Abstract
A genome-wide scan was carried out on a segregating F2 population of rats derived from reciprocal intercrosses between two inbred strains of rats, Fisher 344 (F344) and Wistar Kyoto (WKY) that differ significantly in their behavioral coping responses to stress measured by the defensive burying (DB) test. The DB test measures differences in coping strategies by assaying an animal’s behavioral response to an immediate threat. We have previously identified three X-linked loci contributing to the phenotypic variance in behavioral coping. Here we report on six significant autosomal quantitative trait loci (QTL) related to different behaviors in the DB test:one for the number of shocks received, three for number of prod approaches, one for latency to bury, and one pleiotropic locus affecting both approach and latency. These QTL contributing to different aspects of coping behaviors show that the effect of genotype on phenotype is highly dependent on lineage. The WKY lineage was particularly influential, with five out of the six QTL affecting coping behavior only in rats of the WKY lineage, and one locus affecting only those in the F344 lineage. Thus, epigenetic factors, primarily of WKY origin, may significantly modulate the genetic contribution to variance in behavioral responses to stress in the DB test.
Keywords: Coping, defensive burying, epigenetic, lineage, linear modeling, QTL, stress
INTRODUCTION
It is widely accepted that complex traits are influenced by multiple genes (Flint, 2003). Quantitative trait loci (QTL) analysis provides a method for identifying associations between genotypic and phenotypic variance in a genetically segregating and phenotypically heterogenous population. This technique is used to identify regions along the genome where polymorphic genes that influence complex traits might lie (Lander and Botstein, 1989; Lander and Schork, 1994). Complex traits, in addition to being multigenic, are also influenced by epigenetic and environmental factors (Hofmann, 2003).
Epigenetic and environmental influences, in some cases, can override genetic effects (Francis et al., 2003) while in other studies, genetic contribution to the trait is strong even in the presence of epigenetic modulators (Ahmadiyeh et al., 2004). Some of these epigenetic influences, such as mothering styles, can be faithfully transmitted by recipients of such care to future generations through non-genetic means (Champagne and Meaney, 2001; Francis et al., 1999a; Meaney, 2001). Since previous studies in rats demonstrate that differences in maternal care can result in different stress-response and fear-related behavioral profiles in adulthood (Caldji et al., 1998; Liu et al., 1997), controlling for maternal care via reciprocal breeding in a behavioral genetic study could potentially identify some of these epigenetic effects.
In the present study, we report on a genome-wide analysis of QTLs in a segregating F2 population of rats derived from two inbred strains (F344 and WKY) that differ significantly in their coping responses to stress as measured by the defensive burying (DB) test. We employed a reciprocal breeding strategy to obtain the F1 generation consisting of the two subpopulations. The genetic composition at autosomal loci is identical in the two F1 subpopulations while the X and Y chromosomes and cytoplasmic factors are distinct. Then, we maintained the lineage by mating F1 littermates to generate the intercross population, where the F2 subpopulations have different grandmothers.
Based on naturalistic observations of rodents’ behaviors in response to predators in the wild, the DB test measures differences in coping strategies (Koolhaas et al., 1999; Korte et al., 1992; Pare, 1994; Sluyter et al., 1996; Treit, 1985; Treit et al., 1981) by assaying an animal’s behavioral response to an immediate threat with ethological validity (Treit, 1991). In the DB test, the animal unexpectedly receives a mild electric shock, which represents the immediate danger. In response to this threat, rats can adopt different defensive behavioral strategies. They can further explore the prod by approaching and even receiving further shocks. Alternatively, they can actively avoid further shocks by burying the prod with the bedding material. WKYs, who show passive coping behaviors in the DB test, approach the shock prod more, but bury less than F344s.
Using this same sample of F2 rats, we have previously identified three X-linked QTL that are associated with coping styles in the DB test:Coping-1, on the proximal end on the X Chr related to behaviors of latency to bury and duration of burying the electrified prod, and Approach-1 and Approach-2 on the distal end of the X Chr, related to the number of prod approaches. This latter locus was significantly influenced by lineage (Ahmadiyeh et al., 2003). We have also determined that pup-directed maternal behaviors (licking-grooming, arched-back nursing, no contact, and neglect) are significantly different between F344 and WKY inbred strains (Ahmadiyeh et al., 2004). Thus it is conceivable that the behavioral differences observed between these two inbred strains and behavioral variation within the F2 population are not only a reflection of underlying genotypic differences, but also of epigenetic effects of maternal factors passed on intergenerationally. If genotypic and epigenetic maternal effects interact to determine coping responses to stress, we hypothesize that the effect of genotype at our mapped loci will be significantly modulated at least in some cases by grandmaternal lineage effects.
MATERIALS AND METHODS
Cross
All animal experimentation was approved by the Northwestern University Animal Care and Use Committee. All animals were maintained in a 14:10 light:dark cycle and kept under constant ambient temperature (21 ± 1°C) with food and water available ad libitum. Parental WKY and F344 animals were obtained from Harlan Sprague–Dawley (Indianapolis, IN) and bred reciprocally (WKY females mated with F344 males and vice versa), pairing one male with two females, to generate 121 F1 animals. Sister–brother breeding of both lineages (WKY mother and F344 mother) of F1s generated 486 F2 generation animals. Pups were weaned at 24 days of age, separated by sex and housed 3–5 animals per cage. At the time of weaning, 5 mm tail samples were collected for genomic DNA isolation. At 13 weeks of age, animals were tested in the DB test.
Defensive Burying Test
The defensive burying test was carried out as described previously (Ahmadiyeh et al., 2003). Briefly, animals are habituated (four cagemates together) to a plexiglass chamber (40 cm square, 60 cm high) with bedding (wood shaving) (7 cm deep, 1 cm below the hole for the prod) for 15 minutes each day, for three consecutive days, between 10:00 AM and 2:00 PM. On the fourth day, a continuously electrified prod is introduced into the chamber, which delivers a shock when the rat touches it. The shock is generated from a shock generator (Lafayette Instruments, San Diego, CA) set at 4.5 mA. Animals are singly and randomly (from the same cage) introduced into the chamber on the fourth day between 10:00 AM and 2:00 PM. The rats typically explore the novel prod and receive a shock, which starts the 15-minute videotaped test. Once shocked, animals typically do not approach the prod, retreat to the back of the cage, and begin spraying bedding toward the prod in an effort to cover it. Behaviors recorded and subsequently scored by an observer blind to the identity of the animal include the latency to begin burying, the total time spent burying (duration of burying), the number of times the rat gets shocked and the number of times a rat approaches the prod (snout within 1.0 cm from prod). All traits were analyzed after taking their logarithms (or loge(x+1)) to reduce skew in distributions.
Genotyping
The genotypes of the 486 F2 animals were determined at 110 simple sequence length polymorphism (SSLP) markers, spaced an average of 16.3 cM (range:2–27 cM) apart across the entire genome. Tail samples were collected at weaning and DNA was isolated using standard phenol–chloroform extraction; genotypes were resolved using autoradiography on polyacrylamide gels or ethidium bromide on agarose gels when F344 and WKY alleles differed by 12 bp or greater. For more details, see genotyping methods described previously (Ahmadiyeh et al., 2003; Shimomura et al., 2001).
Primers
Rat Genome Database (URL:http://rgd.mcw.edu/) was used to determine polymorphisms between WKY and F344 strains. Most of these (polyacrylamide markers) were purchased from Research Genetics, while the markers with greater than 12 bp difference (agarose markers) were purchased through Integrated DNA Technologies (IDT; www.idtdna.com). X-linked markers were described previously (Ahmadiyeh et al., 2003). Supplementary Table S1 lists the markers used in our scan.
Quantitative Genetic Analysis
A sex-averaged genetic map was constructed using R/qtl software (Broman et al., 2003) with allowance for 1% genotyping error rate. This map was used for interval mapping analysis. Single QTL and simultaneous all QTL pair genome scans were carried out using pseudomarker software (Sen and Churchill, 2001). All analyses included the X chromosome. For each phenotype, putative positions of QTLs were scanned in 5 cM increments throughout the genome, generating a LOD score at each scanned position. The pairwise analyses included the X chromosome and failed to identify any significant QTL interactions (at permutation based LOD thresholds ranging from 7.2 to 8.2, multiple test adjusted 0.05 level) and are not discussed further here. In our experience with studies of this size, an interaction would have to explain 2–4% of the total variance to be clearly significant. Although this may seem like a small effect, it is quite a large contribution on top of any main effects that the interacting loci may already contribute. Any gene–gene interactions that are sex-and/or lineage-specific would be even more difficult to detect unless they are very substantial. We cannot rule out the possibility that weak interaction effects are present but undetected in this study.
Significance thresholds for the single QTL genome scans were estimated by permutation analysis (1000 permutations) (Churchill and Doerge, 1994). In order to avoid “illegal” genotypes on the X chromosome and to retain the associations of lineage and sex with the phenotypes, the permutations were restricted to shuffling within the four categories of animals defined by lineage and sex.
Detecting QTL by Model Comparison
In our current study, several different genome scans were generated under different statistical models, including genome scans with (1) sex and grandmaternal lineage as additive covariates; (2) sex as an interacting covariate; (3) grandmaternal lineage as an interacting covariate; (4) sex and grandmaternal lineage simultaneously interacting. Thus, covariates sex and grandmaternal lineage were incorporated into genome scans with both additive and interactive effects of covariates being considered. We also considered a combined covariate with four discrete levels corresponding to the possible combinations of sex and lineage. In contrast to the traditional simple genome scan, our hypothesis testing by model comparisons allowed us to detect QTL that were influenced by sex and lineage effects. As previously described (Ahmadiyeh et al., 2003), these genome scans were based on a linear model that includes an interaction term between the covariate and the QTL. This allows the QTL effect to differ among classes of animals defined by the covariate. For example, a QTL may have an effect in male rats but not in female rats or it may affect both groups but in opposite directions. No additional multiple test adjustments were applied for the covariate based genome scans. The scans are not independent and their purpose is to elucidate sex-and lineage-specific effects of QTLs. Thus, we have applied stringent genome-wide adjusted 0.05 significance criteria to each scan and have reported all QTLs that meet this criterion in at least one scan. Suggestive QTLs are not discussed here, although several can be identified in Figures 1–4.
Figure 1.
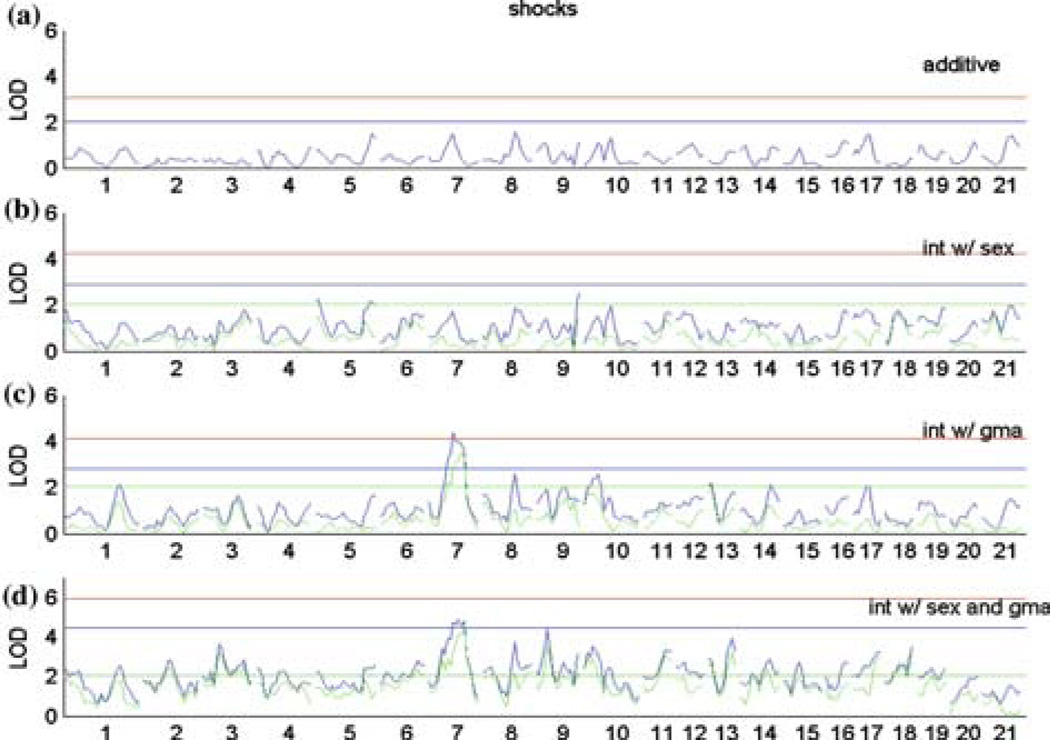
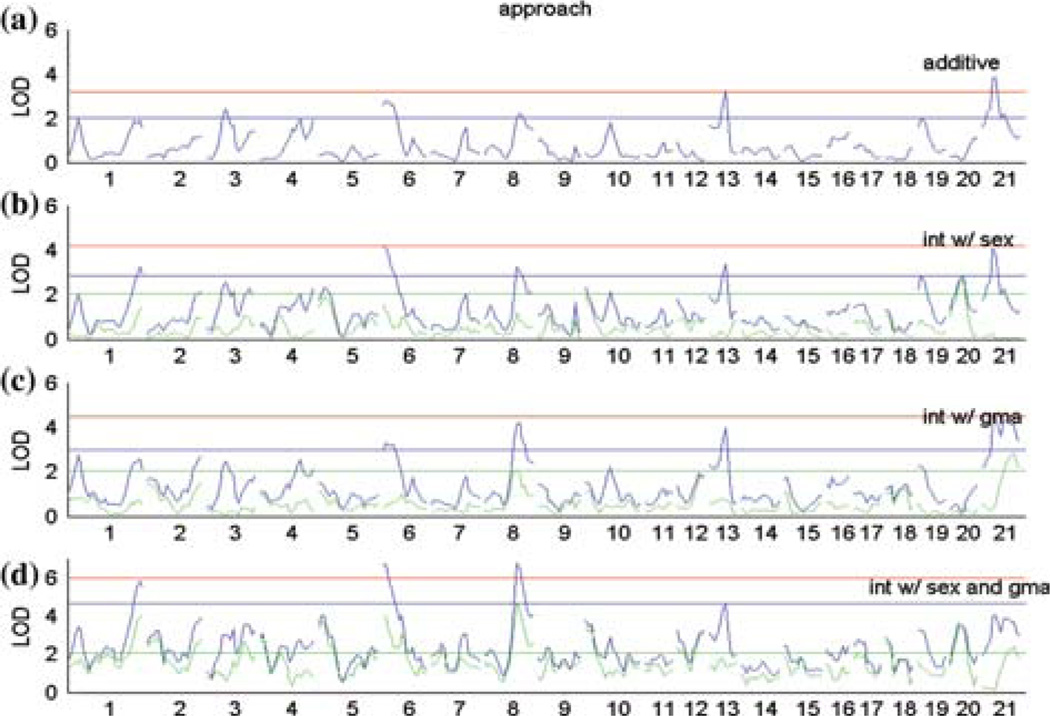
Genome scans of DB-specific traits showing locations where shocks (Fig. 1), approach (Fig. 2), latency (Fig. 3), and duration (Fig. 4) loci lie, each determined by analyzing (a) sex and grandmaternal lineage as additive covariates, (b) sex as an interactive covariate, (c) grandmaternal lineage as an interactive covariate, and (d) sex and grandmaternal lineage simultaneously interacting. X-axis contains representative chromosomal locations, Y-axis displays LOD score. Genome-wide permutation-derived thresholds of significance are shown by red line (significant: p < 0.05), blue line (suggestive: p < 0.63), with green line and green plots representing the interaction only portion of variance in the interaction models.
Figure 4.
Please refer the figure caption of Figure1
Since a thorough analysis of the X-chromosomal loci has been previously published (Ahmadiyeh et al., 2003), these loci are not discussed in this paper. Complete data files and analysis scripts for the analyses carried out here are available at http://www.jax.org/research/churchill. Every autosomal locus that was identified in at least one of the covariate scans at a genome-wide significance level of p < 0.05 was subsequently analyzed within each of the four groups defined by all possible combinations of sex and grandmaternal lineage (F-females, W-females, F-males, W-males, where F denotes F344 grandmaternal lineage and W denotes WKY grandmaternal lineage). A one-way ANOVA F-test was used to establish the significance of phenotypic differences between F344 homozygotes (FF), heterozygotes (FW), and WKY homozygotes (WW) within each stratum. Using stratified allele effects plots we were able to visualize the interplay between genotype, sex and grandmaternal lineage.
RESULTS
Phenotypic differences in DB parameters in parental, F1, and F2 generations have been reported previously (Ahmadiyeh et al., 2003). Here, we only reiterate the major findings as they are related to the genome-wide analysis. In general, F344s are engaged in a more active coping response to the electrified prod than WKYs, with a shorter latency to begin burying, a longer duration of burying, and less prod approaches than the WKYs. There are no differences in the number of shocks received between F344s and WKYs.
Significant sex and/or lineage effects were found in the F2 offspring for every phenotype (Table I). Although some phenotypes such as the number of shocks received and prod approaches showed a significant sex effect, the effect of lineage alone or the sex lineage interaction was more profound explaining a larger percent variance in the approach, latency and duration phenotypes. Even though the largest percent variance explained by the grandmother line (lineage) was only 2.2 (duration), the data demonstrate a strong overall phenotypic effect of grandmaternal lineage.
Table I.
Sex and Lineage Effects in F2 phenotypes
| Log shocks | Log approach | Log latency | Log duration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | %var | F | p | %var | F | p | %var | F | p | %var | F | p |
| Sex | 1 | 1.0 | 3.91 | 0.05 | 1.1 | 4.56 | 0.033 | 0.2 | 0.93 | 0.3 | 0.7 | 2.91 | 0.089 |
| Lineage | 1 | 0.01 | 0.06 | 0.8 | 1.6 | 6.74 | 0.010 | 1.3 | 5.45 | 0.02 | 2.2 | 9.48 | 0.002 |
| sex*lineage | 1 | 0.4 | 1.81 | 0.2 | 0.4 | 1.88 | 0.2 | 1.9 | 8.13 | 0.005 | 1.3 | 5.61 | 0.018 |
| Total | 408 | ||||||||||||
Genome-wide interval mapping analysis using the various covariate models are presented in Figures 1–4 for each phenotype in turn:shocks (Fig. 1), approach (Fig. 2), latency (Fig. 3), and duration (Fig. 4). The four panels of each figure represent models in which sex and lineage are additive covariates (A), sex is an interacting covariate (B), grandmaternal lineage is an interacting covariate (C), and sex and lineage simultaneously interact (D). The top tracing in panels B–D is the full LOD score and the lower tracing (in green) is the component of the LOD attributable to the QTL by covariate interaction term(s).
Figure 2.
Please refer the figure caption of Figure1.
Figure 3.
Please refer the figure caption of Figure1
Table II summarizes the results of these mains-cans showing all significant autosomal loci that achieved a permutation-derived genome-wide level of significance of p < 0.05. We found six significant autosomal QTL (adj. p < 0.05) related to different behaviors in the DB test:one locus for shocks, three for approach, one for latency, and one pleiotropic locus affecting approach and latency. QTL that were retained in the regression model were named. In general, each locus represents a distinct QTL except for the locus on chromosome 6 at 1 cM which is shared by both approach (approach-4) and latency (latency-2) traits. We note that in some cases a QTL that is significant for one trait may achieve a suggestive status for another trait. Thus we believe that some of these loci have pleiotropic effects with respect to the phenotypes measured and the absence of overlap among the QTL selected for different phenotypes is a consequence of our stringent selection criteria. The significant loci of DB phenotypes identified by the multiple regression model (Table II) explained a small percent of the total variance, with the exception of the approach phenotype. Over 20% of the total phenotypic variance in prod approach behavior is explained by the QTL and the QTL and sex and lineage interaction of six loci, including the two previously reported QTL on the X chromosome. This result suggests that the approach trait is truly polygenic in nature.
Table II.
Summary of Significant Autosomal Loci that Influence Behavior in the DB test
| Locus | Marker | Chromosome and map distance (cM) |
Best covariate mainscan model |
LOD** | Pointwise*** significance |
df† | %var with†† | %var w/oe | p-valuef | df§ |
|---|---|---|---|---|---|---|---|---|---|---|
| Shocks-1 | D7Rat68 | Chr7@65cM | trait*lineage | 4.37 | 0.000042 | 4 | 3.8 | 0.5 | 0.0008 | 2 |
| Approach-3 | D1Rat145 | Chr1@135cM | trait*[sex & lineage] | 6.13 | 0.000001 | 8 | 6.2 | 1.7 | 0.0035 | 6 |
| Approach-4 | D6Rat46 | Chr6@1cM | trait*[sex & lineage] | 6.83 | 0.000000 | 8 | 6.8 | 2.7 | 0.0066 | 6 |
| Approach-5 | D8Rat66 | Chr8@60cM | trait*[sex & lineage] | 6.83 | 0.000000 | 8 | 6.9 | 2.1 | 0.0019 | 6 |
| Approach-6 | D13Rat77 | Chr13@30cM | trait additive | 3.35 | 0.000443 | 2 | 3.4 | |||
| Latency-1 | D2Rat188 | Chr2@1cM | trait* lineage | 4.56 | 0.000028 | 4 | 4.9 | 1.8 | 0.0011 | 2 |
| Latency-2 | D6Rat46 | Chr6@1cM | trait additive | 3.55 | 0.000020 | 2 | 3,5 |
LOD scores cannot be compared across different models due to differing degrees of freedom; The genome-wide significance threshold for main effects (including additive and dominant components, 2df) is 3.2; for QTL interacting with a covariate, the thresholds are 4.2 (4df LOD) and 6.1 (8df LOD).
Overall significance of the QTL plus interaction.
df = degrees of freedom.
%var with = variance explained by the QTL and the QTL*Covariate interaction.
%var w/o = variance explained by the QTL with no interaction.
p-value = significance of the interaction term only (from the ANOVA F-test).
df = degrees of freedom associated with the interaction term/F-test.
Allele-effect plots are depicted in Figure 5 for significant loci identified by the genome scans for each phenotype (panels A–D), and show the effect of genotype on phenotype in the F2 generation separated into classes defined by both sex and lineage. The most striking observation from this analysis is that the effect of genotype on phenotype is highly dependent on lineage in every single case. In other words, genotype— whether FF, FW, or WW—often had little or no phenotypic effect except in the context of a particular sex and/or progenitor line. More specifically, we find that the WKY lineage uniquely contributes to the effects of the coping QTL, with five out of our six significant loci appearing only in F2 offspring of the WKY lineage, and one locus appearing in F2 offspring of the F344 lineage.
Figure 5.
Allele-effect plots of F2 generation offspring stratified by sex and lineage showing the phenotypic effect of genotypes at (the marker most tightly linked to) each locus found to be significant in the initial genome scans and represented in Table II. (a) shocks, (b) approach, (c) latency. Genotypes are shown on the X-axis, log transformed phenotypes on the Y-axis, with means ± SEM displayed. One-Way ANOVA was used to detect phenotypic differences between F344 homozygotes (FF), heterozygotes (FW), and WKY homozygotes (WW), with the probability of finding a difference by chance reflected in the p-values. “F-females” denotes F2 females derived from the F344 grandmaternal lineage, “W-females” are F2 females derived from the WKY grandmaternal lineage, “F-males” are F2 males derived from F344 grandmaternal lineage, “W-males” are F2 males derived from WKY grandmaternal lineage. WKY lineage-specific effects are highlighted in pink, while F344 lineage-specific effects are highlighted in yellow.
Allele-effect plots at D7Rat68 on chromosome 7 (Fig. 5a) clearly delineate the effect of different genotypes on shock phenotype, showing that females from a WKY lineage (W-females) are the only group to actually demonstrate a differential effect of genotype at this locus. Approach-3 at D1Rat145, approach-4 at D6Rat46 and latency2 at D6Rat46 loci contributed to the variance of the phenotype in a sex-and lineage-specific manner, by having more influence in males of WKY lineage (Fig. 5). Approach-5 at D8Rat66 and latency-1 at D2Rat188 also showed dramatic lineage effects but these loci affected both males and females of WKY lineage. Finally, the only QTL that shows F344 lineage is approach-6 at D13Rat77.
Many, but not all, QTL showed the expected directions of the genotypic effects on the phenotype. For example, the WKY homozygote at D8Rat66 (approach-5) approached more (WKY profile; Fig. 5B); this effect is seen in both sexes of the WKY lineage. In contrast, some QTL showed opposite directions of effects that could contribute to transgressive segregation in the F2 population:the WKY homozygote at D6Rat46 (approach-4 and latency-2) displays decreased number of approaches and decreased latency but only in males of WKY lineage (F344 profile). Additionally, the effect plot of latency-2 at D6Rat46 (Fig. 5c) illustrates a complex lineage-and sex-specific effect where male heterozygotes of WKY lineage have more extreme phenotypes than WKY homozygotes.
Using the mouse–rat homology maps available at Jackson Laboratories (http://www.informatics.jax.org) and the VCMap comparative mapping program available at the Rat Genome Database at the Medical College of Wisconsin (http://rgd.mcw.edu), we found that several of our loci share conserved synteny with previously identified QTL for emotionality and anxiety in mouse and rat models (Table III).
Table III.
Comparison of Coping QTL in the DB Test with Mouse and Rat QTL for Behavioral Despair, Emotionality and Anxiety
| Phenotype (locus) | Marker | LOD | Mouse synteny ch (cM) | Phenotype | Locus ch (cM) | Cross | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|
| DB shock | D7Rat68 | 4.37 | 15(20) | OFA, EPM, LD box | 15 (20, 24) | H1 × L1 and H2 × L2 | *––†† | Turri et al. (2001a, b) |
| DB approach (approach-3) | D1Rat145 | 6.13 | 19 (36) | TST immobility | 19 (40) | H1 × L1 | 6.2 | Turri et al. (2001a, b) |
| DB approach (approach-4) | D6Rat46 | 6.83 | rat | EPM | D6Mit1 (0) | Lewis × SHR | 2.8 | Ramos et al. (1999) |
| DB approach (approach-4) | D6Rat46 | 6.83 | rat | FST climbing | D6Rat46 | WKY × F344 | 2.57 | Solberg et al. (2004) |
| DB approach (approach-5) | D8Rat66 | 6.83 | rat | Exploratory activity | D8Rat130(57) | BN × WKYA | 5.0 | Moisan et al. (2003) |
| DB latency (latency-1) | D2Rat188 | 4.56 | rat | FST immobility | D2Rat188 | WKY × F344 | 1.94 | Solberg et al. (2004) |
Note: Synteny was estimated based on confidence interval of the specific QTL.
OFA, LOD 12.4.
EPM:open arms, LOD 12.9.
LD box: transitions, LOD 13.5.
LD box:sec to enter light, LOD 19.6.
LD box: time on light side, LOD 24.7.
DISCUSSION
We have genetically mapped different aspects of behavioral coping with stress exhibited in the DB test. Through genome-wide analysis of a segregating F2 population of a WKY × F344 cross, where the WKY animal exhibits a passive coping strategy in multiple behavioral tests, we identified one locus that influences the number of shocks an animal receives, four autosomal loci that influence prod approach and two autosomal loci that influence duration of burying in the DB test. The genetic architecture of coping behavior in this test is complex; these loci were sex-and/or lineage-specific in every case. Several of our QTL show overlapping candidate regions with previously identified QTL for emotionality, anxiety and depressive behavior in the mouse or rat.
Any environmental stimulus could be interpreted as a stressor and the stress-response requires a coping strategy. The concept of coping styles has been debated (Koolhaas et al., 1999), but it seems that individual coping strategies are affected by genetic, developmental, environmental and learned components. Coping is primarily an adaptive response to changes in the environment (Francis et al., 1999b), and the relative benefit of active or passive coping strategies can change depending on alterations in the environment. In the DB test, after receiving the first shock, the animal has primarily two options to avoid further shock. It may retreat to the corner of the cage, or it may actively bury the prod with the bedding material. These two behavioral strategies could be interpreted as passive or active coping responses to stress, and both of these strategies can be considered adaptive in the DB paradigm, since both can eliminate the threat of the prod. However, WKYs, who show passive coping behaviors in the DB test, express stable behavioral characteristics that suggest the continuous presence of behavioral inhibition or despair (Lahmame and Armario, 1996; Lopez-Rubalcava and Lucki, 2000; Pare, 1989; Pare and Redei, 1993; Redei et al., 2001). Thus, this passive coping behavior of WKYs in the DB test is not likely the result of learning a successful coping strategy, but rather a genetically determined predictable stress-response strategy across different behavioral paradigms.
The DB test was originally developed as a test of anxiety (Treit et al., 1981) and has been the focus of extensive research elucidating the ethology, pharmacology and neurobiology of this coping response (De Boer and Koolhaas, 2003). Drugs generally effective in anxiety paradigms decrease burying, while amphetamine, CRF (among others) increase it. Thus, finding that some of our DB loci overlap with activity or anxiety-related QTL in other studies (Table II) suggest that activity, anxiety and coping with the stress of a threat may share genes contributing to their neurobiology. Furthermore, the finding that some of the DB loci mapped to the same markers as QTL found in the same cross for immobility and climbing in the forced swim test, further underline the suggestion that WKYs express a predictable passive response strategy to stress.
It is clear that differences in coping styles between our inbred strains and among our F2 generation were determined not just by different allelic makeup at key loci throughout the genome, but also by the very powerful modifying influence of lineage. Because F344 and WKY dams show significantly different mothering styles, and genetically identical F1 males raised by F344 and WKY mothers show significant behavioral differences in coping styles in the DB test as adults (Ahmadiyeh et al., 2004) maternal behavior passed on intergenerationally could contribute to the lineage effects observed in our present study. This maternal behavior could sensitize those pups from the WKY lineage with a particular genotypic makeup to being particularly susceptible to developing a passive coping response in the DB test. Pups with the same genotypic makeup not exposed to the specificities of the WKY lineage would be protected from showing a passive coping response.
If lineage can have such a pervasive effect on QTL detection in our cross for coping behaviors, it may be operating for other traits as well. One example is the role of mitochondrial inheritance, which could explain some of the patterns of maternal influences on birth weight (Price et al., 1999). Genomic imprinting causes parent of origin effects, and recently it has been shown that trans activation of a normally silent maternal allele can maintain its activation state in the next generation independently of the paternal allele (Herman et al., 2003). Maternal environment was also found to be critical to the detection of several epistatic locus pairs contributing to diabesity in mice (Reifsnyder et al., 2000). Cross-fostering studies confirmed the importance of the postnatal maternal environment in regulating the penetrance of the diabesity gene effects on offspring, and biochemical analyses suggested that this effect was most likely due to differing milk composition. A recent analysis of gene expression QTL and obesity in mice showed how subpopulation analysis, based on differential expression profiles within F2 animals otherwise determined to be phenotypically and genotypically identical, can identify genetic architecture that can include lineage effects (Darvasi, 2003). In our present study, we show that the genotype of our F2 population had little predictive value in the absence of critical information regarding lineage. It is conceivable that the manifestation of many complex traits requires this critical interaction of genetic and non-genetic factors, and that in such cases, the usefulness and predictive value of genotypic information will be best realized when information regarding specific modifying epigenetic variables is also defined and known.
Overall, this study identified several significant QTL affecting behavioral coping with stress, some of which overlap with previously published loci for other behavioral paradigms. All of our QTL in the present study contributed to phenotypic variance in a lineage-specific manner, and in some cases in both lineage-and sex-specific manner, particularly affecting males of the WKY lineage. Thus, epigenetic factors of WKY origin may significantly modulate behavioral responses to stress in the DB test. The capacity for epigenetic factors to modulate the phenotypic expression of genotype has implications for the study of complex traits in animals and humans.
ACKNOWLEDGMENT
This study was supported by NIH grant MH60789 to E.R. J.S.T. is an investigator in the Howard Hughes Medical Institute.
REFERENCES
- Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, et al. X-linked and lineage-dependent inheritance of coping responses to stress. Mamm. Genome. 2003;14:748–757. doi: 10.1007/s00335-003-2292-x. [DOI] [PubMed] [Google Scholar]
- Ahmadiyeh N, Slone-Wilcoxon J, Takahashi J, Redei E. Postnatal maternal environment significantly alters adult coping behaviors. Biol. Psychiat. 2004;55:1069–1074. doi: 10.1016/j.biopsych.2004.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl:QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter:Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog. Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvasi A. Genomics:Gene expression meets genetics. Nature. 2003;422:269–270. doi: 10.1038/422269a. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents:Ethology, neurobiology and psychopharmacology. Eur. J. Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Flint J. Analysis of quantitative trait loci that influence animal behavior. J. Neurobiol. 2003;54:46–77. doi: 10.1002/neu.10161. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999a;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol. Psychiat. 1999b;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Herman H, Lu M, Anggraini M, Sikora A, Chang Y, Yoon BJ, Soloway PD. Trans allele methylation and paramutation-like effects in mice. Nat. Genet. 2003;34:199–202. doi: 10.1038/ng1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA. Functional genomics of neural and behavioral plasticity. J. Neurobiol. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Der Vegt BJ, Van Reenen CG, et al. Coping styles in ani-mals:Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GAH, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm:Effects of ipsapirone. Physiol. Behavior. 1992;52:355–361. doi: 10.1016/0031-9384(92)90284-9. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Armario A. Differential responsiveness of inbred strains of rats to antidepressants in the forced swimming test:Are Wistar Kyoto rats an animal model of subsensitivity to antidepressants? Psychopharmacology. 1996;123:191–198. doi: 10.1007/BF02246177. [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quanititative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Llamas B, Cook MN, Mormede P. Further dissection of a genomic locus associated with behavioral activity in the Wistar-Kyoto hyperactive rat, an animal model of hyperkinesis. Mol. Psychiat. 2003;8:348–352. doi: 10.1038/sj.mp.4001234. [DOI] [PubMed] [Google Scholar]
- Pare WP. “Behavioral Despair” predicts ulceration in WKY rats. Physiol. Behav. 1989;46:483–487. doi: 10.1016/0031-9384(89)90025-5. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol. Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Pare WP, Redei E. Depressive behavior and stress ulcer in Wistar Kyoto rats. J. Physiol. Paris. 1993;87:229–238. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- Price KC, Shibley hyde J, Coe CL. Matrilinear transmission of birth weight in the rhesus monkey (Macaca mulatta) across several gnerations. Obstet. Gynecol. 1999;94:128–134. doi: 10.1016/s0029-7844(99)00269-0. [DOI] [PubMed] [Google Scholar]
- Ramos A, Moisan MP, Chaouloff F, Mormede C, Mormede P. Identification of female-specific QTLs affecting an emotionality-related behavior in rats. Mol. Psychiat. 1999;4:453–462. doi: 10.1038/sj.mp.4000546. [DOI] [PubMed] [Google Scholar]
- Redei EE, Ahmadiyeh N, Baum A, Sasso D, Slone J, et al. Novel animal models of affective disorders. In: Tucker GJ, McKinney W, Saunders WB, editors. Seminars in Clinical Neuropsychiatry. Philadelphia: Saunders Company; 2001. [DOI] [PubMed] [Google Scholar]
- Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Churchill GA. A statistical framework for quantitative trait mapping. Genetics. 2001;159:371–387. doi: 10.1093/genetics/159.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonagressive wild house mice in the shock-probe/defensive burying test. Pharmacol. Biochem. Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Solberg L, Baum A, Ahmadiyeh N, Shimomura K, et al. Sex-and lineage-specific inheritance of depression-like behaviour in the rat. Mamm. Genome. 2004;15(8):648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D. Animal models for the study of anti-anxiety agents:A review. Neurosci. Biobehav. Rev. 1985;9:203–222. doi: 10.1016/0149-7634(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Treit D. Defensive burying: A pharmacolgocial animal model for specific fears? In: Soubrie P, Wid-locker D, editors. Anxiety, Depression, and Mania: Animal Models of Psyrchiatric Disorders. New York: Basel:Karger; 1991. pp. 1–19. [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying:A new paradigm for the study of anxiolytic agents. Pharmacol. Biochem. Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr. Biol. 2001a;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Turri MG, Henderson ND, DeFries JC, Flint J. Quantitative trait locus mapping in laboratory mice derived from a replicated selection experiment for open-field activity. Genetics. 2001b;158:1217–1226. doi: 10.1093/genetics/158.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]