Abstract
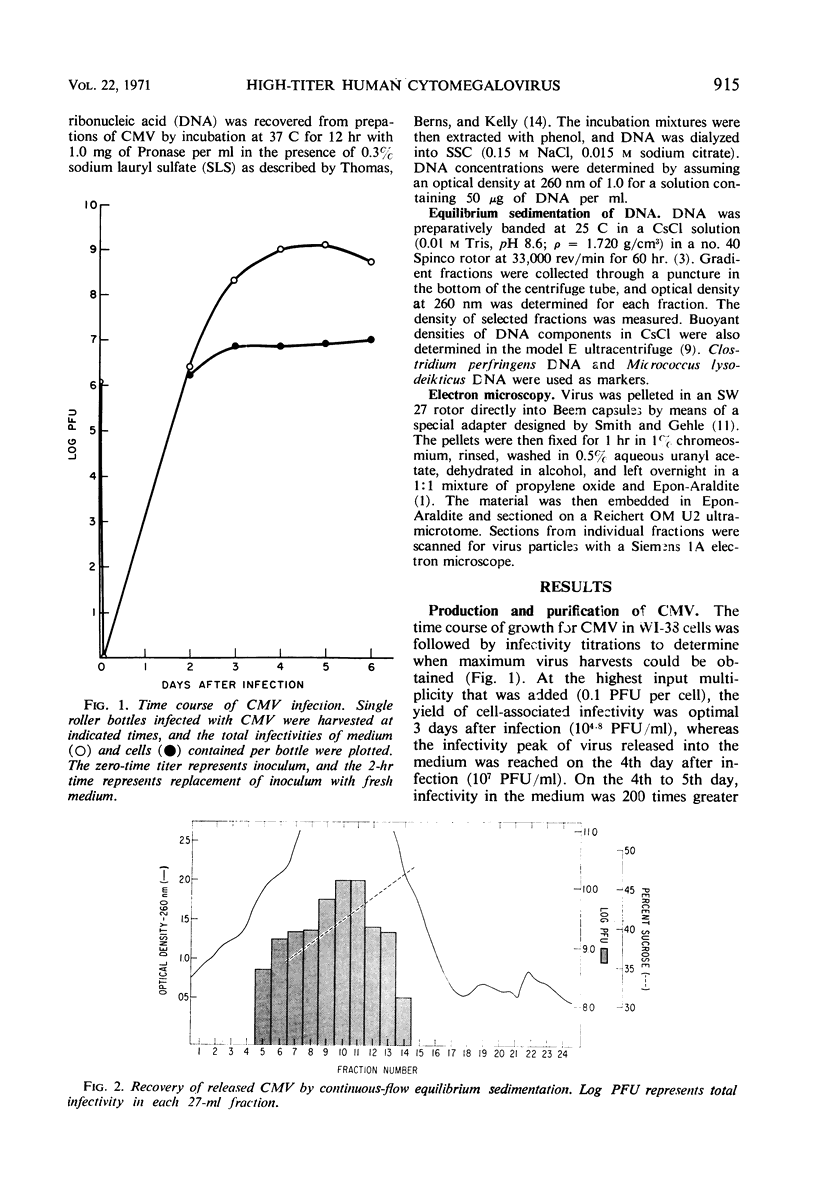
High-titered yields of human cytomegalovirus (CMV), strain AD 169, were produced in WI-38 cells in large roller bottles. Maximum plaque titers were observed by the 4th day after infection at which time infectivity in the medium was 200 times greater than that associated with the cells. Virus released into the medium was recovered by sedimentation in a sucrose gradient in a continuous-flow centrifuge rotor. Maximal viral infectivity was found at a sucrose concentration of 42%, equivalent to a density of 1.18 g/cm3. Deoxyribonucleic acid extracted from these preparations was about 80% viral and 20% cellular as judged by equilibrium centrifugation in cesium chloride density gradients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond H. E., Hall W. T. High-yield isolation of mouse mammary tumor virus. J Natl Cancer Inst. 1969 Nov;43(5):1073–1082. [PubMed] [Google Scholar]
- CRAIG J. M., MACAULEY J. C., WELLER T. H., WIRTH P. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med. 1957 Jan;94(1):4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., LEE A. J. DISCUSSION AND PRELIMINARY REPORTS. THE NUCLEIC ACID OF HUMAN CYTOMEGALOVIRUS. Virology. 1964 May;23:105–107. doi: 10.1016/s0042-6822(64)80014-3. [DOI] [PubMed] [Google Scholar]
- Cline G. B., Nunley C. E., Anderson N. G. Improved continuous flow centrifugation with banding. Nature. 1966 Oct 29;212(5061):487–489. doi: 10.1038/212487a0. [DOI] [PubMed] [Google Scholar]
- Martos L. M., Ablashi D. V., Gilden R. V., Sigüenza R. F., Hampar B. Preparation of immune rabbit sera with neutralizing activity against human cytomegalovirus and varicella-zoster virus. J Gen Virol. 1970;7(2):169–171. doi: 10.1099/0022-1317-7-2-169. [DOI] [PubMed] [Google Scholar]
- McCombs R. M. Concentration and purification of herpesviruses (simplex, cytomegalo, and EB) in a zonal ultracentrifuge. Appl Microbiol. 1969 Apr;17(4):636–638. doi: 10.1128/am.17.4.636-638.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- Plummer G. Comparative virology of the herpes group. Prog Med Virol. 1967;9:302–340. [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. SOME CHARACTERISTICS OF THE DEOXYRIBONUCLEIC ACID FROM HERPES SIMPLEX VIRUS. Virology. 1963 Nov;21:353–361. doi: 10.1016/0042-6822(63)90196-x. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D. Pelleting virus-infected cells for thin-section electron microscopy. Proc Soc Exp Biol Med. 1969 Apr;130(4):1117–1119. doi: 10.3181/00379727-130-33731. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyesh-Melnick M. Interactions of human cytomegalovirus with human fibroblasts. J Bacteriol. 1966 Jan;91(1):213–220. doi: 10.1128/jb.91.1.213-220.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]