Abstract
Dietary supplementation with omega-3 (ω-3) fatty acids is a safe, economical mean of preventive medicine that has shown protection against several neurologic disorders. The present study tested the hypothesis that this method is protective against controlled cortical impact (CCI). Indeed, mice fed with ω-3 polyunsaturated fatty acid (PUFA)-enriched diet for 2 months exhibited attenuated short and long-term behavioral deficits due to CCI. Although ω-3 PUFAs did not decrease cortical lesion volume, these fatty acids did protect against hippocampal neuronal loss after CCI and reduced pro-inflammatory response. Interestingly, ω-3 PUFAs prevented the loss of myelin basic protein (MPB), preserved the integrity of the myelin sheath, and maintained the nerve fiber conductivity in the CCI model. ω-3 PUFAs also directly protected oligodendrocyte cultures from excitotoxicity and blunted the microglial activation-induced death of oligodendrocytes in microglia/oligodendrocyte cocultures. In sum, ω-3 PUFAs elicit multifaceted protection against behavioral dysfunction, hippocampal neuronal loss, inflammation, and loss of myelination and impulse conductivity. The present report is the first demonstration that ω-3 PUFAs protect against white matter injury in vivo and in vitro. The protective impact of ω-3 PUFAs supports the clinical use of this dietary supplement as a prophylaxis against traumatic brain injury and other nervous system disorders.
Keywords: behavior (rodent), brain trauma, inflammation, neurodegeneration, white matter/oligodendrocytes
Introduction
Traumatic brain injury (TBI), with its poor prognosis and long-term consequences, is the leading cause of death among children and young adults. According to the Centers for Disease Control and Prevention, the total annual cost for TBI-related injuries is estimated at 76.5 billion in the United States alone (http://www.cdc.gov/traumaticbraininjury/statistics.html#3). While a cure for this devastating condition still remains elusive, much effort has been invested in translational research to understand and mitigate the clinical symptoms derived from TBI. For example, dietary supplements have garnered increasing attention for both prevention and treatment of brain disorders. Among the many dietary supplements explored to date, omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have shown great promise in conferring protection against several neurologic disorders.1, 2, 3, 4, 5 Moreover, dietary supplementation with ω-3 PUFA, such as fish oil, in humans is extremely safe and can be ingested over long term as a prophylactic measure.6 In other words, healthy individuals can ingest fish oil capsules on a daily basis as a preventive measure towards brain injuries.
Functionally, dietary enrichment with ω-3 PUFAs maintains Ca2+ ion and energy homeostasis,7 decreases cognitive deficits and enhances learning ability during aging,8 and improves the prognosis of ischemic injury, Alzheimer's, and Parkinson's diseases.1, 2, 3 In view of its manifold effects, dietary supplementation with ω-3 PUFAs is likely to also ameliorate deleterious effects of TBI on brain function. To date, there are limited investigations on the impact of dietary supplementation with ω-3 PUFAs on TBI.7, 9 Nevertheless, these studies have consistently shown protection against behavioral deficits and cellular degeneration. However, the effect of ω-3 PUFAs on other facets of TBI, such as secondary inflammatory responses, nerve fiber degeneration, and axonal and myelin sheath damage, have yet to be investigated. These effects are important to study because inflammation and impairments in the conduction of nerve impulses are both likely to severely impair acute patient recovery and long-term neurologic function.
In the present study, we characterized the impact of long-term ω-3 PUFA dietary supplementation on inflammatory responses and white matter injury after controlled cortical impact (CCI) in mice. ω-3 PUFAs were administered in food for 2 months before CCI. This study was designed to test the preventive effects of chronic ω-3 PUFA treatment on future injuries. We measured the extent of microglial activation, release of cytokines, and the expression of inflammatory mediators. In addition, the effects of this dietary alteration on white matter injury and changes in axonal conductivity after CCI were investigated. Finally, we elucidated two parallel protective mechanisms utilized by ω-3 PUFAs to preserve oligodendrocytes in vitro. This protection may account for the in vivo attenuation of CCI-induced white matter injury as well as the improvements in sensorimotor and cognitive functions.
Materials and Methods
Materials
All chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated.
Methods
Animal preparation and omega-3 polyunsaturated fatty acid treatment
Male C57BL/6J mice (Laboratory Animal LLC, Shanghai, China) were housed in groups in a 12-hour light–dark cycle with free access to food and water. All procedures were performed according to protocols approved by the Animal Care and Use Committee at Fudan University. Three-week-old mice were fed on a regular diet with an inherently low ω-3 PUFA concentration (0.5%) or the same diet supplemented with ω-3 PUFAs (docosahexaenoic and eicosapentaenoic acids, 15 g/kg, triple strength ω-3 fish oil, Puritan's Pride, Oakdale, NY, USA) for 2 months before TBI experiments were conducted. At the end of the 2 months' supplementation with ω-3 PUFA-enriched diet, the pO2, pCO2, pH, and blood pressure showed no statistical difference with those on regular diet before surgery when measured before and 15 minutes after surgery.
Traumatic brain injury
Mice were randomly assigned to CCI or sham groups before surgery. The surgeon was masked to dietary conditions (regular diet or ω-3 PUFA supplement diet). The CCI procedure was carried out as previously described.10 Sham animals were subjected to all aspects of the protocol (surgery, anesthesia, craniotomy, recovery) except for the trauma. For the TBI surgery, anesthesia was induced by 3% isoflurane in 67% N2/30% O2 mixture in a plastic jar and maintained with 1.5% isoflurane via nose cone. Rectal temperature was maintained at 37±0.5°C by a thermostat-controlled heating blanket during and after surgery. Less than 2% of mice were excluded for symptoms of suffering, e.g., inability to eat and drink without assistance, wound infection, or behavioral manifestations of pain. Of the 150 mice used in this study, 2 mice died from deep anesthesia, 2 mice died from traumatic epilepsy, and 2 mice died from low body weight giving rise to an overall mortality rate of 4%. The time lines for ω-3 PUFA supplementation, TBI, and various post-TBI outcome assessments are illustrated in Supplementary Figure 1.
Wire hanging test
The wire hanging apparatus was comprised of a stainless steel bar (50 cm; 2 mm diameter), resting on two vertical supports and elevated 37 cm above a flat surface. This test was performed as previously described10 by researchers masked to experimental groups.
Grid walking and foot-fault test
The grid walking test is sensitive to deficits in descending motor control. Each mouse was placed on a stainless steel grid floor (20 × 40 cm with a mesh size of 4 cm2) elevated 1 m above the floor. For a videotaped 1-minute-long observation period, the total number of steps was counted. The number of foot-fault errors (when the animals misplaced a forelimb or hind limb such that it fell through the grid) was also recorded for 1 minute.
Cylinder test and the Morris water maze test
The cylinder test and the Morris Water Maze tests were carried out as previously described10 by researchers masked to experimental groups. In the cylinder test, a total of 20 movements were recorded during the 10-minute test. The final score was determined based on the following formula: final score =(non-impaired forelimb movement−impaired forelimb movement)/ (non-impaired forelimb movement+impaired forelimb movement+both movements). This test evaluates forelimb use asymmetry for weight shifting during vertical exploration and provides high reliability even with inexperienced raters. Occasionally, mice with large deficits did not move frequently enough to obtain an adequate number of vertical movements. Typically, these mice would recover in time when the test was performed. To avoid bias, these mice were not scored until they could perform the test. These tests were carried out by researchers masked to the study groups.
In vitro culture
Primary oligodendrocytes were prepared from mixed glial cultures of P1 mouse brains.11 Briefly, microglia were isolated by shaking flasks containing mixed glia for 1 hour at 180 r.p.m. and collected. Fresh media was then added to the flasks and flasks were then shaken overnight at 200 r.p.m. Oligodendrocyte precursor cells were subsequently collected. To culture oligodendrocytes, basal chemically defined medium11 containing 15 nmol/L triiodothyronine and 10 ng/mL ciliary neurotrophic factor were used. To construct microglia–oligodendrocyte cocultures, microglia were grown on removable culture inserts (1 × 104/well). Microglia and oligodendrocytes were cocultured in 24-well transwell systems by adding microglia in the inserts on top of oligodendrocyte cultures in the lower chamber (1 × 105/well). A 10:1 ratio of oligodendrocytes to microglia was plated in accordance with the ratio of these two types of glia in the cerebral cortex in vivo.12
Tissue section preparation
At 35 days post CCI, mice were anesthetized with chloral hydrate (360 mg/kg, intraperitoneal) and transcardially perfused with saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). Brains were subsequently removed and transferred into a 20% sucrose solution in phosphate-buffered saline overnight for cryoprotection. Frozen serial coronal brain sections were sliced at 25 μm on a cryostat (CM1900, Leica, Bensheim, Germany) beginning +1.42 mm from bregma.
Nissl staining
For Nissl staining, the free-floating sections were mounted onto slides and processed through different baths in the following order: chloroform, 30 minutes; acetone, 15 minutes; 100% ethyl alcohol (EtOH), 30 seconds; 95% EtOH, 30 seconds; 70% EtOH, 30 seconds; distilled water, 30 seconds, twice; cresyl violet, 20 minutes; distilled water, 30 seconds, three times; 70% EtOH, 1 minute; 95% EtOH, 1 minute; 100% EtOH, 1 minute; chloroform, 5 minutes; differentiator (95% EtOH, with glacial acetic acid added to a pH of 4.1), 6 minutes; 95% EtOH, 2 minutes, 100% EtOH, 3 minutes, twice; xylene, 2 minutes; xylene, 3 minutes, twice. After staining, sections were mounted with neutral balata and cover slipped.
Measurement of tissue lost
Brain sections were subjected to Nissl staining for histologic assessment of damage and the adjacent sections were processed for immunohistochemical analysis. The Nissl-stained brain slices were photographed (DM5000B, Leica) and lesion volumes measured with ImageJ analysis software (NIH, Bethesda, MD, USA). Percentage lesion volume was calculated by an investigator masked to the study groups with the following formula: [(VC–VL)/VC] × 100, where VC is the volume of contralateral hemisphere (left side) and VL the volume of non-injured tissue in the ipsilateral hemisphere subjected to CCI (right side).
Estimation of the number of viable neurons at hippocampus CA3
To visualize the surviving neurons in the CA3 region of the hippocampus, the abovementioned Nissl-stained brain sections from mice at 35 days post CCI were used for cell counts. For each animal, four serial sections (between −1.34 and −2.18 mm from the bregma) were subjected to cell counts to obtain an average number of viable neurons. For each section, three non-overlapping fields of the CA3 region were captured for counting. The numbers of surviving neurons in the CA3 region were counted in low magnification ( × 20) of a light microscope (Q5701W, Leica, Germany) by a masked observer using Stereo Investigator software (MBF Bioscience, Williston, VT, USA).
Immunohistochemical staining
The two primary antibodies used were mouse anti-SMI32 antibody (non-phosphorylated neurofilament H monoclonal, Covance, Princeton, NJ, USA) and rabbit anti-myelin basic protein 1 (MBP-1) antibody (Millipore, Billerica, MA, USA). Brain sections derived from mice at 35 days post CCI were incubated with each of the primary antibodies in turn for 1 hour at 37°C each and then at 4°C overnight. After three washes in phosphate-buffered saline, sections were incubated in a mixture of anti-rabbit secondary antibody conjugated with DyLight 488 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and anti-mouse secondary antibody conjugated with DyLight 594 (Jackson ImmunoResearch Laboratories) for 1 hour at 37°C. Sections were then counterstained with 4',6-diamidino-2-phenylindole (Thermo Scientific, Pittsburgh, PA, USA) for 2 minutes at room temperature followed by mounting with Fluoromount-G (Southern Biotech, Birmingham, AL, USA).
For the staining of activated microglia, sections through the cortex and striatum at 24 hours post injury were first incubated with rabbit anti-Iba-1 antibody (Wako, Tokyo, Japan) for 1 hour at 37°C and then at 4°C overnight. The following day, sections were incubated with anti-rabbit secondary antibody conjugated with DyLight 594 for 1 hour at 37°C as above. Sections were then mounted with Fluoromount-G as above.
For densitometric analysis, a computerized camera-based NIH Image-analysis system (available at: http://rsb.info.nih.gov/nih-image/) was used as previously described.13 Briefly, areas of interest in the damaged peripheral zone were digitally captured as TIFF images at equivalent exposure times. The images were then binarized and segmented under a consistent threshold (50%). Next, the total black pixels per image were counted. To account for the differences in fluorescent intensity between non-injured regions in immunostained sections, pixel values were calculated as the ratio of injury in the ipsilateral (IP) relative to the intact contralateral (CL) hemisphere (IP: CL=lesion: intact hemisphere). The results used for analysis were therefore presented as pixels of IP: CL.
Tissue processing and total RNA extraction
At 24 hours post CCI, mice were killed by decapitation under deep chloral hydrate (360 mg/kg, intraperitoneal) anesthesia. The brain was quickly removed and the ipsilateral hemisphere subjected to CCI was isolated, snap-frozen on dry ice, and stored at −80°C. During total RNA preparation, the brain sample was first homogenized by glass dounce homogenizer before actual extraction with Trizol (Applied Biosystems, Grand Island, NY, USA) in accordance with the manufacturer's protocol. The amount of total RNA was quantified by ultraviolet spectrophotometry.
Reverse transcription and semi-quantitative real-time polymerase chain reaction
Reverse transcription was conducted with a RT reagent kit (Promega, Madison, WI, USA) in accordance with the manufacturer's protocol. One μg of total RNA was used to synthesize the first strand of cDNA. Polymerase chain reaction analyses were performed with gene-specific primers (sequences listed below), using the endogenous control of glyceraldehyde-3-phosphate dehydrogenase messenger (mRNA) levels. Polymerase chain reaction was performed using SYBR green PCR Master Mix (Tiangen Biotech, Beijing, China). Real-time data were analyzed with a Mastercycler realplex analysis system (Eppendorf, Hamburg, Germany). All samples were assayed in triplicate. Thermal cycling conditions were set according to the manufacturer's recommendations. Relative quantification of target mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase expression (from the same sample) with the comparative cycle threshold (Ct) method.14 The relative fold change of target gene expression (from mice on a regular diet and an ω-3 PUFA-enriched diet) versus sham, respectively was expressed as 2−ΔΔCt (setting sham as 1), where ΔΔCt=ΔCt test animal−ΔCt calibration animal. Four animals in the sham group were randomly chosen as the calibration samples. The ΔCt was defined as Ct target−Ct glyceraldehyde-3-phosphate dehydrogenase.15 The sequences of the primers pair for various genes are as follows:
GAPDH: 5′-GTGAAGGTCGGTGTGAACGG-3′ and 5′-GTTTCCCGTTGATGACCAG-3′
IL1-α: 5′-CCAAAGTTCCTGACTTGTTTG-3′ and 5′-GAAGGTGAAGGTGGACATC-3′
IL1-β: 5′-CTGTCCCTGAACTCAACTGTG-3′ and 5′-GTCCTCATCCTGGAAGCTCC-3′
TNF-α: 5′-GATCGGTCCCAACAAGGAGG-3′ and 5′-GCTGGTACCACCAGTTGGTTG-3′
COX-2: 5′GACAGATCAGAAGCGAGGACCTG-3′ and 5′-GTAGATCATGTCTACCTGAGTG-3′
iNOS: 5′-GGAAGTTTCTCTTCAGAGTC-3′ and 5′-CGATGGAGTCACATGCAGC-3′.
Compound action potential measurements
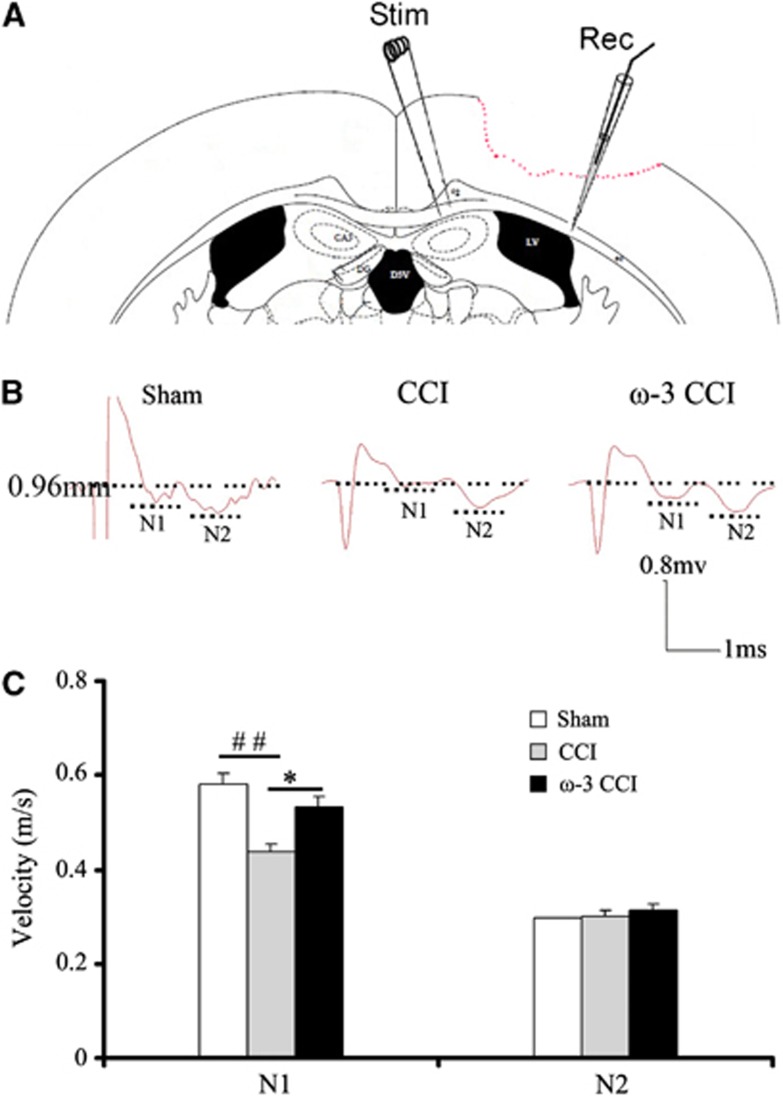
Compound action potentials (CAPs) in the corpus callosum were evaluated as previously described.16, 17 The mice were anesthetized with isoflurane (5%), decapitated, and the brains were quickly removed. Coronal slices (450 μm) were cut on a Vibratome and placed in artificial cerebrospinal fluid (aCSF; NaCl 130 mmol/L, KCl 3.5 mmol/L, Na2H2PO4 1.25 mmol/L, MgSO4 1.5 mmol/L, CaCl2 2 mmol/L, NaHCO3 24 mmol/L, glucose 10 mol/L; pH 7.4) pre-gassed with a mixture of 95% O2/5% CO2. The sections were incubated for 1 hour at room temperature and then placed in the recording chamber where they were submerged and perfused with artificial cerebrospinal fluid (22°C) at a constant rate (3 to 4 mL/minute) for the remainder of the experiment. Recordings were made at room temperature (22°C) to differentiate the myelinated (N1) and unmyelinated (N2) components of the CAPs. A bipolar, tungsten stimulating electrode (intertip distance, 0.5 mm) was lowered into the corpus callosum approximately 1 mm lateral to the midline. A glass extracellular recording pipette (1 to 3 MΩ tip resistance when filled with CSF) was lowered into the corpus callosum at approximately 0.96 mm from the stimulating electrode. Evoked CAPs were recorded using an Axoscope 10 (1 mA, 10v). The amplitude of the CAPs was measured from standardized input–output curves from each brain slice at bregma −1.22 mm.
Quantitative analysis was performed on waveforms that represented the average of four successive sweeps in each of the two slices per mouse. The difference from the first positive peak to the first trough was determined as the amplitude of the myelinated fibers (N1), while the difference between the second peak and the second trough was determined as the amplitude of the slower conducting non-myelinated fibers (N2). To analyze the velocity of the N1 and N2 components, the CAP conduction time was measured. The conduction velocity was expressed as m/second.
Electron microscopic studies
To morphologically demonstrate myelinated and non-myelinated fiber damage in the corpus callosum after TBI, all three groups of TBI mice were subjected to cardiac perfusion with 4% paraformaldehyde+2.5% glutaraldehyde in a 0.1 mol/L sodium phosphate buffer at 35 days post-TBI and the brains blocked to include regions of the corpus callosum matching those used for electrophysiological assessment. These brain slices were fixed by immersion in the same buffered aldehyde solution as the in vivo cases. Blocked tissues were then embedded in agar and 40 μm Vibratome sections generated for each sample. All sections were osmicated, stained in uranyl acetate, dehydrated, and embedded in Medcast Resin (Ted Pella Inc., Redding, CA, USA), using the flat mounting technique with plastic slides and coverslips. Regions of interest within the corpus callosum were then identified, cut out, mounted on plastic studs, and serially sectioned with a diamond knife. The resulting thin sections were collected on formvar-coated slotted grids and stained with lead citrate.17 Using a Philips CM120 electron microscope (Royal Dutch Philips Electronics Ltd., Amsterdam, Holland, The Netherlands), sections were screened and representative images acquired at × 4,800 on film. Given that no international criteria have been established to describe the pathologic sequelae in axons after TBI, fibers were assessed for evidence of any structural/subcellular perturbation over time, such as degradation of the myelin sheath, ultrathin myelin sheath, and morphologic changes in neurofilaments.
Statistical analysis
Data are expressed as the mean and s.e.m. and were analyzed by analysis of variance followed by post hoc Bonferroni/Dunn tests. The difference in means between two groups was assessed by the two-tailed t test. P<0.05 was considered statistically significant.
Results
Omega-3 Fatty acid Supplementation Protected against Motor Deficits Induced by Controlled Cortical Impact
Inflammation is one of the main contributing factors to cellular degeneration, leading to the manifestation of neurobehavioral deficits post-TBI. In substantiation, dietary supplementation with docosahexaenoic acid (DHA), a component of fish oil for 12 to 30 days ameliorated cognitive deficit7 and axonal injury18 from fluid percussion or impact acceleration injuries. However, the impact of long-term dietary supplementation with complex ω-3 PUFAs such as the combined ingredients in fish oil on TBI prognosis had never been explored.
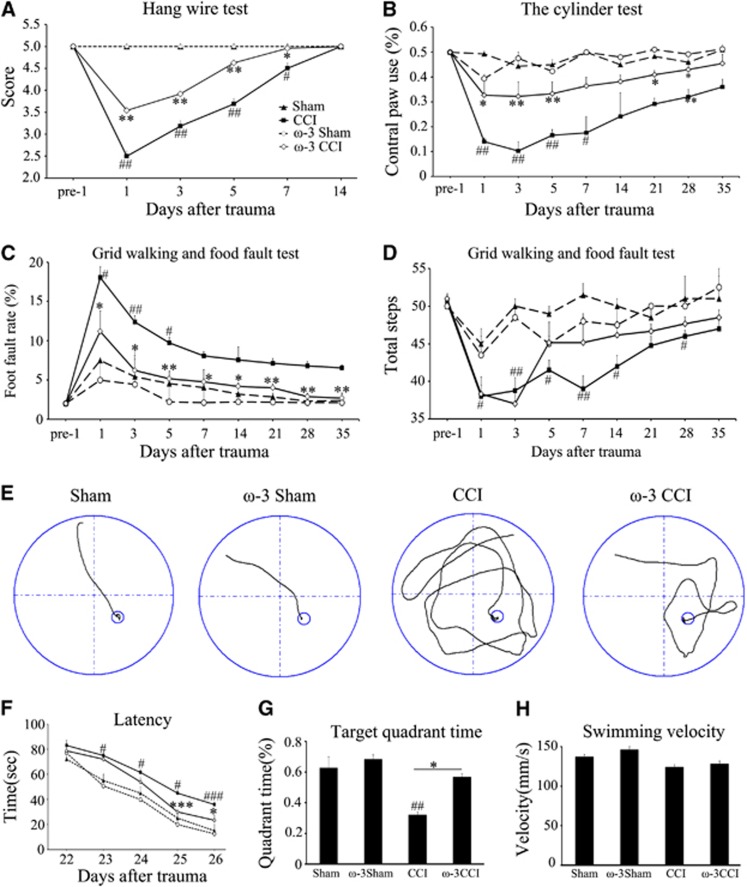
In this study, we characterized the protective effect of complex ω-3 PUFAs on TBI-induced gray and white matter injuries. To achieve this, two groups of 3-week-old (corresponding to human adolescent) C57BL/6J male mice were fed with high ω-3 PUFAs or regular diet for 60 days. Afterwards, the profiles of ω-3 and ω-6 PUFAs in these rats were examined by chromatography. As expected, this dietary regimen elicited significant increases in various ω-3 but not ω-6 PUFAs, resulting in a net elevation of the ω-3/ω-6 ratio (Supplementary Figure 2). No change in body weight was observed (Supplementary Figure 3). Mice from each dietary group were then subjected to CCI (velocity, 3.5 m/second; duration, 150 millisecond; depth, 1.5 mm). After 24 hours, sensorimotor deficits were determined via the wire hanging, cylinder, and grid walking tests. In the wire hanging test, mice that underwent CCI experienced a significant drop in scores followed by gradual recovery whereas sham exhibited virtually no decrease. In comparison, mice fed with the ω-3 PUFA-enriched diet performed better with a consistently higher score throughout the first 14 days post injury (Figure 1A). In addition to enhanced myodynamia, the ω-3-enriched diet reduced limb asymmetry after CCI, as indicated by the cylinder test (Figure 1B) at 5 weeks post injury. The enhanced sensorimotor performance in mice with ω-3 PUFA-enriched diets was also reflected in the grid walking and foot-fault tests (Figures 1C and 1D). Taken together, these tests indicate that sensorimotor deficits are ameliorated by ω-3 PUFA supplementation.
Figure 1.
Omega-3 polyunsaturated fatty acid (ω-3 PUFA) dietary supplementation conferred protection against short- and long-term behavioral deficits induced by controlled cortical impact (CCI). Performance comparison for the first 14 days post injury between mice on different diets; (A) hanging wire test demonstrating that mice on ω-3-enriched PUFA diets experienced smaller decreases in scores after CCI than those on a regular diet; (B) cylinder test showing that mice on a regular diet relied less on the contralateral paw than those on the ω-3-enriched diet; (C and D) grid walking foot-fault test indicating that mice fed with ω-3 PUFAs exhibited lower foot fault rates and stepped more frequently. All data are presented as mean±s.e.m., n=10, *P<0.05, **P<0.01 versus CCI; #P<0.05, ##P<0.01 versus sham; (E) the swim path of a typical mouse in each treatment condition in the Morris water maze test; (F) duration required to reach the platform in the water maze test 22 to 26 days post injury; (G) average time spent in the same quadrant as the target was located; and (H) average swimming speed for mice in the Morris water maze. All data are presented as mean±s.e.m., n=10, *P<0.05, **P<0.01 versus CCI; #P<0.05, ##P<0.01 versus sham.
Attenuation of Cognitive Deficits and Neuronal Degeneration but not Macroscopic Cortical Tissue Damage
We determined whether ω-3 PUFAs can attenuate long-term cognitive deficits by recording the time required to locate a platform submerged in water from 22 to 26 days post injury (Figure 1F). There was a gradual improvement in cognitive performance over time after CCI for all groups of mice. However, mice on ω-3 PUFA-enriched diets exhibited less cognitive dysfunction than those on a regular diet. The memory deficits after CCI in mice on a regular diet were manifested as less time spent in the quadrant where the platform was located. These animals swam in all four quadrants, unlike mice with ω-3 enrichment (Figures 1E and 1G). By comparison, mice from both groups swam at a comparable speed during the Morris water maze test (Figure 1H) suggesting that this difference in swimming pattern is not due to TBI-induced muscle atrophy but likely to reflect direct changes in cognitive functions.
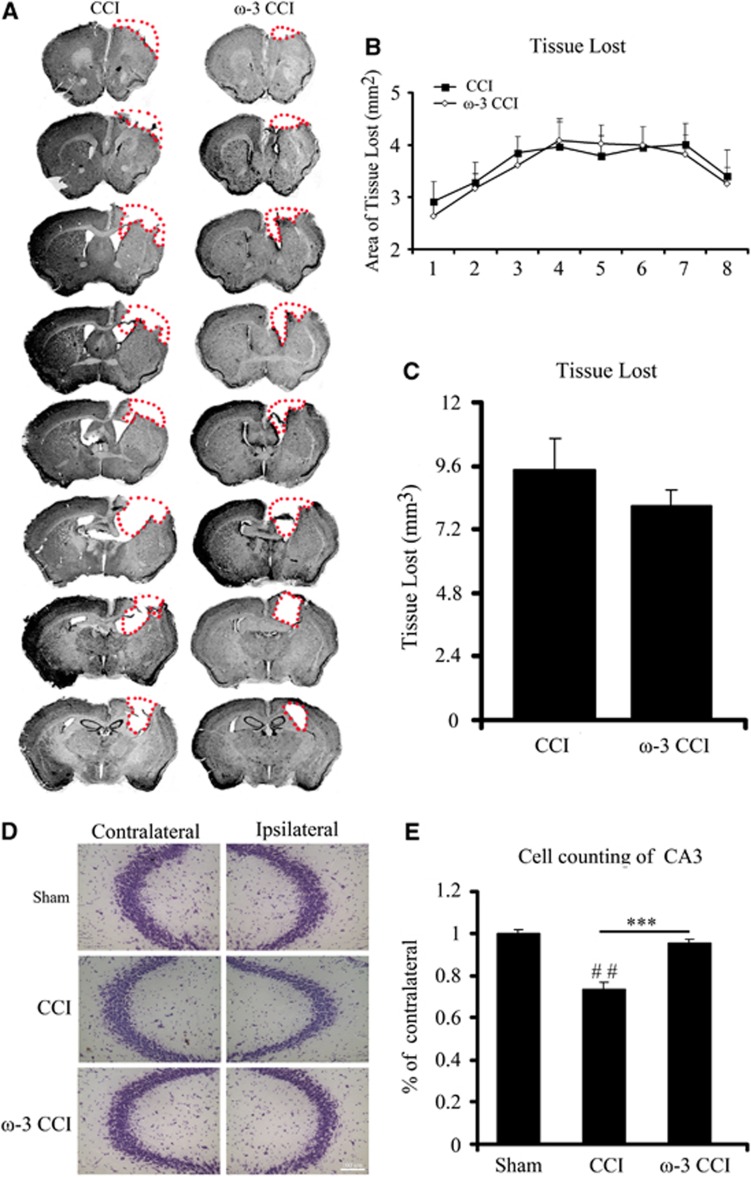
Tissue loss at the impacted region is a hallmark of CCI injury. In the present report, we visualized tissue damage in mice with cresyl violet staining. Eight consecutive sections through the cortex of mice on regular or on ω-3 PUFA-enriched diets were Nissl stained 5 weeks after CCI (Figure 2A). Despite the attenuation in motor and cognitive deficits observed before killing, there was only a marginal decrease in the area of tissue loss in mice fed with ω-3 PUFAs in most sections across the entire rostrocaudal extent of the lesion (Figure 2B). Mice with ω-3 PUFA-supplemented diets exhibited slightly less tissue volume loss than those on a regular diet, but the difference was not statistically significant (Figure 2C).
Figure 2.
Dietary supplementation with omega-3 polyunsaturated fatty acids (ω-3 PUFAs) decreased degeneration of CA3 neurons because of controlled cortical impact (CCI) but had no significant effect on gross tissue loss in the cortex. (A) Eight consecutive brain sections beginning at +1.42 mm from bregma and progressing every 0.3 mm through the entire territory of tissue loss were stained with cresyl violet at 35 days post injury; (B) surface area of tissue loss in eight brain sections from the two groups of mice reveal no significant difference at any rostrocaudal level between mice fed a regular diet and those fed ω-3 PUFAs; (C) quantification of tissue loss in CCI and ω-3 CCI mice at 35 days post injury (n=8 per group). ω-3 CCI mice exhibited less tissue loss (0.7882 mm3 to 0.6748 mm3) but the difference was not statistically significant; (D) change in number of viable neurons in the CA3 region of the hippocampus visualized by cresyl violet staining; (E) quantification of viable neurons in the CA3 region of the hippocampus of mice from the three experimental groups show that the ω-3-enriched diet completely prevented neuronal loss in this region in response to CCI. All data are presented as mean±s.e.m., n=8, ***P<0.01 versus CCI; ##P<0.01 versus sham.
However, when viable neurons within the CA3 region of the hippocampus were counted, ω-3 PUFA supplementation significantly increased the number of surviving neurons by 20% (Figures 2D and 2E). On the other hand, there was no statistically significant difference in the number of viable neurons within the CA1 region of these mice when compared with those on regular diet (data not shown). Although this protection may seem slight, it is important to note that the hippocampal cell number was brought back up almost to control, sham values with ω-3 PUFA supplementation (Figure 2E).
Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids Mitigated Inflammatory Responses Elicited by Controlled Cortical Impact
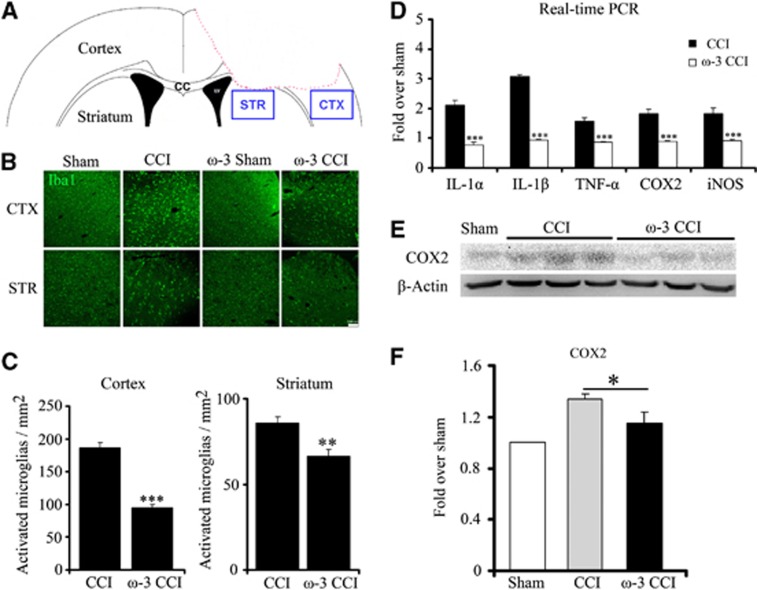
The absence of statistically significant decrease in tissue loss due to ω-3 PUFAs is unlikely to account for the observed attenuation in neurobehavioral impairments. We hypothesized that the motor and cognitive behavioral recovery due to ω-3 PUFAs may be attributed to the decrease in inflammatory response and white matter injury elicited by CCI in light of the anti-inflammatory properties of ω-3 PUFAs. As expected, ω-3 PUFA-fed mice exhibited lower inflammatory responses and cytokine release, especially in the tissue around the lesion site (region around the red dotted line, Figure 3A) and the striatum. The number of microglia (stained positive with Iba-1) in the ipsilateral cortex and striatum was significantly elevated after CCI injury. However, the number of activated microglia was lower in mice fed with enriched ω-3 PUFA diet (Figures 3B and 3C) indicating a diminished inflammatory response. In addition, mRNA levels of multiple cytokines (IL1-α, IL1-β, and TNFα) and of COX-2 and iNOS measured at 24 hours post injury in the ipsilateral cortex were also decreased because of ω-3 PUFA supplementation. In contrast, mice on regular diet harbored higher mRNA levels of the cytokines, COX-2 and iNOS (Figure 3D). As expected, the elevated COX-2 mRNA levels paralleled higher protein levels of COX-2 in the same region as semi-quantified by western blot analysis (P<0.05; Figure 3F). It is likely that the protein levels of cytokines and iNOS exhibited a similar parallel increase. These findings demonstrate that mice on ω-3 PUFA-enriched diets exhibited less remarkable inflammatory responses than those on a regular diet.
Figure 3.
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) suppressed the controlled cortical impact (CCI)-induced activation of microglia in the cortex and striatum. (A) The peri-lesion location in the cortex and striatum where microglia activation was examined is indicated by a blue box; (B) ω-3-enriched dietary supplementation protected against microglial activation in the cortex and striatum compared with the regular diet. Activated microglia were visualized by Iba1 positive staining. Scale bar, 100 μm; (C) quantification of the number of activated microglia in both regions in mice fed on regular and ω-3-enriched diets. All data are presented as mean±s.e.m., n=6, ***P<0.01, **P<0.05 versus CCI; (D) ω-3-supplemented mice have a lower expression level of inflammation-related genes (IL1-α, IL1-β, TNF-α, COX-2, and iNOS), as determined by real-time polymerase chain reaction (RT-PCR). All data are presented as mean±s.e.m., n=6, ***P<0.01 versus CCI; (E) the lower inflammatory response in mice fed with ω-3-enriched diets is also reflected in reduced endogenous COX-2 expression levels by western blot analysis; (F) quantification of the difference in COX-2 protein levels normalized to β-actin. All data are presented as mean±s.e.m., n=6, *P<0.05 versus CCI.
Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids Prevented Neurofilament Dissolution and the Loss of Myelin Basic Protein
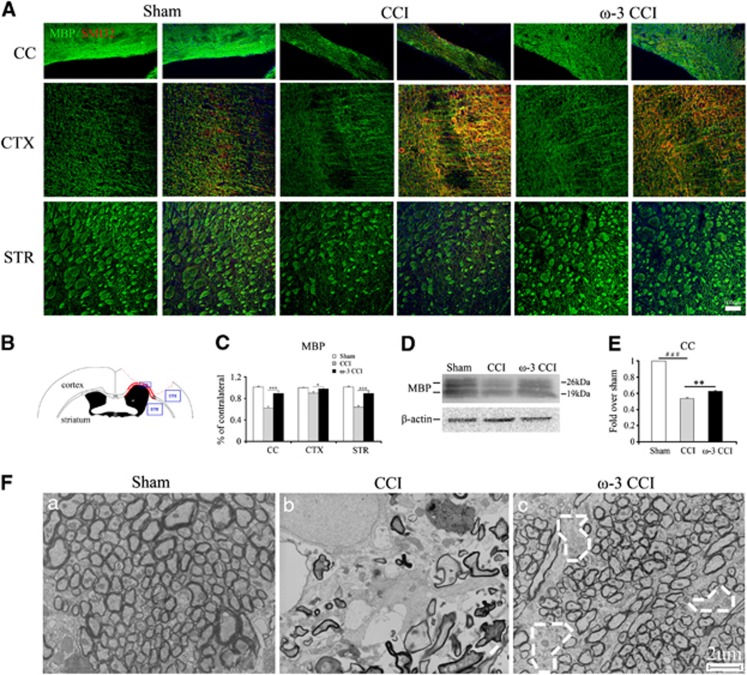
Damage to the myelin sheath and to axons can be visualized by loss of MBP and increases in non-phosphorylated neurofilament (SMI32) staining, as has been reported in TBI.19 Therefore, we stained the corpus callosum, cortex, and striatum for MBP and SMI32 (location indicated in Figure 4B) 35 days post injury in sham mice or CCI mice on ω-3 PUFA-enriched and regular diets (Figure 4A). White matter injury induced by CCI was reflected in elevated SMI32 levels and decreased MBP levels compared with sham animals. We quantified the fluorescent intensity of MPB and SMI32 immunostaining and found a significant higher level of MBP in mice on enriched ω-3 PUFA diets in all three regions (Figure 4C). The higher levels of MBP in the corpus callosum in mice fed with the ω-3 PUFA diet was further confirmed by western blot analysis (Figure 4D). Densitometric quantification of MBP levels showed that ω-3 PUFA-enriched mice retained a higher level of MBP, suggesting a reduction in white matter injury (Figure 4E).
Figure 4.
Attenuation of white matter injury in mice on enriched omega-3 polyunsaturated fatty acid (ω-3 PUFA) diets at 35 days post injury. (A) Double immunofluorescent staining for SMI32 (red, non-phosphorylated neurofilament protein) and MBP (green, myelin basic protein), counterstained with nuclear 4',6-diamidino-2-phenylindole in the corpus callosum, cortex, and striatum. Mice on the ω-3-enriched diet exhibited greater neurofilament integrity (lower SMI32 staining) and higher levels of MBP post injury. Field magnification is at × 20 and scale bar, 100 μm; (B) diagram indicating the locations of the peri-lesion regions photographed after immunostaining; (C) quantification of MBP levels in the corpus callosum, cortex, and striatum as a percentage of contralateral fluorescent staining. All data are presented as mean±s.e.m., n=6, ***P<0.01, *P<0.05 versus controlled cortical impact (CCI); (D) western blot analysis reveals attenuated loss of MBP protein in the corpus callosum in mice on ω-3-enriched diets compared with mice on regular diets; (E) quantification of the change in MBP levels in the corpus callosum by western blot analysis reveals a statistically significant difference between the ω-3-enriched diet and regular diet groups. All data are presented as mean±s.e.m., n=5, **P<0.01 versus CCI, ###P<0.01 versus sham; (F) morphologic changes in the nerve fibers of the corpus callosum 35 days post CCI as visualized by electron microscopy. Controlled cortical impact induced severe degeneration of nerve fiber bundles compared with sham whereas ω-3-supplemented mice exhibited greater structural integrity. A few scattered cavities with localized fiber degeneration are outlined in white. Scale bar, 2 μm (amplification, × 4800).
Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids Preserved Nerve Fibers and Signal Conduction along the Fibers
The changes in SMI32 and MBP after CCI suggested that ω-3 PUFA diets have the capacity to ameliorate white matter injury. To verify this finding, regions of the corpus callosum (at bregma −0.46 mm) were dissected from mice in all groups 35 days post injury to examine the ultrastructure of the white matter via electron microscopy (Figure 4F). In sham animals, closely packed intact fibers were abundant. After CCI, marked fiber degeneration was observed, leaving sparsely distributed fibers behind. However, mice on the enriched ω-3 PUFA diet had more intact fibers and exhibited resistance to injury. The few cavities outlined with white dotted lines indicate the location of degenerating fibers in the ω-3 PUFA group (Figure 4F, panel c). In summary, ω-3 PUFAs attenuated myelin sheath damage induced by CCI.
In addition to the ultrastructural examination, we measured the changes in action potential transmission in the corpus callosum. Evoked compound action potentials (CAPs) were used to determine white matter injury associated with axonal conduction deficits. Typically, evoked CAPs yield a biphasic waveform comprised of an initial segment (N1, represents fast-conducting myelinated axons) followed by a second segment (N2, represents the slower-conducting unmyelinated axons) in adult rats.16 The setup to evoke CAPs is shown in Figure 5A. With this technique, changes in nerve function can be ascertained. In damaged axons or impaired myelin sheaths, the conduction velocity slows down. The biphasic waveforms after evoked CAPs are shown in Figure 5B. Controlled cortical impact decreased the N1 segment magnitude, suggesting a slowdown in conduction velocity. Dietary supplementation with ω-3 PUFAs increased the magnitude of the N1 segment, indicating a higher conduction velocity and a greater preservation of myelinated axons than the regular diet group. However, there was no significant change in the magnitude of N2 segments, suggesting that demyelinated axons had rapidly degenerated.
Figure 5.
Compound action potentials (CAPs) of myelinated fibers at 35 days post controlled cortical impact (CCI). (A) Stimulating and recording electrodes were positioned at the corpus callosum as shown in this diagram; (B) representative traces (0.96 mm from the stimulating point) of the evoked CAPs from all the experimental groups at 35 days post injury; (C) the synaptic velocity of the myelinated (N1) and unmyelinated (N2) axons in the corpus callosum was calculated. The synaptic velocity of the myelinated (N1) axons in the corpus callosum in the CCI group was decreased relative to mice in the sham group and omega-3 (ω-3) CCI group. There was no statistical difference in synaptic velocity within the unmyelinated (N2) axons in the corpus callosum between all three groups. n=5 for each group. All data are presented as mean±s.e.m.,*P<0.0 versus CCI; #P<0.05, ##P<0.01 versus sham.
In short, the conduction velocity for myelinated axons was slowed down by 30% after CCI, corresponding to a severe degeneration in conducting fibers. However, this decrease was attenuated by ω-3 PUFA enrichment (Figure 5C) and paralleled a higher number of fibers in this group (Figure 4).
Oligodendrocyte Protection is Mediated via Direct Effect and Suppression of Inflammatory Response
Thus far, we have shown that ω-3 PUFA dietary supplementation mitigates inflammatory responses and attenuate white matter injury. However, it is uncertain whether these two processes have a causal relationship or are independently elicited by the elevation in dietary ω-3 PUFAs. To address this issue, we mimicked our in vivo paradigm with the following in vitro paradigm. Because of long-term partake of ω-3 PUFAs raised DHA (Supplementary Figure 1), we examined the effect of DHA pretreatment on oligodendrocyte viability after treatment with AMPA. We chose AMPA because it is one of the most widely used excitotoxins in studies involving oligodendrocyte viability.20, 21, 22, 23, 24 Persistent activation of AMPA receptors would damage oligodendrocytes by calcium overload, modeling the acute increase in extracellular glutamate and the resultant excitotoxicity seen in experimental models of TBI.25, 26, 27, 28
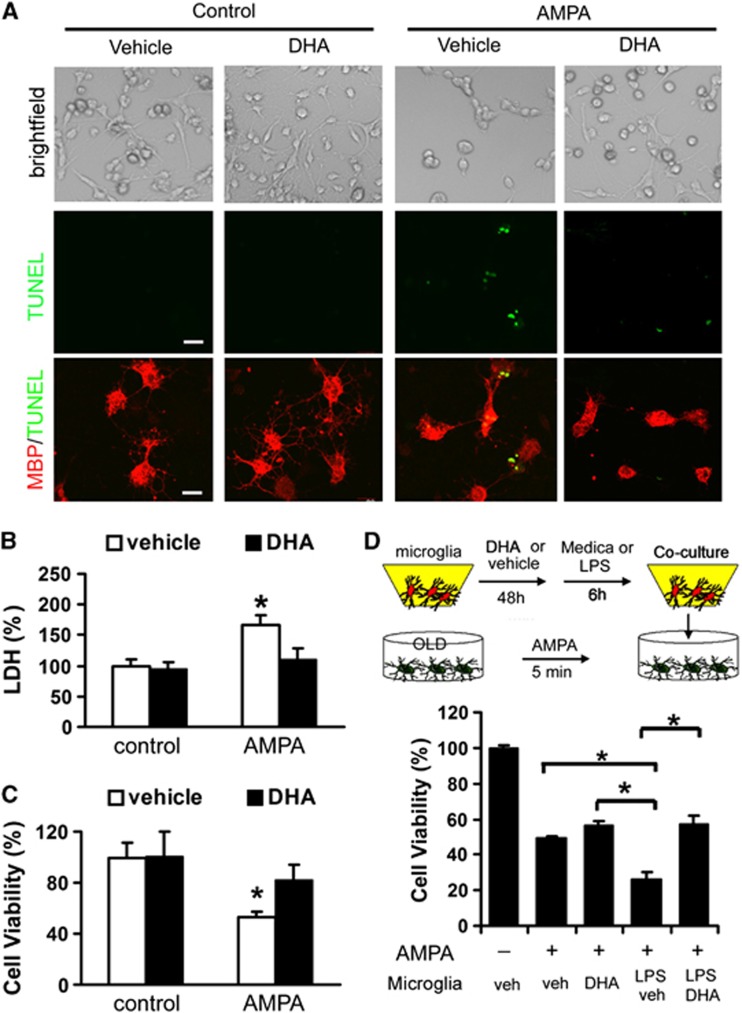
Pretreatment with 20 μmol/L of DHA showed no detectable impact on basal viability but attenuated AMPA-induced degeneration of mature oligodendrocytes as shown by terminal deoxynucleotidyl transferase dUTP nick end labeling staining and immunostaining for MBP as a mature oligodendrocyte marker (Figure 6A). The LDH assay for necrotic loss of membrane integrity and the MTT viability assay for mitochondrial activity both revealed that pretreatment with DHA inhibited the demise of oligodendrocytes in response to AMPA (Figures 6B and 6C). Taken together, these results verify that DHA has a direct protective effect on oligodendrocytes.
Figure 6.
Docosahexaenoic acid (DHA) protected primary oligodendrocytes against 2-amino-3-(5-methyl-3-oxo-1, 2-oxazol-4-yl) propanoic acid (AMPA)-induced cell death. (A) Primary oligodendrocyte (OLD) cultures were pretreated with DHA (20 μM) for 48 hours before AMPA (25 μM, 5 minutes). Media was then replaced with complete growth medium and the cultures were maintained for another 24 hours. The uppermost panels reveal that DHA attenuated AMPA-induced cell death but had no effect on basal cell density. The middle panels show a decrease in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells undergoing apoptosis and the lower panels show that cells undergoing apoptosis were also stained with the mature oligodendrocyte marker MBP. Scale bar, 20 μm; (B) LDH assay for loss of membrane integrity indicates that DHA pretreatment ameliorated cellular necrosis elicited by AMPA. All data are presented as mean±s.e.m., n=4, *P<0.05 versus vehicle; (C) MTT assay for mitochondrial activity reveals higher cell survival after AMPA with DHA pretreatment. All data are presented as mean±s.e.m., n=4, *P<0.05; (D) primary microglia in culture inserts were pretreated with DHA (20 μM) or vehicle for 48 hours followed by lipopolysaccharide (LPS) (100 ng/mL) for 6 hours to elicit activation. Oligodendrocyte cultures were then subjected to AMPA (25 μM, 5 minutes). The activated microglia-in-inserts were added to oligodendrocyte cultures immediately after AMPA. Oligodendrocyte cell viability was quantified by the MTT assay. All data are presented as mean±s.e.m., n=4, *P<0.05 versus vehicle.
We also tested the hypothesis that DHA-pretreated microglia would have fewer toxic effects on oligodendrocytes in cocultures. We subjected microglia cultures to 48 hours of pretreatment with DHA followed by activation with LPS for 6 hours. Microglia cultures without DHA treatment but activated by LPS functioned as controls. LPS-treated microglia was subsequently cocultured with oligodendrocytes subjected to AMPA to induce cell death. Microglia pretreated with DHA elicited less degeneration of oligodendrocytes than those microglia not pretreated with DHA (Figure 6D). This result suggested that DHA treatment can also indirectly protect oligodendrocytes by mitigating microglial activation. On the whole, the results are consistent with the hypothesis that ω-3 PUFAs attenuate white matter injury both by direct protection of oligodendrocyte viability and indirect protection of oligodendrocytes against toxic microglial activation.
In sum, dietary enrichment with ω-3 PUFAs attenuated short- and long-term behavioral deficits, neuronal degeneration in the CA3 region, as well as attenuating inflammatory responses. ω-3 PUFAs also preserved oligodendrocytes in the corpus callosum. Finally, our in vitro results indicate that protection of oligodendrocytes is mediated by direct actions as well as indirect actions involving the suppression of microglial activation. Therefore, ω-3 PUFAs can fortify cells to resist TBI-induced damage.
Discussion
Traumatic brain injury is a prevalent neurologic disorder that elicited gray and white matter injury. In many TBI models, significant loss of oligodendrocytes was observed as soon as 3 days post injury in the external capsule and corpus callosum.29, 30 In addition, a reduction of myelin staining in these areas substantiated a loss of matured oligodendrocytes implicating the ongoing of demylination besides the demise of oligodendrocyte progenitor cells. Therefore, attenuation in oligodendrocyte degeneration should improve the prognosis of TBI.31 In the present study, long-term dietary supplementation with ω-3 PUFAs elicited robust protection against sensorimotor and cognitive deficits in our murine TBI model. This protective dietary strategy has already shown much success in other paradigms6, 32, 33 and can be used in conjunction with moderate hypothermia, which has shown some success in preventing oligodendrocytes degeneration.34 The neurobehavioral recovery of ω-3 PUFAs-fed mice in our TBI model may be attributed to the decrease in neuronal degeneration in the ipsilateral CA3 region, the attenuation in inflammatory responses and the decrease in white matter injury. This is the first demonstration of protection against TBI-induced white matter injury and inflammatory response by dietary supplementation with ω-3 PUFAs. Our results reveal a new preventive strategy for neurologic disorder.
Typically, improvement in motor and cognitive functions in TBI models is attributed to a significant decrease in macroscopic cortical tissue loss.10, 35, 36 However, long-term dietary supplementation with ω-3 PUFAs did not significantly prevent tissue loss at the impact region. Nonetheless, ω-3 PUFAs completely abolished neuronal loss in the CA3 region of the hippocampus. There are three possible explanations for this discrepancy. First, the hippocampus is located in the penumbra of concussive damage, whereas the cortex is directly impacted and lies at the core of damage. Thus, the damage in the core may simply be too severe for protection in our model. Second, perhaps differences between groups were too subtle to be visualized by a macroscopic assay of gross tissue loss. In contrast, the higher resolution afforded by the hippocampal neuronal counts may afford higher sensitivity. A third explanation is that increased astrocytosis in the CCI-alone group masked any changes in neuronal tissue loss between the ω-3 PUFA supplemented and regular diet-fed groups. Although it remains unclear whether the prevention of behavioral deficits is directly related to an increase in viable hippocampal neurons or amelioration in white matter injury, hippocampal neuron survival has been linked to learning and memory.37 Hence, the significant increase in viable hippocampal neurons in the CA3 region is likely to be, at least in part, associated with improvements in memory and cognition in our TBI model. Furthermore, the integrity of white matter is likely to preserve interneuronal communication.
In this study, we found that ω-3 PUFA enrichment can protect oligodendrocytes both directly and indirectly. First, ω-3 PUFAs protected cultured oligodendrocytes against AMPA-induced loss of viability. Second, ω-3 PUFAs attenuated microglial activation by LPS and thereby reduced the negative impact of activated microglia on AMPA-induced oligodendrocyte loss. These findings are consistent with the in vivo data showing that the inflammatory response in the cortex and striatum was mitigated in mice on the ω-3-enriched diet. The anti-inflammatory characteristics of ω-3 PUFAs, such as a decrease in COX-2 expression and cytokines, are consistent with many reported studies38, 39, 40 and support the hypothesis that inhibition of acute inflammatory responses may be a viable protective strategy for TBI.
The efficacy of ω-3 PUFAs against TBI has been reported in a few prior studies.7, 9, 18 In those studies, ω-3 fatty acids were able to maintain Ca2+ ion and energy homeostasis as well as increase resistance towards oxidative stress, thereby mitigating the damage from TBI. In the study by Bailes and Mills,18 supplementation with DHA significantly decreased amyloid precursor protein-positive axons in the white matter tract.18 Consistent with this demonstration of protection against white matter injury, our study also showed that ω-3 PUFAs preserved projection fibers in the corpus callosum and retained the morphology of neurofilaments as indicated by SMI32 staining. In addition, our study also suggested that ω-3 fatty acid enrichment protected against myelin sheath damage. We speculate that ω-3 PUFA dietary enrichment may blunt white matter injury in various diseases in addition to TBI.
In summary, we showed that ω-3 PUFA dietary supplementation is a viable strategy to mitigate injury from TBI. ω-3 PUFAs were administered for months before CCI to model the long-term use of fish oil supplements in otherwise healthy humans. Our data support the notion that fish oil supplementation in humans may exert similar prophylactic or preventive actions against future challenges. On the whole, ω-3 PUFAs dietary supplementation conferred both short- and long-term protection against behavioral deficits, neurodegeneration, and white matter injury. White matter injury has not been scrutinized to the same extent as gray matter injury, but also results in severe dysfunction in interneuronal communication across brain regions. The protective effect of ω-3 PUFAs on both gray and white matters makes it a promising candidate against neurodegenerative disorders that impact the cell soma as well as the axon and its myelin sheath. Considered together with the well-known anti-inflammatory properties of ω-3 PUFAs, these findings continue to support the prophylactic health benefits of a diet high in fish products or fish oil.
Acknowledgments
We thank Mr Shiduo Lu for excellent technical assistance with the TBI model.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research is supported in part by the Special Research Funds from Chinese Ministry of Science and Technology to State Key laboratories (to YG and JC), National Institutes of Health/NINDS grants NS36736, NS43802, NS45048 and NS056118 (to JC), and Chinese Natural Science Foundation grants No 30870794, No 81020108021, No 81171149, No 81150110494, and No 81000497 (to YG). JC is a recipient of the VA Research Career Scientist Award, VA Merit Review grant, and the RK Mellon Endowed Chair from UPMC.
Supplementary Material
References
- Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience. 2002;109:231–241. doi: 10.1016/s0306-4522(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Bousquet M, Calon F, Cicchetti F. Impact of omega-3 fatty acids in Parkinson's disease. Ageing Res Rev. 2011;10:453–463. doi: 10.1016/j.arr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Eady TN, Khoutorova L, Atkins KD, Bazan NG, Belayev L. Docosahexaenoic acid complexed to human albumin in experimental stroke: neuroprotective efficacy with a wide therapeutic window. Exp Transl Stroke Med. 2012;4:19. doi: 10.1186/2040-7378-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Wang X, Chen Z, Landman N, Lo EH, Kang JX. Gene transfer of the Caenorhabditis elegans n-3 fatty acid desaturase inhibits neuronal apoptosis. J Neurochem. 2002;82:1360–1366. doi: 10.1046/j.1471-4159.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- Nguemeni C, Delplanque B, Rovere C, Simon-Rousseau N, Gandin C, Agnani G, et al. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol Res. 2010;61:226–233. doi: 10.1016/j.phrs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68 (Suppl 2:S102–S111. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- Wang G, Jiang X, Pu H, Zhang W, An C, Hu X, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10:124–142. doi: 10.1007/s13311-012-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, et al. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Neuroscience: glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Silverstein FS, Skoff R, Barks JD. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr Res. 2002;51:25–33. doi: 10.1203/00006450-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu X, Liu C, Wang S, Zhang W, Gao H, et al. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed JA, DiLeonardi AM, Fox DP, Tessler AR, Raghupathi R. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J Neurotrauma. 2011;28:547–563. doi: 10.1089/neu.2010.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617–1624. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- Mamere AE, Saraiva LA, Matos AL, Carneiro AA, Santos AC. Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. AJNR. A J Neuroradiol. 2009;30:947–952. doi: 10.3174/ajnr.A1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Alberdi E, Sanchez-Gomez MV, Ariz U, Perez-Samartin A, Matute C. Dual-specific phosphatase-6 (Dusp6) and ERK mediate AMPA receptor-induced oligodendrocyte death. J Biol Chem. 2011;286:11825–11836. doi: 10.1074/jbc.M110.153049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219:53–64. doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Rosival V, Belkova L, Martinkova J, Takac A. [Benign lactic acidosis in 2 female diabetics treated with insulin] Vnitr Lek. 1990;36:783–785. [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury. JNeurotrauma. 1997;14:677–698. doi: 10.1089/neu.1997.14.677. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Potts MB, Adwanikar H, Noble-Haeusslein LJ. Models of traumatic cerebellar injury. Cerebellum. 2009;8:211–221. doi: 10.1007/s12311-009-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Flygt J, Djupsjo A, Lenne F, Marklund N.Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat Eur J Neurosci 2013;. doi: 10.1111/ejn.12179[e-pub ahead of print]. [DOI] [PubMed]
- Lotocki G, de Rivero Vaccari JP, Alonso O, Molano JS, Nixon R, Safavi P, et al. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci Lett. 2011;499:143–148. doi: 10.1016/j.neulet.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasadsri L, Wang BH, Lee JV, Erdman JW, Llano DA, Barbey AK, et al. Omega-3 fatty acids for treatment of traumatic brain injury. J Neurotrauma. 2013;30:897–906. doi: 10.1089/neu.2012.2672. [DOI] [PubMed] [Google Scholar]
- Martin A, Boisgard R, Kassiou M, Dolle F, Tavitian B. Reduced PBR/TSPO expression after minocycline treatment in a rat model of focal cerebral ischemia: a PET study using [(18)F]DPA-714. Mol Imaging Biol. 2011;13:10–15. doi: 10.1007/s11307-010-0324-y. [DOI] [PubMed] [Google Scholar]
- Pignier C, Revenaz C, Rauly-Lestienne I, Cussac D, Delhon A, Gardette J, et al. Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res Cardiol. 2007;102:553–564. doi: 10.1007/s00395-007-0676-x. [DOI] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari J, Alonso O, Molano JS, Nixon R, Dietrich WD, et al. Oligodendrocyte vulnerability following traumatic brain injury in rats: effect of moderate hypothermia. Ther Hypothermia Temp Manag. 2011;1:43–51. doi: 10.1089/ther.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PloS one. 2010;5:e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shein NA, Grigoriadis N, Alexandrovich AG, Simeonidou C, Lourbopoulos A, Polyzoidou E, et al. Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury. FASEB J. 2009;23:4266–4275. doi: 10.1096/fj.09-134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory. Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC, Priestley JV, Perry VH, Michael-Titus AT. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J Neurochemistry. 2012;121:738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- Luchtman DW, Meng Q, Song C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson's disease. Behav Brain Res. 2012;226:386–396. doi: 10.1016/j.bbr.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Okabe N, Nakamura T, Toyoshima T, Miyamoto O, Lu F, Itano T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J Stroke Cerebrovasc Dis. 2011;20:188–195. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.