Abstract
Background
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation, bone erosion, and cartilage destruction in the joints. It is increasingly being realized that inflammation might play an important role in inducing bone damage in arthritis. However, there is limited validation of this concept in vivo in well-controlled experimental conditions. We have addressed this issue using the adjuvant arthritis (AA) model of RA.
Methods
In AA, the draining lymph nodes are the main sites of activation of pathogenic leukocytes, which then migrate into the joints leading to the induction of arthritis. We tested the temporal kinetics of mediators of bone damage (e.g., receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin (OPG) and osteopontin (OPN)) and of mediators of inflammation (pro-inflammatory cytokines and chemokines) in the draining lymph node cells (LNC) at different phases of AA, and then examined their inter-relationships.
Results
Our study revealed that, together with cytokines/chemokines, the mediators of bone remodeling also are produced in LNC. Various cytokines/chemokines showed distinct kinetics of expression as well as patterns of correlation with mediators of bone remodeling at different phases of the disease. Pro-inflammatory cytokines such as TNF-α are known to play an important role in bone damage. Interestingly, there was a positive correlation between TNF-α and RANKL, between RANKL and each of the 3 chemokines tested (RANTES, MIP-1α, and GRO/KC), and between TNF-α and RANTES.
Conclusion
Our results in the AA model lend support to the concept of osteo-immune crosstalk during the course of autoimmune arthritis.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily targets the joints, and it is characterized by inflammatory synovitis mediated by leukocytes and pro-inflammatory cytokines secreted by them (1-5). Rat adjuvant arthritis (AA) is a model of polyarthritis, and it is widely used to study the pathogenesis of arthritis as well as to perform preclinical testing of anti-arthritic agents. AA can be induced in Lewis rats by subcutaneous (s.c.) immunization with heat-killed M. tuberculosis H37Ra (Mtb). The draining lymph nodes are the sites of activation of pathogenic leukocytes, which then migrate into the joints leading to the induction of arthritis. It is increasingly being realized that inflammation plays an important role in inducing bone damage in arthritis. Furthermore, the two processes share a common set of mediators, including certain cytokines and chemokines. This functional relationship between inflammation and bone damage is referred to as the “osteo-immune cross-talk”, or “osteoimmunology” (6, 7).
Although it is known that certain mediators of bone remodeling (e.g., receptor activator of nuclear factor kappa-B ligand (RANKL) and osteopontin (OPN)) also play a role in immune responses by influencing antigen-presenting cell (APC)-T cell interactions, there is barely any information regarding the temporal kinetics of expression of these mediators in the draining lymph node cells (LNC) and their inter-relationships with the mediators of inflammation (cytokines and chemokines) in that tissue. The significance of the draining lymph nodes in the pathogenesis of AA is evident from studies showing that the disease can be adoptively transferred to syngeneic naïve recipient rats via the draining LNC of an arthritic rat (8). Furthermore, the removal of lymph nodes during the incubation phase of AA prevents the subsequent development of AA in rats (9). Also, we (10, 11) and others (12) have shown that study of the temporal cytokine profiles during AA can provide useful insights into the role of cytokine balance in disease susceptibility/resistance.
To better understand the interplay between inflammation and bone damage, it is essential that not only the profiles but also the correlations between the levels of mediators of inflammation (cytokines and chemokines) and bone damage (RANKL, osteoprotegerin (OPG), OPN, etc.) are comprehensively examined. Furthermore, from the disease pathogenesis point of view, it is essential to know whether, and to what extent, the mediators of bone damage that are measured in the peripheral lymphoid tissue (the draining lymph nodes in this study) correlate with actual inflammation and bone damage in arthritic joints as measured by arthritic scores, histology, and bone histomorphometry. We have addressed these issues in the present study. We report here the expression profile of defined mediators of bone remodeling, cytokines, and chemokines involved in arthritis, and their inter-relationships during the course of arthritis.
MATERIALS AND METHODS
Animals
Five to six week-old male Lewis (LEW/Hsd) (RT.11) rats were purchased from Harlan Sprague-Dawley (HSD) (Indianapolis, IN, USA) and then maintained in the animal care facility of the University of Maryland School of Medicine, Baltimore. This study was carried out following recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol (# 0511010) was approved by Institutional Animal Care and Use Committee of University of Maryland School of Medicine, Baltimore, MD. All efforts were made to minimize suffering to animals.
Induction and evaluation of adjuvant arthritis (AA)
Lewis rats were immunized s.c. at the base of the tail with l mg/rat heat-killed M. tuberculosis H37Ra (Mtb) (Difco, Detroit, MI) in 200 μL of mineral oil (Sigma-Aldrich). The rats were assessed for signs of arthritis and graded for the severity of arthritis as described previously (13). Briefly, the severity of arthritis was evaluated on the basis of erythema, swelling, and induration of the hind paws and graded on a scale of 0 to 4 as follows: 0 = no erythema or swelling, 1 = slight erythema or swelling of the ankle or wrist, 2 = moderate erythema and swelling at the wrist or ankle, 3 = moderate erythema and swelling at the wrist/metacarpals or ankle/metatarsals, 4 = severe erythema and swelling of the forepaw or hind paw. Different phases of AA were labeled as follows: 0 day (naive), incubation (d 7), onset (d 12), peak (d 18), and regression/recovery (d 25).
Histological examination of hind paws of rats at different phases of AA
The hind paws were harvested from rats on d 0, d 7, d 12, d 18 and d 25 after Mtb immunization and then immersed for 9 d in Cal-Ex Decalcifying solution CS510-1D (Fisher Scientific, Fair Lawn, NJ). Thereafter, the paws were immersed in 70% ethanol for 5 d and then embedded in paraffin, sectioned serially using a microtome, and mounted on microscope slides in the Histology Core, UMB (14). Thereafter, the sections were stained either with hematoxylin and eosin (H&E) or with Safranin-O (15). Histopathological changes in the joints like synovial hyperplasia, pannus formation, and cartilage and bone damage were observed under a microscope (Nikon Eclips E800 Microscope, Nikon Industries Inc. Melville, NY) using the Spot Imaging Software, (Diagnostic Instruments Inc., Sterling Heights, MI) and digital images were obtained (14).
Tartrate-resistant acid phosphatase (TRAP) staining
The unstained sections of hind paws (prepared as described above) were dehydrated in graded concentrations of ethanol and xylene, and fixed for 2 min using 3.7% formaldehyde. After washing with deionized water, the sections were incubated in the reaction mixture (Acid Phosphatase, Leukocyte (TRAP) Kit) at 37°C in a humid and light-protected incubator for 1 h as directed by the manufacturer. Thereafter, the sections were washed 3 times with distilled water, counter-stained with hematoxylin, observed under a microscope using the Spot Imaging Software, and digital images obtained.
Bone histomorphometry
It was performed on the TRAP-stained hind paw sections using the Osteomeasure Bone Histomorphometry system (Osteometrics) linked to a Nikon Eclipse 50i inverted microscope and a Sony CCD video camera. Histomorphometric parameters used follow the recommended nomenclature of the American Society for Bone and Mineral research (16). The analyses were performed on serial transverse sections through the talus (n= 4). Bone volume versus total tissue volume (BV/TV), the number of osteoclasts per tissue area (N.Oc/T.Ar), active resorption per bone surface area based on the ratio of osteoclast surface/bone surface area (Oc.S/BS), and the number of osteoclasts per bone perimeter (N.Oc/B.Pm) were assessed.
Radiographic assessment of arthritis in hind paws of rats
The severity of AA was assessed blindly at different phases of the disease by radiography. High-resolution digital radiographs (40 kV, 12 s) of hind limbs were performed on rats with a Faxitron Digital X-ray system (Faxitron X-Ray, Lincolnshire, IL, USA).
Preparation and antigenic restimulation of lymph node cells (LNC)
Mtb-immunized rats were euthanized at different phases of AA and their draining lymph nodes (para-aortic, inguinal and popliteal) were harvested (n= 3) (17). A single-cell suspension of LNC was prepared in HL-1 serum free medium (Ventrex Laboratories, Portland, ME) supplemented with 2 mM L-glutamine, 100 U/ml penicillin G sodium, and 100 μg/mL streptomycin sulfate. These LNC were cultured (8 × 106 cells/well) in a 6-well plate (Corning Incorporated Corning, NY) for 24 h at 37°C in an atmosphere of 95% air and 5% CO2. Sonicated Mtb (10 μg/mL) was used for restimulation of LNC. Keyhole limpet hemocyanin (KLH) was used as the control antigen. Further, LNC culture supernatant was harvested for testing by a Multiplex assay as elaborated below. In an independent set of experiments, LNC harvested from arthritic rats on d 18 were tested as described above but with the difference that IL-17 (100 ng/mL) was added to a subset of wells containing LNC. LNC culture supernatant was then tested by a Multiplex assay.
Determination of chemokine/cytokine protein expression by Multiplex immunoassay
Multiplex assays were performed at the Cytokine Core Facility (University of Maryland, Baltimore) using the Luminex 100TH analyzer (Luminex Corp., Austin, TX), and the levels of cytokines (as protein) in 24 h culture supernatant of LNC and serum were measured. The Rat chemokine 4-plex kit (Millipore) was used to measure regulated upon Activation, Normal T-cell Expressed, and Secreted (RANTES), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), growth regulated oncogene- keratinocyte chemoattractant (GRO/KC). Similarly, the Rat cytokine 5-plex kit was used to measure TNF-α, IL-6, IL-17, IL-18 and Granulocyte-macrophage colony-stimulating factor (GM-CSF). Single-plex and 3-plex kits were used to measure the levels of RANKL, OPG and OPN.
Statistical analysis
The data were expressed as mean ± SEM. Student’s t-test and ANOVA were used to assess the significance of differences using GraphPad Prism version 4.0. A ‘P’ value of < 0.05 was considered significant.
RESULTS
Clinical, histological and radiological characteristics of AA at different phases of the disease in the Lewis rat
The course of clinical AA
The severity of arthritis was graded in the hind paws. Swelling and erythema of hind paws appeared on d 10-12 after Mtb injection and then progressively increased with time post Mtb-injection until peak, followed by spontaneous regression of inflammation. The mean arthritic scores at different phases of AA are shown in Fig. 1A. Based on the severity of arthritis, the course of AA had the following 5 phases in reference to the day of Mtb injection: d 0, induction; d 7, incubation; d 12, onset; d 18, peak; and d 25, regression/recovery.
Figure 1. Progression of arthritic inflammation and tissue damage in the hind paws of Lewis rats during the course of AA.
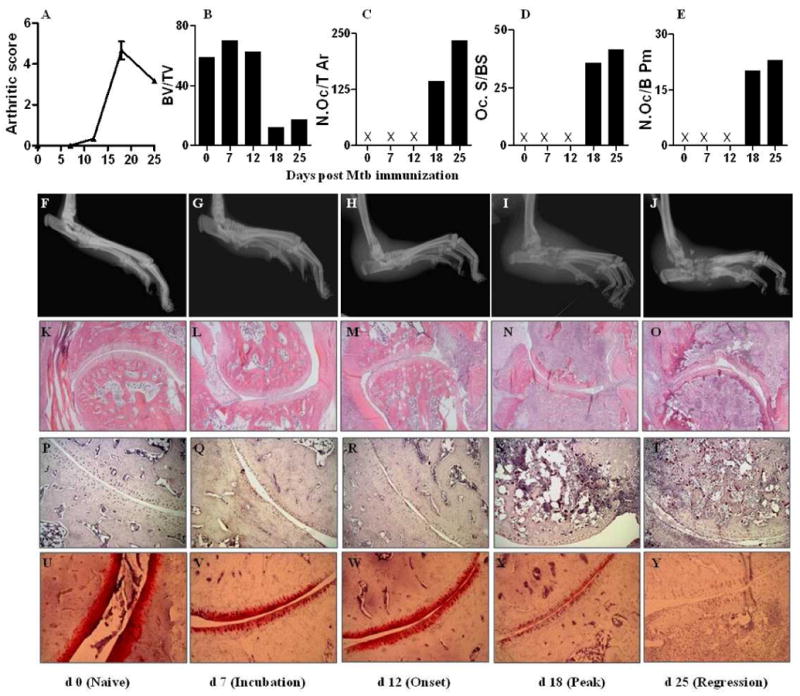
First (Top) panel: (A), Arthritic scores of rats beginning from d 0 (Mtb injection) through d 25; (B-E) Histomorphometric analysis of bone tissue: (B) Bone volume versus total tissue volume (BV/TV), (C) number of osteoclasts per tissue area (N.Oc/T Ar), (D) active resorption per bone surface area based on the ratio of osteoclast surface/bone surface area (Oc.S/BS), and (E) number of osteoclasts per bone perimeter (N.Oc/B Pm). Second panel (F-J), Representative radiographs of hind limbs at different phases of the disease course. Third panel (K-O), Representative micrographs of H&E-stained histological sections (10×) of the hind paw joints showing joint space, pannus containing the mononuclear cell infiltrate, and bone mass at different phases of the disease course. Fourth panel (P-T), representative micrographs of the stained histological sections showing TRAP-positive cells (20×); Fifth (Bottom) panel (U-Y), representative micrographs of the safranin O’-stained histological sections showing proteoglycan content in the cartilage (20×). Mtb, heat-killed M. tuberculosis H37Ra; X- parameter tested but not detectable.
Radiography of hind paws
Hind paws of rats were examined by X-ray on different days after Mtb immunization (Fig. 1F-J). No significant abnormal changes were observed on d 7 compared to d 0 except for minimal soft tissue swelling. By d 12, increased swelling of the soft tissue of the paws was visible along with a decrease in bone density. By d 18, swelling of the paws was aggravated, and bone erosion in the articular facet and the surrounding area was detected. By d 25, soft tissue swelling was still present and bones were found to be fused, especially in the marginal area around the articular facet. The joint space was almost obliterated by then.
Histological examination of hind paws
H&E-stained sections of the hind paws harvested on different days after Mtb immunization were examined (Fig. 1K-O). No inflammation or tissue destruction was detected in histological sections on d 0 and d 7. Pannus formation started appearing on d 12 and its extent continued to increase for the next few days. Marked synovial proliferation, mononuclear cell infiltration and destruction of articular bones were observed on d 12 and d 18 of AA.
Histomorphometry of TRAP-stained paw sections
We examined the subchondral bone loss and osteoclast numbers in the talus of the hind paw joints of rats at different phases of AA using histomorphometry (Fig. 1B-E) of TRAP-stained sections (Fig. 1P-T) as described in detail under methods. There was no significant change in bone volume until d 12, but by d 18 there was a huge reduction in bone volume, and it continued to decrease thereafter. The number of osteoclasts increased from d 18 onwards, and active resorption surface was elevated on d 18 and d 25. There was an increase in osteoclast number/bone perimeter on d 18 and d 25. Safranin ‘O staining revealed that cartilage destruction appeared on d 18 and the cartilage had almost disappeared by d 25 (Fig. 1U-Y).
The profiles of mediators of inflammation and bone damage at different phases of AA
Pro-inflammatory cytokines
There was differential expression of cytokines at different phases of AA (Fig. 2 top panel). TNF-α and IL-6 showed a bell-shaped curve such that these cytokines were expressed at lower levels at early phase of AA, at highest level at the peak phase, and then again at lower levels at the late phase of AA. IL-17 expression had an inverse bell-shaped curve, i.e., highest level of IL-17 was at the incubation phase, lowest level at the time of onset of AA, followed by an increase after d 18 onwards. However, IL-18 showed a gradually increasing profile with progression of AA.
Figure 2. Cytokine/chemokine profiles through the disease course in arthritic rats.
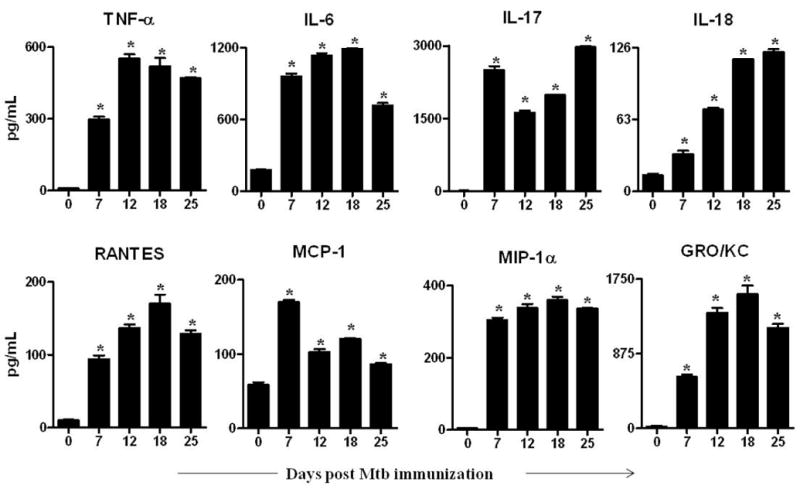
Lymph node cells (LNC) were harvested at each phase of the disease from Mtb-immunized and naïve rats (n= 3 per group) as described under Methods. Thereafter, these cells were restimulated for 24 h with Mtb sonicate (10 μg/mL). The levels of the indicated cytokines (Top panel) and chemokines (Bottom panel) were measured in culture supernatants of LNC using a Multiplex assay and the results were expressed as pg/mL. *, p<0.05, comparing the level at each phase of arthritic rats with that of naïve rats.
Chemokines
We tested 4 different chemokines in LNC culture supernate (Fig. 2 bottom panel). A bell-shaped curve was observed for the levels of RANTES and GRO/KC; a gradual increase from d 7, peak level at d 18, and a decline in levels thereafter. However, a biphasic curve was observed for MCP-1, with increased production at incubation phase (d 7), decreased production at onset (d 12), a slight increase at peak (d 18), followed by reduced expression at d 25. There was an increase in MIP-lα at d 7 but no change thereafter.
Mediators of bone remodeling
The expression profiles of these mediators are shown in Fig. 3. A bell-shaped curve was observed for RANKL with increased production at d 7, highest level at Onset (d 12), and then a gradual decline at later stages of AA (Fig. 3). In contrast, OPG was elevated at early stages but decreased after onset (d 12). As expected, the RANKL/OPG ratio was low at early phase and then increased gradually in the late phase. There was no change in GM-CSF production until d 7, but slightly increased expression found at d 12, followed by high levels on d 18 and d 25. There was increased expression of OPN on d 0, followed by a decline thereafter.
Figure 3. The profile of mediators of bone remodeling through the disease course.
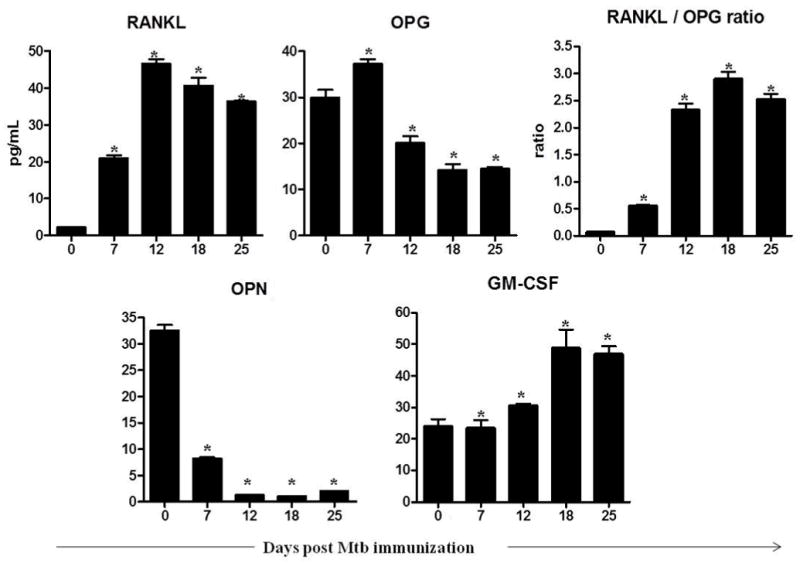
The culture supernates of LNC were prepared as described in the legend to Figure 2. The levels of the indicated mediators of bone remodeling in these culture supernatants were measured using a Multiplex assay, and the results were expressed as pg/mL. RANKL, receptor activator of nuclear factor kappa-B ligand; OPG, osteoprotegerin; GM-CSF, granulocyte-macrophage colony-stimulating factor; OPN, osteopontin; Mtb, heat-killed M. tuberculosis H37Ra. *, p<0.05, comparing the level at each phase of arthritic rats with that of naïve rats.
Correlations among mediators of arthritic inflammation and bone remodeling
The correlation coefficient (r2) was determined for a given pair of biomolecules from different subgroups (cytokines, chemokines and mediators of bone remodeling), and from the same subgroup. The results are summarized in Table I and described below.
Table I.
Correlations among mediators of arthritic inflammation and bone remodeling
| Mediators of bone damage | Cytokines | Chemokines | Arthritic score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RANKL | OPG | GM-CSF | TNF-α | IL-6 | IL-17 | IL-18 | RANTES | MCP-1 | MIP1-α | GRO/KC | |||
| Mediators of bone damage | RANKL | - | + | + | + | + | + | + | NS | + | + | + | |
| OPG | - | - | - | NS | NS | - | - | NS | NS | - | - | ||
| GM-CSF | + | - | + | NS | NS | + | + | NS | + | + | + | ||
| Cytokines | TNF-α | + | - | + | + | + | + | + | NS | + | + | + | |
| IL-6 | + | NS | NS | + | + | + | + | + | + | + | NS | ||
| IL-17 | + | NS | NS | + | + | + | + | + | + | + | NS | ||
| IL-18 | + | - | + | + | + | + | + | NS | + | + | + | ||
| Chemokines | RANTES | + | - | + | + | + | + | + | NS | + | + | + | |
| MCP-1 | NS | NS | NS | NS | + | + | NS | NS | + | NS | NS | ||
| MCP-1α | + | NS | + | + | + | + | + | + | + | + | NS | ||
| GRO/KC | + | - | + | + | + | + | + | + | NS | + | + | ||
+, positive correlation (p< 0.05); -, Negative correlation (p< 0.05); NS, not significant (p> 0.05)
Mediators of bone remodeling and cytokines
A positive correlation was observed between RANKL and each of the pro-inflammatory cytokines (TNF-α, IL-6, IL-17, IL-18) tested (Table I). In contrast, there was a negative correlation between OPG and TNF-α/IL-18. However, no significant correlation was observed between OPG and IL-6/IL-17. Interestingly, GM-CSF showed a pattern of correlation with the pro-inflammatory cytokines that was similar to RANKL but opposite to that of OPG.
Mediators of bone remodeling and chemokines
RANKL/GM-CSF each showed a positive correlation with RANTES, MIP-1α, and GRO/KC. In contrast, OPG showed a negative correlation with both RANTES and GRO/KC.
Cytokines vs. chemokines
TNF-α, IL-6, IL-17 and IL-18 showed a positive correlation with all chemokines tested (RANTES, MCP-1, MIP-1α, GRO/KC), except for no significant correlation of TNF-α and IL-18 with MCP-1.
Arthritic scores vs. mediators of bone damage/cytokines/chemokines
RANKL and GM-CSF showed a positive correlation with arthritic score. In contrast, OPG showed a negative correlation with the disease score. Among the cytokines/chemokines, TNF-α, IL-6, IL-18, RANTES, and GRO/KC showed a positive correlation with arthritic scores.
IL-17–induced cytokines and chemokines in LNC
To assess the validity of some of the statistical correlations, we stimulated LNC with IL-17 in vitro and tested for the expression of cytokines and chemokines (Fig. 4). Stimulation of LNC with Mtb in the presence of IL-17 resulted in increased production of cytokines such as TNF-α and IL-18, and it also elevated the levels of chemokines such as GRO/KC, MIP-1α, and RANTES.
Figure 4. IL-17-induced pro-inflammatory cytokines and chemokines in lymphoid cells.
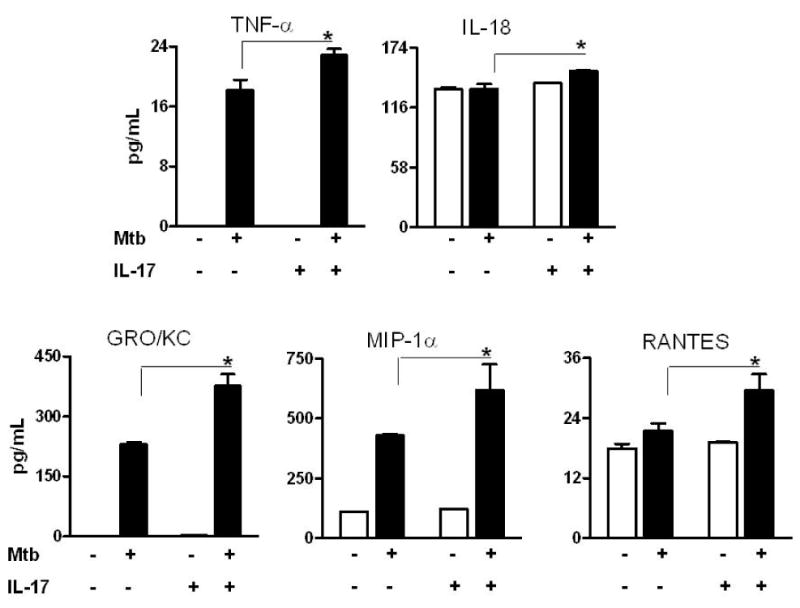
LNC harvested from an arthritic rat on d 18 of AA were stimulated with IL-17 in the presence or absence of Mtb. Culture supernatants were collected after 24 h and measured for indicated cytokines and chemokines using a Multiplex assay. The results were expressed as pg/mL. p<0.05, comparing IL-17-stimulated and control samples. Abbreviations are the same as in Figure 3.
DISCUSSION
Immunization with heat-killed Mtb induces T cell-mediated arthritis (AA) in Lewis rats. Cells in the draining lymph nodes play an important role in the initiation and development of arthritis. The disease-inducing events start with a pro-inflammatory burst of immune activities in the draining lymphoid organs, generating a local pool of arthritogenic cells, which then migrate into the joints leading to arthritis in peripheral joints (8). The inflammation thus induced triggers the production of mediators and the activation of subsets of cells (e.g., osteoclasts) involved in bone damage. This interplay among the mediators of inflammation and bone damage is referred to as the “osteo-immune crosstalk”. Previous reports highlighting such a crosstalk have mostly been focused on the effect of a particular cytokine on stimulating the expression of a specific bone mediator, and that too, frequently on a single day in the course of a disease, for example at the peak of the disease. In this context, there is barely any information on the kinetics of mediators of bone damage in the draining LNC during the course of arthritis, or on the relationships between the kinetics of cytokines/chemokines versus that of the mediators of bone damage. We have addressed these critical issues in the present study.
Our study has revealed at least two important aspects of the mediators of osteo-immune crosstalk in the draining LNC: 1) Together with cytokines/chemokines, several mediators of bone remodeling are also produced in LNC; we propose that these mediators might be recruited to the target organs (joints) and thereby contribute to the disease process in AA. 2) Certain cytokines/chemokines show high correlation with bone mediators in early phase of AA, while others correlate well only in late phase of AA; however, a third group shows good correlation throughout the course of arthritis. These differential correlations might have functional implications.
Effect of cytokines on bone mediators
In arthritis, inflammation and tissue destruction result from complex cell-cell interactions. Cytokines such as TNF-α, IL-6 and IL-17 are the major pro-inflammatory mediators, which play an important role in inflammation and bone damage (18-21). Our results show a strong positive correlation between TNF-α and RANKL, supporting the existing literature (22, 23). It is clear from our data that both TNF-α and RANKL are produced at incubation period of AA and reach highest level at the onset of AA, followed by a slight decrease during the peak phase of AA. This trend also supports the likely synergistic effect of TNF and RANKL in up-regulating RANK expression as reported by others (23). Other investigators have shown that IL-18 together with TNF-α stimulates osteoclastogenesis by inducing RANKL production in synovial T cells (24). In comparison to RANKL, there was no significant correlation between OPG and any of the pro-inflammatory cytokines tested except for IL-18, which showed a strong negative correlation with AA. We suggest that cytokines produced in LNC mediate bone damage predominantly via induction of RANKL instead of suppression of OPG during the disease course. A similar result was observed when testing synovial-infiltrating cells (SIC) in our previously reported study (25)
It has been reported that IL-17 can induce RANKL expression in the synovium, as tested at the peak phase of arthritis (26). However, our study revealed only a weak positive correlation between IL-17 and RANKL in LNC during the disease course. This weak correlation might be due to the varying level of expression of IL-17 at different time points of the disease. We observed that IL-17 is produced at maximum level at early incubation phase and then the level drops at the onset and peak phases, followed by a sudden rise at the regression phase of AA. In contrast, RANKL reaches its maximum level at the onset of AA and then drops at the regression phase of the disease. Besides IL-17, IL-6 can also induce RANKL production (27) and this is supported by a strong correlation between these two mediators throughout the disease course. Another cytokine GM-CSF is known to aggravate arthritis (28). In our study, GM-CSF increased with disease progression in AA, as did IL-18, but its levels did not correlate with any of the pro-inflammatory cytokines except for IL-18. As expected, we observed a strong negative correlation between RANKL and OPG, but a moderately negative correlation between GM-CSF and OPG. Further, OPN showed a prominent decrease with progression of AA.
Effect of chemokines on bone mediators
Chemokines play an important role in the development of arthritis. Chemokines may either have a direct or an indirect effect on bone mediators. The former by inducing them, the latter by either recruiting cells that produce cytokines (or bone mediators), or by regulating a cytokine which then can alter bone mediators. Chemokines such as RANTES, MCP-1, MIP-1α and GRO/KC have been shown to be elevated in LNC and splenic adherent cells (SAC) of arthritic rats(29, 30). In AA, elevated levels of RANTES are found in the peripheral blood at the onset of the disease, whereas MCP-1 is first detected in the synovial tissue and later detected in the peripheral blood on d 18 (peak phase of AA) (31). However, there is limited information on the expression kinetics of RANTES and MCP-1 in the lymphoid tissue during the entire course of AA. Here we report that RANTES and MCP-1 are expressed in the lymphoid tissue even before the onset of AA. The levels of RANTES and GRO/KC showed a gradual increase through the disease course until the peak phase, whereas MCP-1 had a biphasic expression profile. In comparison, MIP-1α levels were almost constant after a sudden elevation at the incubation phase of AA. Further, RANTES positively correlated with both the disease score and the extent of bone damage. RANTES and MIP-1α stimulate the development and motility of RANKL-dependent bone-resorptive osteoclasts (32). In support of that report, our results show a strong positive correlation between RANKL and the 3 chemokines tested (RANTES, MIP-1a, and GRO/KC), whereas OPG failed to show any significant correlation with the expression of these chemokines. Further, RANKL induces RANTES and MCP-1 production to promote osteoclast fusion and forms a platform for osteoclastogenesis (33).
Effect of cytokines on chemokines
Cytokines can effectively modulate the expression of certain chemokines. TNF-α showed a strong positive correlation with RANTES expression, whereas IL-17 had a weak correlation with RANTES. Though currently we do not know the significance of this correlation between IL-17 and RANTES, but it supports an earlier study where it was reported that IL-17 inhibited TNF-α -induced RANTES expression in synovial fibroblasts (34). As per that study, levels of TNF-α, IL-6, and IL-18 exhibited strong positive correlation with that of RANTES, MIP-1α and GRO/KC, indicating that cytokines and chemokines cooperate to propagate the disease process. The observed correlations were validated by our results of in vitro stimulation of LNC with IL-17. IL-17 not only induced the expression of TNF-α and IL-18, but also of chemokines such as RANTES, MIP-1α and GRO/KC. In view of the above correlation, it is tempting to speculate a reverse effect, i.e., chemokines influencing the production of pro-inflammatory cytokines. However, to the best of our knowledge, there are no reports documenting the direct effects of chemokines on cytokine production in arthritic milieu.
In conclusion, LNC expressed not only pro-inflammatory cytokines and chemokines but also mediators of bone remodeling during the course of AA. Statistical analysis showed a significant correlation between cytokines/chemokines and bone remodeling mediators, as well as between cytokines and chemokines. Our results lend support to the concept of osteo-immune crosstalk during the course of autoimmune arthritis in the AA model. These results are of direct relevance to human RA not only for advancing our understanding of the pathogenesis of autoimmune arthritis, but also for developing novel approaches for the treatment of arthritis.
Acknowledgments
This work was supported by grant # R01AT004321 from the National Institutes of Health, Bethesda, MD, USA. The authors thankfully acknowledge help of Lisa Hester for Multiplex assays, and Carla Hebert for helping in staining procedures. We also thank Shivaprasad Venkatesha, Hua Yu and Brian Astry for helpful discussions.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest with any aspect of the work reported in this manuscript.
References
- 1.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31(12):927–40. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorman CL, Cope AP. Immune-mediated pathways in chronic inflammatory arthritis. Best Pract Res Clin Rheumatol. 2008;22(2):221–38. doi: 10.1016/j.berh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hahn B. A pathophysiologic approach to the clinical management of arthritis and pain: current and future implications. J Clin Rheumatol. 2004;10(3 Suppl):S3–4. doi: 10.1097/01.rhu.0000130683.61892.b2. [DOI] [PubMed] [Google Scholar]
- 4.Lipsky PE. Rheumatoid arthritis. In: Fauci EB A, Kasper D, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison’s Principles of Intenrnal Medicine. 17. New York: McGraw Hill New York, NY, USA; 2008. pp. 2083–92. [Google Scholar]
- 5.Steiner G, Tohidast-Akrad M, Witzmann G, et al. Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxford) 1999;38(3):202–13. doi: 10.1093/rheumatology/38.3.202. [DOI] [PubMed] [Google Scholar]
- 6.Gravallese EM. Bone destruction in arthritis. Annals of the rheumatic diseases. 2002;61(Suppl 2):ii84–6. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 8.Holm BC, Lorentzen JC, Bucht A. Adjuvant oil induces waves of arthritogenic lymph node cells prior to arthritis onset. Clin Exp Immunol. 2004;137(1):59–64. doi: 10.1111/j.1365-2249.2004.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newbould BB. Role of Lymph Nodes in Adjuvant-Induced Arthritis in Rats. Annals of the rheumatic diseases. 1964;23:392–6. doi: 10.1136/ard.23.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Shin EK, Kim YH, et al. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Invest. 2009;39(9):819–27. doi: 10.1111/j.1365-2362.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 11.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J Biol Chem. 2011;286(4):2817–25. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolina M, Bolon B, Middleton S, et al. The evolving systemic and local biomarker milieu at different stages of disease progression in rat adjuvant-induced arthritis. J Clin Immunol. 2009;29(2):158–74. doi: 10.1007/s10875-008-9238-8. [DOI] [PubMed] [Google Scholar]
- 13.Moudgil KD, Chang TT, Eradat H, et al. Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. J Exp Med. 1997;185(7):1307–16. doi: 10.1084/jem.185.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YH, Rajaiah R, Lee DY, et al. Suppression of ongoing experimental arthritis by a chinese herbal formula (huo-luo-xiao-ling dan) involves changes in antigen-induced immunological and biochemical mediators of inflammation. Evid Based Complement Alternat Med. 2011;2011:642027. doi: 10.1155/2011/642027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. The Journal of bone and joint surgery. 1997;79(10):1452–63. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286(17):15138–46. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163(9):5049–55. [PubMed] [Google Scholar]
- 19.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strand V, Kavanaugh AF. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):ii110–iii6. doi: 10.1093/rheumatology/keh202. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Calatrava MJ, Prieto-Potin I, Roman-Blas JA, Tardio L, Largo R, Herrero-Beaumont G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res Ther. 2012;14(3):R149. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276(1):563–8. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 24.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Annals of the rheumatic diseases. 2004;63(11):1379–86. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanjundaiah SM, Venkatesha SH, Yu H, Tong L, Stains JP, Moudgil KD. Celastrus and Its Bioactive Celastrol Protect against Bone Damage in Autoimmune Arthritis by Modulating Osteoimmune Cross-talk. J Biol Chem. 2012;287(26):22216–26. doi: 10.1074/jbc.M112.356816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubberts E, van den Bersselaar L, Oppers-Walgreen B, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003;170(5):2655–62. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 27.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47(11):1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 28.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Annals of the rheumatic diseases. 1997;56(6):364–8. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanjundaiah SM, Lee DY, Ma Z, et al. Modified huo-luo-xiao-ling dan suppresses adjuvant arthritis by inhibiting chemokines and matrix-degrading enzymes. Evid Based Complement Alternat Med. 2012;2012:589256. doi: 10.1155/2012/589256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesha Shivaprasad H, BCA, Nanjundaiah Siddaraju M, Yu Hua, Moudgil Kamal D. Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines. Bioorganic & Medicinal Chemistry. 2012 doi: 10.1016/j.bmc.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szekanecz Z, Halloran MM, Volin MV, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis and rheumatism. 2000;43(6):1266–77. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res. 2004;19(12):2065–77. doi: 10.1359/JBMR.040910. [DOI] [PubMed] [Google Scholar]
- 33.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280(16):16163–9. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 34.Schnyder B, Schnyder-Candrian S, Pansky A, et al. IL-17 reduces TNF-induced Rantes and VCAM-1 expression. Cytokine. 2005;31(3):191–202. doi: 10.1016/j.cyto.2005.02.012. [DOI] [PubMed] [Google Scholar]


