Abstract
A single case study recently documented one woman’s ability to recall accurately vast amounts of autobiographical information, spanning most of her lifetime, without the use of practiced mnemonics (Parker, Cahill, & McGaugh, 2006). The current study reports findings based on eleven participants expressing this same memory ability, now referred to as Highly Superior Autobiographical Memory (HSAM). Participants were identified and subsequently characterized based on screening for memory of public events. They were then tested for personal autobiographical memories as well as for memory assessed by laboratory memory tests. Additionally, whole-brain structural MRI scans were obtained. Results indicated that HSAM participants performed significantly better at recalling public as well as personal autobiographical events as well as the days and dates on which these events occurred. However, their performance was comparable to age- and sex-matched controls on most standard laboratory memory tests. Neuroanatomical results identified nine structures as being morphologically different from those of control participants. The study of HSAM may provide new insights into the neurobiology of autobiographical memory.
Keywords: Autobiographical memory, Highly superior autobiographical memory, Brain morphology
1. Introduction
Highly Superior Autobiographical Memory (HSAM) is a newly described ability in which individuals are able to recall events from their personal past, including the days and dates on which they occurred, with very high accuracy. Previously termed “hyperthymestic syndrome,” it was first studied in an individual referred to as A.J. (Parker, Cahill, & McGaugh, 2006). HSAM is distinct from other types of superior memory as participants with this ability perform autobiographical remembering without the apparent use of mnemonic skills. Typically, individuals with superior memory encode and retrieve domain-specific and/or relatively meaningless information utilizing strategies acquired through practice (e.g., street maps of entire cities, pi out to 22,514 decimal places, and long displays of words or digits; Ericsson, Delaney, Weaver, & Mahadevan, 2004; Hunt & Love, 1972; Gordon, Valentine, & Wilding, 1984; Wilding & Valentine, 1997). Even the extreme memory abilities of one of the most famous mnemonists, patient S., described by Luria (1968), did not entail autobiographical remembering. Patient S. described living his personal life “as in a haze” (p. 159).
Since the publication of Parker et al. (2006) numerous individuals have contacted our research group professing either to have HSAM, or to know someone who does. We have identified ten new HSAM participants (in addition to A.J.) utilizing a screening process developed to identify HSAM characteristics.
Here we report a detailed analysis of both cognitive function and brain structure of the eleven HSAM participants (including A.J.). Cognitive assessment involved a battery of memory tests both general in nature and specific to autobiographical memory. We examined potential differences in the neuroanatomy of the HSAM participants, as compared to that of age- and sex-matched controls, using structural magnetic resonance imaging (MRI). A substantial literature indicates that changes in human brain structure can be associated with changes in behavior, including memory (Golestani, Paus, & Zatorre, 2002; Bohbot, Lerch, Thorndycraft, Iaria, & Zijdenbos, 2007; Boyke, Driemeyer, Gaser, Buchel, & May, 2008; Draganski et al., 2006; Fujie et al., 2008; Scholz, Klein, Behrens, & Johansen-Berg, 2009). For the present project, four neuroanatomical methods were used. The first two, Voxel Based Morphometry Grey-Matter (VBM-GM) and Voxel Based Morphometry White-Matter (VBM-WM) allowed for the comparison, between groups, of the local concentration of grey and white matter found in any given voxel throughout the brain (Ashburner & Friston, 2000). The third, Tensor Based Morphometry (TBM) was used to detect group-related differences in the shape of regions of the brain (Chung et al., 2001). The fourth, Diffusion Tensor Imaging-Fractional Anisotropy (DTI-FA) allowed for a means of quantifying and comparing differences in white-matter structure (Beaulieu, 2009; Moseley et al., 1990). We present here the results of these cognitive and neuroanatomical analyses.
2. Materials and methods
A multi-step, Institutional Review Board (IRB) approved process was developed to identify and test HSAM participants. Individuals, who contacted us proclaiming to have HSAM, were screened over the telephone and if they met criteria, were formally consented. One hundred and fifteen adults, claiming to have HSAM, were screened with the Public Event Quiz (the first screening quiz, described below). Forty-one of those adults scored well enough to advance to the 10 Dates Quiz (the second screening quiz, described below). Thirty-six of the forty-one adults were screened with the 10 Dates Quiz (five did not respond when contacted) and 31 passed it (achieving a score of 65% or above). Eleven of these adults (4 females, 7 males, age range 27–60, average age = 43; six right-handed, three left-handed, and two ambidextrous) came to the laboratory for an interview, during which participants discussed their memory ability, cognitive testing was performed and detailed anatomical data, via a structural MRI, were collected. A behavioral questionnaire, designed to determine possible common qualities of the HSAM participants, was administered via the telephone at a later time point. Each of these procedures is discussed below in detail. All research data were collected through a protocol approved by the University of California, Irvine IRB and informed written consent was obtained from all eleven participants.
Three different sets of age- and sex-matched controls were used for the screening, cognitive battery and MRI. All were adults recruited actively via contacts in the adjacent community, of whom none claimed to have HSAM or other superior memory abilities. All gave written informed consent in compliance with the IRB of the University of California, Irvine for behavioral testing and the usage of MRI scans.
Screening Controls
Thirty age- and sex-matched controls (15 males, 15 females, age range 26–67, average age = 43.9) were screened for the study using the Public Events Quiz of which 13 were given the 10 Dates Quiz (6 males, 7 females age range 28–62, average age = 50).
Cognitive Battery Controls
Fifteen age- and sex-matched controls (5 males, 10 females, age range 23–56, average age = 36.8) received the cognitive battery.
MRI Controls
Structural MRI data was compiled from nineteen age- and sex-matched healthy controls previously scanned by a collaborating research group (10 males, 9 females, age range 23–66). Controls were determined, via the Edinburgh Handedness Inventory, to be right-handed (see Section 3).
2.1. Screening procedures
The Public Events Quiz consisted of thirty questions presented over the telephone. It contained two types of questions: fifteen asked for the date of a given significant public event that took place within the individuals’ lifetime (e.g., When did Jimmy Carter win the Nobel Peace prize?); fifteen asked for the significant public event that took place on a given date that fell within the individual’s lifetime. In addition, for all 30 questions, individuals were asked to state the day of the week the date fell on. The order of presentation of the two types of questions was interchanged. The significant public events given were selected from five different categories: Sporting events, political events, notable negative events, events concerning famous people and holidays. The participant received one point for each correctly identified category (i.e., the event, the day of the week, the month, the date and the year) and could achieve a total of 88 possible points. Percentages scored were calculated for each individual claiming to have HSAM as well as each screening control. A score of 50% or above qualified an individual claiming to have HSAM to advance to the second even more challenging round of screening, the 10 Dates Quiz.
The 10 Dates Quiz consisted of ten computer generated random dates, ranging from the individuals’ age of fifteen to the day of testing. It was administered via the telephone with no time limits. Individuals were asked to provide three different categories of information for each of the 10 dates generated: (1) the day of the week; (2) a description of a verifiable event (i.e. any event that could be confirmed via a search engine) that occurred within ± one month of the generated date; (3) a description of a personal autobiographical event the individual participated in. One point was awarded for the correct day of the week, for giving a verifiable event confirmed as true, and/or for giving a personal autobiographical event. A maximum of three possible points per date could be achieved (thirty points total). The percentage scored for each category as well as the total score, the average of all three categories, was calculated. A total score of 65% or above qualified the individual as an HSAM participant and for further, in person, behavioral and neuroanatomical testing.
2.2. Cognitive battery
Following the screening procedure, eleven HSAM participants were brought to the laboratory and examined with a cognitive battery consisting of thirteen behavioral tests assessing autobiographical memory, various types of learning and memory, obsessional tendencies hand dominance and depression levels. The choice of tasks was driven by our prior experience with HSAM individuals and by our desire to assess different aspects of their memory ability. The battery took approximately an hour and a half to complete. Three participants failed to complete the entire battery. The number of participants who took each test is detailed in the results.
2.2.1. Autobiographical Memory Task (AMT)
Autobiographical memory was assessed following a modified cued-recall procedure based on Pohl, Bender, and Lachmann (2005). Each participant was asked to recall five specific personal events, chosen such that answers could be verified for accuracy. Participants had no prior knowledge of which personal events would be asked. The five specific events were: First day at university; First day of elementary school; 18th birthday celebration; Address and description of the first place they resided after moving out of parents’ house; Last final exam in college.
Participants were asked to recall verbally each event in as much detail as possible and encouraged to include details such as dates, weather, names of others present and location. At the conclusion of the test, participants were asked to supply the following items for verification of the accuracy of their memories: College transcripts, correspondence from first address, kindergarten or 1st grade class photo, pictures from 18th birthday celebration, diaries and calendars.
Two separate scores were devised, one for ‘AMT Verifiable Details Score’ and one for ‘AMT Total Details Score’. Details for the ‘AMT Verifiable Details Score’ were verified via personal documents, calendars, or web searches (using sites such as historical weather databases, Google Maps and news articles) and given one point if accurate. See Appendix A for examples that illustrate the verification process. The percentage correct out of all verifiable details was calculated. Details for the ‘AMT Total Details Score’ were not verified. One point was given regardless of whether a detail could or could not (e.g., conversations, thoughts, emotions etc.) be verified by the researcher.
2.2.2. Names to faces
The recall of names paired with faces was assessed following a modified procedure based on Morris, Jones, and Hampson (1978). Participants were shown fourteen unknown faces (seven male, seven female), for two seconds each. Each face was verbally assigned a first and last name. All faces were presented in frontal position with a neutral expression. Immediately following the study phase, the faces were shown in the same order and participants were asked to recall the name previously associated with each face. The facial images were selected from the Wechsler Memory Scale-III (WMS-III) Faces subtest. Names were generated using an online random name generator that uses US census data to randomly generate male and female names (http://www.kleimo.com/random/name.cfm). One point was given for a correctly given first and last name paired with the appropriate face (maximum of 2 points per face).
2.2.3. Visual memory
Visual memory was assessed following a procedure developed by Marks (1973). Participants were shown a stimulus consisting of a set of 15 unrelated objects for 20 s (Appendix B). Following the distraction question, “Would you like a drink of water?” they were asked five questions regarding the objects and their locations. If they were unable to answer a question, a prompt directly followed consisting of three options, one of which was the correct answer. A correct answer without a prompt was given two points. A correct answer with a prompt was given one point.
2.2.4. Forward and backward digit span
Auditory short-term recall was assessed following a modified version of the WMS-III digit span. Participants were presented with a series of number sequences that increased in length, incrementally with every correct response. After each individual number sequence, participants were asked to either repeat it verbatim or in reverse order. The test was concluded after two consecutive incorrect responses. One point was given for each correctly repeated sequence.
2.2.5. Visual reproduction
Visual memory was assessed following a modified version of the WMS-III Visual Reproduction Subtest. Participants were given ten seconds to look at an abstract design, and then asked to draw the design from memory on a blank sheet of paper. Scores were assigned according to the WMS-III with a maximum of thirty possible points.
2.2.6. Logical memory test
Memory for story content, presented verbally, was assessed using a modified version of the WMS-III Logical Memory Subtest. Free-recall and recognition tests were administered. Participants were read Story A of the WMS-III Logical Memory by the examiner and immediately after asked to verbally recall the story in as much detail as possible. One point was given for each correctly recalled key word or phrase. HSAM performance for the free-recall portion was compared with that of MRI Controls (see Section 3). Immediately following the free-recall portion HSAM participants and Cognitive Battery Controls were asked 15 yes or no questions about the story (see Appendix C for examples). A point was given for each correct answer.
2.2.7. Verbal paired associates
Verbal memory was assessed following a modified version of the WMS-III. Participants were read a series of eight word pairs at a rate of one word pair per three seconds. Immediately following the eight pair list, they were prompted with the first word of a given pair and instructed to give the correct corresponding word. The order of first words presented during the test phase differed from the order of the original word pairs (Appendix D). One point was given for each correct answer.
2.2.8. Leyton Obsessional Inventory Score-Short Form (LOI-SF)
Common obsessional symptoms were assessed using the LOI-SF (Mathews, Jang, Hami, & Stein, 2004) as prior encounters with a number of the individuals demonstrated a potential obsessional component. The LOI-SF self-report inventory consists of thirty “Yes/No” questions assessing the presence or absence of obsessional symptoms focusing on concerns of contamination and honesty, the presence of repeating behaviors, uncomfortable thoughts/doubts and checking behaviors, allotting too much attention to detail, strictness with one’s conscience and routine, taking a long time to dress, hang up and put away clothing, and lastly belief in extremely unlucky numbers. Participants could score a maximum of thirty points. Approximately half of the questions were reverse scored to account for simple response biases.
2.2.9. Edinburgh Handedness Inventory (EHI)
Hand dominance in everyday activities was assessed using the EHI (Oldfield, 1971). Participants were given a copy of the EHI and asked to self-report hand use preferences.
2.2.10. Becks Depression Inventory II (BDI-II)
The severity of depression was assessed using the BDI-II. HSAM participants were given a copy of the BDI-II and asked to complete 21 multiple-choice self-report questions relating to symptoms of depression. Note that HSAM performance was compared with that of MRI Controls (see Section 3).
2.3. Behavioral questionnaire
Following the interview and the cognitive battery, a behavioral questionnaire was administered to the eleven HSAM participants via the telephone. It consisted of 43 questions concerning the nature of their memory and behaviors, such as their knowledge about the calendar and dates, their obsessive tendencies, and what they believe influences their recollection of events. We present here the most consistent and salient responses both from this questionnaire and from the general interview process.
2.4. Acquisition of structural MRI data
The eleven HSAM participants were examined by Magnetic Resonance Imaging at the University of California, Irvine on a Philips Achieva 3T scanner equipped with an 8-channel phased-array head coil. Nineteen age- and sex-matched MRI controls were scanned on the same device during the same time frame, but drawn from a separate study. High-resolution anatomical images were acquired using T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) protocol (TR 11 ms, TE 4.6 ms, FA 18 degrees, 200 sagittal slices, 320 × 264 matrix, FOV 240 mm × 150 mm, isotropic voxel resolution of 0.75 mm). Diffusion-weighted (DW) images were acquired using a diffusion-weighted spin-echo sequence (TR 12.88 s, TE 48.69 ms, FA 90 degrees, 60 axial slices, 128 × 128 matrix, FOV 256 mm × 256 mm, voxel resolution of 1.8 × 1.8 × 2 mm, 32 gradient directions at b = 800 s/mm2, one at b = 0 s/mm2, and one acquisition with isotropic gradients at b = 800 s/mm2). Because the controls took part in a separate study that dictated alignment of the scans with the principal axis of the hippocampus, these images were acquired at an angulation of 30 degrees with respect to the AC–PC plane. As a consequence, approximately 2 cm of the superior frontal lobe and 4 cm of the posterior cerebellum were not included in the scans. Besides the images, the following variables were collected as covariates: group label (participant or control), gender, age (at time of examination), and intracranial volume.
2.5. Analysis of structural MRI data
The T1-weighted MR imaging data were aligned with the stereotactic coordinate system and interpolated to an isotropic voxel size of 1 mm using a fourth-order b-spline method (Kruggel & von Cramon, 1999). All head images were mapped into the space of the MNI-152 atlas using nonlinear registration with a cross-correlation similarity metric (Vercauteren, Pennec, Perchant, & Ayache, 2009). Next, registered images were intensity-corrected and averaged. The brain was extracted from the average to yield the brain template A.
A mask of the intracranial volume was generated from each head data set by a registration approach and used to extract the brain (Hentschel & Kruggel, 2004). Data were corrected for intensity inhomogeneities using a newly developed technique that estimates the gain field by comparing the global intensity distribution with regional ones, resulting in an intensity-corrected image of the intracranial space. Data were segmented by a fuzzy approach into three classes yielding a set of three probability images (Zhang, Brady, & Smith, 2001). Each voxel received a probability for belonging to the intensity class 0: cerebrospinal fluid, CSF; (1) grey matter, GM or (2) white matter, WM. As a result, gross compartment volumes (in ml) for GM, WM, CSF, and intracranial volume were obtained for all participants. All intensity-corrected brain images were nonlinearly registered with brain template A, and averaged to yield the brain template B in MNI space. Finally, nonlinear transformations to template B were computed, and the resulting deformation field stored for each participant and control.
From this deformation field, the determinant of the first partial derivative (J) was computed at each voxel. This value can be used to detect local shape changes (J > 1 for locally expanding areas, J < 1 for locally contracting areas, and ~1 for no change, translations and rotations). Data were smoothed using a Gaussian filter (σ = 2, FWHM of 4.7 mm), and log-transformed. Linear regression models were computed for each voxel, using log (J) as a dependent variable, and variables group, age, gender, and intracranial volume as independent variables. Computation was restricted to a tissue mask with a combined GM and WM probability p > 0.25. As a result, the significance of group-related shape differences, expressed as voxel-wise z-scores, were obtained. Note that negative z-scores correspond to a local contraction in the deformation maps of participants vs. controls and positive scores correspond to a local expansion. This technique is commonly denoted as “tensor-based morphometry” (TBM, Chung et al., 2001).
Next, the probabilistic GM (and WM) segmentation obtained above were warped into in MNI space using the stored deformation fields, smoothed using a Gaussian filter (σ = 2, FWHM of 4.7 mm) and logit-transformed. Linear regression models were computed for each voxel, using the transformed probability as a dependent variable, and variables group, age, gender, and intracranial volume as independent variables. Computation was restricted to a GM(and WM) mask with a GM (and WM) probability p > 0.25. As a result, the significance of group-related differences in GM (and WM) probability expressed as voxel-wise z-scores, were obtained. Note that negative z-scores correspond to a locally lower concentration for participants vs. controls and positive numbers reflect higher concentrations. This technique is commonly denoted as “voxel-based morphometry” (VBM-GM and VBM-WM, Ashburner & Friston, 2000).
Finally, we examined group-related differences in white matter fiber structures based on DW imaging data. Scan data in Philips PAR-REC format were converted into BRIAN format, and image volumes corresponding to all gradient directions were corrected for motion artifacts by affine registration with the gradient-free (T2)-weighted image volume. Diffusion tensors were computed from the registered DW images using a nonlinear procedure including anisotropic noise filtering (Fillard, Pennec, Arsigny, & Ayache, 2007). Tensors were converted into fractional anisotropy (FA) values. Diffusion Tensor Imaging-Fractional Anisotropy (DTI-FA) allowed for a measurement of the diffusivity of water molecules along white matter fiber bundles. FA is a measure of the anisotropy of the white-matter microstructure in the human brain, specifically the coherence of fiber bundles (Beaulieu, 2009; Moseley et al., 1990).
Resulting FA images were mapped into MNI space using the nonlinear deformation field computed above. Data were smoothed using a Gaussian filter (σ = 2, FWHM of 4.7 mm), and linear regression models were computed for each voxel, using FA as a dependent variable, and variables group, age, gender, and intracranial volume as independent variables. Computation was restricted to a tissue mask with a WM probability p > 0.5. As a result, the significance of group-related differences in FA, expressed as voxel-wise z-scores were obtained. Note that negative (positive) z-scores correspond to a locally lower (higher) coherence of WM fibers of participants vs. controls (method DTI-FA).
The statistical maps resulting from the four methods were correct for multiple comparisons using the theory of excursion sets in Gaussian random fields (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1993). Maps were thresholded by an absolute z-score of 2.5, and clusters were assessed for their significance. Only clusters with p < 0.05 were retained, and characterized by their size (in mm3), mean position in Talairach space, peak and mean z-score.
VBM-GM, VBM-WM and TBM used the same T1-weighted MRI acquisition and therefore may provide correlated results. DTI-FA is based on DW MRI data, and is therefore considered independent from the previous methods.
3. Results
3.1. Public Events Quiz and 10 Dates Quiz results
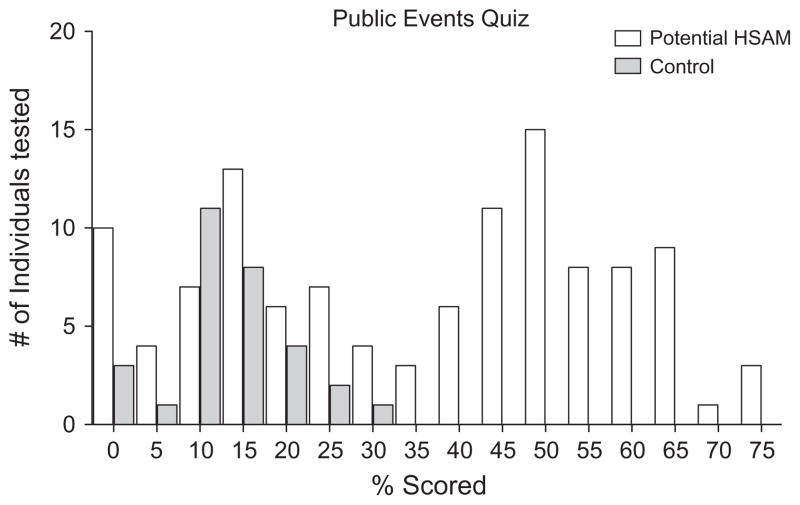
A histogram of the percentage scored on the Public Events Quiz by individuals claiming to have HSAM (n = 115, white bars) and screening controls (n = 30, grey bars) is presented in Fig. 1. A bimodal distribution resulted suggesting the presence of two separate populations within those individuals claiming to have HSAM. A notable number of these individuals performed at a level indistinguishable from that of controls, falsely self-identifying their autobiographical memory as superior. Forty, of the initial 115 individuals claiming to have HSAM, passed our criterion achieving a score of 50% or higher on this test. The eleven, who would eventually become the HSAM participants, achieved an average score of 56.5% which was significantly higher than the average score of 12.9% achieved by the screening controls (t(39) = 19.61, p < .001).
Fig. 1.
Histogram of the percent-correct scores on the Public Events Quiz by individuals who contacted us claiming to have HSAM (white) and by age- and sex-matched controls who did not contact us directly (grey).
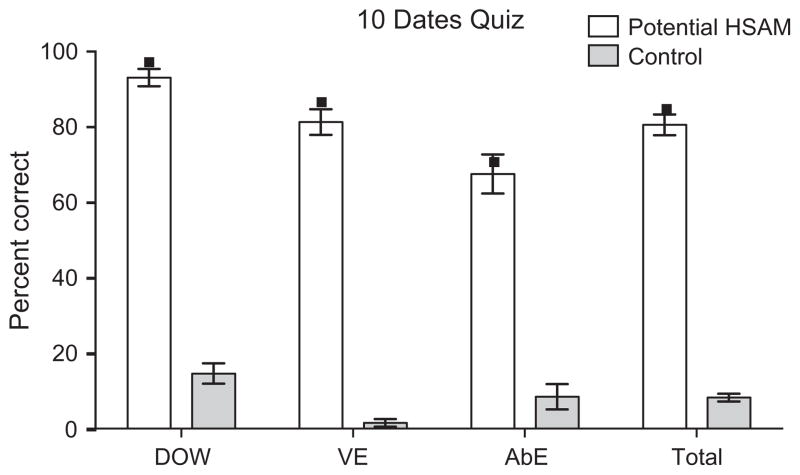
Performance on the 10 Dates Quiz (mean ± standard error of the mean) achieved by the individuals claiming to have HSAM (n = 36, white bars) as well as screening controls (n = 13, grey bars) is presented in Fig. 2. Individual data points represent the average score the eleven HSAM participants received in each category. On average, they correctly produced the day of the week 97% of the time, a verifiable event 87% of the time, and an autobiographical event 71% of the time, scoring an average of 85% on the quiz as a whole (total score) (see Fig. 2). Controls correctly produced the day of the week 14.6% of the time, a verifiable event 1.5% of the time, and an autobiographical event 8.5% of the time, scoring an average of 8.2% on the quiz as a whole (total score). For each of these categories, the eleven HSAM participants scored significantly higher than the controls (t(22) = 25.8, 18.2, 6.4, and 20.46 respectively, all p’s < .001).
Fig. 2.
Performance on the 10 Dates Quiz for individuals who contacted us claiming to have HSAM and scored 50% or higher on the Public Events Quiz (white, n = 36) and for age- and sex-matched controls (grey, n = 13). The total percentage scored (mean ± standard error of the mean) on all three categories combined (total) along with the percentage scored (mean ± standard error of the mean) on each individual category, day of the week (DOW), verifiable event (VE), and autobiographical event (AbE), are shown. Single data points indicate the average score achieved in each category by the eleven HSAM participants.
3.2. Cognitive battery results
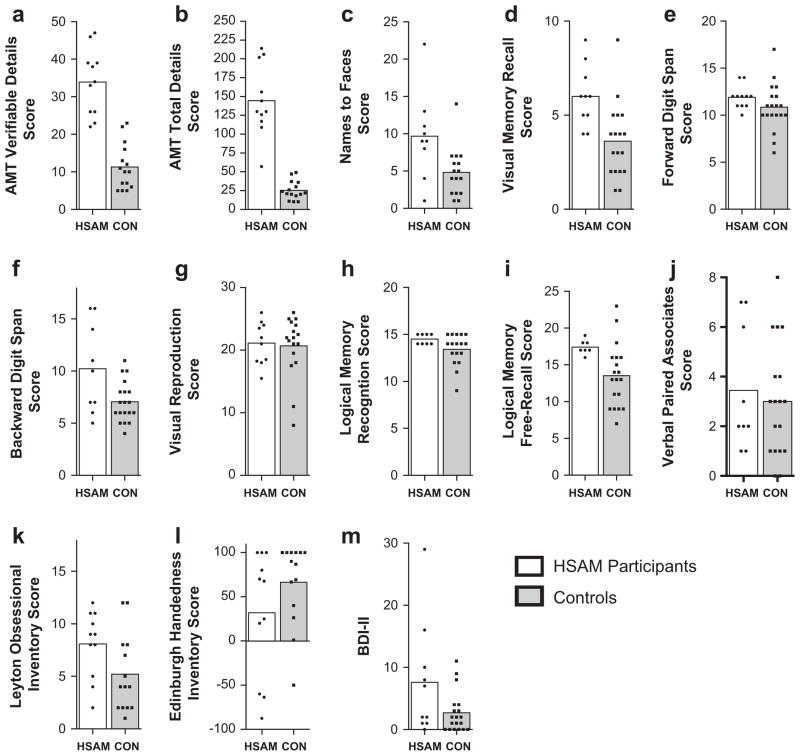
The mean performance of the participants now characterized as having HSAM (n = 11, white bars) and the controls (n = range 15–19, grey bars) on each of the 13 cognitive battery tests are presented in Figs. 3a–m. Three HSAM participants failed to complete the entire battery, either due to a lack of time or a lack of willingness to complete it. Note, in Fig. 3, MRI control group data is presented in the logical memory free-recall score (i) and BDI-II (m), while cognitive battery control group data is presented in the rest.
Fig. 3.
Compilation of the mean results from the cognitive battery. Individual data points indicated the scores achieved by the HSAM (white) and control participants (grey) in each respective test. Specific tests are labeled on the Y-axis (see Section 3 text for details).
Fig. 3a and b shows the results from two analyses of the Autobiographical Memory Test. Fig. 3a shows the mean recall of verifiable details for the eleven HSAM participants and retrieval of events from the control group (unverified). Pertaining to the control participants, only items that could theoretically be verified (excluding e.g. conversations, thoughts, emotions, etc.) were scored and all were treated as accurate. Even with this bias towards assumed accuracy for the controls, the mean score for the HSAM participants (33.91) was significantly greater than that for controls (11.33; Mann–Whitney p < 0.001). Ten out of eleven HSAM participants scored 100% correct in the verifiable details they recalled, while one made a single error resulting in a score of 32 out of 33 or 97% correct. Fig. 3b shows the mean recall of the total details (both verified and unverified) for eleven HSAM participants and the control group. The recall score summing any details for HSAM participants (145) was significantly greater than that for controls (25.4; Mann–Whitney p < 0.01).
Fig. 3c shows the mean recall of names to faces for nine HSAM participants and the control group. The mean score for HSAM participants (9.67) was significantly greater than that for controls (4.81; Mann–Whitney p < 0.017).
Fig. 3d shows the mean recall of image details for ten HSAM participants and the control group on the visual memory test. The mean score for HSAM participants (6.00) was significantly greater than that for controls (3.61; Mann–Whitney p < 0.002).
Fig. 3e shows the mean recall of digit span forward for 11 HSAM participants and the control group. The mean score for HSAM participants (11.91) was not significantly greater than that for controls (10.84; Mann–Whitney p > 0.085).
Fig. 3f shows the mean recall of backward digit span for nine HSAM participants and the control group. The mean score for HSAM participants (10.22) was not significantly greater than that for controls (7.05; Mann–Whitney p > 0.068), although a trend towards significance is apparent. From the figure, it is clear that the distribution of the HSAM participants had a large variance. Several of these individuals indicated that the difficulty of the test prompted them to convert these numbers into dates, which may have inflated both the group mean and variance (see Section 4).
Fig. 3g shows mean visual reproduction scores for ten HSAM participants and the control group. The mean score for HSAM participants (21.13) was not significantly greater than that for controls (20.68; Mann–Whitney p > 0.931).
Fig. 3h shows the mean logical memory recognition test scores for eight HSAM participants and the control group. The mean score for HSAM participants (14.50) was not significantly greater than that for controls (13.41; Mann–Whitney p > 0.102). However, these results could be attributable to ceiling effects (maximum points achievable is 15). This test was designed for utilization in clinical settings to measure memory deficits, not superior memory.
Fig. 3i shows the mean logical memory free-recall test scores for seven HSAM participants and the MRI control group. The mean score for HSAM participants (17.43) was significantly greater than that for controls (13.53; Mann–Whitney p < 0.006).
Fig. 3j shows the mean results for verbal paired associates test for nine HSAM participants and the control group. The mean score for HSAM participants (3.44) was not significantly greater than that for controls (3.00; Mann–Whitney p > 0.710).
Fig. 3k shows the mean score on the Leyton Obsessional Inventory Score-Short Form for the eleven HSAM participants and the control group. The mean score for HSAM participants (8.09) was significantly greater than that for controls (5.21; Mann–Whitney post hoc analysis p < 0.047).
Fig. 3l shows the mean score on the Edinburgh Handedness Inventory for the eleven HSAM participants and the control group. The mean score for HSAM participants (31.96) was not significantly greater than that for the cognitive battery controls (66.42; Mann–Whitney p > 0.211). The mean score for HSAM participants (31.96) was significantly greater than that for MRI controls (89.27; Mann–Whitney p < 0.021, data not shown). It is worth noting that being left-handed may be over-represented in the HSAM population. The probability that at least five of the eleven HSAM participants would be non-right-handed is less than 1% (binomial probability, assuming a 10% base rate in the general population).
Fig. 3m shows the mean score on the Becks Depression Inventory II (BDI-II) for ten HSAM participants and the MRI control group. The mean score for HSAM participants (7.60) was not significantly greater than that for the MRI controls (2.68; Mann–Whitney analysis p > 0.136). HSAM participant and control means both fell within the minimal depression range (score of 0–13) as indicated by normative values.
3.2.1. Awareness of memory
The self-reported age at which the HSAM individuals became aware of their ability to remember events from most days of their life was 10.5 years old. On average, they became aware of their highly developed knowledge of dates relative to others at the age of 11.6.
3.2.2. Mental calendar
HSAM participants stated that they enjoy thinking about dates, dating events and going over them in their mind. All exhibited an extensive knowledge of the calendar. All could report leap years, patterns within the calendar, and the fact that the calendar repeats itself every 28 years. All could quickly and accurately give the day of the week for a given date, provided that the date fell within their lifetime. When questioned about the use of strategies to achieve the correct answer, participants responded in only one of two ways: First, and most commonly, they stated that the answer comes to them automatically (they “just know it”). Second, and much less commonly, they stated that they recalled a nearby date and/or memory for which the day of the week and date was known and deduced the answer. All, with the exception of one participant, either struggled in an effort to come up with the correct day of the week for a date outside their lifetime or could not arrive at it (indicating that there is no way they would know the answer as they did not experience the day). Only one participant did so seemingly automatically, stating he is unsure of how he achieves this feat and that he could do so even before he became aware of the calendric pattern. Notably, the dates he was able to arrive at the day of the week for, while remote, did not span across centuries outside of his lifetime as is typical of calendar calculators (see Section 4). The few others who have this ability use the second method mentioned above to arrive at their answer. Further, if the date is far-removed from their lifetime, they apply their knowledge of the 28- year calendar cycle and leap years to derive the answer. Invariably, when a date is outside their lifetime they tend to be uncertain of its accuracy.
HSAM participants view their knowledge of dates as highly important to their daily lives (rating this importance on average at an 8.3, 1 being not important, 10 being very important), but owning and using calendars is of little to no interest (on average rating the importance of calendars at a 4.7, 1 being not important, 10 being very important) and they rarely, if ever, study or memo- rize calendars (on average rating the amount they study calendars per week at a 1.1, 1 being they never study or memorize calendars, 10 being they study and memorize calendars every day). Thus, while an inherent interest does appear to play a role, intentionally studying/memorizing calendar structures/dates does not appear to explain either their extensive calendar knowledge or autobiographical memory.
3.2.3. Diaries
Three of the eleven HSAM participants kept diaries during some portion of their life. One participant (AJ) kept a detailed and extensive diary for most days of her life from the age of 10 to the age of 34 (described by Parker et al. (2006)). The “diary” of the second participant consists of two or three bullet points of events from the day, written on calendars, for nearly every day from her age of 13 to 38. The third kept a diary only in junior high school.
3.2.4. Obsessive tendencies
Nine of the eleven HSAM participants reported that they hoard items, need organization in their physical environment, and/or are germ-avoidant. These reported behaviors included a need to organize childhood toys, CD collections, movie collections, and/or articles of clothing in a precise and/or complicated order. They described their excessive collections of stuffed animals, newspapers, TV guides, CDs, mugs, and/or hats, along with an inability to discard these items. They expressed aversions to touching public doorknobs, restaurant utensils, items that are near or have touched the ground, and/or a need to wash their hands excessively. The Leyton Obsessional Inventory-Short Form (Mathews et al., 2004) indicated that HSAM participants express significantly more obsessional tendencies than controls (see Fig. 3j).
3.3. Results from structural MRI
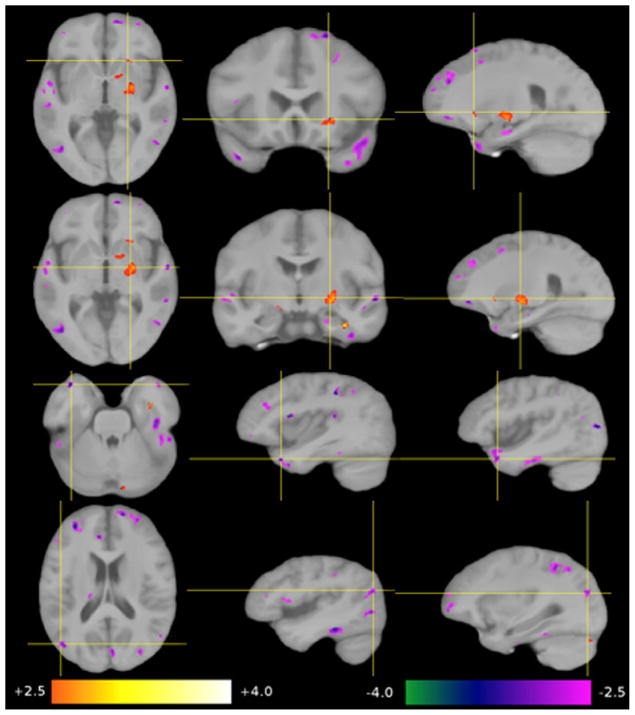
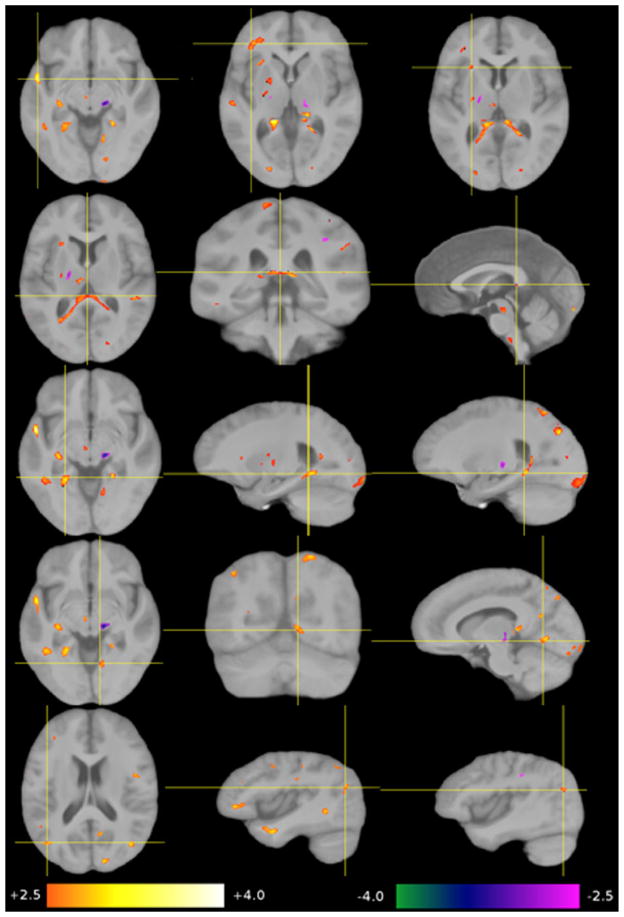
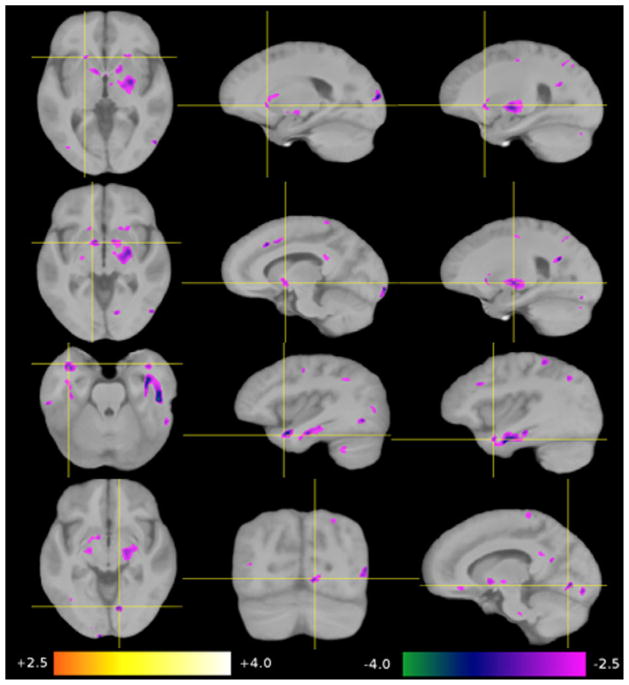
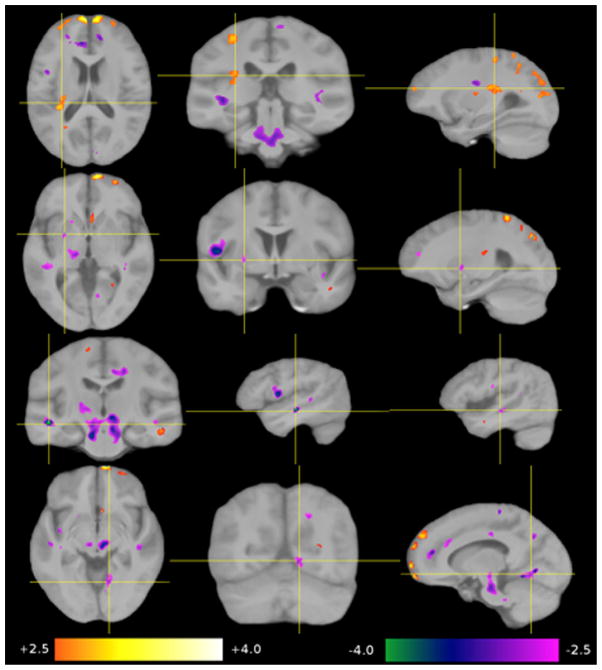
Nine regions-of-interest (ROI 1–9) were selected from the four analyses, voxel based morphometry-grey matter (VBM-GM), voxel based morphometry-white matter (VBM-WM), tensor based morphometry (TBM) and Diffusion Tensor Imaging-Fractional Anisotropy (DTI-FA). The first three methods revealed changes in size and shape in six of the nine regions while DTI-FA revealed an increase in white matter coherence in five of the nine regions (see Tables 1 and 2). Statistical maps were overlaid on the group-average image (template B). The significance of the group-related regressor is expressed as a z-score and color-coded (+2.5 orange to +4.0 white, −4.0 green to −2.5 magenta). Differences are shown for HSAM participants relative to controls (see Figs. 4–7). Due to the considerable inter-individual variability, and the local smoothing applied before generating the statistical maps, results have a limited spatial precision. Thus, we refer to this positional uncertainty by the term “in the vicinity of.” If we refer to a structure that is not directly visible in a given modality, we use the term “in overprojection with” (e.g., the arcuate fascicle). Because we are comparing two “healthy” groups here, we choose the term “contraction” instead of “atrophy,” and “expansion” instead of “growth” to describe group-related shape differences.
Table 1.
Regions of interest (ROIs) 1–9 showed the most consistent and meaningful group-related differences across the four analyses (VBM-GM, VBM-WM, TBM, and DTI-FA). Arrows indicate an increase (decrease) in the corresponding measure relative to controls. Left (L), Right (R), Bilateral (B) hemispheres.
| # | ROI | VBM-GM | VBM-WM | TBM | DTI |
|---|---|---|---|---|---|
| 1 | Uncinate Fascicle | – | – | – | L↑ |
| 2 | Forceps Major | – | – | – | B↑ |
| 3 | Parahippocampal Gyrus | – | – | – | B↑ |
| 4 | Posterior Insula | – | – | L↑ | – |
| 5 | Anterior Putamen & Caudate surrounding Anterior Limb of Internal Capsule | R↑ | B↓ | L↓ | – |
| 6 | Posterior Pallidum | R↑ | R↓ | – | – |
| 7 | Anterior & Middle Temporal Gyrus | B↓ | B↓ | L↓ | – |
| 8 | Lingual Gyrus | – | R↓ | R↓ | R↑ |
| 9 | Intraparietal Sulcus | B↓ | – | – | B↑ |
Table 2.
Compilation of anatomical group differences as determined by methods VBM-GM, VBM-WM, TBM, and DTI-FA. Each of the nine regions of interest (ROI) are specified along with their corresponding center in Talairach coordinates, their maximum z score (zmax), mean z score (zmean), and supra-threshold size in mm3 (Size), given for both hemispheres.
| # | ROI | Method | Left Hemisphere
|
Right Hemisphere
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | zmax | zmean | Size | Position | zmax | zmean | Size | |||
| 1 | Uncinate Fascicle | DTI | (−53, 5, −10) | 4.714 | 3.163 | 958 | – | – | – | – |
| 2 | Forceps Major | DTI | (0, −38, 10) | 4.138 | 2.914 | 2015 | (0, 32, 10) | 4.138 | 2.914 | 2015 |
| 3 | Parahippocampal Gyrus | DTI | (−26, −50, −5) | 3.633 | 2.940 | 416 | (22, −47, −6) | 3.499 | 2.851 | 444 |
| 4 | Post. Insula | TBM | (−34, −31, 21) | 3.656 | 2.818 | 1060 | – | – | – | – |
| 5 | Ant. Putamen & Caudate and Ant. Limb of Internal Capsule | VBM-GM | – | – | – | – | (22, 17, 0) | 2.833 | 2.617 | 103 |
| VBM-WM | (−12, 2, 2) | −3.096 | −2.735 | 695 | (14, 3, 2) | −2.917 | −2.648 | 468 | ||
| TBM | (−18, 13, 2) | −3.187 | −2.711 | 187 | – | – | – | – | ||
| 6 | Post. Pallidum | VBM-GM | – | – | – | – | (24, −13, 0) | 3.379 | 2.756 | 3866 |
| VBM-WM | – | – | – | – | (22, −13, 2) | −3.516 | −2.568 | 841 | ||
| 7 | Ant. & Middle Temporal Gyrus | VBM-GM | (−42, 17, −26) | −4.131 | −2.927 | 495 | (46, 16, −21) | −3.927 | −2.841 | 1658 |
| VBM-WM | (−42, 6, −21) | −4.229 | −2.953 | 856 | (46, −8, −21) | −4.681 | −2.903 | 3830 | ||
| TBM | (−51, −21, −8) | −3.187 | −2.711 | 187 | – | – | – | – | ||
| 8 | Lingual Gyrus | VBM-WM | – | – | – | – | (11, −78, −2) | −3.518 | −2.840 | 238 |
| TBM | – | – | – | – | (11, −71, −1) | −3.984 | −2.885 | 661 | ||
| DTI | – | – | – | – | (10, −69, −4) | 4.006 | −2.861 | 372 | ||
| 9 | Intraparietal Sulcus | VBM-GM | (−46, −77, 22) | −3.736 | −2.894 | 357 | (32, −84, 22) | −3.620 | −2.903 | 164 |
| DTI | (−44, −74, 24) | 3.981 | 2.908 | 277 | (40, −75, 22) | 3.556 | 2.896 | 122 | ||
Fig. 4.
Significant group-related differences in GM concentrations (VBM-GM). Top row: a lower GM concentration in the vicinity of the right anterior ventral putamen, the anterior limb of the internal capsule and caudate. Second row: a higher GM concentration in the right posterior pallidum. Third row: a lower GM concentration in the anterior portions of the middle temporal gyrus on both sides, also called temporal tip/BA 38. Bottom row: a lower GM concentration in the banks of the posterior intraparietal sulcus in both hemispheres (BA7).
Fig. 7.
Significant group-related increases in FA (DTI-FA). Top row: in the left uncinate fascicle, as seen in white matter of the left superior temporal gyrus, the inferior frontal gyrus, and the left anterior external capsule. Second row: in the forceps major, traversing the mid-sagittal plane through the splenium of the corpus callosum. Third row: in the WM supplying the parahippocampal gyrus on both sides. Fourth row: in the right lingual gyrus. Bottom row: in the vicinity of intraparietal sulcus on both sides.
3.3.1. VBM-GM
Four regions differed in GM concentration in the HSAM participants compared to controls (see Table 1 and Fig. 4). A higher GM concentration was detected in the vicinity of the right anterior putamen and caudate surrounding the anterior limb of the internal capsule (ROI 5) and in the right posterior pallidum (ROI 6). A relative lower GM concentration was found for bilateral regions of the anterior pole and the adjacent middle temporal gyrus on both sides (ROI 7) and bilateral regions at the banks of the intraparietal sulcus on both sides (ROI 9).
3.3.2. VBM-WM
Four regions were found with a lower WM concentration in the HSAM participants (see Table 1 and Fig. 5): at both sides of the anterior limb of the internal capsule (ROI 5), the right posterior pallidum (ROI 6), the anterior pole and the adjacent middle temporal gyrus on both sides (ROI 7), and the right lingual gyrus (ROI 8).
Fig. 5.
Significant group-related lower WM concentrations (VBM-WM). Top row: in several small bilateral regions in vicinity of the anterior putamen, caudate and anterior limb of the internal capsule, in correspondence with regions in Fig. 4, top. Second row: in the posterior pallidum on both sides, in correspondence with regions in Fig. 4, second row. Third row: in the bilateral anterior portions of the middle temporal gyrus. Bottom row: in the right lingual gyrus.
3.3.3. TBM
Four regions with significant shape differences were found in the HSAM participants (see Table 1 and Fig. 6). A region of local expansion was detected in the left posterior insula, presumably in overprojection with the arcuate fascicle (ROI 4). A relative contraction was detected in the anterior limb of the internal capsule of the left side (ROI 5), adjacent areas in the temporal WM on both sides (ROI 7), and the right lingual gyrus (ROI 8).
Fig. 6.
Significant group-related shape differences (TBM). Top row: a local expansion of the posterior insula in overprojection with the arcuate fascicle. Second row: a local contraction of the left anterior putamen and the anterior limb of the internal capsule. Third row: a local contraction of middle portion of the middle temporal gyrus (left > right) in the depths of the superior temporal sulcus. Bottom row: a local contraction of the right lingual gyrus.
3.3.4. DTI-FA
Five regions of increased FA were detected in the HSAM participants (see Table 1 and Fig. 7): in the WM of the left anterior temporal lobe, external capsule and fronto-basal WM, all in overprojection with the uncinate fascicle (ROI 1), occipito-occipital fibers traversing the mid-sagittal plane through the splenium of the corpus callosum (ROI 2), the WM supplying the parahippocampal gyrus on both sides (ROI 3), the right lingual gyrus (ROI 8), and at the banks of the intraparietal sulcus in both hemispheres (ROI 9).
4. Discussion
4.1. Background and behavioral observations
This investigation identified and studied, both behaviorally and neuroanatomically, a group of individuals who have HSAM. Our findings have revealed commonalities among HSAM participants in both behavioral and neuroanatomical domains. Additionally, some light has been shed on what may and may not enable HSAM participants to achieve such remarkable levels of autobiographical and public event recall.
Our testing procedures, as well as the formal AMT, have verified that all HSAM participants not only have a rich personal autobiographical event memory, but also have an extensive repertoire of public event information, all of which are tied to dates. Public event information is generally stored as concept-based knowledge, in which the information remembered is unrelated to specific autobiographical experiences. HSAM participants’ public event information seems to be tied to specific autobiographical experiences; they typically know the date and where they were when they learned about the public event. Westmacott and Moscovitch (2003) have speculated that semantic concepts associated with autobiographical experiences are given a distinct status in long-term memory. They emphasize an interaction and overlap between the two memory systems, theorizing that autobiographical significance (the association of a concept with contextual episodic detail and specific personal memories) contributes to the content and organization of semantic memory, ultimately leading to the enhancement of semantic recall. It is in the realm of possibility that a majority of HSAM participants’ concept knowledge is tied to personally relevant events, resulting in the enhancement of their public event knowledge and a blurring between the distinction of concept knowledge that is truly semantic and concept knowledge that is more episodic and contextual in nature.
HSAM participants are not “calendar calculators.” None of the participants possess the rare ability, found in some autistic savants, to readily specify the day of the week for dates spanning across centuries. Such savants are often described as being extremely interested in using and memorizing calendars as well as socially withdrawn and unskilled at communicating with others (for review see Howe & Smith, 1988). In contrast, HSAM participants have a limited interest in using and/or memorizing calendars, are socially adept and easily make eye contact/appropriate conversation. Further, for the typical HSAM participant, the range of dates for which the days of the week “just come to them” automatically and effortlessly (along with details of what happened on that day), is limited to dates within their lifetime, and in particular after around the age of ten (our one exception, although the dates are outside his/her lifetime, they do not span across centuries). This calendric ability is a unique and defining characteristic of the HSAM population. We speculate that this ability allows for the application of a temporal order to their memory, an organization that possibly facilitates the retrieval of details from their daily life.
As first described by Parker et al. (2006) with patient A.J. and further documented here, HSAM participants tend to exhibit a degree of obsessive-like behavior. However, how/whether these tendencies contribute to their highly superior autobiographical memory abilities remains unclear. The diagnostic criterion for obsessive–compulsive disorder (OCD) includes recurrent ideas, thoughts, impulses or images (obsessions) that are experienced as intrusive, cause marked anxiety and interfere with a person’s daily function. Repetitive behaviors or mental acts (compulsions) are performed in order to decrease distress associated with these obsessions (American Psychiatric Association, 2000). Although HSAM participants have yet to undergo clinical diagnosis of OCD, the LOI-SF has pointed to non-mnemonic OCD symptoms of intrusive behavior. However, HSAM participants typically do not view their memories as excessively intrusive, persistent and/or unwanted or as disruptive of their daily life (rating an average of 3.5 out of 10, 1 indicating their memories are not intrusive, and 10 indicating they are highly intrusive). Their memory does not distract them from ongoing tasks, nor does it hinder their ability to plan future ones (rating an average of 2.7 and 2.6, respectively, 1 being no difficulty staying present/thinking about the future and 10 being high levels of difficulty staying present/thinking about the future). As a group they view their autobiographical memory ability as a positive attribute (rating an average of 8.4, 1 being negative and 10 being positive). Thus, while they have frequent recollections of past events, it does not appear, in a clinical sense, that their memory qualifies as a true obsessive characteristic.
There are, however, a few indications that their autobiographical memory abilities and obsessive tendencies might be linked to some degree. Nine HSAM participants reported that they organize their memories chronologically or by categories (e.g., knowing which event occurred on which date or knowing all the times they have been to a particular restaurant), allowing for a mental organization over the high number of memories they can recall. They also reported habitually recalling their memories, a seemingly compulsive tendency. Every night before bed one participant recalls what occurred on that day X number of years ago. Another recalls, while stuck in traffic, as many days possible from a certain year. Another wakes up every morning and reviews whether there are any upcoming anniversaries or birthdates to be congratulated. Three (of these nine) HSAM participants reported intentionally documenting their memories as a means of coping with the vast number they can remember. One claimed to be “obsessed with writing things down” as a means of off-loading memories/thoughts from his/her mind. Another voiced that writing allows him/her to “process the memories better.” The third “feels an obsession/compulsion to keep it [the memories] fresh.” The other six reported habitually recalling their memories as a way of lulling themselves to sleep, a pastime, or as a means of staying on top of important events. We should note that the habitual recall of their memories is not done in some effort to intentionally rehearse them as a means to improve them. Rather, external or internal cues will often automatically lead to retrieval of the information. In and of itself, this is not at all irregular (as this is a fundamental process of memory). However, for them, there is an incredible abundance of detailed autobiographical information available to be cued, without substantial effort.
Intentional rehearsal does not appear to be the sole means by which HSAM participants achieve their rich repertoire of memories, though rehearsal may certainly contribute to the preservation of information acquired. Instead, they appear to have some inherent ability to retain and retrieve vast amounts of public and autobiographical events, well beyond what one may expect from simple rehearsal.
Although HSAM participants were unequivocally superior in the recall of autobiographical events, they were no different in their performance on digit-span forward, verbal-paired associates and visual reproduction. These results are important because they support the premise that HSAM is specific to the domain of recalling events that are autobiographical in nature, as opposed to having strong memory generally. The recollection of laboratory based, to-be-remembered, materials should not be considered comparable to the recollection of events from a participant’s life. This notion is supported by the substantial differences in patterns of neuronal activation found in studies comparing episodic memory for laboratory tasks to autobiographical memory of past experiences (Gilboa, 2004; McDermott, Szpunar, & Christ, 2009). In contrast to their performance on the tasks mentioned above, HSAM participants performed significantly better than controls on the Logical Memory Test free-recall and Names-to-Faces task. Although there was no explicit report of the use of strategies, a relevant mnemonic could have been applied in both cases. The Logical Memory Test free-recall and Names-to-Faces task both have clear links to autobiographical memory; recalling details from a short fictitious narrative of another’s life is similar to recalling details from one’s own life. It is possible HSAM participants are applying the same strategies to acquire significantly more details in both cases. In relation to the Names-to-Faces task, connecting a name to a face is a rather important social tool and a common autobiographical occurrence.
HSAM participants’ performance on the backward, but not forward, digit-span task approached being statistically different. Informal questioning after these tests revealed the tendency of HSAM participants to use date-related mnemonics for the more challenging backwards digit span task (e.g., “4, 1, 8, 3, 9” becomes April 18 and March 9), while applying no mnemonics to the forward digit span task. The backwards task was described (as it often is) as being particularly challenging, which may have led them to adopt such a strategy. Controls depended on short-term memory for forward presented digits and attempted to mentally reverse the numbers when asked to repeat the digits in reverse order.
The performance of the HSAM individuals on the formal behavioral tests suggests that, in the main, they do not possess a domaingeneral, highly effective ability to encode and retrieve new information. Instead, it is more domain-specific as even those tests in the cognitive battery in which they outperform controls can be viewed in a personal, autobiographical manner. Whether this is related to the way in which they initially represent the information (e.g., a particularly effective encoding scheme for autobiographical information that does not generalize to other domains) or whether it is the result of different post-encoding processes remains to be determined. In terms of further characterizing HSAM a more comprehensive battery assessing other cognitive functions, such as executive/motor functioning and/or intellectual ability would be useful.
4.2. Discussion of anatomical results
Four standard structural imaging analyses were applied in this study: three complementary methods for quantifying grey and white matter structure (VBM-GM, VBM-WM and TBM) and one distinct method for assessing white matter alone (DTI-FA). We identified nine brain regions that differed most consistently and meaningfully across analyses in the HSAM participants as compared to controls in terms of grey matter/white matter concentration, regional shape, or white matter tract coherence (see Tables 1 and 2 and Figs. 4–7). While our analyses identified directionality of change among the specified regions, we refrain from interpreting it. Concluding that higher GM concentrations in healthy adults correlate with improvements in behavior or memory is tempting, but conclusive evidence on this topic has yet to be demonstrated (e.g. Van Petten, 2004). Here we highlight the network of regions that were identified as morphologically different and discuss their possible relevance to HSAM participants’ memory abilities and obsessional tendencies.
Anatomical analyses revealed structural differences in the region of the inferior and middle temporal gyri and temporal pole (BA 20, 21 and 38, respectively), the anterior insula, and the parahippocampal gyrus, (BA 36) of the HSAM participants. These regions have been identified both through a comprehensive meta-analysis (Svoboda, McKinnon, & Levine, 2006) and a number of neuroimaging studies (Fink et al., 1996; Steinvorth, Corkin, & Halgren, 2006; Andreasen et al. 1995; Gilboa, 2004; Levine et al., 2004; Maguire, 2001; Markowitsch, 1995) as contributing to a hypothesized autobiographical memory network. This is certainly consistent with the phenomenal autobiographical memory performance of the HSAM participants. The right temporal pole (BA 21,BA 38) and medial temporal gyrus (BA 21) in addition to the parahippocampus and right anterior insula have been shown, using PET, to be primarily engaged during affect-laden autobiographical memory ecphory (cued recovery of a past event eliciting an imagined image or representation of the information; Fink et al., 1996). This parallels HSAM participants’ extensive memory database, which seemingly retains a high number of retrieval cues stimulating the recollection of vivid and often emotional memories. Patients with lesions to the left medial temporal region and the bilateral middle temporal gyri demonstrated retrograde memory loss, for both public and personal autobiographical facts and events (Barr, Goldberg, Wasserstein, & Novelly, 1990; Kapur, Ellison, Smith, McLellan, & Burrows, 1992). Thus it seems likely that HSAM participants’ highly reliable and richly detailed recollection of personal as well as public events could depend in some manner on these regions.
Anatomical analyses revealed structural differences in the region of the inferior parietal sulcus. This region is thought to be involved in attention mediated perceptual binding, allowing for the rich array of interrelated elements, that make up an episodic memory, to be conjoined into a common memory representation (Uncapher, Otten, & Rugg, 2006). Furthermore, the inferior parietal lobule is a core region associated with the brain’s so called “default mode network,” displaying activation during tasks, such as autobiographical memory retrieval, in which the individual’s focus is internal (Buckner, Andrew-Hanna, & Schacter, 2008). Also, it is commonly recruited during the construction and elaboration of past and future events (Addis, Wong, & Schacter, 2007). Taking these elements into consideration, it is plausible that this region is contributing to HSAM participants’ exceptional autobiographical memory.
The white matter findings from the present study are particularly intriguing. Increased DTI-FA indicates amplified white matter tract coherence, suggesting greater efficiency in the transfer of information between connecting brain regions (Wahl et al., 2007; Yasmin et al., 2008). Further, studies have indicated possible connections between increased FA values and behavior, demonstrating positive correlations between surpluses/deficits in FA values and behavioral improvements/shortfalls in both episodic memory and training-related domains (Fujie et al., 2008; Scholz et al., 2009).
A majority of the brain regions exhibiting increased FA values in the HSAM participants have been identified in previous lesion, structural imaging and/or functional imaging studies as being critical to autobiographical encoding and/or retrieval. Thus, it might be that a more robust white matter tract system may contribute to HSAM participants’ autobiographical memory abilities. DTI analysis revealed increased FA in three portions of the uncinate fascicle, a tract creating a cortico-cortical loop connecting the postero-lateral temporal and ventrolateral frontal cortices, from the temporal pole to the orbital gyri (Kier, Staib, Davis, & Bronen, 2004). As proposed by Markowitsch (1995), the uncinate fascicle guides and channels information flow to the prefrontal cortex and transmits preprocessed information back to the temporal cortex for final representation. Its centrality to the recall of declarative information from long-term memory storage has been inferred from cases of patients suffering from principally retrograde, as opposed to anterograde, memory disturbances after combined temporo-polar and ventrolateral prefrontal damage (Kapur et al., 1992; Markowitsch et al., 1993). Levine et al. (1998) proposed its preferential involvement in the retrieval of autobiographical information and its possible contribution to autonoetic awareness following the examination of the memory of a patient suffering traumatic brain injury to the uncinate fascicle and surrounding areas. Steinvorth et al. (2006), observed preferential activation in the two regions conjoined by the uncinate fascicle, the orbital gyrus and middle temporal gyrus by means of fMRI of healthy adults performing an autobiographical ecphory task. Fujie et al. (2008) acquired DTI on amnestic mild cognitive impairment (aMCI) patients and revealed a strong correlation between a decrease in FA of the uncinate fascicle and a decrease in episodic memory performance on a verbal memory task. Finally, Schott et al. (2011) using a combination of fMRI and DTI of healthy adults demonstrated that the uncinate fascicle links active regions of the vl-PFC to the MTL at encoding and that its density is positively correlated with free-recall performance. The strength of the anatomical connectivity of the UF between the PFC and MTL could ostensibly contribute to interindividual differences in episodic memory performance of healthy humans. Although the procedure does not specifically test ecphory or autonoetic awareness, its implication for the contribution of the uncinate fascicle to HSAM is clear. In relation to HSAM participants, the correlation between individual FA values of the uncinate fascicle and autobiographical memory performance, on a test sensitive enough to detect gradations in autobiographical memory ability, would provide valuable insight into the contribution of the uncinate fascicle to HSAM.
The caudate and lentiform nucleus have been associated with skills and habit memory (Packard & Knowlton, 2002; Poldrack & Packard, 2003) as well as OCD (Radua & Mataix-Cols, 2009). While these regions have been associated with simple memory recognition (McDermott et al., 2009), less is known about their relation to autobiographical- or recollection-based memory. Thus, our present findings may be more so the result of the apparent obsessive tendencies and memory habits of the HSAM participants. Patients with OCD have been previously shown to have a tendency towards increases in regional grey matter volumes in bilateral lenticular nuclei (specifically the anterior putamen, extending to the caudate nuclei) (for meta-analysis see Radua & Mataix-Cols, 2009). While we are hesitant to ascribe a definite link due to the lack of clinical diagnoses of HSAM participants with OCD, we do propose the possibility of common underlying mechanisms. Findings of morphological differences in the anterior putamen, caudate and posterior pallidum and behavioral trends towards obsessive tendencies as well as significantly higher LOI-SF scores have lead us to this line of thought.
The data do not, of course, allow the conclusion that all regions detected in our analyses contribute to autobiographical memory or the autobiographical memory network. However, it is striking that many regions identified as structurally different in our HSAM participants overlap with regions that have been implicated in previous autobiographical memory studies (see neurosynth.org, Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). Such findings suggest that structural changes found in the HSAM population may contribute to the more efficient use of the same “hardware.” It is not known of course, whether the anatomical differences observed in our analyses are enabling or resulting from HSAM participants’ memory performance. Subsequent research on children with HSAM may help to answer this important question.
Acknowledgments
This research was supported by NIH 1RO1 AG034613 (C.E.LS.), the Gerard Family Trust (J.L.M), Unither Neurosciences, Inc. (J.L.M) NIH 5RO1 MH12526 (J.L.M).
We wish to give a very special thank you to Nancy Collett and our team of undergraduate researchers whose ideas, dedication and support greatly facilitated the progress of this research project. We are also incredibly appreciative of the HSAM as well as control participants who opened their lives to us and devoted themselves so enthusiastically to this project.
Appendix A. Autobiographical memory test
A.1. Examples illustrating verification process
A subject recalled that their first day of college fell on Wednesday, September 6th, 1978. Using the individual’s college transcripts the date of matriculation was verified. In this example 4 points were assigned: 1 point for the day, Wednesday; 1 point for the month, September; 1 point for the day of the month the 6th; 1 point for the year, 1978.
A subject, recalling their first night in their own apartment, described the building as a brownstone, with exposed brick. A search of the address using Google Maps revealed a street level picture of the building confirming the participant’s description. Thus a point was added to the score for this correct detail.
A subject recalled the temperature during his 18th birthday, was about 55–60 degrees, and overcast. Using historical weather databases, one point was awarded after confirming that was the correct weather range during that day in that region of the world. A subject recalled a dinner event on the last day of college. The date and name of the particular restaurant was verified using the subject’s personal diaries, which listed the date and description of the events of the day. Two points were awarded; 1 point for the memory of the dinner; the 2nd point for the name of the restaurant.
A subject described an outfit worn on her first day of college as an orange, burlap wraparound. The school newspaper took a cameo photograph of the subject on the first day of class that was featured in the paper. Two points were awarded for fabric and style, color could not be confirmed because the paper was printed in black and white.
Appendix B. Visual memory
B.1. Instructions to subjects
“I’m going to present you with a picture. After I cover it up, I’m going to ask you a series of questions about the picture.”
B.2. Image

Questions. (Marks, 1973):
-
“What number was written on the golf ball?”
Prompt: four, five or six?
-
“What was in the bottom right-hand corner?”
Prompt: clock, scissors, or siphon?
-
“What was the time on the clock?”
Prompt: ten to seven, ten to ten or ten to four?
-
“What was the ballerina standing on?”
Prompt: tiptoes, with one foot on the floor, or with both feet on the floor?
-
“What was directly below the suitcase?”
Prompt: bicycle, candle, or books?
Appendix C. Logical Memory Test
C.1. WMS-III instructions to subjects
“I am going to read a short story to you. Listen carefully and try to remember it just the way I say it, as close to the same words as you can remember. When I am through, I want you to tell me everything I read to you. You should tell me all you can remember even if you are not sure. Are you ready?”
Subject listens to story
“Tell me everything you can remember about this story. Start at the beginning”
C.2. Story a narrative
“Anna Thompson of South Boston, employed as a cook in a school cafeteria, reported at the police station that she had been held up on State Street the night before and robbed of fifty-six dollars. She had four small children, the rent was due, and they had not eaten for two days. The police touched by the woman’s story, took up a collection for her.”
C.3. Examples of questions
“Was the woman’s name Diana Thompson?”
“Was the story setting in South Boston?”
“Was the woman a cook?”
“Did she work in a restaurant?”
Appendix D. Verbal paired associates
D.1. Original list order
Truck – Arrow
Insect – Acorn
Reptile – Clown
Bank – Cartoon
Star – Ladder
Raccoon – Paper
Rose – Bag
Elephant – Glass
D.2. WMS III instructions to subjects
“I am going to say a word and then say another word that goes with it. I will say a whole list of words like that. Listen carefully because when I am finished I will say the first word, and I want you to tell me the word that goes with it. For example, if the word pairs were Fruit-West, Gold-Walk, then when I say the word Fruit, you would answer (pause) West. When I say the word Gold, you would answer (pause) Walk. Do you understand?”
D.3. Recall/response
Bank (Cartoon)
Reptile (Clown)
Star (Ladder)
Rose (Bag)
Elephant (Glass)
Truck (Arrow)
Insect (Acorn)
Raccoon (Paper)
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. p. 943. text rev. [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, et al. Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphomentry – the methods. Neuro Image. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28(3):243–255. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. In: Diffusion MRI: from quantitative measurement to in vivo neuroanatomy. Johansen-Berg H, Behrens TEJ, editors. London: Academic Press; 2009. p. 576. [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. The Journal of Neuroscience. 2007;27(38):10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. The Journal of Neuroscience. 2008;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrew-Hanna JR, Schacter DL. The brain’s default network anatomy, function, and relevance to disease. Annals of the New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Paus T, Cherif C, Colins DL, Giedd JN, et al. A unified statistical approach to deformation-based morphometry. Neuro Image. 2001;14:595–606. doi: 10.1006/nimg.2001.0862. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn GH, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson KA, Delaney PF, Weaver G, Mahadevan R. Uncovering the structure of a memorists superior “basic” memory capacity. Cognitive Psychology. 2004;49:191–237. doi: 10.1016/j.cogpsych.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Fillard P, Pennec X, Arsigny V, Ayache N. Clinical DT-MRI estimation, smoothing, and fiber tracking with long-Euclidean metrics. IEEE Transactions on Medical Imagining. 2007;26(11):1472–1482. doi: 10.1109/TMI.2007.899173. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. The Journal of Neuroscience. 1996;16(13):4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1(3):210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fujie S, Namiki C, Nishi H, Yamada M, Miyata J, Sakata D, et al. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2008;26:432–439. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory – One and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Gordon P, Valentine E, Wilding J. One man’s memory: A study of a mnemonist. British Journal of Psychology. 1984;75:1–14. [Google Scholar]
- Hentschel S, Kruggel F. Determination of the intracranial volume: A registration approach. In: Yang G-Z, Jiang T, editors. International workshop on medical imaging and augmented reality (MIAR). Lecture notes in computer science. Berlin: Springer; 2004. pp. 3150pp. 253–260. [Google Scholar]
- Howe MJ, Smith J. Calendar calculating in ‘idiots savants’: How do they do it? British Journal of Psychology. 1988;79:371–386. doi: 10.1111/j.2044-8295.1988.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Hunt E, Love T. How good can memory be? In: Melton AW, Martin E, editors. Coding processes in human memory. Washington, DC: Winston-Wiley; 1972. pp. 237–260. [Google Scholar]
- Kapur N, Ellison D, Smith MP, McLellan DL, Burrows EH. Focal retrograde amnesia following bilateral temporal lobe pathology. A neuropsychological and magnetic resonance study. Brain. 1992;115:73–85. doi: 10.1093/brain/115.1.73. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR American Journal of Neuroradiology. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Kruggel F, von Cramon DY. Alignment of magnetic-resonance brain datasets with the stereotactical coordinate system. Medical Image Analysis. 1999;3(2):175–185. doi: 10.1016/s1361-8415(99)80005-x. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, Mcintosh AR, Toth JP, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Luria AR. The mind of a mnemonist: A little book about a vast memory. Cambridge: Harvard University Press; 1968. [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London, Series B. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Research Reviews. 1995;21:117–127. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Calabrese P, Liess J, Haupts M, Durwen HF, Gehlen W. Retrograde amnesia after traumatic injury of the fronto-temporal cortex. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:988–992. doi: 10.1136/jnnp.56.9.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DF. Visual imagery differences in the recall of pictures. British Journal of Psychology. 1973;64:17–24. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Jang KL, Hami S, Stein MB. The structure of obsessionality among young adults. Depression and Anxiety. 2004;20(2):77–85. doi: 10.1002/da.20028. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Morris PE, Jones S, Hampson H. An imagery mnemonic for the learning of people’s names. British Journal of Psychology. 1978;69:335–336. [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Parker ES, Cahill L, McGaugh JL. A case of unusual autobiographical remembering. Neurocase. 2006;12(1):35–49. doi: 10.1080/13554790500473680. [DOI] [PubMed] [Google Scholar]
- Pohl RF, Bender M, Lachmann G. Autobiographical memory and social skills of men and women. Applied Cognitive Psychology. 2005;19:745–759. [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder. The British Journal of Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niklas C, Kaufmann J, Bodammer NC, Machts J, Schütze H, et al. Fiber density between rhinal cortex and activated ventrolateral prefrontal regions predicts episodic memory performance in humans. PNAS. 2011;108:5408–5413. doi: 10.1073/pnas.1013287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuro Image. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: An fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between the hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vercauteren T, Pennec X, Perchant A, Ayache N. Diffeomorphic demons: Efficient non-parametric image registration. Neuro Image. 2009;45(1):56–571. doi: 10.1016/j.neuroimage.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. The Journal of Neuroscience. 2007;27(45):12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Memory & Cognition. 2003;31(5):761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Wilding J, Valentine E. Superior memory. Hove, East Sussex: Psychology Press; 1997. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, et al. Diffusion abnormalities of the uncinate fasciculus in Alzheimer’s disease: Diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50:293–299. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]