Abstract
Microglial activation in crossing white matter tracts is a hallmark of noncystic periventricular leukomalacia (PVL), the leading pathology underlying cerebral palsy in prematurely born infants. Recent studies indicate that neuroinflammation within an early time-window can produce long-lasting defects in oligodendroglial maturation, myelination-deficit, as well as disruption of transcription factors important in oligodendroglial maturation. We recently reported an ischemic mouse model of PVL, induced by unilateral neonatal carotid artery ligation, leading to selective long lasting bilateral myelination deficits, ipsilateral thinning of the corpus callosum, ventriculomegaly, as well as evidence of axonopathy.
Here, we report that permanent unilateral carotid ligation on postnatal day 5 (P5) in CD-1 mice induces an inflammatory response, as defined by microglial activation and recruitment, as well as significant changes in cytokine expression (increased IL-1b, IL-6, TGF-b1, and TNF-a) following ischemia. Transient reduction in counts of oligodendrocyte progenitor cells (OPCs) at 24 and 48 hours post-ischemia, a shift in OPC cell size and morphology towards the more immature form, as well as likely migration of OPCs were found. These OPC changes were topographically associated with areas showing microglial activation, and OPC counts negatively correlated with increased microglial staining.
The presented data shows a striking neuroinflammatory response in an ischemia-induced model of PVL, associated with oligodendroglial injury. Future studies modulating the neuroinflammatory response in this model, may contribute to a better understanding of the interaction between microglia and OPCs in PVL and open opportunities for future therapies.
Keywords: Infants, inflammation, ischemia, microglia, neonatal, oligodendrocyte progenitor, white matter
INTRODUCTION
Extreme prematurity, defined by gestational age of ≤ 28 weeks or birth weight < 1500 g, affects up to 2 percent of all newborns in the United States [1]. A high incidence of adverse neurologic outcomes in this group calls for intensified research in neuroprotective options [2,3]. Up to 20% of these infants develop cerebral palsy [4] and about 50% will develop cognitive, behavioral, attention, or socialization deficits of variable degree [5–7].
The main underlying neuropathology in this population is perinatal white matter injury (PWMI), also known as periventricular leukomalacia (PVL), which is defined as dysmyelination, neuroaxonal injury, and astrogliosis, as well as microglial activation [3,8,9]. The etiology of PWMI is thought to be multifactorial and encompasses perinatal infectious processes, systemic and neuroinflammation, ischemia-reperfusion, hypoxia and excitotoxicity, combined with developmental vulnerability of cellular structures, especially late oligodendrocyte progenitors and immature oligodendrocytes [5,10–12].
Epidemiological studies strongly suggest that inflammation is associated with PWMI. Furthermore, it is postulated that active inflammatory processes may prevent regeneration and/or exacerbate brain damage in the premature infant, and sensitize the brain to further injury [13–15]. While adequate glial response to injury is essential to repair [16] and regeneration, studies in radiation injury [17] and traumatic brain-injury [18–21] show long lasting microglial activation and increased expression of inflammatory cytokines for several months to years. Interestingly, a study of 7-year-old children with CP born prematurely showed elevated basal levels of tumor necrosis factor alpha (TNF-a) in the plasma and in supernatants of peripheral blood mononuclear cells, supporting the notion of a prolonged inflammatory response in CP [22]. This concept broadens the possible therapeutic window in CP, and blocking such persistent inflammation could be of therapeutic value [23].
It has been shown that during prenatal brain development in humans, clusters of microglia are located at axonal crossroads (at crossing fibers between the internal capsule and thalamus, cingulum and corpus callosum and other regions) at sites vulnerable to PWMI in very preterm babies during time-periods critical for PWMI [8,24]. Microglial activation could be deleterious or neuroprotective, depending on the timing in relation to an initial insult, the lesion-type, and the lesion-location [25], and may be triggered by excitotoxic, inflammatory, hypoxic, or hypoxic-ischemic events [26–29]. Also, in normal rodents, clusters of activated microglia were detected transiently within white matter tracts (cingulum, internal and external capsules) during early postnatal development, resembling the distribution found in the normal human brain [8,28–30]
Late oligodendrocyte progenitor cells (OPCs) exhibit selective vulnerability in PWMI [11,31], and it has been shown that activated microglia can have deleterious effects on OPC survival and also inhibit maturation of these progenitors into myelin-producing oligodendrocytes in vitro [32–35]. Microglia can be a source of many inflammatory mediators, including cytokines such as TNF-a, chemokines, reactive oxygen/nitrogen species, as well as glutamate, which all have been demonstrated to be detrimental to oligodendroglial development in cell culture [3,8]. Expression of the interleukins, Interleukin-1 beta (IL-1b), IL-2, and IL-6, and of TNF-a has been demonstrated in microglia in neonates with PWMI [36–38].
In terms of oligodendrocyte maturation, the development of the newborn rodent brain is equivalent to the human fetal brain at 6 to 7 months gestation. Late OPCs prevail between postnatal days 2 (P2) and P7 in rodents, and at gestational weeks 23 to 32 in humans [39]. Based on this pattern of development, we recently reported a mouse model of PWMI [40] by performing permanent unilateral carotid artery ligation on P5 (day of birth defined as P1) in CD-1 mice and found selective vulnerability of the white matter with myelin pallor, reduced mature oligodendrocyte counts, as well as evidence of axonal injury in the corpus callosum, similar to reports from autopsy cases of PVL [41].
We then hypothesized that ischemia in our model elicits an inflammatory response, consisting of cytokines, chemokines and adhesion molecules, as well as active inflammatory cells, especially microglia. Because of the developmental migration pattern and clusters of microglia within the white matter, we further hypothesized that neuroinflammatory effects on the oligodendroglial lineage could be observed by correlating temporospatial patterns of microglia and OPCs.
The objectives of this study were (1) to quantify the temporospatial response of microglia within the white matter in our P5 ischemic model of PWMI and their spatial relationship to OPCs; (2) to quantify the cytokine response relevant to PWMI within the white matter; (3) to quantify and categorize OPC cell morphology and proliferation, and the temporospatial pattern of change following injury; (4) to evaluate the spatial correlation between white matter areas with microglial clusters and OPC death.
MATERIALS AND METHODS
Animals
This study was approved by the Johns Hopkins Animal Care and Use Committee (protocol no. MO09M422). In all, 21 CD-1 litters of 9 to 11 pups each were purchased from Charles River Laboratories (Wilmington, MA, USA) at P3. The day of birth was defined as P1.
Carotid Artery Ligation
On P5 pups were placed in an incubator at 35°C for 15 to 303 minutes and were then anesthetized with isoflurane (4% induction 1% to 1.2% maintenance), and the right common carotid artery was ligated (right hemisphere referred to as ipsilateral, left hemisphere as contralateral). Pups recovered at 36°C for 30 to 603 minutes and were returned to the dam. Rectal temperatures were 36°C±0.5°C before surgery and 34.5°C±1°C postoperatively; surgery time was standardized to 12 to 153 minutes.
Histology
A subset of on P5 ligated animals were examined at 24h (P6, n=10), 48h (P7, n=8) and 16 days (P21, n=8) post ligation. They were compared to P6 (n=9), P7 (n=8) and P21 (n=8) naïve controls. Mice were anesthetized and perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in phosphate buffer for 12 to 18 hours, and the brains were cryoprotected in sucrose and cryostat-sectioned at 40 μm. Sections were mounted onto 10 slides in parallel, each slide having sections with 400 μm displacement. All sections and images were identified using a standardized numbering system, so that antigen expression in adjacent sections of the same animal stained with different antibodies could be correlated. Slides were incubated in blocking solution, followed by primary antibody incubation at 4°C: anti-platelet derived growth factor receptor-α (PDGFRa) antibody (BD Biosciences, San Jose, CA, USA, 1:500) for detection of OPCs, anti-ionized calcium binding adaptor molecule 1 antibody (Iba-1, Wako, Richmond, VA, USA, 1:2.000) for detection of microglia, anti-Ki67 (Abcam, MA, USA, 1:500) for visualization of proliferation and anti-CD68 (Abcam, MA, USA, 1:100) for detection of microglia during developmental migration.
Fluorescent secondary antibodies were used to detect anti-PDGFRa, anti-Ki67+ and anti-CD68, and all sections were counterstained with DAPI. The Iba-1 antigen–antibody complex was visualized using an ABC ELITE kit (Vector Labs, Burlingame, CA, USA). Adjacent sections were stained using Nissl to aid in outlining anatomic structures.
To investigate the relationship between microglia and OPCs in our injury model, microglia were evaluated using Iba1 immunohistochemistry and their presence quantified. Since exact counting of migrating microglia within the corpus callosum proved to be difficult, we decided to quantify the fractional area stained (referred to as proportional target area) as a means of quantitative measurement of microglial activation (described below). Corresponding sections were stained for OPC presence (PDGFRa), as well as proliferation (Ki67). In the OPC population we quantified cell count, cell size, as well as morphology, while differentiating proliferating (Ki67+) from non-proliferating OPCs.
Brightfield image acquisition and quantification
Images of anatomically defined regions in Iba-1 immunostained brain-sections were acquired using a Zeiss Axio Imager microscope (Zeiss Microimaging, LLC, Thornwood, NY, USA) with brightfield imaging using standardized illumination and image acquisition settings. Bright-field images were exported as loss-free compressed TIFF files to MCID Core (InterFocus Imaging Ltd, Cambridge, UK) for semi-automated analysis. For quantification of microglia and improved acquisition of microglial processes, Z-stack images (4 layers with 5 μm section thickness) were taken under a 20x objective in anatomically defined areas within anti-Iba1 stained brain-sections. Z layers were collapsed using Zeiss Axiovision’s wavelet algorithm and mosaic images were stitched together using Zeiss Axiovision software. Anatomical areas of interest were chosen: At the rostrocaudal level of the anterior commissure: mid corpus callosum (Mid-CC), supraventricular corpus callosum (SVWM), the roof of anterior subventricular zone (ASVZ-r); at the level of the rostral hippocampus: area encompassing the supraventricular external capsule and the roof of posterior subventricular zone (EC/PSVZ-r, Figure 1).
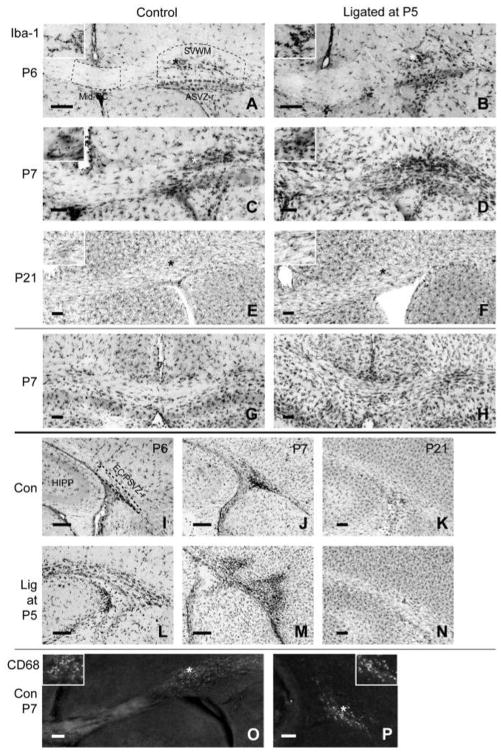
Figure 1. Microglial distribution and morphology in developing white matter in controls and after P5 carotid artery ligation.
Iba1 immunohistochemical staining of microglia is shown at the level of the anterior commissure (A–F) and at the level of the rostral hippocampus (G–N). 24h to 48h after ligation (P6–P7), there is a notable increase in microglial staining in several regions: mid-corpus callosum (Mid-CC), clusters within the supraventricular white matter (SVWM), the roof of anterior subventricular zone (ASVZ-r), and the external capsule combined with the roof of posterior subventricular zone (EC/PSVZ-r). CD68+ cells were found in controls (O,P) and ligated mice (not shown) in clusters within the SVWM at anterior levels (O) and in the EC/PSVZ-r at posterior levels (P) at P6–P7 but not at P21, indicating that these regions are migrational zones for invading monophagocytic cells in the developing brain [8,30]. Dashed lines indicate the areas examined for quantitative analysis (* indicates the location of higher magnification inset, HIPP, hippocampus; scale bar = 50 μm for A–O, 10 μm for P).
Threshold-based target detection was performed; images were reviewed, artifacts excluded and the proportional target area (PTA) for Iba-1 stained images calculated. The PTA is defined as the fractional area stained, e.g. the areal fraction of Iba-1 positive pixels within a measured target area, which in case of Iba-1 IHC is a reflection of either the number and/or size of microglia within an outlined region. This method has been shown to be a reproducible quantitative tool for assessment of monochrome histological stains, with less user dependent variability than unbiased stereology (Donnelly et al, 2009). Furthermore, for Iba1 PTA we calculated a mid corpus callosum/supraventricular white matter ratio (for left and right hemispheres separately), assuming that as microglial migration progresses from the clusters in supraventricular white matter towards the mid corpus callosum, this ratio would increase.
OPC cell count and morphology quantification
Fluorescent images were acquired using the Zeiss Axio Imager M5 microscope with ApoTome functionality using structured illumination to optimize resolution. Using this technique the total exposure of the specimen is slightly greater because the grid projection is typically not completely opaque. However, the resolution is comparable to that achieved by either confocal or deconvolution techniques. To reduce bias due to bleaching of the fluorescent antibody all images used for analysis were acquired the first time the slides were illuminated under the microscope. Mosaic settings, Z-stack settings, and anatomical regions were defined and set in the microscope software prior to acquisition to reduce illumination time and thereby bleaching using the robotic microscope stage. Fluorescent channels switched automatically and were acquired sequentially for each field of view, in the same order for each sample. Regions to be acquired were selected based on the DAPI staining. All images and channels were post-processed in the Zeiss Axiovision software following standardized protocols for optimal quantification. For verification of correct regional identification, overview mosaic images of the DAPI channel of each analyzed brain section were acquired using a 10x microscope-objective.
To understand the pattern of OPC cell damage in ligated animals, we outlined the PDGFRa + cells and measured the cell length/height ratio as well as the cell area. For quantification of OPCs and their proliferation, images were taken under a 40x objective in anatomically defined regions for anti-PDGFRa/anti-Ki67+/DAPI+ triple-stained sections, with 5 Z-stack layers at 3 μm intervals. Within the selected regions PDGFRa + cells and PDGFRa+/Ki67+ cells were manually counted and their density (cell count per area) used for statistical analysis. All PDGFRa+ cell counts were normalized to an area of 10,000 μm2. The following anatomic regions were selected: at the rostrocaudal level of the anterior commissure, we selected Mid-CC limited to mid 440 micrometers, ASVZ-r excluding one ependymal cell layer, and SVWM; at the level of the rostral hippocampus, we selected the PSVZ-r and EC as two separate regions identified by DAPI staining (Figure 3).
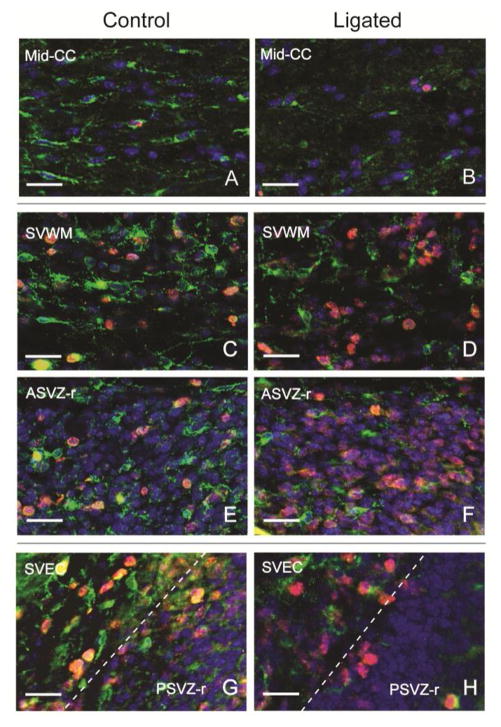
Figure 3. Oligodendrocyte progenitor cell morphology and proliferation in developing white matter and subventricular zone in controls and after P5 right carotid artery ligation.
Triple immunostained images (PDGFRa in green, Ki67 in red, and DAPI in blue) of the mid corpus callosum (Mid-CC, A,B), the supraventricular white matter (SVWM, C,D), the roof of the anterior and posterior subventricular zone (ASVZ-r, and PSVZ-r, E–H), and the external capsule (EC, G,H) are shown in control and ligated animals. All animals were ligated on P5. Mid-CC, ASVZ-r and SVEC images were obtained from animals sacrificed after 24h and paired with age-matched controls. The images of SVWM were selected from a representative animal 48h after ligation and an age-matched control. In control mice most OPCs (PDGFRa+) in the Mid-CC show more branched processes than in ligated animals, and only few were proliferating (PDGFRa +/Ki67+, A). Clusters of OPCs are seen in SVWM and EC with a high number of proliferating OPCs (C,G). Interestingly, these two regions correspond to areas where dense microglial clusters were noted at the same age (Figure 1). The proliferating OPCs appear rounder, with fewer branched processes, and have a larger nucleus when compared to non-proliferating OPCs in the same areas, reflecting a more immature stage. In ligated animals, there is a notable decrease in the number of OPCs in the Mid-CC (B), SVWM (D), and PSVZ-r (H); in addition, surviving OPCs in Mid-CC appeared rounder with smaller nucleus and cytoplasm and fewer branched processes, likely representing a less mature OPC population. (Scale bars = 25 μm)
To quantify morphological changes in OPCs 24h and 48h after ligation, PDGFRa positive cells were outlined manually in images of Mid-CC using the Zeiss Axiovision software. Cell area and cell aspect ratio, defined as cell width (along the corpus callosum) divided by cell height, were measured.
Quantitative reverse transcriptase polymerase chain reaction (qrt-PCR)
For qrt-PCR, P5 ligated pups and age-matched controls were examined at 6h (n = 10 controls, 11 ligated), 12h (n = 11 controls, 11 ligated), 24h (n = 16 controls, 18 ligated), 48h (n = 17 controls, 17 ligated), and 16 days (P21) post ligation (n = 8 controls, 7 ligated). To understand whether cytokines may be involved in the pathology leading to OPC death and/or affecting oligodendrocytic maturation, we measured the RNA expression levels of IL-1beta, IL-6, TNF-a and TGF-beta1 at 6h, 12h, 24h, 48h, and 16 days after ligation, as well as the expression levels of IL-2 and Interferon-gamma 48h after ligation. Total RNA was extracted from a 1 mm thick mid-sagittal section of fresh frozen control and ligated mouse brains (excluding the cerebellum) using the RNeasy Lipid Tissue Mini Kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer’s instructions. RNA quantity was measured using the Nanodrop ND-1000 UV Spectrometer (Nanodrop Technologies, Wilmington, DE) and 0.5–1 μg of DNase-treated total RNA was used to generate complementary DNA (cDNA) using Superscript III™ Reverse transcriptase (Invitrogen, Carlsbad, Calif, USA) following manufacturer’s instructions. Predesigned Taqman primers were used in conjunction with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, Calif, USA) to assay the expression of IL-1beta (Mm01336189_m1), IL-2 (Mm00434256_m1), IL-6 (Mm00446190_m1), INF-gamma (Mm99999071_m1), TNF-a (Mm00443258_m1), TGF-beta1 (Mm01227699_m1) and eukaryotic 18S rRNA (4310893E). The reactions were run and analyzed using an ABI PRISM 7900 Real-Time PCR system (Applied Biosystems, Foster City, Calif, USA). Samples were run in triplicate and the results averaged. Relative levels were normalized against 18S rRNA. All analyses were performed blinded to study conditions.
Statistical analysis
Data is expressed as the mean ± standard deviation for each group of mice. Two-way ANOVAs were performed to determine any effects of ligation status and of time after ligation on the measured variables in each analyzed region and to evaluate any interaction between ligation status and the time after ligation. The 6h–48h time points were used as acute outcome measures, while the 16 day post ligation (P21) time point was used as a late outcome measure and therefore, separate ANOVA tests were performed for this latter time point. In addition, unpaired t-tests were performed to evaluate any significant differences between the means of measured variables in control and ligated groups at each time point after ligation. Correlations between OPCs and microglia were evaluated using Pearson’s correlation coefficient. For all figures *p < 0.05, **p < 0.01 and ***p < 0.001 were used. Graphs were plotted and statistics assessed using the program GraphPad Prism 5.0 (GraphPad Software) and IBM SPSS 19.
RESULTS
Right-sided unilateral carotid artery ligation was performed in 102 mice on P5; 89 littermates were used as normal controls. The mortality rate of the ligation procedure was 8.8% (9 of 102) and was caused by either rupture of the carotid artery or failure to recover from anesthesia. Postsurgical death occurred in 2 mice, both within the first 48 hours, increasing the total mortality to 10.7%.
Microglial migration and morphology in white matter during development
Regions of interest for our analysis were selected after a qualitative evaluation of microglial and OPC distribution at 24h and 48h after ligation, as well as in age matched controls (Figure 1). These areas include at the level of the anterior commissure the mid corpus callosum (Mid-CC), the supraventricular corpus callosum (SVWM), and the roof of anterior subventricular zone (ASVZ-r), and at the level of the rostral hippocampus the supraventricular external capsule (EC), as well as the roof of posterior subventricular zone (PSVZ-r). The external capsule was of particular interest, based on our prior finding of persistent dysmyelination in this area.
At P6 and P7, dense clusters of Iba1 positive cells were seen in the SVWM and ASVZ-r (Figure 1A–D), and in the EC/PSVZ-r (Figure 1I, J, L, M) in control and ligated animals. Such clusters were first described by Pío del Río Hortega as “fountains of microglia” [42]. The morphology of microglia changed with distance away from the cluster region. Microglia closer to the dense clusters appeared round with dense staining and short processes, but those at a greater distance were elongated with longer processes. It is important to note, that these dense clusters were also seen in control sections. In addition, these cells were CD68+ not only in ligated but also in controls at P6 and P7 but no longer on P21, suggesting that these areas are physiological/developmental migrational zones of monophagocytic cells (Figure 1O,P). Interestingly, the regions of these clusters were also the regions with the highest density of OPCs (PDGFRa+) outside the subventricular zone.
At P6, other white matter areas like the Mid-CC showed only few microglia (Figure 1A,B), but by P7, there was a higher microglial distribution throughout the white matter, with larger and more branched microglial processes (Figure 1C,D). By P21, Iba1 staining revealed a microglial population with uniform morphology throughout the corpus callosum with longer, thinner and more highly branched cell processes (Figure 1E,F,K,N). The clusters of microglia observed at P6–P7 were no longer present at P21. Furthermore, at P21, there was no CD68 staining in control or ligated animals suggesting that the systemic migration of monophagocytic cells is no longer present at this later stage of development.
Microglial response within the corpus callosum after unilateral ligation
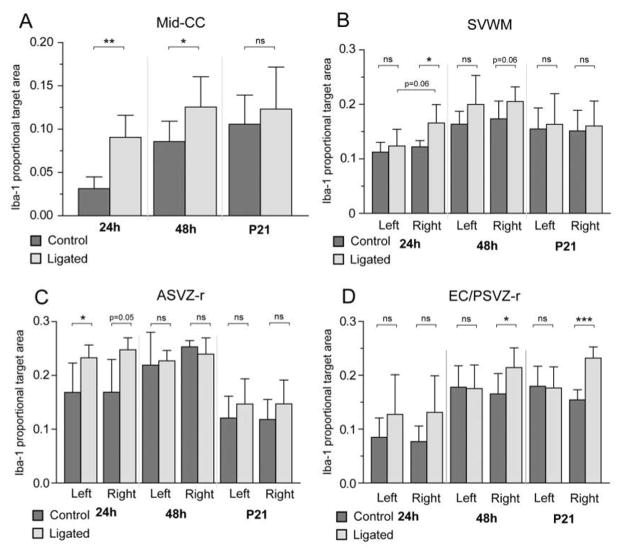
Activation of microglia was quantified by measuring Iba1+ proportional target area (PTA), which reflects changes in the number and/or morphology of microglia. Ligation at P5 significantly increased Iba1 PTA in Mid-CC, on the ligated side in SVWM and EC/PSVZ-r, and bilaterally in ASVZ-r (Figure 2, ANOVA). In all of these regions, there was also a significant effect of ‘time after ligation’ on Iba1 PTA. Although there was no significant interaction between ligation and time after ligation, post-hoc t-tests showed that ligation significantly increased Iba1 PTA in Mid-CC at 24h and 48h after ligation but not at P21 (Figure 2A). At 24h after ligation, Iba1 PTA was increased on the ligated side of SVWM and bilaterally in ASVZ-r. A significant increase in Iba1 PTA was found in EC/PSVZ-r on the ligated side at 48 hours after ligation and remained increased in this area only through P21 (16 days post ligation).
Figure 2. Microglial activation after P5 right carotid artery ligation.
Activation of microglia was quantified by measuring the Iba1+ proportional target area (PTA) within mid corpus callosum (Mid-CC, A), the supraventricular white matter (SVWM, B), the roof of the anterior subventricular zone (ASVZ-r, C), and the external capsule combined with the roof of posterior subventricular zone (EC/PSVZ-r, D) at P6/24h after ligation (n = 5 controls, 6 ligated), P7/48h after ligation (n = 7 controls, 7 ligated), and P21/16 days after ligation (n = 5 controls, 4 ligated). In ligated animals, there was a significant increase of Iba1 in Mid-CC (ANOVA, p < 0.001), right SVWM (ANOVA, p < 0.05), bilateral ASVZ-r (ANOVA, left p < 0.05; right p < 0.05), and right EC/PSVZ-r (ANOVA, p < 0.01) across all time points while a marginally significant interaction was found between ligation status and time after ligation only in ASVZ-r (ANOVA, p = 0.05). Post-hoc t-tests showed significant increases in Iba1 in ligated animals compared to controls in Mid-CC at 24h and 48h after ligation (A), but only at 24h after ligation in the right SVWM (B) and bilateral ASVZ-r (C). In the EC/PSVZ-r (D), Iba1 PTA was significantly increased on the ligated side at 48h after ligation, and in this area only, remained significantly increased through P21. Error bars represent 1 standard deviation.
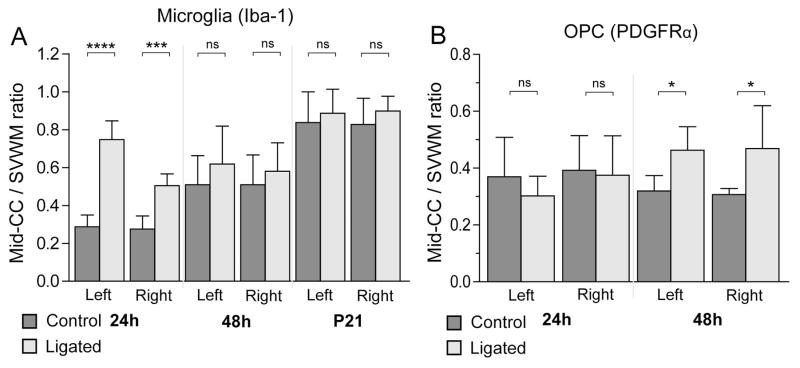
Since we observed transient increases in microglial PTA in regions such as the mid corpus callosum after ligation, we hypothesized that ligation could also be accelerating microglial migration from clusters in the SVWM into other regions of the white matter. Therefore, we calculated an Iba1 PTA Mid-CC/SVWM ratio (for left and right hemisphere separately), assuming that with increased microglial migration this ratio would be increased, as more microglia would leave the clusters and move into other regions of the corpus callosum. Iba1 Mid-CC/SVWM ratios increased bilaterally in ligated animals, but this effect was transient (Figure 5A, ANOVA p < 0.01, ligation*time after ligation p < 0.01). There was a highly significant bilateral increase in this ratio in ligated animals at 24h after ligation, but at 48h and 16 days (P21) after ligation this ratio was comparable in controls and ligated mice, suggesting accelerated microglial migration from SVWM towards the Mid-CC in injured mice.
Figure 5. Shift in regional ratios suggests migration of microglia and OPCs.
The ratio, Iba1 PTA in mid corpus callosum (Mid-CC)/Iba1 PTA supraventricular white matter (SVWM), was examined at 24h, 48h, and 16 days (P21) after ligation as a measure of migration (n = 6 per group at each age, A). At 24h after ligation, there was a highly significant bilateral increase in this ratio in ligated animals, but 48h after ligation this ratio was comparable in controls and ligated mice, suggesting accelerated microglial migration from SVWM towards the mid-CC in injured mice. A similar ratio was calculated using OPC count Mid-CC/SVWM (n = 6 per group at each age, B), which showed a significant bilateral increase only at 48h after ligation. Since the OPC count in Mid-CC was reduced at 24h after ligation but had normalized at 48h after ligation (see Figure 4), this increased ratio is likely reflecting migration of OPCs from the SVWM to Mid-CC.
OPC qualitative assessment
To evaluate OPC morphology and proliferation, sections immunostained for PDGFRa and Ki67 were examined. In control mice at P6, many OPCs (PDGFRa+) in the Mid-CC showed elongated cell bodies, branched processes, a small nucleus and only a few were proliferating (PDGFRa+/Ki67+, Figure 3A). Clusters of OPCs were seen in SVWM and EC with a high number of proliferating OPCs (Figure 3C,G). Interestingly, these two regions correspond to areas where dense microglial clusters were noted at the same age (Figure 1). The proliferating OPCs appeared rounder, with fewer branched processes, and had a larger nucleus when compared to non-proliferating OPCs in the same areas, reflecting a more immature stage. In ligated animals, there was a notable decrease in the number of OPCs in the Mid-CC (Figure 3B), SVWM (Figure 3D), and PSVZ-r (Figure 3H). Furthermore, within the Mid-CC, the surviving OPCs appeared rounder with smaller nucleus and cytoplasm and fewer branched processes and were not Ki67+, likely representing a less mature OPC population that is not proliferating.
OPC response to unilateral ligation
Quantitative analysis of OPCs was carried out on P6 and P7, ages at which OPCs are still the predominant developmental stage of the oligodendroglial lineage.
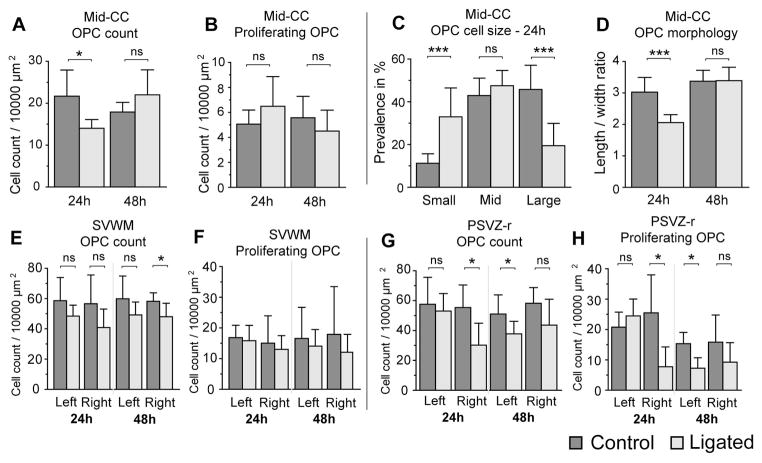
Cell counts and proliferation: In Mid-CC there was no significant overall effect of ligation on OPC counts, however, there was a significant interaction between ligation status and time after ligation, and post-hoc t-tests showed a significant reduction in ligated mice at 24h after ligation (Figure 4A). There was no significant effect of ligation on OPC proliferation in this area (Figure 4B). Ligation significantly decreased OPC counts across all times examined in bilateral SVWM and in PSVZ-r on the ligated side (using ANOVA, Figure 4E&G). Post-hoc t-tests showed significantly reduced OPC counts on the ligated side in PSVZ-r at 24h after ligation (Figure 4G) and in SVWM at 48 after ligation (Figure 4E). Ligation significantly reduced proliferating OPC counts in right PSVZ-r (Figure 4H), but not in any other assessed region (using ANOVA). Post-hoc t-tests showed significantly lower proliferating OPC counts in PSVZ-r on the ligated side at 24h and on the contralateral side at 48h after ligation (Figure 4H).
Figure 4. Oligodendrocyte progenitor cell response after P5 right carotid artery ligation.
The total number of OPCs (PDGFRa + cells) and proliferating OPCs (PDGFRa +/Ki67+ cells) per 10,000 mm2 were determined in the mid corpus callosum (Mid-CC, A&B), the supraventricular white matter (SVWM, E&F) and the external capsule (EC, K&L) at 24h after ligation/P6 (n = 8 controls, 7 ligated) and 48 hours after ligation/P7 (n = 6 controls, 7 ligated). In the Mid-CC, ANOVA showed no significant overall effect of ligation on OPC counts, however, there was a significant interaction between ligation status and time after ligation (ANOVA, p < 0.01), and post-hoc t-tests showed a significant reduction in ligated mice at 24h after ligation (A). There was no significant effect of ligation on proliferating OPC count in this area (B). Ligation significantly decreased OPC counts across all time points examined in bilateral SVWM (ANOVA, left, p < 0.05; right, p < 0.05), and in right PSVZ-r (ANOVA, p < 0.01); post-hoc t-tests showed significantly reduced OPC counts in right PSVZ-r at 24h after ligation (K) and in right SVWM at 48h after ligation (E). Ligation significantly reduced proliferating OPC counts in right PSVZ-r (L), but not in any other assessed region (ANOVA, p < 0.01); and post-hoc t-tests showed significantly lower proliferating OPC counts in PSVZ-r on the ligated side at 24h after ligation and on the contralateral side at 48h after ligation. Since the OPCs in Mid-CC appeared to have a different morphology in ligated animals compared to controls (Figure 3), OPCs were manually outlined and average cell size (C) and length/width ratio (D) were calculated in this region at 24h and 48h after ligation, as well as in age matched controls. Small, mid, and large cells were defined as 20–50 μm2, 50–70 μm2 and 70+ μm2 respectively. Interestingly, this analysis showed significantly smaller OPCs with a smaller length/hight ratio (reflecting rounder appearnce) in Mid-CC of ligated animals compared to age matched controls at 24h after ligation. Error bars represent 1 standard deviation.
OPC size and shape analysis: OPC size and morphology (which reflects maturation) were examined in Mid-CC, away from the proliferative zones. At 24h after ligation, we found significantly altered OPC size distribution and OPC-morphology compared to age matched control animals. In ligated mice, there were significantly more small cells (20 to 50 μm2) than in controls, but significantly fewer large cells >70 μm2 (Figure 4C). At 24h after ligation the OPC length/width ratio was significantly less in ligated animals than in age-matched controls (Figure 4D). However at 48h after ligation there was no significant difference in the size or shape of OPCs in between ligated and control mice.
As noted above, in ligated animals we found a transient reduction in total OPC count in Mid-CC 24h after ligation, but at 48h after ligation this number had fully recovered. No significant changes in cell proliferation were seen in Mid-CC, but there was a significant reduction of total OPC counts in the SVWM of ligated animals compared controls at 48h after ligation. We therefore postulated that OPCs may migrate from SVWM to the Mid-CC, replenishing that population of OPCs. As a measure of migration, the ratio of ratio between OPC counts in Mid-CC/SVWM was calculated. In ligated animals, there was a significant bilateral increase in this ratio at 48h after ligation (Figure 5B), implying a higher rate of OPC migration from the SVWM clusters towards Mid-CC. It is of interest to note that the change in this index of migration for microglia (Figure 5A) precedes the change in this index for OPCs.
Relationship between OPC and microglia after unilateral ligation
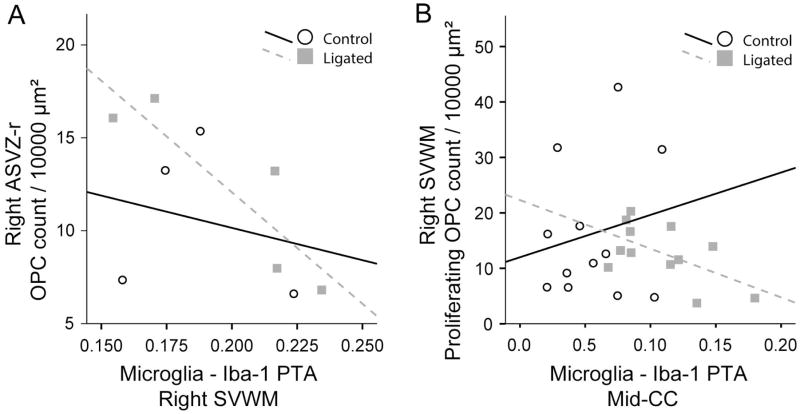
In ligated animals, most areas with increased microglial activation or migration showed fewer OPCs or proliferating OPCs; we, therefore, used Pearson’s correlation to examine associations between Iba1 PTA values and total OPC or proliferating OPC counts. At 48h after ligation, there was a significant negative correlation between ASVZ-r OPC counts and the adjacent SVWM Iba1 PTA on the ligated side (Figure 6A); in posterior regions (EC/PSVZ-r) there was a similar trend which did not gain significance (r = −0.45, p=0.15). Because the strongest effect of ligation on Iba1 PTA was in Mid-CC, we evaluated whether the microglial response in this region would correlate with any regional changes in OPC counts. At 24h and 48h post ligation there was a significant negative correlation between proliferating OPC counts in the SVWM (ligated side) and Mid-CC Iba-1 in ligated animals but not in controls (Figure 6B).
Figure 6. Correlation between microglial activation and OPC counts and proliferation.
Pearson’s correlations were performed to look for associations between Iba1 PTA values and total OPC or proliferating OPC counts at 24h and 48h after ligation. At 24h after ligation, there was a significant negative correlation between ASVZ-r OPC counts and the adjacent SVWM Iba1 on the ligated side (r= −0.88, p < 0.05, n= 5, A). Such correlation was not significant in control animals (n= 4). At 24h and 48h after ligation, there was a significant negative correlation between SVWM proliferating OPC counts (ligated side) and Mid-CC Iba1 in ligated animals (r= −0.58, p < 0.05, n=12) but not in controls (n=12).
Pro-inflammatory cytokines upregulation
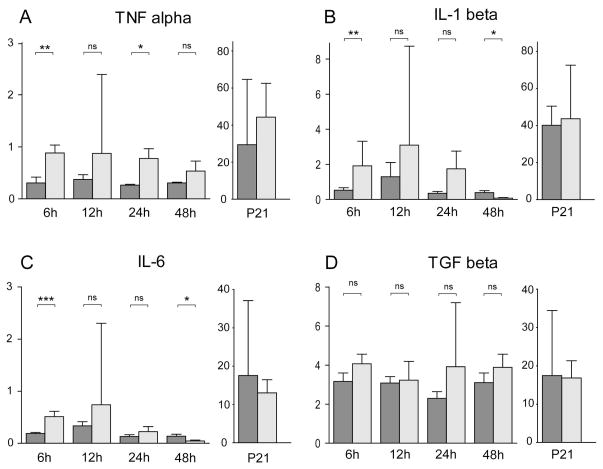
Using qrtPCR, we measured mRNA expression levels for the pro-inflammatory cytokines, TNF-a, IL-1b, and IL-6, and for the growth factor TGF-b1 normalized to 18S rRNA in ligated and control mice 6h, 12h, 24h and 48h after ligation, as well as on P21. IL-2 and IFN-gamma mRNA levels were measured 48h after ligation but there was no expression detected in ligated or control brains. In ligated mice, there was significant increase in TNF- a gene expression across 6h–48h after ligation (ANOVA p = 0.001). Although there was no significant interaction of ligation and time after ligation, t-tests showed a significant increase at 6h and 24h (Figure 7A). IL-1b and IL-6 showed a trend for increased gene expression in ligated animals across 6h–48h (ANOVA: IL-1b, p = 0.06; IL-6, p = 0.09); t-tests showed an initial significant increase of IL-1 and IL-6 at 6h, followed by a significant decrease at 48h (Figure 7B,C). In ligated animals, there was an overall increase in TGF-b1 gene expression across 6h–48h after ligation (ANOVA p=0.03, Figure 7D), but there was no interaction of ligation and time after ligation, and unpaired t-tests did not reveal significant differences at any individual time point. Interestingly, no significant differences were found in the expression of any of the cytokines 16 days post ligation (at P21) between ligated and controls (Figure 7).
Figure 7. Brain cytokines gene expression in controls and after P5 carotid artery ligation.
Quantitative real-time PCR was performed to measure RNA expression of Tumor Necrosis Factor-a (TNF-a, A), Interleukin 1 beta (IL-1b, B), Interleukin 6 (IL-6, C), and Transforming Growth Factor beta 1 (TGF-b1, D) in mid-sagittal brain sections in ligated animals and age matched controls at 6h (n = 10 controls, 11 ligated), 12h (n = 11 controls, 11 ligated), 24h (n = 16 controls, 18 ligated), 48h (n = 17 controls, 17 ligated) after ligation, and at P21 (16 days after ligation, n = 8 controls, n = 7 ligated). RNA expression values are normalized to 18S rRNA and scaled to the measured cytokines. In ligated animals across 6h–48h, there was a significant increase in TNF-a gene expression (ANOVA, p = 0.001), while t-tests showed a significant increase at 6h and 24h in the ligated animals compared to controls (A). IL-1b and IL-6 showed a trend for increased gene expression in ligated animals across 6h–48h (ANOVA: IL-1b, p = 0.06; IL-6, p = 0.09); t-tests showed an initial significant increase of IL-1b and IL-6 at 6h, followed by a significant decrease at 48h (B, C). In ligated animals, there was an increase in TGF-b1 gene expression across 6h–48h (ANOVA p=0.03, p < 0.05), but there was no interaction of ligation and time after ligation, and unpaired t-tests did not reveal significant differences at any individual time point (D). Error bars represent 1 standard deviation.
DISCUSSION
Microglial activation at axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants [25,43,44]. Recent studies indicate that neuroinflammation can produce long-lasting defects in oligodendroglial maturation, myelination-deficits, an increase in oligodendroglial progenitors, as well as a disruption of the expression of transcription factors important in oligodendroglial maturation [3,45]. The concept of ongoing neuroinflammation predisposing a patient to further injury, or preventing repair or regeneration after an initial insult may open possibilities for new therapies within a broader time-window [23] and calls for adapted animal models to test experimental therapies. We recently reported an ischemic model of periventricular leukomalacia with long lasting bilateral myelination defects as assessed at two months of age [40]. We used this model to study whether ischemia would result in neuroinflammation and whether changes in this inflammatory response would be associated with oligodendrocyte progenitor cell injury. Here we report that ischemia on P5 in CD-1 mice induces a region-specific neuroinflammatory response as defined by microglial activation and recruitment/migration along the white matter, as well as changes in mRNA levels of a subset of pro-inflammatory cytokines. We find that microglial response is altered at least up to 16 days after the initial ischemic insult in the external capsule of the ischemic hemisphere, where the most significant longterm myelination deficits are seen at two months of age [40]. Along with these topographic changes in microglial activation, we found a transient reduction in OPC counts and proliferating OPC count. Interestingly, in addition to a reduction in total number of OPCs, as defined by PDGFRa staining, quantitative morphologic analysis suggested that most OPCs seen in the post-ischemic brain were less differentiated cells (smaller, rounder cells with fewer processes) when compared to controls. Finally, we show that the striking microglial response following ischemia was significantly associated with the reduction of OPCs.
Non-infectious insults to the CNS like hypoxia-ischemia, stroke and exposure to excitotoxicity have been shown to induce inflammatory reactions in the immature brain involving inflammatory cells, mainly microglia/macrophages. While the mechanisms for microglial activation in infectious settings have been well studied, the initial signals after sterile tissue damage are less well characterized [43,46,47]. Microglia play important roles during brain development that regulate apoptosis, vascularization, axonal development and myelination [5,48]. In humans they become increased in the forebrain at 16–22 weeks of gestation, and reach peak prominence in cerebral white matter in the third trimester [44,49,50]. At P6 and P7 we could detect CD68+ (presumed to be recently blood-derived) macrophage/microglial clusters within the supraventricular corpus callosum and the external capsule (Figure 1), as has been shown during early postnatal development in rodents and the normal human brain [28–30]. Interestingly these macrophage/microglial clusters were in near proximity to the site of OPC proliferation (Figures 1, 3). One should note that late OPCs prevail between P2 and P7 in rodents, and at gestational weeks 23 to 32 in human fetuses [39].
Microgliosis is a prominent feature of PWMI in humans and expression of interleukin IL-1b, IL-2, IL-6, and TNFa was demonstrated on microglia–macrophages in autopsy samples from neonates with PWMI [8,36–38]. In the presented model, we found that the microglial response 24h after ligation, and in some regions also 48h after ligation, was not restricted to the ligated hemisphere, but was bilateral. We further observed a temporary significant shift of microglial staining between the supraventricular clusters and the mid corpus callosum which was stronger on the non-ligated side, suggesting recruitment/migration of microglia from microglial clusters to other white matter regions (Figure 5). This finding is in line with our prior report [40] of white matter abnormalities and axonopathy in the bilateral corpus callosum at two months following unilateral neonatal ischemia. On P21 however, highly significant microgliosis among the regions of interest was only detected in the external capsule of the ipsilateral hemisphere in ligated animals (Figures 1, 2), where ligation of the internal carotid artery caused the most severe longterm myelination deficits in our model [40].
We detected a transient significant increase in gene expression of inflammatory cytokines IL-1b, IL-6 and TNFa in the brain from 6h after ligation. The question, whether and how the neuroinflammatory response has deleterious effects on OPC proliferation, survival and development, has been suggested in several studies, but is not yet completely addressed [34,35,51]. Studies have shown that activated microglia can release or up-regulate various cytokines, chemokines, and enzymes including IL-1b, TNF-a, IL-6, TGF-b1, macrophage-colony stimulating factors (MCSF), and iNOS [52]. A recent study showed that systemic IL-1b injection between P1–P5 leads to longterm myelination deficits in Swiss mice while an increase in PDGFRa+ OPCs was seen at P5 [45]. Apart from cytokine induced toxicity [3] and possible disruption of oligodendroglial maturation process [45], high levels of NO generated by iNOS can damage neighboring neurons/axons by inhibiting mitochondrial respiration and increasing glutamate release from neurons and glia. NO is also oxidized to reactive oxygen species (ROS), to which developing oligodendroglia show great vulnerability [28,35,53].
The developmental vulnerability of oligodendroglial progenitors due to impaired antioxidant defenses, increased expression of calcium permeable glutamate receptors (AMPA receptors mainly on cell body, NMDA receptors mainly on cell processes) and glutamate transporters, as well as susceptibility to cytokine-triggered injury has been established [54–56]. While we show that after a transient decrease, there is recovery in OPC counts at 48h after ligation in the mid corpus callosum, morphologic analysis of the same region revealed a significant shift in cell size towards smaller less mature cells (Figure 4). We conclude that ischemia leads to a shift toward less mature OPCs, a finding that is in line with suggested maturational arrest of oligodendrocytes in PVL. Regional OPC cell count ratios between sites of OPC proliferation (SVWM) towards areas with more mature OPCs (Mid-CC) revealed a significant shift at 48 after ligation, showing relatively more cells in the mid corpus callosum than in age-matched controls (Figure 5). This may indicate OPC migration from sites of proliferation to sites of injury in ligated animals. In the current study, we did not assess the intercellular matrix and the role of hyaluronan [57], chondroitin sulfates and other proteoglycans, which may be blocking the differentiation of OPCs, as shown in multiple sclerosis models and autopsy samples, [58,59] and but we plan to investigate these in future studies.
Considering the strong proximity of supraventricular areas of OPC proliferation (Figure 3) to microglial clusters (Figure 1) within the white matter, as well as the slower recovery of OPC cell count on P7 in these regions, in contrast to the mid corpus callosum (Figure 4), it is also plausible that the proximity to activated microglia may contribute to decreased OPC counts after ligation. Following this notion we found that microglial Iba1 PTA increase in the dense SVWM cluster was associated with decreased OPC counts in adjacent SVZ. Furthermore, decreased OPC proliferation in the white matter on the ligated side was associated with increased Iba1 PTA in mid corpus callosum, which appears to be a global measure of microglial response after ligation. These correlations were not seen in control brains. While we cannot ascertain a causal relationship, it is conceivable that the activated microglia may release cytokines, inflammatory products or have phagocytic activity that could result in reduced OPC cell counts. Many inflammatory mediators and products which have been demonstrated in microglia in neonates with PWMI [36–38] and have been shown to be detrimental for oligodendroglial development in cell culture [3,8].
The interaction between microglia and OPCs in PWMI is complex and requires further investigation. Cell culture studies with endotoxin activated microglia and OPCs have demonstrated that microglia may secrete factors which, depending on the experimental setting and activator, increase or reduce OPC survival [32,58,60]. However, activated microglia increase survival and reduce apoptosis of mature oligodendrocytes, independent of an activating agent [51]. Microglia have been shown to secrete many growth/trophic factors such as brain-derived neurotrophic factor, bFGF, NGF, IGF-1, TGF-b, and ciliary neurotrophic factor [61–63], most of which also support oligodendroglial survival and differentiation. They can also generate free radicals, secrete injurious pro-inflammatory cytokines, and contribute to excitotoxicity [5,8]. Thus, microglia play a dual role in maintenance of tissue homeostasis and repair. A recent study using the mouse cuprizone model, where remyelination occurs spontaneously after toxin-induced primary demyelination, examined genome-wide gene expression analysis of microglia from the corpus callosum during demyelination and remyelination and characterized a microglial phenotype that supports remyelination that was present at the onset of demyelination and persisted throughout remyelination. These microglia expressed cytokines and chemokines that activate and recruit endogenous OPCs to the lesion site and deliver trophic support during remyelination [64].
While we show increased microglial activation along with increased expression of pro-inflammatory cytokines, one limitation in our study is that we did not further characterize whether the activated microglia are pro- or anti-inflammatory. It is possible that the microglial response seen in our study during the late phase (P21) involves different mechanisms than the initial microglial activation, and is perhaps more a reparative or trophic response rather than destructive. Along with this notion is the fact that none of our pro-inflammatory cytokines were still up-regulated at this late stage. Future studies with this model will need to address the role of neuroinflammatory priming and the potential for tertiary injury [23] in older animals. Another limitation of this study is that astrocytes could be an important part of the neuroinflammatory process in this model [56,65], but while we showed ipsilateral astrogliosis in this model at P60 [40], at early postnatal ages our assessment using the GFAP marker proved insensitive, as it also stained progenitor cells and radial glial cells (data not shown). We are planning to utilize newer, more specific astrocyte markers in our future studies to further characterize the role of astrocytes following acute ischemia.
In summary, we report an ischemia-induced neonatal mouse model of PVL in which we find significant neuroinflammation and selective vulnerability of the white matter. Temporospatial study of the interaction of microglia and OPCs after injury, as well as the cytokine-profile, indicate that neuroinflammation plays an important role in ischemia-induced oligodendroglial injury. Future studies utilizing immunomodulatory agents in this ischemic model can aid in a better understanding of the diverse roles of neuroinflammation within the vulnerable developmental period of PWMI and open possibilities for new protective therapies.
Acknowledgments
The authors would like to thank Ms. Patrice Carr, and Ms. Karen Connor for their kind assistance with the immunohistochemial studies. This study was funded by the National Institute of Health (K08NS063956 to A.F., R01NS028208 to M.V.J., R01NS061969 to A.C.), Child Neurology Foundation (to A.F.), and the Miracle for Megan Foundation (to A.F.).
References
- 1.National Center for Health Statistics, final natality data. Retrieved August 23, 2012 from www.marchofdimes.com/peristats.
- 2.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011 Apr;10:372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe JJ. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann Neurol. 2011 Oct;70:525–529. doi: 10.1002/ana.22533. [DOI] [PubMed] [Google Scholar]
- 4.Hamrick SEG, Miller SP, Leonard C, Glidden DV, Goldstein R, Ramaswamy V, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004 Nov;145:593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009 Jan;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil. 2005 Apr;38:130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007 Oct;12:363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat. 2010 Jun 11; doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatemi A, Wilson MA, Johnston MV. Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 2009 Dec;36:835–858. vii. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002 Jan 15;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007 Feb;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 12.Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010 Jun;6:328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- 13.Dammann O. Persistent neuro-inflammation in cerebral palsy: a therapeutic window of opportunity? Acta Paediatr. 2007 Jan;96:6–7. doi: 10.1111/j.1651-2227.2007.00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005 Dec;58:821–828. doi: 10.1002/ana.20662. [DOI] [PubMed] [Google Scholar]
- 15.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009 Sep;24:1119–1126. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009 Dec;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mildner A, Schlevogt B, Kierdorf K, Böttcher C, Erny D, Kummer MP, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J Neurosci. 2011 Aug 3;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007 Nov;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- 19.Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006 Dec;80:308–322. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010 Dec;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 21.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011 Sep;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 22.Lin C-Y, Chang Y-C, Wang S-T, Lee T-Y, Lin C-F, Huang C-C. Altered inflammatory responses in preterm children with cerebral palsy. Ann Neurol. 2010 Aug;68:204–212. doi: 10.1002/ana.22049. [DOI] [PubMed] [Google Scholar]
- 23.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012 Jun;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 24.Judas M, Rados M, Jovanov-Milosevic N, Hrabac P, Stern-Padovan R, Kostovic I. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol. 2005 Dec;26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- 25.Verney C, Pogledic I, Biran V, Adle-Biassette H, Fallet-Bianco C, Gressens P. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J Neuropathol Exp Neurol. 2012 Mar;71:251–264. doi: 10.1097/NEN.0b013e3182496429. [DOI] [PubMed] [Google Scholar]
- 26.Tahraoui SL, Marret S, Bodénant C, Leroux P, Dommergues MA, Evrard P, et al. Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 2001 Jan;11:56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallard C, Welin A-K, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003 Feb;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 28.Baud O, Daire J-L, Dalmaz Y, Fontaine RH, Krueger RC, Sebag G, et al. Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Pathol. 2004 Jan;14:1–10. doi: 10.1111/j.1750-3639.2004.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivier P, Baud O, Evrard P, Gressens P, Verney C. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005 Nov;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- 30.Monier A, Adle-Biassette H, Delezoide A-L, Evrard P, Gressens P, Verney C. Entry and Distribution of Microglial Cells in Human Embryonic and Fetal Cerebral Cortex. Journal of Neuropathology and Experimental Neurology. 2007 May;66:372–382. doi: 10.1097/nen.0b013e3180517b46. [DOI] [PubMed] [Google Scholar]
- 31.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002 Jan 15;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipović R, Zecević N. Interaction between microglia and oligodendrocyte cell progenitors involves Golli proteins. Ann N Y Acad Sci. 2005 Jun;1048:166–174. doi: 10.1196/annals.1342.015. [DOI] [PubMed] [Google Scholar]
- 33.Miller BA, Crum JM, Tovar CA, Ferguson AR, Bresnahan JC, Beattie MS. Developmental stage of oligodendrocytes determines their response to activated microglia in vitro. J Neuroinflammation. 2007;4:28. doi: 10.1186/1742-2094-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, et al. Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res. 2010 Jun;88:1632–1644. doi: 10.1002/jnr.22335. [DOI] [PubMed] [Google Scholar]
- 35.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010 Mar 17;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997 Aug;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 37.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sébire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001 May 22;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 38.Kadhim H, Tabarki B, Prez CD, Rona A-M, Sébire G. Interleukin-2 in the pathogenesis of perinatal white matter damage. Neurology. 2002 Apr 9;58:1125–1128. doi: 10.1212/wnl.58.7.1125. [DOI] [PubMed] [Google Scholar]
- 39.Craig A, Ling Luo N, Beardsley DJ, Wingate-Pearse N, Walker DW, Hohimer AR, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003 Jun;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 40.Fatemi A, Wilson MA, Phillips AW, McMahon MT, Zhang J, Smith SA, et al. In vivo magnetization transfer MRI shows dysmyelination in an ischemic mouse model of periventricular leukomalacia. J Cereb Blood Flow Metab. 2011 May; doi: 10.1038/jcbfm.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008 Apr;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bechmann I, Rappert A, Priller J, Nitsch R. The Microglial Component [Internet] In: Dermietzel R, Spray DC, Nedergaard iken, editors. Blood-Brain Barriers. Wiley-VCH Verlag GmbH & Co. KGaA; 2007. pp. 167–188. [cited 2012 Aug 22] [Google Scholar]
- 43.Dommergues M-A, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 44.Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006 Dec;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- 45.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011 Oct;70:550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 46.Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000 Jul;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- 47.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012 Apr;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 48.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010 Nov 11;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 49.Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000 Aug;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 50.Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005 Apr 4;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 51.Miller BA, Crum JM, Tovar CA, Ferguson AR, Bresnahan JC, Beattie MS. Developmental stage of oligodendrocytes determines their response to activated microglia in vitro. J Neuroinflammation. 2007;4:28. doi: 10.1186/1742-2094-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001 Sep 1;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005 Jul 12;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipton SA. Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol. 1996 Oct;6:507–517. doi: 10.1111/j.1750-3639.1996.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 55.Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, et al. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002 Apr;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 56.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2008 März;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Back SA, Tuohy TMF, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005 Sep;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 58.Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience. 2006 Jun 19;140:87–100. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 59.Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011 Oct;119:176–188. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- 60.Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006 Apr;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004 Mar;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 62.Palacios N, Sánchez-Franco F, Fernández M, Sánchez I, Cacicedo L. Intracellular events mediating insulin-like growth factor I-induced oligodendrocyte development: modulation by cyclic AMP. J Neurochem. 2005 Nov;95:1091–1107. doi: 10.1111/j.1471-4159.2005.03419.x. [DOI] [PubMed] [Google Scholar]
- 63.Talbott JF, Cao Q, Bertram J, Nkansah M, Benton RL, Lavik E, et al. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Exp Neurol. 2007 Mar;204:485–489. doi: 10.1016/j.expneurol.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012 Feb;60:306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 65.Sen E, Levison SW. Astrocytes and developmental white matter disorders. Ment Retard Dev Disabil Res Rev. 2006;12:97–104. doi: 10.1002/mrdd.20106. [DOI] [PubMed] [Google Scholar]