Abstract
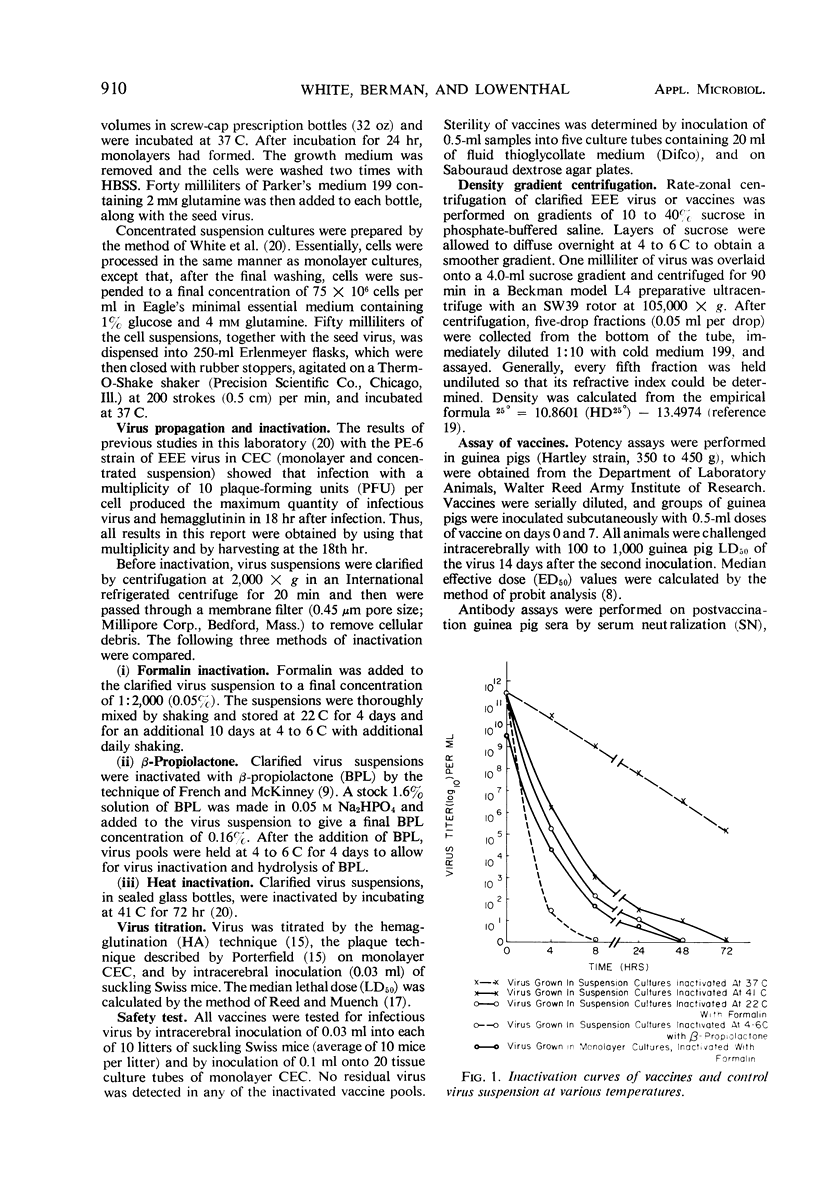
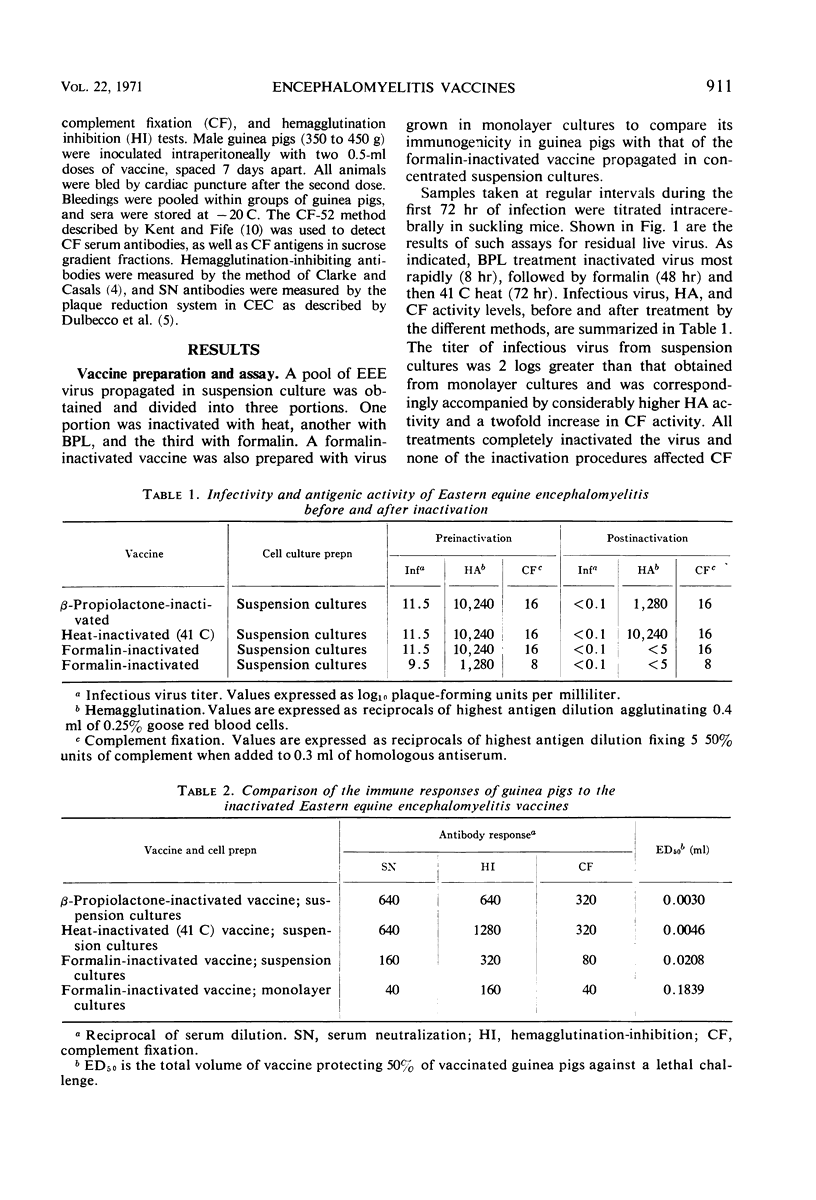
Eastern equine encephalomyelitis vaccines were prepared with virus propagated in stationary monolayer cultures and in concentrated suspension cultures of primary chick embryo cells. Virus pools for vaccine preparation were inactivated by three different methods: 0.05% formalin, 41 C heat, and 0.16% β-propiolactone. Heat-and β-propiolactone-inactivated vaccines maintained high hemagglutinating titers in the fluid state for at least 10 months, whereas formalin-inactivated vaccines lost their hemagglutinating activity within a few hours after treatment. The hemagglutinin of β-propiolactone-inactivated virus particles was more dense than the hemagglutinin of the parent virus particles, as determined by sucrose density gradient centrifugation. The increase in density may be due to the degradation or removal of the lipid from the virus envelope. When administered to guinea pigs, all three vaccines stimulated hemagglutination-inhibiting, complement-fixing, and neutralizing antibodies and afforded protection against a live virus challenge. Test results showed that vaccines prepared with virus propagated in concentrated suspension cultures were more immunogenic and stimulated greater serologic responses in guinea pigs than vaccines derived from monolayer-propagated virus. The β-propiolactone-inactivated vaccine was most protective, the heat-inactivated (41 C) vaccine next, and the formalin-inactivated vaccine least potent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Hoffman E. J., Brown A. Fractionation of Eastern equine encephalitis virus by density gradient centrifugation in CsCl. J Virol. 1968 Oct;2(10):972–978. doi: 10.21236/ad0832598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S., Lowenthal J. P., Sorrentino J. V., White A. B. A safety test for Eastern equine encephalomyelitis vaccine. Appl Microbiol. 1967 Jul;15(4):968–969. doi: 10.1128/am.15.4.968-969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binn L. N., Sponseller M. L., Wooding W. L., McConnell S. J., Spertzel R. O., Yager E. H. Efficacy of an attenuated western encephalitis vaccine in equine animals. Am J Vet Res. 1966 Nov;27(121):1599–1604. [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Eckels K. H., Harrison V. R., Hetrick F. M. Chikungunya virus vaccine prepared by Tween-ether extraction. Appl Microbiol. 1970 Feb;19(2):321–325. doi: 10.1128/am.19.2.321-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., SULKIN S. E. Effect of beta-propiolactone on complement-fixing antigens of St. Louis encephalitis virus. Proc Soc Exp Biol Med. 1957 May;95(1):112–115. doi: 10.3181/00379727-95-23138. [DOI] [PubMed] [Google Scholar]
- FRENCH G. R., MCKINNEY R. W. USE OF BETA-PROPIOLACTONE IN PREPARATION OF INACTIVATED ARBOVIRUS SEROLOGIC TEST ANTIGENS. J Immunol. 1964 May;92:772–778. [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- LOWENTHAL J. P., BERMAN S., GROGAN E. W. Eastern equine encephalomyelitis vaccine prepared in cell cultures. Science. 1961 Aug 25;134(3478):565–566. doi: 10.1126/science.134.3478.565. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M. STUDIES ON THE STRUCTURE OF A HEMAGGLUTINATING COMPONENT OF A GROUP A ARBO VIRUS (SINDBIS). Virology. 1964 Aug;23:573–581. doi: 10.1016/0042-6822(64)90241-7. [DOI] [PubMed] [Google Scholar]
- Maire L. F., 3rd, McKinney R. W., Cole F. E., Jr An inactivated eastern equine encephalomyelitis vaccine propagated in chick-embryo cell culture. I. Production and testing. Am J Trop Med Hyg. 1970 Jan;19(1):119–122. doi: 10.4269/ajtmh.1970.19.119. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. A simple plaque-inhibition test for the study of arthropod-borne viruses. Bull World Health Organ. 1960;22:373–380. [PMC free article] [PubMed] [Google Scholar]
- Reitman M., Tribble H. R., Jr, Green L. Gamma-irradiated Venezuelan equine encephalitis vaccines. Appl Microbiol. 1970 May;19(5):763–767. doi: 10.1128/am.19.5.763-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


