Abstract
Accurate imaging of ischemic penumbra is crucial for improving the management of acute stroke patients. T2* magnetic resonance imaging (MRI) combined with a T2*oxygen challenge (T2*OC) is being developed to detect penumbra based on changes in blood deoxyhemoglobin. Using 100% O2, T2*OC-defined penumbra exhibits ongoing glucose metabolism and tissue recovery on reperfusion. However, potential limitations in translating this technique include a sinus artefact in human scans with delivery of 100% OC and relatively small signal changes. Here we investigate whether an oxygen-carrying perfluorocarbon (PFC) emulsion can enhance the sensitivity of the technique, enabling penumbra detection with lower levels of inspired oxygen. Stroke was induced in male Sprague-Dawley rats (n=17) with ischemic injury and perfusion deficit determined by diffusion and perfusion MRI, respectively. T2* signal change was measured in regions of interest (ROIs) located within ischemic core, T2*OC-defined penumbra and equivalent contralateral areas during 40% O2±prior PFC injection. Region of interest analyses between groups showed that PFC significantly enhanced the T2* response to 40% O2 in T2*-defined penumbra (mean increase of 10.6±2.3% compared to 5.6±1.5% with 40% O2, P<0.001). This enhancement was specific to the penumbra ROI. Perfluorocarbon emulsions therefore enhances the translational potential of the T2*OC technique for identifying penumbra in acute stroke patients.
Keywords: acute stroke, BOLD contrast, brain imaging, focal ischemia, MRI
Introduction
Stroke has been known since antiquity yet the only notable treatment success has come from the NINDS trial in 19951 showing a therapeutic benefit from acute administration of recombinant tissue plasminogen activator (tPA). The restoration of blood flow through thrombolytic therapy during the first few hours from stroke onset has resulted in benefit for stroke patients because of the presence of hypoperfused, metabolically active penumbra tissue, the salvage of which is associated with a better outcome.2
The time window for existence of penumbra varies among individuals and relates to factors including the status of collateral flow, patient age, coexistent metabolic abnormalities, blood pressure, and other confounding variables. With evidence for survival of penumbral tissue for up to 48 hours in some cases,3 it is clear that the availability of a clinical imaging technique to accurately identify penumbra on an individual patient basis rather than using an arbitrary time limit (recently extended out to 4.5 hours from stroke onset4), could result in increased and or safer use of tPA.
In current standard clinical practice, stroke patients have a CT scan to confirm ischemic stroke and exclude hemorrhage, with no further acute imaging to confirm the existence of penumbra. Thus, the decision on whether to thrombolyse is based principally on the clock and not on the status of the affected tissue in each patient. One consequence of this is very low treatment numbers for patients receiving tPA (<7%) in emergency departments despite a third of the patients arriving within 3 hours of stroke onset.5, 6
Many patients are currently ineligible for thrombolysis with tPA because of unknown time of stroke onset (data from International Stroke Trial indicates wake-up stroke accounts for 29.6% of patients7) or presentation beyond the current time window for treatment. In addition, treating patients with tPA within 4.5 hours from stroke who have no penumbra is subjecting these patients to the unnecessary risks of thrombolysis, especially symptomatic intracranial hemorrhage, which ranged from 6.4% in the NINDS trial 1 to 19.8% in the ECASS I trial,8 and anaphylactoid reactions in 1.9%.9
The death of tissue in ischemic stroke is ultimately due to the lack of aerobic metabolism, causing failure of cell energetics required to maintain cell structure and function. The gold standard technique for penumbra detection is positron emission tomography where the tissue is identified as a metabolically active region with reduced cerebral blood flow (CBF) and an increased oxygen extraction fraction.10 However, this technique has not been adopted clinically because of high costs, radiation exposure, and lack of availability making it impractical. The ubiquitous nature of magnetic resonance imaging (MRI) combined with its high resolution and ability to provide structural, physiologic, and metabolic data without the need for ionizing radiation makes it an ideal alternative in acute stroke diagnosis. Much attention has focused on the potential of using the mismatch between the lesion on the diffusion-weighted image (DWI), identifying injured tissue with cell swelling, and a larger region of perfusion deficit on the perfusion image (PI) (PI/DWI mismatch).11
This indirect technique has been used for assessment of penumbra and patient recruitment in clinical trials, but has not been adopted as standard clinical practice in assessing acute stroke patients. Issues still under discussion12, 13 include threshold setting for defining the acute ischemic lesion on DWI and the perfusion deficit on PI,14, 15 the optimum method for image analyses and interpretation, whether or not the perfusion deficit includes benign oligemia (not at risk) and if penumbral tissue extends into the DWI-defined lesion.
Perfusion image/diffusion-weighted image mismatch assumes that the DWI-defined lesion represents the irreversibly damaged ischemic core, but there is continuing debate about the validity of this assumption. Based on the criteria set out by the Oxford Centre for Evidence-Based Medicine, a systematic review16 concluded that DWI hyperintensity as a surrogate marker of ischemic core was inconsistent and given a grade D (the lowest grade indicating a low level of evidence and inconsistency within the literature). Diffusion-weighted image-defined lesions can extend beyond the perfusion deficit (reanalysis of EPITHET data),17 which is likely to signify restoration of flow to this tissue prior to the scan. Diffusion-weighted image lesions both outside and within the perfusion deficit can resolve, particularly if treatment occurs within 4.5 hours18 or 6 hours15 from stroke onset. In both cases, DWI reversibility was associated with a favorable outcome strongly suggesting that penumbra extends into the acute DWI lesion. Highlighting the uncertainty surrounding PI/DWI mismatch, a recent reanalysis of EPITHET and DEFUSE clinical trial data concluded that clinically relevant diffusion lesion reversal is rare, and often transient, concluding that the acute diffusion lesion is generally a reliable signature of infarct core.19 Therefore, PI/DWI mismatch continues to be the subject of methodological refinements in threshold setting and image processing to address limitations, and should therefore be applied cautiously when used to identify ischemic penumbra.
Our group have developed a new, more direct MRI technique where penumbra is identified as a region of increased T2* signal change during a transient oxygen challenge (OC).20 Blood oxygen influences on T2* signal was originally demonstrated by Ogawa et al, using a hypoxic challenge,21 and is based on the paramagnetic properties of deoxyhemoglobin.22 Following stroke, oxygen supply is limited within the compromised but metabolically active penumbra tissue. This leads to an increased oxygen extraction fraction within the penumbra with an ensuing increase in the deoxy:oxyhemoglobin ratio. During OC, the increased oxygen delivered will convert deoxyhemoglobin to oxyhemoglobin resulting in an increase in T2* signal, which will be greater within the penumbra than in surrounding tissues. T2* oxygen challenge identifies a region within the perfusion deficit, which displays several features of penumbra: histologic evidence of normal neuronal morphology, ongoing glucose metabolism, and tissue recovery on prompt reperfusion.20, 23, 24
The T2*OC technique was initially developed using 100% normobaric hyperoxia in a rat stroke model and has already shown translational potential for clinical stroke diagnostic imaging with no adverse effects encountered in acute stroke patients.25 However, 100% inhaled oxygen through a mask is associated with susceptibility artefacts over the frontal lobes due to increased levels of paramagnetic gaseous oxygen in the paranasal sinuses.25, 26 Reducing the percentage of inhaled oxygen to 50% or lower26 should eliminate these artifacts. However, this will also reduce the amount of oxygen carried by the blood during OC with a resulting decrease in the size of T2* signal change, making it more difficult to distinguish penumbra from the surrounding tissue compartments.27 In a recent review of oxygen imaging using MRI,28 the T2*OC technique was highlighted for generating interesting results that merited further investigations but had limitations, particularly the low signal changes in humans and the alterations in local physiology. As hemoglobin saturation is >98% on leaving the lungs, the technique primarily depends on the ability of plasma to carry and deliver oxygen to the tissues during the OC. However, plasma is a poor carrier of oxygen (0.003 mL O2/100 ml of blood per mm Hg PO2).29 A potential solution to overcome these limitations would be the use of a more efficient oxygen carrier than plasma.
Perfluorocarbons (PFCs) are fluorinated hydrocarbons with respiratory gas carrying capacity superior to that of hemoglobin.30 They are hydrophobic in nature and therefore are emulsified for intravenous use. Perfluorocarbon particle size within emulsions is very small (∼0.2 μm compared to ∼7 μm for erythrocytes), enabling better infiltration through the microcirculation improving oxygen supply to poorly perfused tissue. These characteristics coupled with the release of oxygen by simple diffusion down a partial pressure gradient make PFCs an ideal agent for overcoming the current limitations of translating T2*OC to humans. Thus, the aim in this study was to establish whether intravenous PFC could enhance the sensitivity of the T2*OC technique for penumbra detection, and allow penumbra detection with a lower fraction of inspired oxygen (FiO2).
Materials and Methods
Model of Middle Cerebral Artery Occlusion
Experiments were performed under license from the UK Home Office, were subject to the Animals (Scientific Procedures) Act, 1986, and were approved by the University Ethical Review Panel. Experiments have been carried out and reported in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Male Sprague-Dawley rats (338±32 g, n=17, Harlan, Bicester, UK) had free access to food and water and were maintained on a 12-hour light-dark cycle. Isoflurane (5%) in N2O:O2 (70%:30%) was used for induction of anesthesia and, following tracheostomy, was lowered during further surgery (2.5% to 3%) and maintenance of animals throughout the protocol (2% to 2.5%). N2O:O2 was replaced with medical air supplemented with ∼5% O2 (26% O2: normoxia) prior to middle cerebral artery occlusion (MCAO). Animals were artificially ventilated and body temperature maintained at 37°C. Femoral arteries were cannulated with polythene tubing (Portex, Smiths Medical, Hythe, UK: external diameter 0.96 mm; internal diameter 0.58 mm) for mean arterial blood pressure measurement and blood gas analysis to maintain physiologic stability. A femoral vein was cannulated for administration of the PFC emulsion. Mean arterial blood pressure and heart rate were continuously recorded (AcqKnowledge, Biopac Systems, Goleta, CA, USA). Middle cerebral artery occlusion was induced with an intraluminal filament. A 3.0 uncoated nylon filament with a heat-induced bulb at the tip (approximately 0.3 mm in diameter), was advanced along the left internal carotid artery for approximately 20 to 21 mm from the bifurcation of the external carotid artery until resistance was felt. Following, MCAO rats were transferred, under the same anesthetic, directly into the magnet bore, fully instrumented for monitoring and control of physiologic variables (blood pressure, temperature, and blood gases). Successful MCAO was confirmed from the initial DWI and PI MRI scans.
Magnetic Resonance Imaging Scanning
Magnetic resonance imaging was performed on a Bruker Biospec (Karlsruhe, Germany) 7T/30 cm system equipped with an inserted gradient coil (121 mm ID, 400 mT/m) and a 72 mm birdcage resonator. Following stroke surgery, the animals were placed in a rat cradle with head secured in position using ear and tooth bars to restrict movement. Brain imaging was acquired using an actively decoupled linear surface receiver coil (2 cm diameter) placed above the head.
Diffusion-weighted image was employed to identify acute ischemic damage: for quantitative determination of the apparent diffusion coefficient (ADC) a multishot spin-echo (echo planar imaging (EPI)) diffusion-weighted scan was used (TE: 22.5 milliseconds, TR: 4,000.3 milliseconds, 4 averages, matrix: 96 × 96, FOV: 25 × 25 mm, 3 directions: x, y, z, B values: 0, 1,000 seconds/mm2, 8 contiguous coronal slices of 1.5 mm thickness, 4-shot EPI).
Perfusion imaging was employed to identify the blood flow deficit: non-invasive, quantitative CBF measurements were carried out within the MCA territory using a form of pseudo-continuous arterial spin labeling based on a train of adiabatic inversion pulses.31 The sequence employs a spin-echo EPI imaging module (TE: 20 milliseconds, TR: 7,000 milliseconds, matrix 96 × 96, FOV 25 × 25 mm, slice thickness 1.5 mm, 16 averages, 4 shots) preceded by 50 hyperbolic secant inversion pulses in a 3-second train. For quantification of CBF maps, a T1-weighted image was acquired, using an EPI inversion recovery sequence (imaging parameters as above, TR 10,000 milliseconds, using 16 inversion times).
The T2* sequence performed during OC was a single shot, gradient echo (EPI) sequence (TE: 20 milliseconds, TR: 10 seconds, matrix 96 × 96, FOV 25 × 25 mm, 8 contiguous slices, 1.5 mm thick, 2 averages, temporal resolution 20 seconds, 75 repetitions). Two coronal slices within middle cerebral artery territory were selected to generate T2* signal change maps, which were used to generate the mean signal change for each region of interest (ROI) across the two slices. Penumbra tissue was defined using a threshold based on the empirical rule: tissue displaying a mean increase in T2*, which was greater than two standard deviations above the contralateral cortex mean.
Perfluorocarbon Emulsion
The PFC used in this study was perfluorodecalin (C10F18) (F2 Chemicals, Lea Town, UK), which is known to have an oxygen solubility of 49 mL/100 mL of aqueous PFC at standard temperature and pressure. The PFC was emulsified for intravenous use in a phosphate buffered solution containing (4% w/v) purified egg yolk lecithin (Lipoid 80 S Egg Lecithin, Ludwigshafen, Germany). The final emulsion for intravenous use was 40% w/v perfluorodecalin and was prepared by Professor Julian Eastoe, an expert in emulsion chemistry at the University of Bristol. The mean droplet diameter within our emulsion was analyzed using dynamic light scattering and was shown to be similar to that reported in the literature (∼0.2 μm).
Experimental Protocol
Following transfer of the animal into the magnet bore, a period of stabilization (∼20 minutes) was allowed during which arterial blood gases, blood pressure, ECG, and respiration were monitored to ensure hemodynamic stability and values were within the normal physiologic range. During the initial hour after MCAO, DWI and PI were carried out to confirm the presence of ischemic injury and CBF deficit. At a mean time of 92 minutes following MCAO, animals were randomized to one of the following groups for MRI studies aimed at identifying ischemic penumbra using T2* OC. (i) 1.5 mL PFC intravenous followed by an increase in FiO2 to 0.4 (n=7); (ii) an increase in FiO2 to 0.4 (n=7). The T2* scan was started with the animal on normoxic ventilation. Following ∼4 minutes (baseline), O2 was increased to 40% during which T2* scanning was continued to enable acquisition of the peak signal change. Arterial blood samples were collected for blood gas analysis prior to the baseline scanning and during the OC.
In a separate group of 3-stroke animals, following PFC injection (1.5 mL intravenous), and 3 minutes baseline T2* scanning (normoxia), FiO2 was increased first to 0.4 for 5 minutes and then to 1.0 for further 4 minutes. This provided additional information on the maximum T2* signal change achievable in the presence of PFC with 40% and 100% O2.
Image Analysis
Apparent diffusion coefficient, CBF, and T2* percentage signal change maps during OC were generated using ImageJ software (http://rsb.info.nih.gov/ij/). Apparent diffusion coefficient maps (see Figure 1A (i)) generated from DWI scans revealed ischemic injury following MCAO. Cerebral blood flow maps (see Figure 1A(v)) generated using arterial spin labeling revealed hypoperfused tissue. Data on the time course and magnitude of T2* signal change during OC were generated for the following ROIs as shown in Figures 1A and 1B, (iv). (1) Presumed penumbra as defined by thresholded T2* percentage signal change; (2) contralateral cortex; (3) ischemic core in caudate nucleus within the thresholded ADC lesion; (4) a corresponding contralateral region manually designated by researcher. In selecting ROIs, the mean T2* percentage signal change (with standard deviation) was measured in the dorsolateral–contralateral cortex, avoiding any large veins and venous sinuses. The T2* penumbra ROI was automatically derived from a threshold: tissue with a percentage signal change ⩾ the contralateral ROI mean+two standard deviations. The ischemic core ROI was selected as a region within the thresholded ADC lesion for the corresponding slice. A representative contralateral region was manually drawn avoiding areas of high signal change on T2* maps because of the presence of large veins, meaning that the region was not always an exact mirror of the ipsilateral ROI.
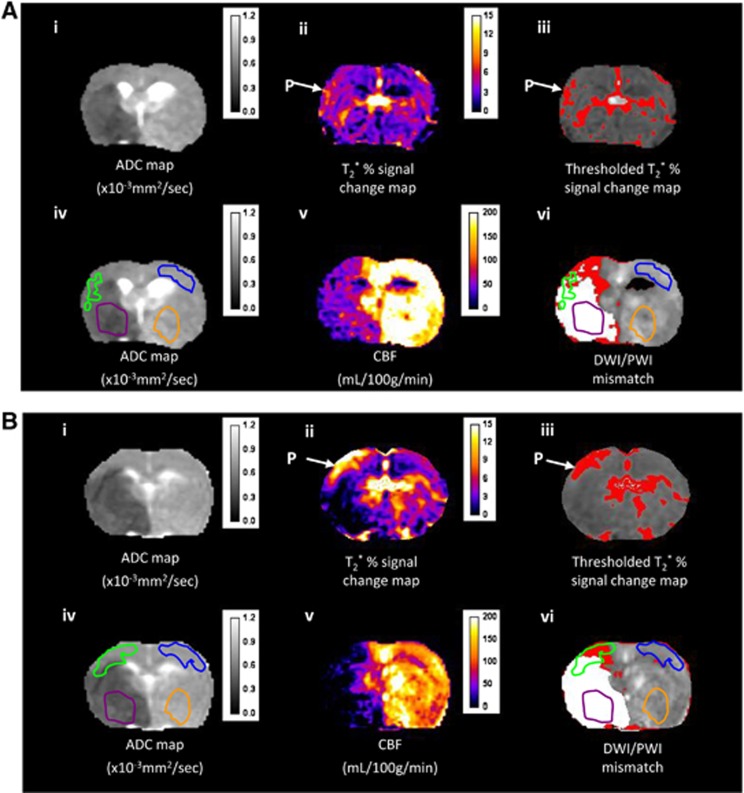
Figure 1.
Detection of the penumbra (P) using T2* oxygen challenge OC with a 0.4 fraction of inspired oxygen (FiO2) (A) or 0.4 FiO2 following 1.5 ml perfluorocarbon (PFC) intravenous (B). (i) Apparent diffusion coefficient (ADC) map prior to OC; (ii) T2* % signal change map; (iii) the corresponding thresholded image; (iv) ADC map with selected regions of interests (ROIs) superimposed (green, T2* P; blue, contralateral cortex; purple, ischemic core; and orange, equivalent contralateral ROI); (v) quantitative cerebral blood flow (CBF) map for the same slice measured at baseline prior to PFC injection and OC; and (vi) the thresholded diffusion-weighted image (DWI) lesion (white shading) and perfusion image-DWI mismatch (red shading) with the four ROIs superimposed.
The ROIs were analyzed on two coronal slices within the middle cerebral artery territory and graphs showing the time course of T2* signal change were generated for each ROI (see Figures 2A and 2B). T2* percentage signal change maps were produced by comparing the peak signal during the OC protocol with the mean signal during baseline (first 4 minutes of scan prior to OC). The difference in signal was divided by the mean baseline signal and multiplied by 100 to give percentage signal change maps.
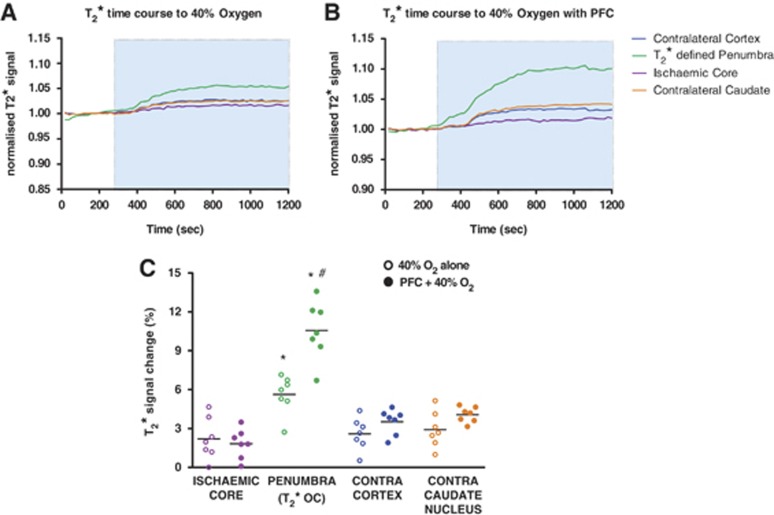
Figure 2.
Graphs showing mean time course data (n=7 per group) of T2* signal change to 0.4 fraction of inspired oxygen (FiO2) (A) or perfluorocarbon (PFC) +0.4 FiO2 (B) in the four regions of interests (ROIs); blue shading indicates the period of oxygen challenge (OC) with 0.4 FiO2. (C) Peak T2* signal changes to 0.4 FiO2 with and without prior administration of PFC in the four ROIs. Horizontal bars indicate means. *indicates significantly greater signal change in T2*-defined penumbra (P) compared to all other ROIs. #indicates significantly enhanced T2* response in the P to 40% oxygen following PFC when compared to 40% oxygen alone.
An estimate of penumbral tissue was also generated based on DWI–PI mismatch area (Figure 1) over the same two coronal slices within the MCA territory. Quantitative ADC maps in units of square millimeter per second were prepared and a reduction of 16.5% in the ADC of the mean contralateral value (excluding ventricles) was used as a threshold to determine ischemic lesion volume.32 Cerebral blood flow maps were produced to identify the perfusion deficit area, which was calculated using a threshold of a 57% reduction of the mean contralateral CBF.33 Thresholded ADC and CBF maps were co-registered to allow assessment of the PI–DWI mismatch (see Figures 1A and 1B (vi)).
Statistics
Statistical analysis was performed using GraphPad Prism (Version 4.03, La Jolla, CA, USA). All data are presented as mean±s.d. Comparison of physiologic variables before and during OC, and of OC-induced T2* signal change in penumbra, contralateral cortex, and ischemic core ROIs were assessed using a 2-tailed paired Student's t-test. Data from different groups were assessed using a 2-tailed unpaired Student's t-test. P<0.05 was considered statistically significant.
Results
Physiologic Variables
Physiologic data are shown in Table 1. Prior to initiation of OC, with or without prior PFC injection, physiologic variables in all animals were within the normal physiologic range. Oxygen challenge (FiO2 0.4) induced a small, significant increase in mean arterial blood pressure and an approximately two-fold increase in PaO2 in both groups, consistent with an earlier report.27 As expected, the increase in PaO2 was less than the 3.5- to 4.5-fold increase reported previously with 100% O2.20, 27 No significant changes were evident in either heart rate or PaCO2 during 40% OC in either group.
Table 1. Physiologic data (n=4–7).
| Baseline | 40% O2 | Baseline | PFC+40% O2 | |
|---|---|---|---|---|
| MABP (mm Hg) | 90.8±7.2 | 94.5±8.3a | 82.8±11.2 | 90.2±12.0a |
| Heart rate (b.p.m.) | 372±15 | 365±14 | 373±19 | 367±20 |
| PaO2 (mm Hg) | 89.2±5.5 | 167±27.7a | 87.5±5.4 | 182±11.2a |
| PaCO2 (mm Hg) | 36.4±7.9 | 35.8±8.3 | 34.7±6.0 | 35.0±3.3 |
b.p.m., beats per minute; MABP, mean arterial blood pressure; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; PFC, perfluorocarbon.
The table shows physiologic data before (baseline) and during oxygen challenge (0.4 fraction of inspired oxygen) in the presence or absence of PFC (1.5 mL, intravenous). Data are expressed as mean±s.d.
Significant increase in MABP and PaO2 (P<0.05) compared to the corresponding baseline value, Student's paired t-test.
Perfluorocarbons Enhance Signal Change to 40% Oxygen in Presumed Penumbra
Data from the T2* percentage signal change maps, analyzed over two coronal slices displayed a heterogeneous pattern of T2* signal change during 40% OC (Figure 1A(ii) and B(ii)). In the ipsilateral hemisphere, the ischemic core ROI showed the smallest percentage change in T2* signal, while the adjacent dorsolateral cortex (thresholded T2*-defined penumbra Figures 1A(iii) and 1B(iii)) displayed significantly greater changes during 40% OC both with and without prior PFC treatment (Figure 2C). Likewise, when compared to percentage changes in the contralateral cortex ROI and the contralateral caudate nucleus ROI, the thresholded T2*-defined penumbra displayed significantly greater changes in T2* signal in both groups (Figure 2C).
When comparing ROI signal changes between groups, it was evident that PFC significantly enhanced the T2* response to 40% O2 in the thresholded penumbra (mean increase of 10.6±2.3% compared to 5.6±1.5% with 40% O2, P<0.001, Figure 2C). This enhancement in the T2* signal change to OC is illustrated in Figure 1B(ii) compared with Figure 1A(ii) and was specific to the penumbra ROI with other ROIs showing similar signal changes between groups.
40% and 100% O2 Produce Matching Regions of Perfluorocarbon-Enhanced Oxygen Challenge-Defined Penumbra
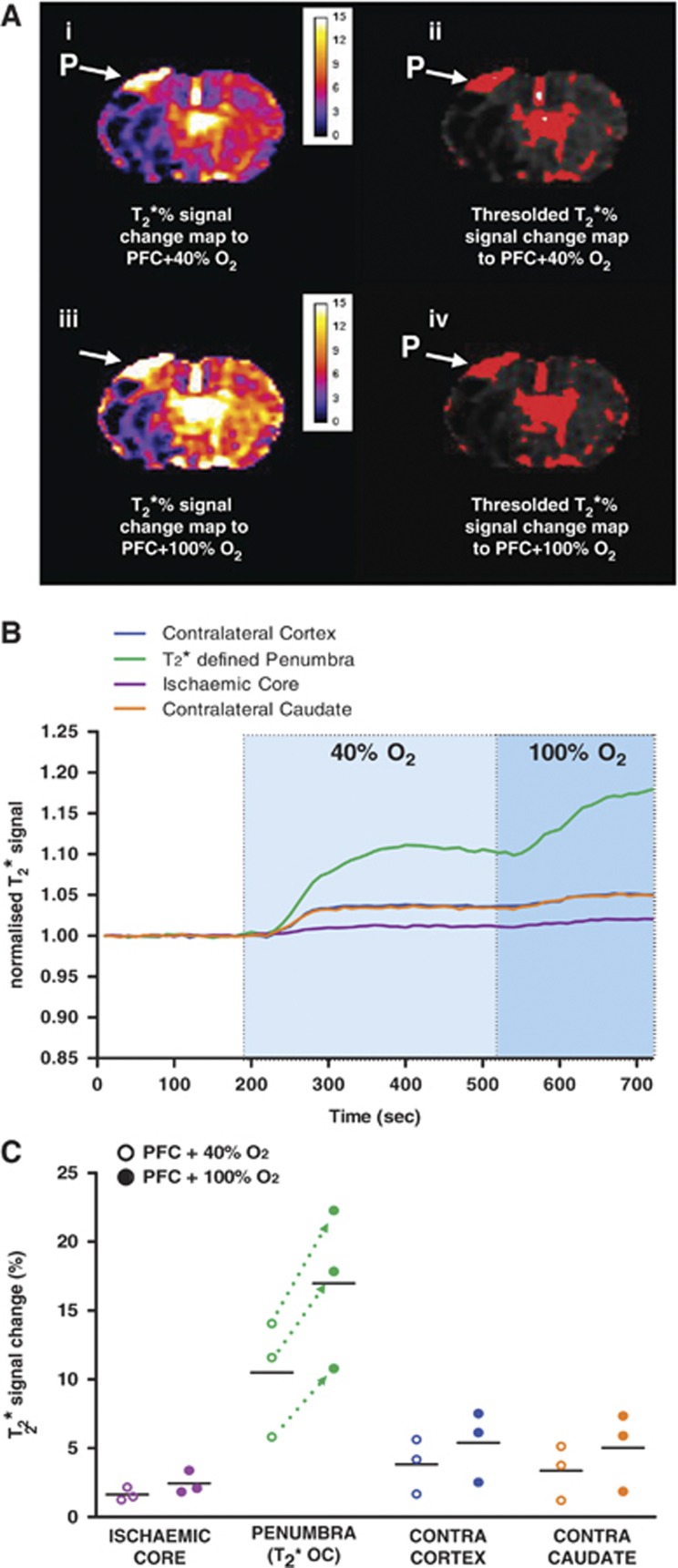
From images produced in the experiments where the level of oxygen during OC was further increased from 40% O2 to 100% O2, T2* signal change maps displayed a similar pattern to those seen previously (Figure 3A). The magnitude of signal change in the penumbra ROI was increased upon switching from 40% to 100% O2 (mean increase of 10.48±4.23% at 40% O2 to 16.96±5.79% at 100% O2, Figures 3B and 3C). Of note, the volume of presumed penumbra identified by PFC+OC was similar for both 40% and 100% O2 OC (Figure 3A(ii) versus Figure 3A(iv)). The mean volume over the two coronal slices analyzed was 6.81±6.35 mm3 with 40% O2 versus 6.92±6.9 mm3 with 100% O2.
Figure 3.
(A) (i) Detection of the penumbra (P) from the T2* % signal change map using 0.4 fraction of inspired oxygen (FiO2) following 1.5 mL perfluorocarbon (PFC) intravenous; (ii) the corresponding thresholded image; (iii) detection of the P in the same animal from the T2* % signal change map following an increase in FiO2 to 1.0; (iv) the corresponding thresholded image. (B) Graph showing mean (n=3) time course of T2* signal change in regions of interests (ROIs) to 0.4 FiO2 (light blue shaded area) and 1.0 FiO2 (darker blue shaded area) following administration of PFC. (C) Peak T2* signal change in ROIs to 0.4 and 1.0 FiO2 following administration of PFC. Dotted lines indicate further increase in T2* within the P ROI on increasing FiO2 from 0.4 to 1.0 following PFC. Horizontal bars indicate means.
T2* Oxygen Challenge-Defined Penumbra Compared to Perfusion Image–Diffusion-Weighted Image Mismatch
Regions identified as penumbra using the T2*OC technique did not identically correspond to the region of PI–DWI mismatch. This is illustrated in Figures 1A and 1B (vi) where it can be seen that the T2*OC-defined penumbra overlaps with the thresholded ADC-defined region of ischemic injury. Also, there are regions of thresholded perfusion deficit that lie out with the region of T2*OC-defined penumbra.
Discussion
The T2*OC technique is based on the different magnetic properties (and influence on T2* signal) of deoxyhemoglobin (paramagnetic) and oxyhemoglobin (diamagnetic), the increased deoxyhemoglobin levels in the vasculature of the penumbra, and the change in deoxyhemoglobin:oxyhemoglobin ratio achieved by delivering an OC. This technique thus enables detection of penumbra based on the tissue response to an OC and is independent of time from stroke onset. Potential healthcare benefits of employing this technique would arise through more informed treatment decisions based on available penumbra, not just time from stroke onset. For example, patients who display significant penumbra beyond the current time window for tPA or for whom stroke onset time is unknown, could be considered for thrombolysis, while those displaying spontaneous recanalisation or no penumbra within 4.5 hours of stroke onset would not receive thrombolysis and be spared the potential risk of hemorrhage.
Evaluation of the technique has shown that T2*OC-defined penumbra (rat MCAO, using 100% O2) displays a physiologic level of glucose metabolism,23 recovers if promptly reperfused,24 and displays 55% of neurons with a normal morphology as shown by histologic analysis at ∼4 hours post stroke.20 The technique has also been successfully reproduced by another group.34
In the current study, we have addressed limitations encountered during subsequent translation of the technique to the clinic. First, the presence of a susceptibility artefact, due to the paramagnetic effect of 100% O2 within the airways and paranasal sinuses, which obscures and distorts parts of the brain, particularly the frontal lobes.25, 26 We have subsequently shown that T2*OC can delineate the different tissue compartments in a rodent stroke model using lower levels of O2 (40% O2)27 but this results in a significant reduction in T2* signal change within the penumbra, potentially decreasing the sensitivity of the technique.28 Second, smaller T2* signal changes to 100% O2 were detected in the clinical study.25 To overcome these problems, we have introduced an intravenous PFC emulsion to increase the oxygen carrying capacity of the blood. Perfluorocarbons have been shown to improve oxygen delivery to ischemic cerebral tissue in preclinical stroke and brain trauma models.35, 36
A lower FiO2 (0.4) with PFC delivery should remove the artefact from the airways on human scans and minimize the influence of the OC on blood pressure and CBF.27 In addition, the lower FiO2 may improve access for patients where high-flow oxygen is contraindicated, such as those with existing pulmonary disease.
Results presented here show that PFC+40% O2 significantly enhanced T2* signal within the penumbra, compared to 40% O2 alone (Figures 1 and 2C). Indeed, the magnitude of signal change to PFC+40% O2 in this study (10.6±2.2%) was slightly higher than the T2* signal change recorded in previous studies using 100% O2 (9.2±3.9%23, 8.4±4.1%24, 8.6±3.7%27). In experiments where FiO2 was increased from 0.4 to 1.0 during the OC, (Figure 3) an additional increase in the T2* signal change occurred in the presence of PFC (16.96±5.79%), demonstrating potential to further increase the sensitivity, if required. Although the signal was enhanced by 100% O2, the volume of penumbra identified did not change, supporting the validity of the technique.
The PFC-induced enhancement in the difference in T2* MRI signal between tissue identified as penumbra and normal tissue (Figure 2) reflects the increased oxygen delivery to the tissue. Our hypothesis for the mechanistic basis of penumbra detection with T2*OC is based on differences in deoxyhemoglobin levels within the vasculature of the penumbra and surrounding tissue due to differences in the oxygen extraction fraction and cerebral blood volume. PaO2 within the penumbra vasculature is low because of the low hemoglobin saturation.37 Reduced oxygen delivery results in an increased oxygen extraction fraction within penumbra, effectively increasing the deoxy:oxyhemoglobin ratio on the capillary/venous side of the circulation compared to normal brain. Factors other than deoxy:oxyhemoglobin ratio could also be contributing to the increased signal seen within the penumbra. The sigmoidal shape of the hemoglobin dissociation curve provides some indication for the differences in T2* signal seen within the penumbra as compared to normal tissues and the disproportionate increase with increasing oxygen delivery. In Figure 4, penumbra and contralateral cortex tissue pO2 data, recorded prior to and during 40% OC,27 have been plotted onto the hemoglobin dissociation curve for Sprague-Dawley rats.38 The slope of the curve is steepest at oxygen tensions below 60 mm Hg where even relatively small increases in oxygen tension will result in a significant increase in arterial hemoglobin oxygen saturation (SaO2), a reduction in deoxy:oxyhemoglobin ratio, and consequently an increase in T2* signal (change in tissue R2* (R2*=1/T2*) varies linearly with changes in cerebral blood oxygen saturation).39, 40 Given that penumbral tissue has a low partial pressure of oxygen (pO2, 15 mm Hg27), it maps onto the steep part of the hemoglobin dissociation curve. A relatively small increase in pO2 (11 mm Hg with 40% O227) maps to a proportionately greater influence on SaO2 and therefore T2* signal change in penumbra than in contralateral cortex, which has a higher baseline pO2 (35 mm Hg27).
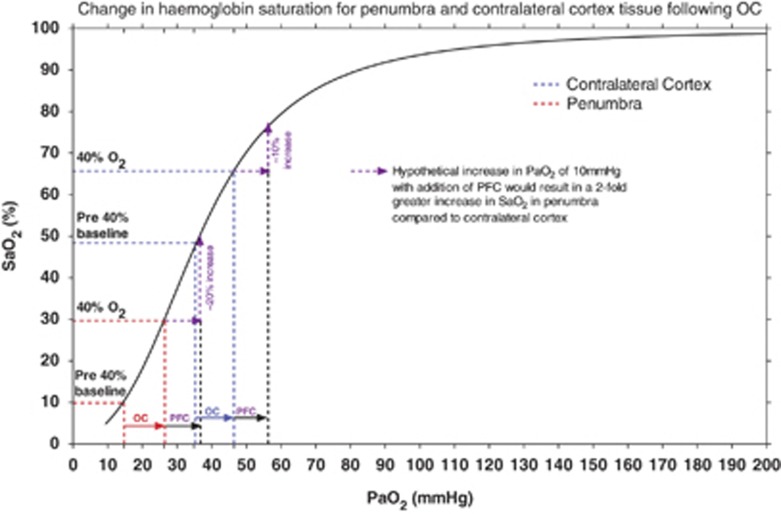
Figure 4.
Standard hemoglobin dissociation curve (black line) for Sprague-Dawley rats (modified from Cartheuser 38) where arterial hemoglobin saturation (SaO2%) is plotted against partial pressure of oxygen in arterial blood (PaO2). Brain tissue pO2 data have been superimposed from one of our earlier studies (brain tissue pO2 was measured simultaneously in the presumed penumbra (P) and corresponding contralateral cortex using implanted Oxyflo/Oxylite probes19) to emphasize the expected SaO2 changes associated with 40% O2 oxygen challenge (OC) and perfluorocarbon (PFC)+40% O2 in the contralateral cortex and P.
The increased amount of O2 carried by the PFC would move PaO2 to the right in both the penumbra and normal tissues as compared to 40% O2 alone. For example, if PFC increased pO2 by a further 10 mm Hg in both penumbra and contralatreral cortex (Figure 4), the additional increase in PaO2 within the penumbra occurs within the steepest part of the curve resulting in an approximate 20% increase in SaO2. However, in contralateral cortex, a similar increase would result in an approximate 10% increase in SaO2. Therefore, the amount of hemoglobin that is saturated within the penumbra vasculature during PFC+40% O2 OC would be greater than with 40% OC alone and this would contribute to the greater signal increase seen within penumbra on T2* imaging thereby enabling it to be visualized as a separate compartment.
We appreciate that a limitation of the current study is that the T2* response to OC was not measured simultaneously with levels of oxy- and deoxyhemoglobin to generate SaO2 values in the penumbra. However, funding is being sought for a subsequent study using simultaneous 2-dimensional optical imaging spectroscopy with MRI to test our hypothesis for the mechanistic basis of T2*OC penumbra detection.
In summary, the addition of intravenous PFC to the T2*OC technique has the potential to improve technique sensitivity for identification of the ischemic penumbra in stroke patients. The addition of the PFC offers improvements by significantly enhancing the T2* signal response and enabling penumbra detection at lower levels of inspired oxygen, thereby avoiding artefacts arising from oxygen in the airways and sinuses. Additionally, by delivering more oxygen to this tissue at risk, PFC with maintained hyperoxia could potentially be investigated as an adjunctive or alternative to reperfusion-based therapies, supporting penumbral survival not only during the imaging session but also during the critical hours following stroke onset.
Acknowledgments
We would like to thank Professor Julian Eastoe for his expertise in producing the PFC emulsion used in this study and Lipoid (Ludwigshafen, Germany) for their gift of Egg Lecithin which was used in emulsion preparation. We would also like to acknowledge Drs Ian Piper and Martin Shaw from Clinical Physics, University of Glasgow for discussions on the mechanistic basis of the technique.
The authors declare no conflict of interest.
Footnotes
This work was supported by an award from Scottish Enterprise, Proof of Concept (POC 8-LSM019).
References
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Perez A, Restrepo L, Kleinman JT, Barker P, Beauchamp N, Wityk RJ. Patients with diffusion-perfusion mismatch on magnetic resonance imaging 48 hours or more after stroke symptom onset: clinical and imaging features. J Neuroimaging. 2006;16:329–333. doi: 10.1111/j.1552-6569.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Wahlgren N, Grond M, Hennerici M, Lees KR, Mikulik R, et al. Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol. 2010;9:866–874. doi: 10.1016/S1474-4422(10)70165-4. [DOI] [PubMed] [Google Scholar]
- Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The “Golden Hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd AG, Hoffman A, Grant R, Campbell JT, Lowe D. Stroke thrombolysis in England, Wales and Nothern Ireland: how much do we do and how much do we need. J Neurol Neurosurg Psychiatry. 2011;82:14–19. doi: 10.1136/jnnp.2009.203174. [DOI] [PubMed] [Google Scholar]
- Moradiya Y, Janjua N.Presentation and outcomes of "Wake-Up Strokes" in a large randomized stroke trial: analysis of data from the international stroke trial J Stroke Cerebrovasc Dis 2012PMID22939198(in press). [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162:1281–1284. [PMC free article] [PubMed] [Google Scholar]
- Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Sobesky J. Comparison of PET and DWI/PWI-MRI in acute ischemic stroke. Keio J Med. 2008;57:125–131. doi: 10.2302/kjm.57.125. [DOI] [PubMed] [Google Scholar]
- Coutts SB, Simon JE, Tomanek AI, Barber PA, Chan J, Hudon ME, et al. Reliability of assessing percentage of diffusion-perfusion mismatch. Stroke. 2003;34:1681–1683. doi: 10.1161/01.STR.0000078840.96473.20. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, et al. EPITHET investigators. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40:1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR, Am J Neuroradiol. 2009;30:1206–1212. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakane Y, Christensen S, Brekenfeld C, Henry MA, Churilov L, Parsons MW, et al. EPITHET: Positive result after reanalysis using baseline diffusion-weighted imaging/perfusion-weighted imaging co-registration. Stroke. 2011;42:59–64. doi: 10.1161/STROKEAHA.110.580464. [DOI] [PubMed] [Google Scholar]
- Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, et al. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke. 2012;43:2986–2991. doi: 10.1161/STROKEAHA.112.661009. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW, et al. EPITHET-DEFUSE investigators. J Cereb Blood Flow Metab. 2012;32:50–56. doi: 10.1038/jcbfm.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosh C, Brennan D, McCabe C, Macrae IM, Holmes WM, Graham DI, et al. Potential use of oxygen as a metabolic biosensor in combination with T2*-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008;28:1742–1753. doi: 10.1038/jcbfm.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci USA. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CA, McCabe C, Gallagher L, Lopez-Gonzalez Mdel R, Holmes WM, Condon B, et al. Stroke penumbra defined by an MRI-based oxygen challenge technique: 1. Validation using [14C]2-deoxyglucose autoradiography. J Cereb Blood Flow Metab. 2011;31:1778–1787. doi: 10.1038/jcbfm.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CA, McCabe C, Gallagher L, Lopez-Gonzalez Mdel R, Holmes WM, Condon B, et al. Stroke penumbra defined by an MRI-based oxygen challenge technique: 2. Validation based on the consequences of reperfusion. J Cereb Blood Flow Metab. 2011;31:1788–1798. doi: 10.1038/jcbfm.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani KA, Santosh C, Brennan D, McCabe C, Holmes WM, Condon B, et al. T2*-weighted magnetic resonance imaging with hyperoxia in acute ischemic stroke. Ann Neurol. 2010;68:37–47. doi: 10.1002/ana.22032. [DOI] [PubMed] [Google Scholar]
- Pilkinton DT, Gaddam SR, Reddy R. Characterization of paramagnetic effects of molecular oxygen on blood oxygenation level-dependent-modulated hyperoxic contrast studies of the human brain. Magn Reson Med. 2011;66:794–801. doi: 10.1002/mrm.22870. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Deuchar GA, McCabe C, Robertson CA, Holmes WM, Santosh C, et al. Influence of 100% and 40% oxygen on penumbral blood flow, oxygen level, and T2*-weighted MRI in a rat stroke model. J Cereb Blood Flow Metab. 2011;31:1799–1806. doi: 10.1038/jcbfm.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Kondering U, Baron JC. Oxygen imaging by MRI: can blood oxygen level-dependent imaging depict the ischemic penumbra. Stroke. 2012;43:2264–2269. doi: 10.1161/STROKEAHA.111.632455. [DOI] [PubMed] [Google Scholar]
- Law R, Bukwirwa H. The physiology of oxygen delivery. Update Anaesth. 1999;10:1–2. [Google Scholar]
- Clark LC, Jr, Becattini F, Kaplan S. The physiological effects artificial blood made from inert organic oxygen solvents. Ala J Med Sci. 1972;9:16–29. [PubMed] [Google Scholar]
- Moffat BA, Chenevert TL, Hall DE, Rehemtulla A, Ross BD. Continuous arterial spin labeling using a train of adiabatic inversion pulses. J Magn Reson Imaging. 2005;21:290–296. doi: 10.1002/jmri.20268. [DOI] [PubMed] [Google Scholar]
- Lo EH, Pierce AR, Mandeville JB, Rosen BR.Neuroprotection with NBQX in rat focal cerebral ischemia. Effects on ADC probability distribution functions and diffusion-perfusion relationships Stroke 199728439–446.; discussion 446–447. [DOI] [PubMed] [Google Scholar]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004;55:207–212. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Huang S, Du F, Duong TQ. Probing ischemic tissue fate with BOLD fMRI of brief oxygen challenge. Brain Res. 2011;1425:132–141. doi: 10.1016/j.brainres.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty WP, Levasseur JE, Sun D, Spiess BD, Bullock MR.Perfluorocarbon emulsion improves cerebral oxygenation and mitochondrial function after fluid percussion brain injury in rats Neurosurgery 2004541223–1230.; discussion 1230. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Sun D, Daugherty WP, Spiess BD, Bullock MR. Effect of perfluorocarbons on brain oxygenation and ischemic damage in an acute subdural hematoma model in rats. J Neurosurg. 2005;103:724–730. doi: 10.3171/jns.2005.103.4.0724. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Cartheuser CF. Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comp Biochem Physiol Comp Physiol. 1993;106:775–782. doi: 10.1016/0300-9629(93)90396-l. [DOI] [PubMed] [Google Scholar]
- Prielmeier F, Nagatomo Y, Frahm J. Cerebral blood oxygenation in rat brain during hypoxic hypoxia. Quantitative MRI of effective transverse relaxation rates. Magn Reson Med. 1994;31:678–681. doi: 10.1002/mrm.1910310615. [DOI] [PubMed] [Google Scholar]
- Lin W, Paczynski RP, Celik A, Kuppusamy K, Hsu CY, Powers WJ. Experimental hypoxemic hypoxia: changes in R2* of brain parenchyma accurately reflect the combined effects of changes in arterial and cerebral venous oxygen saturation. Magn Reson Med. 1998;39:474–481. doi: 10.1002/mrm.1910390318. [DOI] [PubMed] [Google Scholar]