Abstract
Background Malignant tenosynovial giant cell tumors (GCTs) are extremely rare, and their etiology is unknown. However, this type of malignancy is associated with high metastasis and mortality rates. Therefore, the treatment of choice is wide excision.
Case Description A 66-year-old man complained of tingling and loss of sensation in the left hand, caused by a tumor that compressed the median nerve. The tumor was excised. Histopathologic examination revealed a ganglion cyst. Two years later, the patient visited our clinic with recurrent and similar complaints of the left hand. This time, however, the lesion turned out to be a malignant tenosynovial GCT and was treated by amputation of the forearm.
Literature Review Since 1979, only 37 malignant tenosynovial GCTs have been reported in literature. Follow-up of these patients showed that 11 patients died of the disease, 4 patients were still living with the disease, and 14 patients had no evidence of disease after treatment. The other seven patients were lost to follow-up, and one patient died of other causes. In these 37 patients, a high incidence of lymph node metastasis (41%) and a high mortality rate (30%) were seen.
Clinical Relevance Although this malignant tenosynovial GCT is very rare, high mortality rates have been observed because of the high incidence of lymph node metastases. Therefore, more awareness has to be created, to recognize and treat this tumor timely.
Keywords: malignant giant cell, carpal tunnel syndrome
Giant cell tumors (GCTs) of the soft tissues are, based on their site of origin, divided into nontenosynovial and tenosynovial. Nontenosynovial GCTs are less common, and their biological behavior varies from borderline malignant to full-blown malignant. Tenosynovial GCTs, also known as GCTs of the tendon sheath or pigmented villonodular synovitis are nearly always benign, with a strong capacity for local recurrence because of their pattern of growth.1,2 We present a case of malignant tenosynovial GCT in the carpal tunnel and a review of the literature.
Case Report
A 66-year-old man, with tingling and a loss of sensation in the left hand, was treated in our center for a ganglion cyst in the carpal tunnel of the left hand (Fig. 1). This ganglion was excised and the carpal tunnel way opened. Two years later the patient presented with a recurrence of the same complaints: tingling and loss of sensation in the left hand during the night. Palpation revealed a mass of 6 × 4 cm in the left wrist in the region of the carpal tunnel that was proximal to the carpal ligament. This was confirmed by ultrasound (Fig. 2). Subsequently, an excision was planned. During the operation, two separate masses, indicating two separate tumors, were seen, the larger of which was attached to the median nerve and the smaller to the ulnar nerve (Fig. 3). Both tumors were attached to the nerves but had not invaded the nerve fascicle. The affected nerves were dissected away. Both lesions were excised. Histopathologic examination revealed a cellular lesion with areas compatible with a tenosynovial GCT. The tumor contained plump spindle-shaped cells with a variable degree of atypia. Nuclear pleomorphism was present, as well as (atypical) mitoses. This, in combination with the region involved, led to the diagnosis of malignant tenosynovial GCT. Flow cytometry was not used because you do not use this in solid tumors. Also, no immunohistochemical studies were performed because you only need thesewhen the morphology is uncertain. In this case, the morphology was clear and the classification of the tumor could be made (i.e., a malignant GCT).
Fig. 1.
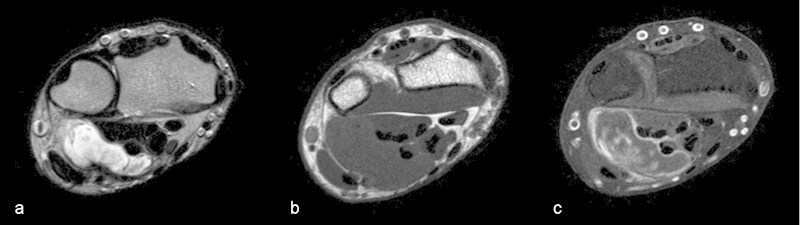
Axial MRI images. Sharply delineated mass on the ulnovolar side of the wrist partially encasing the flexor tendons, hyperintense on T2 (a), hypointense in T1 (b), and with edge enhancement on T1 with spectral presaturation with inversion recovery (SPIR) (c) after injection of gadolinium.
Fig. 2.
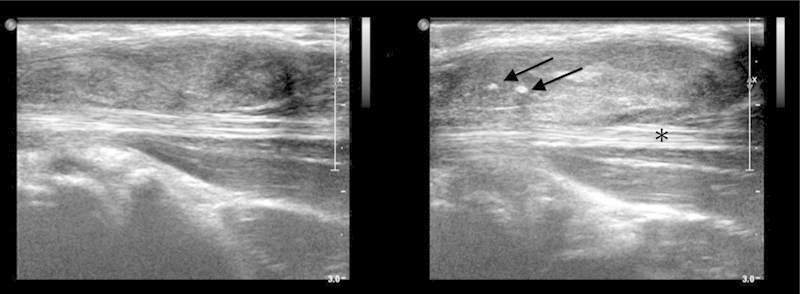
Ultrasound image. More or less sharply delineated inhomogeneous hypoechoic mass, with some small calcifications (arrows), encasing part of the flexor tendons (asterisk). It resembles extensive synovitis and is not hypervascular.
Fig. 3.
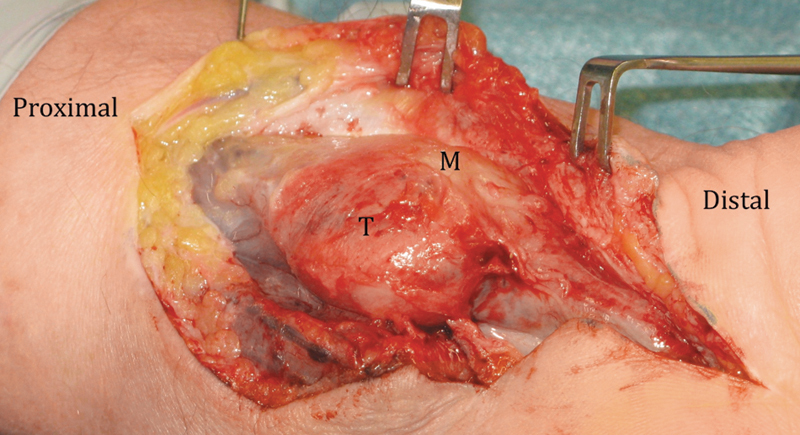
Volar view of the left carpal tunnel, demonstrating the malignant GCT surrounding the median nerve. T, tumor; M, median nerve.
Three weeks after the re-excision, a MRI scan of the region involved showed an inhomogeneous mass. It was impossible to differentiate between residual disease and scarlike tissue (Fig. 4). To perform a re-excision with free margins, the median and ulnar nerve and all flexor tendons would have had to be excised. This would have resulted in a dysfunctional hand; therefore, a forearm amputation was performed. We staged the patient for metastases; no lymph node metastates were seen. Histopathologic examination revealed scarring with remnants of the malignant tenosynovial GCT. Staging computed tomography (CT) of the thorax and ultrasonography of the axilla did not reveal any evidence of metastases.
Fig. 4.
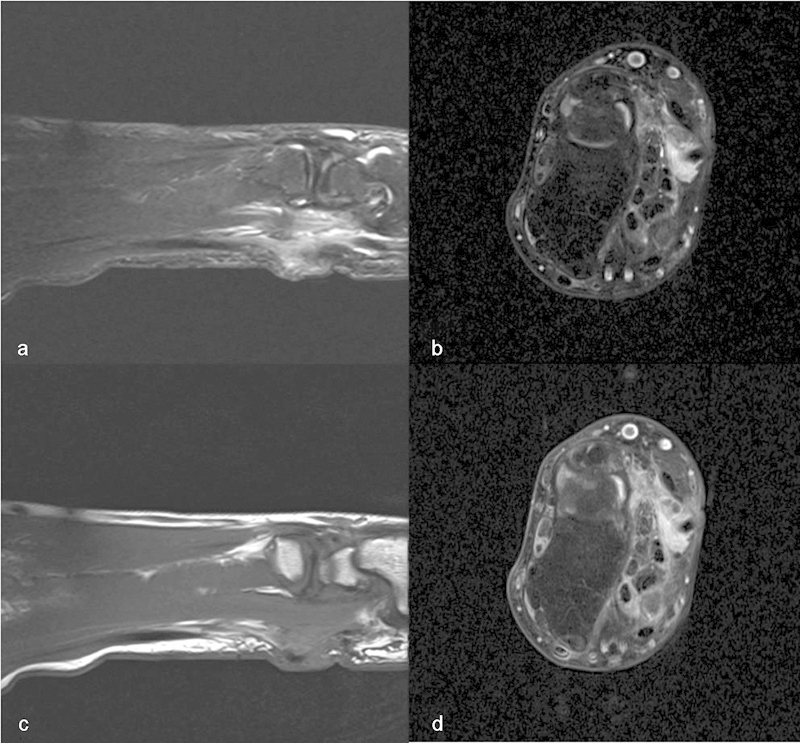
MRI Inhomogeneous mass on the volar side of the wrist, encasing part of the flexor tendons. Hyperintense on T1 SPIR (a), FatSat T2 (b), T1 proton density (PD) (c), and strong inhomogeneous enhancement on FatSat T1 (d) after injection of gadolinium.
During the follow-up period of two years, there was no evidence of local recurrence or metastatic disease.
Discussion
Tenosynovial GCTs are nearly always benign. Their malignant counterparts are extremely rare. Diagnosis is made on histological examination. Only 37 malignant tenosynovial GCTs have been reported in the literature since 1979, and these are summarized in Tables 123.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17
Table 1. Review of the literature on MGCTs: patients who died of disease (DOD) or were living with disease (LWD).
| Reference | Age | Sex | Site | Treatment | Metastasis | Follow-up |
|---|---|---|---|---|---|---|
| Nielsen and Kiaer, 19893 | 67 | M | Knee (L) | AM, RT | B, ST | DOD (8 m) |
| Shinjo et al, 19934 | 72 | F | Hip (R) | EX, AM, CT | Lungs, viscera | DOD (5 m) |
| Bertoni et al, 19975 | 57 | M | Foot (R) | EX (2x), AM, RT | LN, ST, lungs | DOD ( year 9 m) |
| 74 | F | Knee (R) | EX (2x) | LN | DOD (3 years 5 m) | |
| 71 | F | Cheek (L) | EX (6x), RT | Lungs, brain | DOD (10 years 1 m) | |
| 12 | M | Thigh | EX | B, lungs | DOD (5 years) | |
| Kalil and Unni, 19986 | 21 | F | Ankle (R) | EX (3x), AM, RT | LN, lungs | DOD ( year) |
| Somerhausen and Fletcher, 20007 | 58 | F | Elbow | EX, AM | Lungs | DOD (2 years 11 m) |
| Bhadra et al, 20078 | 65 | M | Knee (L) | EX (2x), RT, CT | B, lungs | DOD (2 years 7 m) |
| Li et al., 20089 | 45 | M | Ankle | EX | B | DOD (10 m) |
| Imakiire et al., 201110 | 57 | F | Knee (L) | EX (3x), RT, CT | B | DOD (2 years 9 m) |
| Carstens and Howell, 197911 | 48 | F | Foot (R) | EX (2x), AM, RT | LN, ST | LWD (8 years 5 m) |
| Ushijima et al, 198612 | 59 | M | Knee (R) | EX (6x), AM, RT, CT, IT | LN, B, ST | LWD (4 years 4 m) |
| Li et al., 20089 | 39 | F | Forearm | AM, RT | LN, lungs | LWD ( year 5 m) |
| 68 | M | Supracubital | EX | LWD (3 years 7 m) |
DOD: n = 11, follow-up 5-121 months, mean 34 (32.2) months, median 31 months.
LWD: n = 4, follow-up 17-101 months, mean 53 (35.1) months, median 48 months.
Abbreviations: AM, amputation; CT, chemotherapy; EM, embolization; EX, excision; F, female; IT, immunotherapy; M, male; m, months; RT, radiotherapy; y, years.
Table 2. Review of the literature on MGCTs: patients with no evidence of disease (NED).
| Reference | Age | Sex | Site | Treatment | Metastasis | Follow-up |
|---|---|---|---|---|---|---|
| Choong et al, 199513 | 35 | F | Popliteal (L) | EX (7x), AM, RT | Stump, ST | NED (50 years) |
| Bertoni et al, 19975 | 59 | M | Knee (L) | AM | NED (13 years) | |
| 37 | F | Knee (L) | EX (12x), AM, RT | Stump | NED (49 years) | |
| 79 | F | Ankle (L) | EX (2x), AM (2x), RT | Stump | NED (13 years) | |
| 38 | F | Ankle (L) | AM | NED (3 years) | ||
| Somerhausen and Fletcher, 20007 | 50 | F | Paravertebral | EX, EM | NED ( year 1 m) | |
| 26 | F | Knee | EX, RT, EM | NED (6 m) | ||
| Wu et al, 200414 | 27 | M | Forearm (R) | EX (4x), RT, CT | NED(2 years) | |
| Bhadra et al, 20078 | 72 | F | Knee (L) | EX, AM | NED (6 m) | |
| 53 | F | Ring finger (R) | EX (2x) | NED (8 m) | ||
| Li et al, 20089 | 78 | M | Knee | EX (2x) | NED (3 years) | |
| 52 | M | Suprapopliteal | EX | NED (12 m) | ||
| 67 | F | Lower leg | EX | NED (8 m) | ||
| 46 | F | Thigh | EX, RT | LN | NED (10 m) |
NED: n = 14, follow-up 6-600 months, mean 119 (207.9) months, median 19 months.
Abbreviations: AM, amputation; CT, chemotherapy; EM, embolization; EX, excision; F, female; IT, immunotherapy; M, male; m, months; RT, radiotherapy; y, years.
Table 3. Review of the literature on MGCTs: patients who died of other causes (DOC) or with unknown outcome.
| Reference | Age | Sex | Site | Treatment | Metastase | Follow-up |
|---|---|---|---|---|---|---|
| Layfield et al, 200015 | 65 | F | Hip (R) | EX, RT | DOC | |
| Somerhausen and Fletcher, 20007 | 42 | M | Hand | AM | Unknown | |
| 21 | F | Thigh | EX | Unknown | ||
| 50 | M | Knee | EX, AM | LN | Unknown | |
| 33 | F | Sacrococcygeal | EX | Unknown | ||
| Layfield et al, 200015 | 24 | M | Knee (R) | EX (5x), AM, RT | Stump, ST | Unknown |
| Oda et al, 200716 | 53/F | F | L5-S1 | EX (2x), CT | LN | Unknown |
| Yoon et al, 201117 | 29 | M | Cheek (R) | EX (2x) | Lungs | Unknown |
DOC: n = 1, follow-up 21 months.
Abbreviations: AM, amputation; CT, chemotherapy; EM, embolization; EX, excision; F, female; IT, immunotherapy; M, male; m, months; RT, radiotherapy; y, years.
Of these 37 patients, follow-up outcome was known in 30 patients; one patient had died of other causes (DOC). Of the remaining 29 patients, 11 patients died of disease (DOD), 4 patients were living with disease (LWD) and 14 patients had no evidence of disease (NED) since their last treatment, with a follow-up of 6 months to 49 years. All 15 DOD and LWD patients had metastases, with a high incidence of lymph node metastases, unusual for soft tissue sarcomas (41%). Lymph node metastases were also found in one of the 14 NED patients and two of the 7 patients for whom the follow-up outcome was unknown.
The first choice of treatment is wide excision, resection, or amputation, depending on the extensiveness of the tumor. The use of radiotherapy in combination with surgery for soft tissue sarcomas is supported by phase III clinical trials and is based on two premises: that microscopic foci of residual disease can be destroyed by radiotherapy and that less radical surgery can be performed when surgery and radiotherapy are combined.2 The optimal sequencing of surgery and radiation is a subject of considerable controversy and debate. Compared with postoperative radiotherapy, preoperative radiotherapy does not significantly improve survival. Although it involves a generally lower radiation dose and a small field size, with reduced risks for long-term complications such as edema and fibrosis, it also increases the risk of wound healing problems. These problems were found to be twice as common with preoperative radiotherapy as with postoperative radiotherapy.2,18,19 Chemotherapy, whether or not multidrug, is not effective for malignant tenosynovial GCTs. Because GCTs express colony-stimulating factor-1 (CSF1), targeted drug treatment with CSF1 inhibitors, such as imatinib mesylate (Gleevec®, Novartis), has become an option.20,21 This is supported by several other clinical studies that show promising results in the treatment of benign GCTs. However, such data are not available for malignant GCTs, whether or not of tenosynovial origin.
We recommend that every surgeon who comes across a patient with this kind of tumor should check the lymph nodes because of the high incidence of lymph node metastasis. Although surgical excision is the first choice of treatment, each individual patient has to be evaluated to choose the most appropriate additional treatment based on location, size, and risks.
Reporting these cases and their clinical course will lead to a better understanding of these tumors. Awareness and vigilance are necessary when treating a patient with an atypical lump in the wrist and hand region.
Footnotes
Conflict of Interest No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
References
- 1.Rubin B P. Tenosynovial giant cell tumor and pigmented villonodular synovitis: a proposal for unification of these clinically distinct but histologically and genetically identical lesions. Skeletal Radiol. 2007;36(4):267–268. doi: 10.1007/s00256-006-0249-3. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S W, Goldblum J R. St Louis, MO: Mosby; 2008. Soft Tissue Tumors; pp. 769–788. [Google Scholar]
- 3.Nielsen A L, Kiaer T. Malignant giant cell tumor of synovium and locally destructive pigmented villonodular synovitis: ultrastructural and immunohistochemical study and review of the literature. Hum Pathol. 1989;20(8):765–771. doi: 10.1016/0046-8177(89)90070-1. [DOI] [PubMed] [Google Scholar]
- 4.Shinjo K, Miyake N, Takahashi Y. Malignant giant cell tumor of the tendon sheath: an autopsy report and review of the literature. Jpn J Clin Oncol. 1993;23(5):317–324. [PubMed] [Google Scholar]
- 5.Bertoni F, Unni K K, Beabout J W, Sim F H. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis) Am J Surg Pathol. 1997;21(2):153–163. doi: 10.1097/00000478-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kalil R K, Unni K K. Malignancy in pigmented villonodular synovitis. Skeletal Radiol. 1998;27(7):392–395. doi: 10.1007/s002560050405. [DOI] [PubMed] [Google Scholar]
- 7.Somerhausen N S, Fletcher C D. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am J Surg Pathol. 2000;24(4):479–492. doi: 10.1097/00000478-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bhadra A K, Pollock R, Tirabosco R P. et al. Primary tumours of the synovium. A report of four cases of malignant tumour. J Bone Joint Surg Br. 2007;89(11):1504–1508. doi: 10.1302/0301-620X.89B11.18963. [DOI] [PubMed] [Google Scholar]
- 9.Li C F, Wang J W, Huang W W. et al. Malignant diffuse-type tenosynovial giant cell tumors: a series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32(4):587–599. doi: 10.1097/PAS.0b013e318158428f. [DOI] [PubMed] [Google Scholar]
- 10.Imakiire N, Fujino T, Morii T. et al. Malignant pigmented villonodular synovitis in the knee - report of a case with rapid clinical progression. Open Orthop J. 2011;5:13–16. doi: 10.2174/1874325001105010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castens H P, Howell R S. Malignant giant cell tumor of tendon sheath. Virchows Arch A Pathol Anat Histol. 1979;382(2):237–243. doi: 10.1007/BF01102878. [DOI] [PubMed] [Google Scholar]
- 12.Ushijima M, Hashimoto H, Tsuneyoshi M, Enjoji M. Giant cell tumor of the tendon sheath (nodular tenosynovitis). A study of 207 cases to compare the large joint group with the common digit group. Cancer. 1986;57(4):875–884. doi: 10.1002/1097-0142(19860215)57:4<875::aid-cncr2820570432>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Choong P F, Willén H, Nilbert M. et al. Pigmented villonodular synovitis. Monoclonality and metastasis—a case for neoplastic origin? Acta Orthop Scand. 1995;66(1):64–68. doi: 10.3109/17453679508994643. [DOI] [PubMed] [Google Scholar]
- 14.Wu N L, Hsiao P F, Chen B F, Chen H C, Su H Y. Malignant giant cell tumor of the tendon sheath. Int J Dermatol. 2004;43(1):54–57. doi: 10.1111/j.1365-4632.2004.01833.x. [DOI] [PubMed] [Google Scholar]
- 15.Layfield L J, Meloni-Ehrig A, Liu K, Shepard R, Harrelson J M. Malignant giant cell tumor of synovium (malignant pigmented villonodular synovitis) Arch Pathol Lab Med. 2000;124(11):1636–1641. doi: 10.5858/2000-124-1636-MGCTOS. [DOI] [PubMed] [Google Scholar]
- 16.Oda Y, Takahira T, Yokoyama R, Tsuneyoshi M. Diffuse-type giant cell tumor/pigmented villonodular synovitis arising in the sacrum: malignant form. Pathol Int. 2007;57(9):627–631. doi: 10.1111/j.1440-1827.2007.02150.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoon H J, Cho Y A, Lee J I, Hong S P, Hong S D. Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):e30–e36. doi: 10.1016/j.tripleo.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan B, Davis A M, Turcotte R. et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 19.Tseng J F, Ballo M T, Langstein H N. et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209–1215. doi: 10.1245/s10434-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 20.Cassier P A, Gelderblom H, Stacchiotti S. et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118(6):1649–1655. doi: 10.1002/cncr.26409. [DOI] [PubMed] [Google Scholar]
- 21.Ravi V, Wang W L, Lewis V O. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol. 2011;23(4):361–366. doi: 10.1097/CCO.0b013e328347e1e3. [DOI] [PubMed] [Google Scholar]


