for national planning purposes as well as increasing the cost effectiveness and coordination of programmes where different strategies are deployed, and where there have been previous interventions which will impact on the goals of the LF programme.
Supporting Information
PRISMA checklist.
(DOC)
PRISMA flowchart.
(DOCX)
LF prevalence database.
(XLSX)
Funding Statement
The study was supported by a grant from the Department for International Development (DFID) and GlaxoSmithKline (GSK) for research on the elimination of lymphatic filariasis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) (2010) Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. WHO/HTM/NTD/PCT/2010.6. Geneva: WHO.
- 2.World Health Organization (WHO) (2011) Position statement on managing morbidity and preventing disability in GPELF. WHO/HTM/NTD/PCT/2011.8. Geneva: WHO.
- 3. World Health Organization (WHO) (2011) Global programme to eliminate lymphatic filariasis: progress report. Wkly Epidemiol Rec 87: 346–356. [PubMed] [Google Scholar]
- 4. Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T (2009) Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect Genet Evol 10: 159–177. [DOI] [PubMed] [Google Scholar]
- 5. Hotez PJ, Asojo OA, Adesina AM (2012) Nigeria: “Ground Zero” for the High Prevalence Neglected Tropical Diseases. PLoS Negl Trop Dis 6: e1600 doi:10.1371/journal.pntd.0001600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Addiss D (2010) Global Alliance to Eliminate Lymphatic Filariasis. The 6th Meeting of the Global Alliance to Eliminate Lymphatic Filariasis: A half-time review of LF elimination and its integration with the control of other neglected tropical diseases. Parasit Vectors 3: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zouré HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, et al. (2011) The geographic distribution of Loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis 5: e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boussinesq M (2006) Loiasis. Ann Trop Med Parasit 100: 715–731. [DOI] [PubMed] [Google Scholar]
- 9. Gardon J, Gardon-Wendel M, Demanga ND, Kamgno J, Chippaux JP, et al. (1997) Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa infection. Lancet 350: 18–22. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization (WHO) (2011) WHO position statement on integrated vector management to control malaria and lymphatic filariasis. Wkly Epidemiol Rec 86: 121–127. [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) (2011) World Malaria Report. WHO Global Malaria Programme, Geneva, WHO. Available: http://www.who.int/malaria/world_malaria_report_2011/en/. Accessed 11 November 2012.
- 12.World Health Organization (WHO) (2012) Provisional strategy for interrupting LF transmission in loiasis-endemic countries. WHO/HTM/NTD/PCT/2012.6. Geneva, WHO.
- 13.Uniting to Combat Neglected Tropical Diseases (2012) London Declaration on Neglected Tropical Diseases. Available: http://unitingtocombatntds.org/downloads/press/ntd_event_london_declaration_on_ntds.pdf. Accessed 27 January 2013.
- 14. Molyneux DH, Nantulya VM (2004) Linking disease control programmes in rural Africa: a pro-poor strategy to reach Abuja targets and millennium development goals. BMJ 328: 1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molyneux DH, Hotez PJ, Fenwick A, Newman RD, Greenwood B, et al. (2009) Neglected tropical diseases and the Global Fund. Lancet 373: 296–299. [DOI] [PubMed] [Google Scholar]
- 16. Manga L (2002) Vector-control synergies, between ‘Roll Back Malaria’ and the Global Programme to Eliminate Lymphatic Filariasis, in the African Region. Ann Trop Med Parasitol 96 (Suppl 2) 129–132. [DOI] [PubMed] [Google Scholar]
- 17. van den Berg H, Kelly-Hope LA, Lindsay SW (2013) Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect Dis 13: 89–94. [DOI] [PubMed] [Google Scholar]
- 18. Kelly-Hope LA, Molyneux DH, Bockarie MJ (2013) Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity. Parasit Vectors 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Kelly-Hope L (2011) Nigeria Anopheles vector database: an overview of 100 years' research. PLoS One 6: e28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federal Ministry of Health (2008) Federal Ministry of Health, National Malaria Control Programme, Abuja, Nigeria. Strategic Plan 2009–2013. Available: http://nmcpnigeria.org/f/Nigeria%20Annex%201_National%20Malaria%20Control%20Strategic%20Plan%202009-2013.pdf. Accessed 11 November 2011.
- 21.Emukah E, Graves PM, Mosher AW, Rakers L, Miri E, et al.. (2009) Long lasting insecticidal nets alone can reduce transmission of lymphatic filariasis in south east Nigeria. Abstract Book American Society of Tropical Medicine and Hygiene 58th Annual Meeting: November18–22, 2009, Washington DC.
- 22. Blackburn BG, Eigege A, Gotau H, Gerlong G, Miri E, et al. (2006) Successful integration of insecticide-treated bed net distribution with mass drug administration in Central Nigeria. Am J Trop Med Hyg 75: 650–655. [PubMed] [Google Scholar]
- 23. The CDI Study Group (2010) Community-directed interventions for priority health problems in Africa: results of a multicountry study. Bull World Health Organ 88: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly-Hope LA, Thomas BC, Bockarie MJ, Molyneux DH (2011) LF in the Democratic Republic of Congo; micro-stratification overlap mapping (MOM) as a prerequisite for control and surveillance. Parasit Vectors 18: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization/African Programme for Onchocerciasis Control (WHO/APOC) (2011) 15 years of APOC, 1995–2010. African Programme for Onchocerciasis Control, Ouagadougou, Burkina Faso.
- 26. World Health Organization (WHO) (2010) Report of the External mid-term Evaluation of the African Programme for Onchocerciasis Control, Geneva, WHO. [Google Scholar]
- 27. Amazigo UV, Leak SGA, Zoure HGM, Njepuome N, Lusamba-Dikassa PS (2012) Community-driven interventions can revolutionise control of neglected tropical diseases. Trends Parasitol 28: 231–238. [DOI] [PubMed] [Google Scholar]
- 28. Kyelem D, Sanou S, Boatin B, Medlock J, Coulibaly S, et al. (2003) Impact of long-term ivermectin (Mectizan) on Wuchereria bancrofti and Mansonella perstans infections in Burkina Faso: strategic and policy implications. Ann Trop Med Parasitol 97: 827–838. [DOI] [PubMed] [Google Scholar]
- 29. Kyelem D, Medlock J, Sanou S, Bonkoungou M, Boatin B, et al. (2005) Short communication: impact of long-term (14 years) bi-annual ivermectin treatment on Wuchereria bancrofti microfilaraemia. Trop Med Int Health 10: 1002–1004. [DOI] [PubMed] [Google Scholar]
- 30. Tekle A, Elhassan E, Isiyaku S, Amazigo UV, Bush S, et al. (2012) Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: first evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasit Vectors 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) (2012) African Programme for Onchocerciasis Control (APOC); Country Profiles – Nigeria. Available: http://www.who.int/apoc/countries/nga/en/index.html. Accessed 11 November 2012.
- 32.World Health Organization (WHO) (2012) Eliminating soil-transmitted helminthiasis as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. WHO/HTM/NTD/PCT/2012.4 Geneva, WHO.
- 33.The Global Atlas of Helminth Infection (2012) Available: www.thiswormyworld.org.Accessed 11 November 2012.
- 34. Njepuome NA, Hopkins DR, Richards Jr FO, Anagbogu IN, Pearce PO, et al. (2009) Nigeria's war on terror: fighting dracunculiasis, onchocerciasis, lymphatic filariasis, and schistosomiasis at the grassroots. Am J Trop Med Hyg 80: 691–698. [PubMed] [Google Scholar]
- 35.Federal Ministry of Health (2012) Nigeria Master Plan for Neglected Tropical Diseases (NTDs) 2013–2017. Government of the Federal Republic of Nigeria, Abuja.
- 36.Central Intelligence Agency (2012) The World Factbook. Available: https://www.cia.gov/library/publications/the-world-factbook/geos/ni.html. Accessed: 9 November 2012
- 37.Work Health Organization (WHO) (2010) Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphatic filariasis: a manual for national elimination programmes. WHO/HTM/NTD/PCT/2011.4
- 38.NGA GEOnet Names Server (GNS) Available: http://earth-info.nga.mil/gns/html/namefiles.htm. Accessed: 02 October 2012.
- 39.Directory of Cities and Towns in the World, Global Gazetteer Version 2.1 Available: http://www.fallingrain.com/world/NI/index.html. Accessed: 02 October 2012.
- 40.National Population Commission (NPC) [Nigeria], National Malaria Control Programme (NMCP) [Nigeria], and ICF International. 2012. Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: NPC, NMCP, and ICF International.
- 41. Agi PI, Ebenezer A (2009) Observations on Filaria Infection in Amassoma Community in the Niger Delta. Niger J Appl Sci Environ Man 1: 15–19. [Google Scholar]
- 42. Ajero CMU, Nwoke BEB, Okolie NJC, Nwanjo HU, Oze G, et al. (2007) Bancroftian Filariasis in the Niger Delta Area of Eastern Nigeria. Res J Med Sci 1: 113–117. [Google Scholar]
- 43. Anosike JC, Nwoke BEB, Ajayi EG, Onwurili CO, Okoro OU, et al. (2005) Lymphatic Filariasis among the Ezza people of Ebonyi State, Eastern Nigeria. Ann Agric Env Med 12: 181–186. [PubMed] [Google Scholar]
- 44. Anosike JC, Onwuliri COE, Onwuliri VA (2003) Human filariasis in Dass local government area of Bauchi state, Nigeria. Trop Ecology 44: 217–227. [Google Scholar]
- 45.Badaki JA (2010) Parasitological and social aspects of Lymphatic Filariasis in Taraba State. PhD thesis. University of Jos, Jos, Nigeria, Department of Zoology. 137p.
- 46. Braide EI, Ikpeme B, Edet E, Atting I, Kale OO (2003) Preliminary observations on the occurrence of lymphatic filariasis in Cross River State, Nigeria. Nig J Parasitol 24: 9–16. [Google Scholar]
- 47. Dogara MM, Nock HI, Agbede RIS, Ndams SI, Joseph KK (2012) Prevalence of Lymphatic Filariasis In Three Villages In Kano State, Nigeria. Internet J Trop Med 8: 1. [Google Scholar]
- 48. Ebenezer A, Amadi EC, Agi PI (2011) Studies on the microfilaria, antigenemia and clinical signs of bancroftian filariasis in Epie creek communities, Niger Delta, Nigeria. Int Res J Microbiol 2: 370–374. [Google Scholar]
- 49. Eigege A, Richards FO Jr, Blaney DD, Miri ES, Gontor I, et al. (2002) Rapid assessment for lymphatic filariasis in central Nigeria: a comparison of the immunochromatographic card test and hydrocele rates in an area of high endemicity. Am J Trop Med Hyg 68: 643–646. [PubMed] [Google Scholar]
- 50. Ekanem IA, Alaribe AAA, Ekanem AP (2011) Prevalence of Bancroftian Filariasis among Edim Otop sub-urban dwellers in Calabar Municipality of Cross River state, Nigeria. J Appl Pharm Sci 01: 63–67. [Google Scholar]
- 51. Engelbrecht F, Oettl T, Herter U, Link C, Philipp D, et al. (2003) Analysis of Wuchereria bancrofti infections in a village community in northern Nigeria: increased prevalence in individuals infected with Onchocerca volvulus . Parasitol Int 52: 13–20. [DOI] [PubMed] [Google Scholar]
- 52. Iboh CI, Okon OE, Opara KN, Asor JE, Etim SE (2002) Lymphatic filariasis among the Yakurr people of Cross River State, Nigeria. Parasit Vectors 19: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. King JD, Eigege A, Umaru J, Jip N, Miri E, et al. (2012) Evidence for stopping mass drug administration for lymphatic filariasis in some, but not all local government areas of Plateau and Nasarawa States, Nigeria. Am J Trop Med Hyg 87: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mathieu E, Amann J, Eigege A, Richards F, Sodahlon Y (2008) Collecting baseline information for national morbidity alleviation programs: different methods to estimate lymphatic filariasis morbidity prevalence. Am J Trop Med Hyg 78: 153–158. [PubMed] [Google Scholar]
- 55. Mohammad AI (2010) Lymphatic Filariasis Infection Of Children in Zing, Taraba State, Nigeria. NIJOSTEE 3: 1. [Google Scholar]
- 56. Obi RK, Oyibo WA, Okangba CC, Nwanebu FC, Oparaocha ET, et al. (2010) Concurrent Parasitosis in an Onchocerciasis Endemic Community. Asian J Exp Biol Sci 26: 263–270. [Google Scholar]
- 57. Obi RK, Nwanebu FC, Ndubuisi-Nnaji UU, Okangba CC, Braide W, et al. (2011) Endemicity of Lymphatic filariasis in three Local Government Areas of Imo State, Nigeria. Aust J Basic Appl Sci 5: 875–879. [Google Scholar]
- 58. Ojiako OA, Onyeze GOC (2009) Epidemiological and biochemical studies of human lymphatic filariasis and associated parasitoses in Oguta, South-Eastern, Nigeria. Afr J Biochem Res 3: 114–119. [Google Scholar]
- 59. Ojurongbe O, Akinbo JA, Ogiogwa IJ, Bolaji OS, Adeyeba OA (2010) Lymphatic filariasis in a rural community in Nigeria: a challenge ahead. Afr J Med Sci 39: 179–183. [PubMed] [Google Scholar]
- 60. Okon OE, Iboh CI, Opara KN (2010) Bancroftian filariasis among the Mbembe people of Cross River State, Nigeria. J Vector Born Dis 47: 91–96. [PubMed] [Google Scholar]
- 61. Omudu EA, Ochoga JO (2011) Clinical epidemiology of lymphatic filariasis and community practices and Perceptions amongst the ado people of Benue state, Nigeria. Afr J Infect Dis 5: 47–53. [PMC free article] [PubMed] [Google Scholar]
- 62. Omudu EA, Okafor FC (2007) Rapid epidemiological and Socio-cultural appraisal of lymphatic filariasis amongst the Igede ethnic group in Benue State, Nigeria. Nig J Parasitol 28: 118–123. [Google Scholar]
- 63. Richards FO, Eigege A, Miri ES, Kal A, Umaru J, et al. (2011) Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis 5: e1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Targema CN, Onwuliri CO, Mafuya HB, Mwansat GS, Aida A, et al. (2008) Mapping of lymphatic filariasis in Benue State, Nigeria. Nig J Parasitol 29: 55–60. [Google Scholar]
- 65.Targema CN (2010) Studies on the status of Bancroftian filariasis in parts of Benue state, Nigeria. PhD Thesis. University of Jos, Jos, Nigeria. Department of Zoology. 236p.
- 66. Terranella A, Eigiege A, Gontor I, Dagwa P, Damishi S, et al. (2006) Urban lymphatic filariasis in central Nigeria. Ann Trop Med Parasitol 100: 163–172. [DOI] [PubMed] [Google Scholar]
- 67. Uttah E, Ibeh DC (2011) Multiple filarial species microfilaraemia: a comparative study of areas with endemic and sporadic onchocerciasis. J Vector Borne Dis 48: 197–204. [PubMed] [Google Scholar]
- 68. Uttah EC, Simonsen PE, Pedersen EM, Udonsi JK (2004) Bancroftian filariasis in the Lower Imo River Basin, Nigeria. Afr J Appl Zool Environ Biol 6: 65–75. [Google Scholar]
- 69. Uttah EC (2011) Prevalence of endemic Bancroftian filariasis in the high altitude region of south-eastern Nigeria. J Vector Borne Dis 48: 78–84. [PubMed] [Google Scholar]
- 70. Akogun OB (1992) Filariasis in Gongola State Nigeria. I: Clinical and parasitological studies in Mutum-Biyu District. Angew Parasitol 33: 125–131. [PubMed] [Google Scholar]
- 71. Anosike JC (1994) The status of human filariasis in north-western zone of Bauchi State, Nigeria. Appl Parasitol 35: 133–140. [PubMed] [Google Scholar]
- 72. Mbah DC, Njoku OO (200) Prevalence of lymphatic filariasis in Oraeri, Aguata Local Government Area of Anambra State, Nigeria. Nig J Parasitol 21: 95–102 cited in Anosike JC, Nwoke BEB, Ajayi EG, Onwurili CO, Okoro OU, et al (2005) Lymphatic Filariasis among the Ezza people of Ebonyi State, Eastern Nigeria. Ann Agric Env Med 12: 181–186. [PubMed] [Google Scholar]
- 73. Udonsi JK (1986) The status of human filariasis in relation to clinical signs in endemic areas of the Niger Delta. Ann Trop Med Parasitol 80: 425–432. [DOI] [PubMed] [Google Scholar]
- 74. Udonsi JK (1988) Bancroftian filariasis in the Igwun basin, Nigeria: an epidemiological, parasitological, and clinical study in relation to the transmission dynamics. Folia Parasitol (Praha) 35: 147–155. [PubMed] [Google Scholar]
- 75. Udonsi JK (1988) Bancroftian filariasisin the Igwun Basin, Nigeria. An epidemiological, parasitological, and clinical study in relation to the transmission dynamics. Acta Trop 45: 171–179. [PubMed] [Google Scholar]
- 76. Udonsi JK (1988) Filariasis in the Igwun River Basin, Nigeria: an epidemiological and clinical study with a note on the vectors. Ann Trop Med Parasitol 82: 75–82. [DOI] [PubMed] [Google Scholar]
- 77. Ufomadu GO, Nwoke BE, Akoh JI, Sato Y, Ekejindu GO, et al. (1990) The occurrence of loiasis, mansonellosis and wuchereriasis in the Jarawa River Valley, central Nigeria. Acta Trop 48: 137–147. [DOI] [PubMed] [Google Scholar]
- 78. Wijeyaratne PM, Verma OP, Singha P, Osuhor PC, Motha B, et al. (1982) Epidemiology of filariasis in Malumfashi district of northern Nigeria. Indian J Med Res 76: 534–544. [PubMed] [Google Scholar]
- 79. Cobban KM (1959) Analysis of a year's outpatients at University College Hospital, Ibadan. J Trop Med Hyg 62: 129–134. [PubMed] [Google Scholar]
- 80. Courtney BJ (1923) The association of certain common complaints as seen in native hospital patients with the presence of microfilariae in their blood. Trop Med Hyg 26: 87–89. [Google Scholar]
- 81.Ngu VA, Folami AO (1965) J. Nigerian Med. Ass. 2: 160–163 cited in Hawking F (1974) The distribution of human filariasis throughout the world. Part III. Africa. World Health Organization. WHO/FIL/74.124.
- 82. Nwoke BEB, Dozie INS, Jiya J, Saka Y, Ogidi JA, et al. (2006) The prevalence of hydrocoele in Nigeria and its implication on mapping of lymphatic filariasis. Nigerian J Parasitol 27: 29–35. [Google Scholar]
- 83. Coffeng LE, Stolk WA, Zouré HGM, Veerman JL, Agblewonu KB, et al. (2013) African Programme for Onchocerciasis Control 1995–2015: Model-Estimated Health Impact and Cost. PLoS Negl Trop Dis 7 (1) e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tamarozzi F, Tendongfor N, Enyong PA, Esum M, Faragher B, et al. (2012) Long term impact of large scale community-directed delivery of doxycycline for the treatment of onchocerciasis. Parasit Vectors 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S (2011) Filariasis in Africa–treatment challenges and prospects. Clin Microbiol Infect 17: 977–985. [DOI] [PubMed] [Google Scholar]
- 86. Wanji S, Tendongfor N, Nji T, Esum M, Che JN, et al. (2009) Community-directed delivery of doxycycline for the treatment of onchocerciasis in areas of co-endemicity with loiasis in Cameroon. Parasit Vectors 2: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oresanya OB, Hoshen M, Sofola OT (2008) Utilization of insecticide-treated nets by under-five children in Nigeria: Assessing progress towards the Abuja targets. Malar J 7: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organisation (WHO) (2012) Focus on Nigeria Progress and impact series. Country Reports Number 4. April 2012. Roll Back Malaria Partnership. Geneva, WHO.
- 89.The Carter Center (2012) Summary Proceedings 3rd Annual Malaria Control Program Review. Ethiopia and Nigeria: 2012 Available: http://www.cartercenter.org/resources/pdfs/news/health_publications/malaria/2012-summary-proceedings.pdf. Accessed 10 November 2012.
- 90.President's Malaria Initiative (PMI) Nigeria Malaria Operational Plan FY 2013 Available at http://www.pmi.gov/countries/mops/fy13/nigeria_mop_fy13.pdf
- 91. Pedersen EM, Mukoko DA (2002) Impact of insecticide-treated materials on filarial transmission by the various species of vector mosquito in Africa. Ann Trop Med Parasitol 96 (Suppl 2) 91–95. [DOI] [PubMed] [Google Scholar]
- 92. Bøgh C, Pedersen EM, Mukoko DA, Ouma JH (1998) Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med Vet Entomol 12: 52–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
PRISMA flowchart.
(DOCX)
LF prevalence database.
(XLSX)