Abstract
Purpose
Tumours of the calcaneus are exceedingly rare and the correct diagnosis is often missed. X-rays are the standard clinical examination tool and therefore we wanted to discover whether X-rays alone were a sufficient diagnostic tool for these tumours. Diard’s classification was applied to define whether different types of lesions were characteristically distributed in the bone and in addition we analysed whether type and/or duration of symptoms were possible indicators of malignancy.
Methods
Ninety-two patients’ files (59 men and 33 women) were retrospectively reviewed. Seventy-five patients with a mean age at surgery of 28 years (range five to 78) were surgically treated. Parameters analysed were sex, age at surgery, side, type and duration of symptoms, tentative diagnosis, biopsy prior to surgery, operative procedure, recurrence rate, revision and localisation of the lesion according to Diard. For each lesion the first documented radiological diagnosis and—in cases of malignancy—Enneking’s classification was applied.
Results
Discrepancies between the radiological and definitive histological diagnosis occurred in 38 (41 %) of 92 cases. In eight (osteosarcoma n = 5, Ewing’s sarcoma n = 2, metastases n = 1) of 17 malignant cases radiological examination initially gave no evidence of malignancy, resulting in an unplanned excision (“whoops procedure”) in three cases of osteosarcoma. Applying Diard’s system trabecular area 6 (radiolucent area) was highly affected in 64 (80 %) of 80 investigated plain X-rays, whereas areas 1 and 5 were affected in nine (11 %) and 16 (20 %) cases only.
Conclusions
In each case of an osteolytic lesion of the calcaneus a malignant tumour must be ruled out, and thus preoperative plain X-rays in two planes alone are not sufficient and should therefore be followed by magnetic resonance imaging. Applying the Diard system different types of lesions are not characteristically distributed in the bone. Increasing pain for more than ten days without previous trauma should always justify further examinations.
Keywords: Calcaneus, Osteolytic lesions, Malignancy, Diard system, Pain, Enneking classification
Introduction
Tumours of the calcaneus are exceedingly rare, although intraosseous lipomas and simple bone cysts are disproportionately frequent at this site compared to the rest of the appendicular skeleton [1–3]. Due to their rarity, the correct diagnosis is often missed for months and in addition the treatment is often inadequate [4, 5]. Patients with tumours involving the musculoskeletal system often present with a history of trauma, which may mislead the physician when determining the correct diagnosis. Both benign and tumour-simulating processes are asymptomatic at first, since they grow slowly. Thus they are often found incidentally [6].
This retrospective data analysis of 92 patients with osteolytic lesions of the calcaneal bone was initiated to investigate
If X-rays alone were a sufficient diagnostic imaging tool for these lesions.
Furthermore, we wanted to determine whether different types of lesions were characteristically distributed with regard to the calcaneal bone architecture according to Diard et al. [7].
Finally, type and duration of symptoms were analysed as possible indicators of malignancy.
Methods
All documented cases of osteolytic lesion of the os calcis that were admitted to our departments [Berlin (n = 9), Vienna (n = 12), Graz (n = 27), Birmingham (n = 44)] between 1992 and 2011 were included in this study. Accordingly, data from 92 patients (59 men and 33 women; one male patient had bilateral lesions) were acquired. In 77 cases patients underwent surgery (mean age at surgery 28 years; range five to 78). However, not all conservatively treated cases were included in this study, as they often were not documented in the tumour databases. The distribution of definitive diagnoses is shown in Fig. 1.
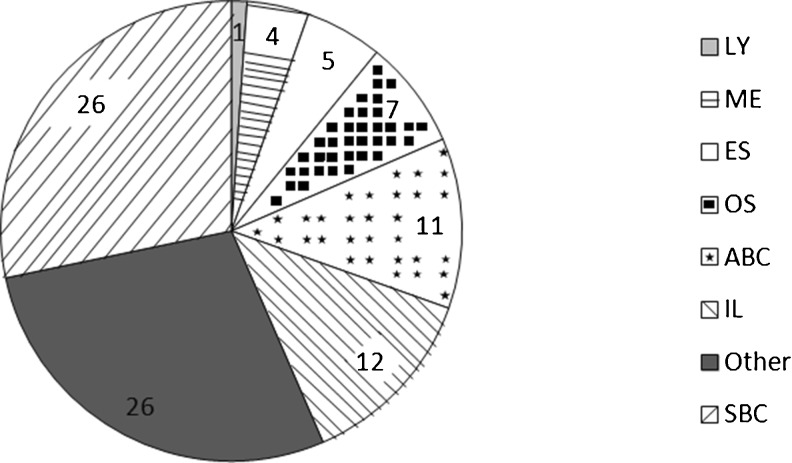
Fig. 1.
Distribution of definitive diagnoses. LY lymphoma, ME metastases, ES Ewing’s sarcoma, OS osteosarcoma, ABC aneurysmatic bone cyst, IL intraosseous lipoma, Other osteomyelitis and osteonecrosis, SBC simple bone cyst. The definitive diagnosis in 75 (81 %) of 92 cases was that of a benign lesion. Of these benign lesions, 26 were diagnosed as simple bone cysts, 26 as osteomyelitis or osteonecrosis, 12 as intraosseous lipomas and 11 as aneurysmal bone cysts. Malignant lesions were present in 17 (18 %) cases, of which 4 patients presented with metastatic disease with known primary site affecting the os calcis (1 female patient with breast cancer and 3 male patients with rectal carcinoma, colon carcinoma and lung cancer, respectively). Furthermore, seven osteosarcomas, five Ewing’s sarcomas and one lymphoma were diagnosed
The patients’ files were retrospectively examined regarding sex, age at surgery, side, type and duration of symptoms, tentative diagnosis, biopsy prior to surgery, operative procedure, recurrence rate, revision and localisation of the lesion according to Diard’s classification. We wanted to analyse type and duration of symptoms especially as possible indicators of malignancy. The tentative diagnosis was defined as the first documented radiological diagnosis. Accordingly, for each lesion the first documented radiological report and—in cases of malignancy—Enneking’s classification [8, 9] for malignant tumours were retrieved from the patients’ files to determine whether tentative radiological and definitive diagnoses corresponded. Tentative radiological diagnoses had been made either by an expert radiologist or an orthopaedic consultant at the local centres, or by external orthopaedic consultants referring the patients. Plain X-rays were classified according to Diard et al. [7] enabling us to define the precise amount of affected trabecular bone areas (Figs. 2, 3, and 4). Intraosseous lipomas were additionally re-examined according to Milgram’s classification [10, 11]. Nineteen patients had been included in previous studies [12–14].
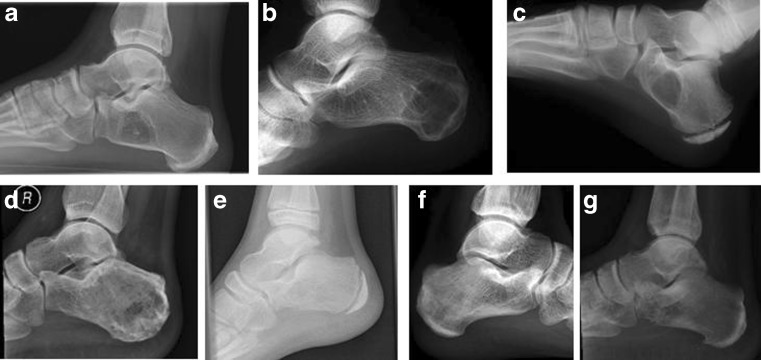
Fig. 2.
Entity, sex/age. a Intraosseous lipoma, male/35; b aneurysmatic bone cyst, female/21; c simple bone cyst, male/11; d giant cell tumour, male/43; e Ewing’s sarcoma, male/17; f osteosarcoma, female/21; g metastatic bronchial carcinoma, male/81
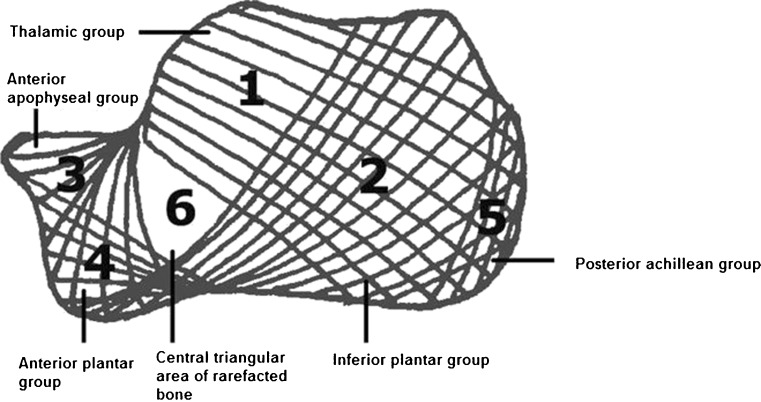
Fig. 3.
Calcaneal trabecular bone architecture. According to Diard et al. [7] the calcaneal trabecular bone architecture is described by six trabecular bone groups. In terms of functionality, the thalamic group (area 1) is the most important one, characterised by a trabeculation arising from the subchondral bone of the subtalar joint spreading on the cortex of the posterior tuberosity with a concave anteroinferior curve. The inferior plantar group (area 2) arises from the inferior cortex and spreads on the cortex of the posterior tuberosity with an anterosuperior curve. The anterior apophyseal group (area 3) is small and arises from the calcaneal cortex next to the tarsal sinus and spreads to the subchondral cortex of the anterior tuberosity. The anterior plantar group (area 4) is much reduced, spreading from the anterior part of the inferior cortex to the cortex of the anterior tuberosity. The posterior or achillean group (area 5) is parallel and close to the posterior cortex strengthening the posterior tuberosity. The first three major groups intertwine and delineate a central triangular radiolucent area (area 6) of rarefacted bone
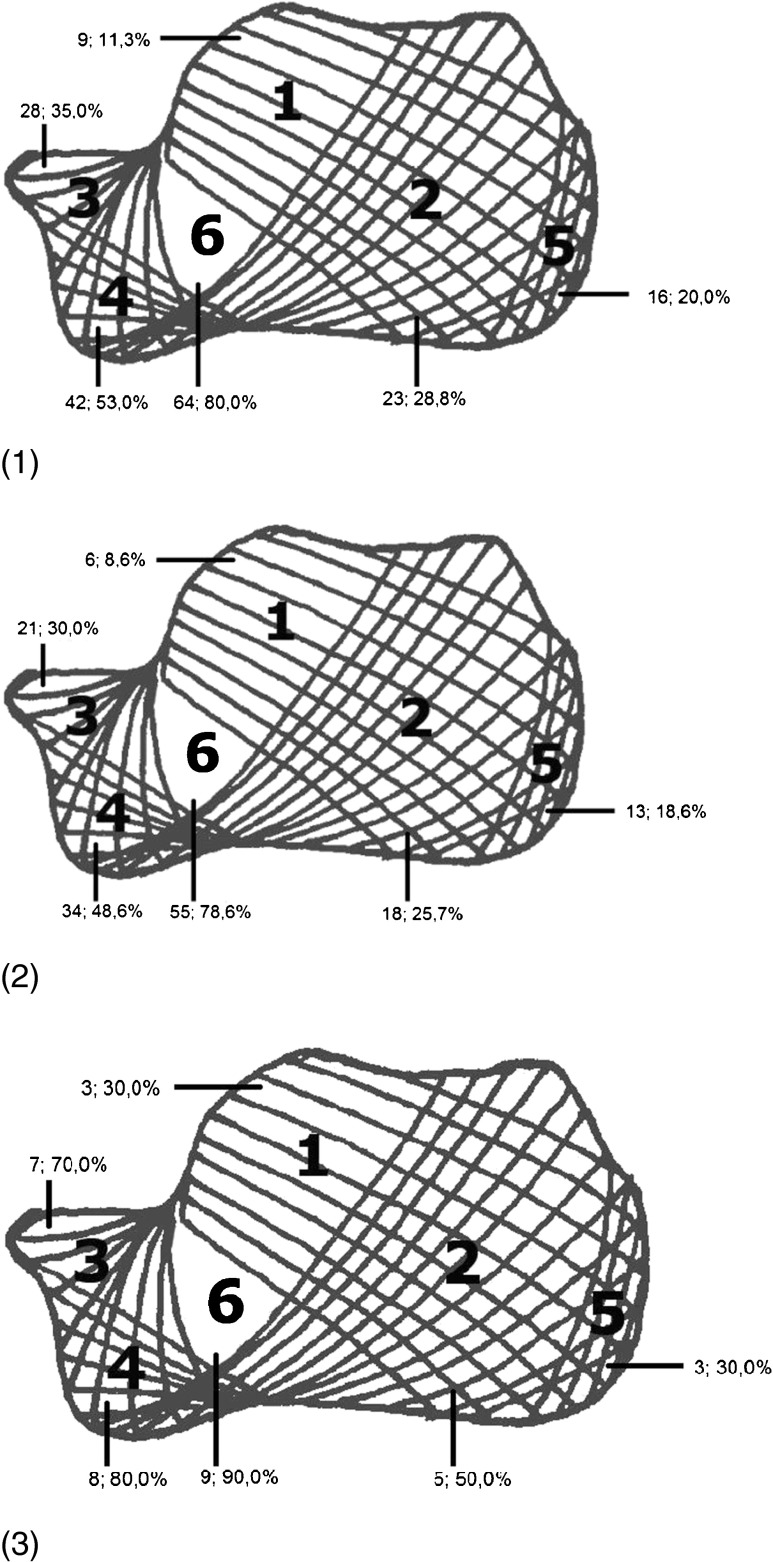
Fig. 4.
Localisation of lesions (calcaneal trabecular bone architecture according to Diard): 1 all lesions, 2 benign lesions, 3 malignant lesions. Trabecular area 6 (radiolucent area) was affected in 64 (80 %) of 80 investigated plain X-rays, whereas areas 1 and 5 only were affected in 9 (11 %) and 16 (20 %) cases, respectively. The lesions were mainly (58 %) located in inferior anterior parts of the calcaneal bone. In 48 (64 %) of 75 benign cases the lesions were restricted either to the inferior anterior part (areas 3, 4 and 6) of the bone, to the central triangular area of rarefacted bone (area 6) or to the anterior plantar bone areas 4 and 6. Malignant lesions were most frequently (68 %) found in the inferior anterior part (areas 3, 4 and 6) of the bone as well
Results
Seventy-nine patients were alive as of October 2011 with a mean follow-up of 4.6 (range 0–14.1) years. Patients’ features are summarised in Table 1.
Table 1.
Patients’ features
| n (total) | Surgical treatment | Side | Sex | Mean duration of symptoms (months) | Surgical procedure | Mean age at surgery (years) | Mean follow-up (years) in patients with surgery | Recurrence rate | Status | |
|---|---|---|---|---|---|---|---|---|---|---|
| Benign cases | 75 | 64 | 36 right, 39 left | 27 F, 48 M | 11.4 (range 1–60) | 62 curettages, 1 amputation, 1 asp/bm | 72.5 (range 5–78). | 4.8 (range 1 month–14 years) | 0 | 64 NED, 7 AWD, 4 LOST |
| Malignant cases | 17 | 13 | 12 right, 5 left | 6 F, 11 M | 17.2 (range 1–60) | 7 curettages, 4 amputations below knee, 2 not documented | 35.3 (range 8–68). For primary malignant cases: 27.1 (range 8–38) | 4.4 (range 0 months–13.8 years). For primary malignant cases: 4.6 (range 0 months–13.9 years) | 3 | 8 DOD, 5 NED, 3 AWD, 1 LOST |
Sixty-nine patients had no evidence of disease and ten were alive with disease (AWD). Five patients were lost to follow-up and eight patients died of disease (four osteosarcomas, three metastases, one Ewing’s sarcoma). Patients with primary malignant disease had a 4.6-year mean follow-up (range 0–13.9). For all malignant conditions, the mean age at surgery was 35.3 years (range 8–68). All patients with benign tumours who underwent surgery remained free of recurrence. Sixty-two curettages were documented as well as one injection of bone marrow and one below knee amputation due to a desmoplastic fibroma. For benign lesions, the mean age at surgery was 72.5 years (range 5–78)
asp/bm aspiration/bone marrowNED no evidence of disease, AWD alive with disease, DOD dead of disease, LOST lost to follow-up
When admitted to our departments, the main current complaint was of pain without prior history of trauma in 53 (66 %) of 80 documented cases (n = 12 not documented). In 15 (18 %) cases the lesions were detected incidentally. Symptoms, if any, preceded the definitive diagnosis for a mean time of 12.6 (range 1–60) months. The mean time in benign lesions was 11.4 (range 1–60; n = 11 not documented) months. For malignant lesions, a mean time of 17.2 (range 1–60; n = 4 not documented) months was documented.
Radiological appearance was suspicious for a malignant lesion in 27 of 89 cases (three patients without X-rays available), and thus those patients underwent open biopsy prior to definitive surgery. In 11 cases histological samples were subjected to intraoperative frozen section analysis. Surgery was performed in 37 cases (n = 2 not documented) without biopsy. Here, local recurrence occurred in three cases, which were then correctly revised to high-grade osteosarcomas.
Tentative radiological diagnosis differed from definitive histological examination in 38 (41 %) of 92 cases (n = 3 radiological diagnoses were not available) (Table 2). Five of seven (n = 2 not documented) cases of osteosarcoma were initially described as benign lesions based on tentative radiological diagnosis and thus resulted in inadequate operative treatment (“whoops procedure” [15]) in three cases. These patients finally underwent amputation surgery due to local recurrence.
Table 2.
Definitive histological diagnosis and corresponding tentative radiological diagnoses
| Definitive diagnosis | n (total) | Tentative radiological diagnosis (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aneurysmatic bone cyst | Ewing’s sarcoma | Intraosseous lipoma | Metastasis | Other/multiplea | Simple bone cyst | Osteosarcoma | Lymphoma | ND | ||
| Aneurysmatic bone cyst | 11 | 7 | – | 1 | – | 1 | 2 | – | – | – |
| Ewing’s sarcoma | 5 | – | 3 | – | – | 2 | – | – | – | – |
| Intraosseous lipoma | 12 | – | – | 6 | – | – | 6 | – | – | – |
| Metastasis | 4 | – | – | – | 3 | 1 | – | – | – | – |
| Other (osteonecrosis and osteomyelitis) | 26 | 3 | – | 2 | 1 | 13 | 7 | – | – | – |
| Simple bone cyst | 26 | 3 | – | 1 | – | – | 22 | – | – | – |
| Osteosarcoma | 7 | 1 | – | – | – | 2 | 2 | – | – | 2 |
| Lymphoma | 1 | – | – | – | – | – | – | – | – | 1 |
For each condition, the tentative radiological diagnosis was retrieved from the patient’s files. Tentative radiological diagnosis differed from definitive histological examination in 38 (41 %) of 92 cases (n = 3 radiological diagnoses were not available). In benign lesions 28 (37 %) of 75 tentative diagnoses differed from the definitive histology. Of 12 intraosseous lipomas, 6 were correctly reported as benign lesions with typical central calcification, whereas, if radiologically missed (n = 6), intraosseous lipomas were suspected to be aneurysmal bone cysts. According to Milgram’s classification [10, 11], intraosseous lipomas were retrospectively classified as two stage I lesions, three stage II lesions and one stage III lesion (n = 6 MRI not available). In three of four cases with metastatic disease plain X-rays were correctly signed out. Imaging showed specific features that were consistent with those of aggressive bone lesion with cloudy, partly osteolytic, partly osteoblastic areas. However, in the case of a 77-year-old male patient with metastatic disease plain X-rays were initially misdiagnosed as stress fracture. In one case of osteosarcoma the time interval between onset of first symptoms and histologically correctly signed out diagnosis was 60 months. This lesion was initially diagnosed as a simple bone cyst and treated with curettage alone in terms of an unplanned excision (whoops procedure [15]) leaving a residual tumour
ND not documented
aMore than one tentative diagnosis was made
To define the affected trabecular area of the calcaneal bone, the classification according to Diard et al. [7] (Fig. 3) was applied in 80 patients (n = 12 plain X-rays were not available). Trabecular area 6 (radiolucent area) was affected in 64 (80 %) of 80 plain X-rays, whereas areas 1 and 5 only were affected in nine (11 %) and 16 (20 %) cases, respectively (Fig. 4).
Discussion
Tumours of the calcaneus are exceedingly rare, although intraosseous lipomas and simple bone cysts are disproportionately frequent at this site compared to the rest of the appendicular skeleton. The data presented herein suggest that a benign lesion may not be routinely deduced from benign-looking radiological features accompanied by a long history of symptoms.
To avoid misdiagnoses, we strongly recommend plain X-rays in two planes and magnetic resonance imaging (MRI) with contrast medium enhancement [5, 7]. Computed tomography (CT) scans can also be useful, because bony structures can be assessed better [6, 26]. At the time of surgery, imaging should not be older than four weeks [16].
When admitted to our departments, the main current complaint was of pain without preceding trauma in close to two thirds of documented cases. To our knowledge, these symptoms may occur in many conditions, ranging from trauma to chronic inflammation to malignant neoplasms. Astonishingly, there was at least a 12-month interval between onset of first symptoms and correct diagnosis in six of 13 documented cases with malignant lesions (mean interval 17.2 months; range one to 60). Delayed treatment usually leads to higher morbidity, often making limb salvage impossible, which is functionally devastating, and can even lead to immediate death from the disease [1]. As the data presented herein reveal Ewing’s sarcoma and osteosarcoma in the majority of malignant cases, malignancy should be ruled out especially in children as these conditions frequently occur in young patients.
A benign asymptomatic lesion is not necessarily an indication for surgery. Often, the best policy for these lesions is a radiological examination after three and nine months, followed by annual examination in the context of a “watch and wait” approach [14]. According to our data, curettage seems to be an adequate treatment option for benign lesions that are symptomatic or at risk of fracture [2, 3, 10, 17–19]. As this paper is based on multicentric data, we can only assume that all treatment regimes for benign lesions were appropriate as there was no control group in this retrospective study. In addition, we assume that our documentation of pathological or incipient fractures in benign lesions is insufficient. In 26 patients with simple bone cysts only two cases were not subjected to surgery, although in previous literature reports pathological fractures are exceptional [20]. The same authors report that simple bone cysts are very rarely symptomatic, which is in complete contradistinction to our data, since all patients with simple bone cysts reported severe pain and swelling. A partial explanation for this inconsistency is the fact that several of our patients had calcaneal spurs that perhaps caused the symptoms, and thus the simple bone cysts were misdiagnosed as symptomatic. Accordingly, we should admit that some benign cases presented herein could also have been treated in terms of a watch and wait approach. However, we did not observe local recurrence for benign lesions in patients treated with curettage. Whenever local recurrence was observed, the patients had been primarily treated in terms of a whoops procedure.
The only adequate treatment for primary malignant bone tumours is wide resection [16]. Concerning the calcaneal bone many authors favour below knee amputation [1, 5, 21–23] after major resection of malignant tumours. While limb salvage for osteosarcoma of long bones is accepted as state-of-the-art treatment, it can rarely be applied for malignant tumours of the calcaneus, due to loss of function [12]. Limb salvage of primary malignant calcaneal tumours is sometimes performed for stage I (low-grade) and stage IIA (high-grade, intracompartmental) lesions according to Enneking [8, 9].
The calcaneal trabecular bone architecture is divided into six trabecular bone groups [7]. Due to this unique architecture a central triangular radiolucent area (area 6) results and may be confused with a cystic lesion, because both entities can have similar radiological features. Diard et al. describe area 6 as fatty pseudocyst [7]; approximately 70 % of all patients show this radiolucent area as an anatomical variant [7]. Correct diagnosis can therefore be demanding even for experienced consultants. On the other hand, our results show that most lesions are at least partially located in this central triangular radiolucent area. However, the classification according to Diard et al. [7] does not support definitive conclusions regarding the true nature of the lesion because our data give no evidence of a characteristic distribution of benign and malignant lesions in the os calcis.
The diagnosis of an osteosarcoma of the calcaneus should be regarded as especially demanding. Even histological examination seems to be limited sometimes, because it does not routinely reveal the correct diagnosis. Several factors may contribute to this, since in contrast to other locations differing biological behaviour of calcaneal osteosarcoma has been noted before [2, 3, 12, 13, 24]. Some authors suggest that osteosarcoma of the foot may represent a distinct subgroup with clinical features that differ highly from conventional osteosarcoma [5, 22]. Clinically, radiographically and histologically they often mimic benign lesions. On the other hand, some benign lesions can have the appearance of an osteosarcoma [5]. Due to their rarity, correct diagnosis of malignant lesions of the foot is often missed for months [4, 5, 21]. Thus, inadequate therapy is instituted. Based on previous data, the mean time interval between onset of symptoms and correct diagnosis is usually more than one year [5, 12, 13, 21]. Case reports of missed osteosarcoma of the calcaneus have been reported [5, 12]. The results of our study appear to be consistent with previous data [25, 26].
To conclude, the results of this paper suggest that:
X-rays alone are insufficient to assess the true nature of all lesions. To obtain the correct diagnosis, preoperative plain X-rays in two planes and MRI with contrast enhancement should be performed in symptomatic lesions.
Most osteolytic lesions seem to have their origin in the central triangular radiolucent area (area 6) according to Diard. However, Diard’s classification does not allow malignancy to be ruled out, because there is no characteristic distribution of benign and malignant lesions in the os calcis.
Increasing pain without trauma for more than ten days should always justify further examinations. If there is any suspicion of malignancy, a biopsy is required to establish the true nature of the lesion prior to operative treatment.
References
- 1.Kilgore WB, Parrish WM. Calcaneal tumors and tumor-like conditions. Foot Ankle Clin. 2005;10:541–565. doi: 10.1016/j.fcl.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Pogoda P, Priemel M, Linhart W, et al. Clinical relevance of calcaneal bone cysts: a study of 50 cysts in 47 patients. Clin Orthop Relat Res. 2004;424:202–210. doi: 10.1097/01.blo.0000128297.66784.12. [DOI] [PubMed] [Google Scholar]
- 3.Radl R, Leithner A, Machacek F, et al. Intraosseous lipoma: retrospective analysis of 29 patients. Int Orthop. 2004;28:374–378. doi: 10.1007/s00264-004-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adkins CD, Kitaoka HB, Seidl RK, Pritchard DJ. Ewing’s sarcoma of the foot. Clin Orthop Relat Res. 1997;343:173–182. doi: 10.1097/00003086-199710000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Biscaglia R, Gasbarrini A, Bohling T, et al. Osteosarcoma of the bones of the foot–an easily misdiagnosed malignant tumor. Mayo Clin Proc. 1998;73:842–847. doi: 10.4065/73.9.842. [DOI] [PubMed] [Google Scholar]
- 6.Windhager R, Kastner N, Leithner A. Benigne Knochentumoren und tumorähnliche Läsionen. Monatsschr Kinderheilkd. 2006;154:20–31. doi: 10.1007/s00112-005-1274-3. [DOI] [Google Scholar]
- 7.Diard F, Hauger O, Moinard M, et al. Pseudo-cysts, lipomas, infarcts and simple cysts of the calcaneus: are there different or related lesions? JBR-BTR. 2007;90:315–324. [PubMed] [Google Scholar]
- 8.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 9.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 10.Milgram JW. Intraosseous lipomas. A clinicopathologic study of 66 cases. Clin Orthop Relat Res. 1988;231:277–302. [PubMed] [Google Scholar]
- 11.Milgram JW. Intraosseous lipomas: radiologic and pathologic manifestations. Radiology. 1988;167:155–160. doi: 10.1148/radiology.167.1.3347718. [DOI] [PubMed] [Google Scholar]
- 12.Leithner A, Bodo K, Scheipl S, et al. Two cases of calcaneal osteosarcomas presenting as aneurysmal bone cysts. Foot Ankle Int. 2004;25:815–818. doi: 10.1177/107110070402501111. [DOI] [PubMed] [Google Scholar]
- 13.Leithner A, Scheipl S, Maurer-Ertl W et al (2007) Osteolytische Läsionen des Calcaneus: eine Serie von 18 Patienten. MOT 1:57–60
- 14.Salzer-Kuntschik M, Brand G, Delling G. Bestimmung des morphologischen Regressionsgrades nach Chemotherapie bei malignen Knochentumoren. Pathologe. 1983;4:135–141. [PubMed] [Google Scholar]
- 15.Marsh E, Egan H, Cave R, Long J, Abudu A, Grimer RJ. Impact of whoops procedure on outcome of primary osteosarcoma. J Bone Joint Surg Br. 2011;93-B(SUPP III):316-a. [Google Scholar]
- 16.Leithner A, Maurer-Ertl W, Windhager R. Biopsy of bone and soft tissue tumours: hints and hazards. Recent Results Cancer Res. 2009;179:3–10. doi: 10.1007/978-3-540-77960-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Hanna SJ, Dasic D, Floyd A. Simple bone cysts of the calcaneus: a report of five cases and a review of the literature. Foot Ankle Int. 2004;25:680–684. doi: 10.1177/107110070402500914. [DOI] [PubMed] [Google Scholar]
- 18.Pogoda P, Priemel M, Catalá-Lehnen P, et al. Kalkaneuszysten: Differentialdiagnose und Therapie. Unfallchirurg. 2004;107:680–689. doi: 10.1007/s00113-004-0783-1. [DOI] [PubMed] [Google Scholar]
- 19.Vergel De Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::AID-CNCR2820691210>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Grumbine NA, Clark GD. Unicameral bone cyst in the calcaneus with pathologic fracture. A literature review and case report. J Am Podiatr Med Assoc. 1986;76:96–99. doi: 10.7547/87507315-76-2-96. [DOI] [PubMed] [Google Scholar]
- 21.Choong PF, Qureshi AA, Sim FH, Unni KK. Osteosarcoma of the foot: a review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70:361–364. doi: 10.3109/17453679908997825. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji Y, Kusuzaki K, Kanemitsu K, et al. Calcaneal osteosarcoma associated with Werner syndrome. A case report with mutation analysis. J Bone Joint Surg Am. 2000;82:1308–1313. doi: 10.2106/00004623-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Wagner A, Venbrocks RA, Fuhrmann RA. Chondrosarcoma of the calcaneus: amputation or resection with limb preservation: a case report. Foot Ankle Int. 2007;28:1090–1094. doi: 10.3113/FAI.2007.1090. [DOI] [PubMed] [Google Scholar]
- 24.Chou LB, Malawer MM. Osteosarcoma of the calcaneus treated with prosthetic replacement with twelve years of followup: a case report. Foot Ankle Int. 2007;28:841–844. doi: 10.3113/FAI.2006.0841. [DOI] [PubMed] [Google Scholar]
- 25.Young PS, Bell SW, MacDuff EM, Mahendra A. Primary osseous tumors of the hindfoot: why the delay in diagnosis and should we be concerned? Clin Orthop Relat Res. 2013;471(3):871–877. doi: 10.1007/s11999-012-2570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anninga JK, Picci P, Fiocco M, Kroon HMJA, Vanel D, Alberghini M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2012;462(1):109–120. doi: 10.1007/s00428-012-1339-3. [DOI] [PubMed] [Google Scholar]