Abstract
Aging is a degenerative process resulting in compromised tissue maintenance and increased susceptibility to diseases, such as cancer. Recent advancements support the notion that aging is a highly regulated process governed by evolutionarily conserved pathways. In mammals, tissue-specific adult stem cells (ASCs) persist throughout the lifetime to maintain and repair tissues. While reduced ASC self-renewal is thought to contribute to compromised tissue maintenance, increased self-renewal of cancer stem cells (CSCs) may lead to tumorigenesis. It is speculated that genetic regulators of aging, such as sirtuins, are likely to impinge upon the ASC compartments to regulate tissue maintenance and tumorigenesis. In this review, we discuss the emerging evidence linking sirtuins to normal and malignant ASC self-renewal, tissue maintenance, and tumorigenesis.
Keywords: sirtuin, hematopoietic stem cell (HSC), leukemia, leukemic stem cell (LSC), chronic myelogenous leukemia (CML)
Introduction
Loss of homeostasis and decreased ability to regenerate after injury are hallmarks of aging. Tissue homeostasis and regeneration after injury are maintained by rare populations of tissue-specific adult stem cells (ASCs). ASC function decreases with age, contributing to the loss of tissue homeostasis. It is speculated that genetic regulators of aging, such as sirtuins, are likely to function in the ASC compartment to modulate tissue maintenance. Here, we review the regulation of sirtuins in ASC self-renewal and tissue homeostasis with a focus on the hematopoietic system (Fig. 1) and discuss how this regulation is hijacked by cancer stem cells (CSCs) to drive cancer development and their resistance to therapies (Figs. 1 and 2).
Figure 1.
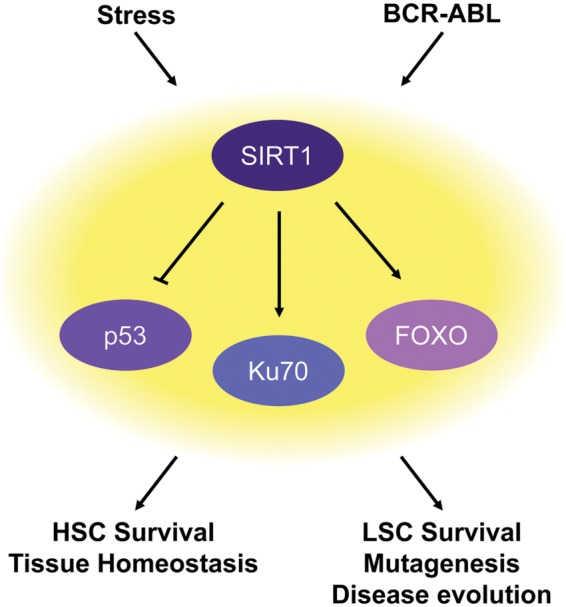
SIRT1, tissue maintenance, and tumorigenesis. SIRT1 mediates the stress response in hematopoietic stem cells (HSCs) and promotes HSC survival and tissue homeostasis. This stress response pathway is hijacked by leukemic stem cells (LSCs) and promotes LSC survival and disease evolution.
Figure 2.
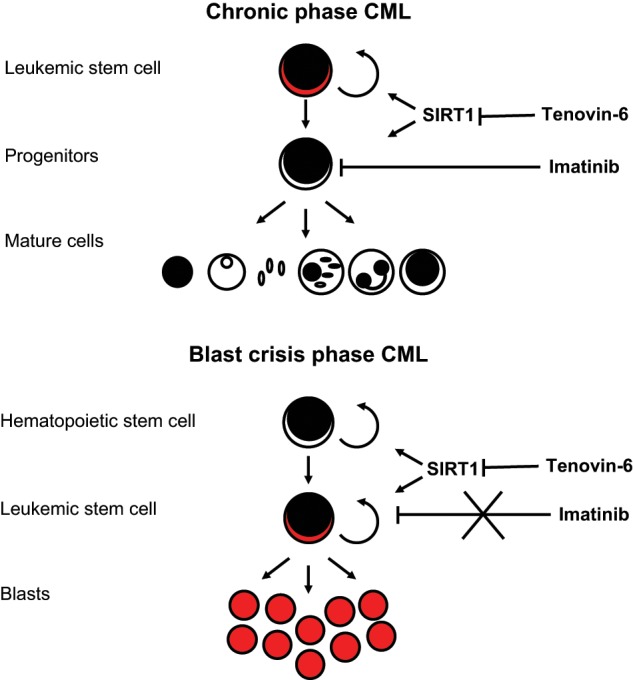
SIRT1 as a therapeutic target for chronic myelogenous leukemia (CML). CML is supported by leukemic stem cells (LSCs) that produce the nontumorigenic cells that make up the bulk of the tumor. SIRT1 is active in LSCs during both chronic and blast crisis phases of CML. Imatinib, a tyrosine kinase inhibitor (TKI), can control CML by targeting the differentiated cancer cells. However, it does not affect CML LSCs. A combination of TKI treatment plus inhibition of SIRT1 can effectively eradicate CML LSCs.
Aging and Conserved Regulators of Aging
Aging is a fundamental process of life associated with the decline of cellular and tissue functions over time. When homeostasis and repair are reduced to the point that cellular and tissue integrity and function are lost, physiological decline begins, and aging is manifested.1 Thus, the gradual and complex process of aging can be considered the outcome of an imbalance between damage and repair.2 Supporting this explanation, many of the diseases of the elderly—osteoporosis, anemia, and sarcopenia—result as an imbalance between tissue loss and replacement.1 Aging is closely associated with increased disease susceptibility and risk of mortality.3 In fact, old age is the greatest risk factor for many diseases. Understanding how we age may allow us to slow the aging process and thus limit the prevalence of many age-associated diseases all at once.
As many cellular processes change and decline with age, one of the most challenging tasks in understanding aging is to parse the causes of cellular and tissue aging from the many changes that accompany it. The daunting complexity of aging originally led people to believe that the process of aging was passive and entropic. However, we now know that, like other cellular processes, the process of aging is regulated by signaling pathways and transcription factors.4 The original hint that aging might be a regulated process came from the observation that different species have vastly different life spans, suggesting that aging could be genetically regulated.5 Work with model organisms like Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster has confirmed this and provided evidence that genetic and environmental interventions can extend the life span as well as health span, which is the period of life when one is generally healthy and free of chronic illnesses.
Many of the pathways identified in model organisms to extend the life span, and thus regulate aging, are involved with nutrient-sensing or stress responses. Under conditions of plenty, when food is readily available and stress levels are low, these pathways promote reproduction and growth. However, under stressful conditions, where food is scarce and conditions are harsh, these pathways change their activity and promote cell protection and maintenance instead. It is thought that the mutation of genes involved in these pathways can increase longevity because they activate pathways that protect cells from stress. Dietary restriction (DR), a reduction in food intake without malnutrition, is one environmental intervention shown to increase the life span across many different species.6 DR was originally believed to enhance longevity by reducing cellular respiration and limiting the rate at which cellular damage accumulated. However, it is now clear that the longevity response associated with DR is regulated by various nutrient-sensing pathways, such as the target of rapamycin (TOR), AMP kinase, sirtuins, and insulin/insulin-like growth factor (IGF-1) signaling pathways.4 These nutrient-sensing pathways control cellular stress response pathways including DNA damage, proteostasis, autophagy, as well as mitochondrial function, redox, and metabolism and have emerged as regulators of aging, with their function being conserved across many different species.2
Sirtuins
Sirtuins link the metabolic state of the cell to stress response pathways and thus age-related phenotypes. Sirtuins are proteins with deacetylase and/or ADP-ribosyltransferase activities that require the cellular nutrient nicotinamide adenine dinucleotide (NAD+) to perform their functions.7-9 This requirement for NAD+ allows sirtuins to sense the cellular metabolic state and tailor their activity to the needs of the cell. Silent information regulator 2 (SIR2), the founding member of the sirtuin family, was originally identified in S. cerevisiae. Under DR or fasting conditions when NAD+ levels are high, SIR2 activity is increased; conversely, under nutrient-rich conditions when NAD+ levels are low, SIR2 activity is limited.10-12 Expression of SIR2 was found to have an inverse relationship with a replicative life span. Overexpression of SIR2 increased the number of divisions a mother yeast cell could complete, thus slowing aging, while deletion of SIR2 decreased the number of divisions and shortened the life span.13 The life span extension activity of SIR2 is conserved across other model organisms, including C. elegans, D. melanogaster, and Mus musculus.14-16 However, the role of sirtuins in longevity regulation has recently become hotly debated.17,18
There are 7 mammalian SIR2 homologs (SIRT1-SIRT7) that are found in various cellular compartments. SIRT1, the mammalian ortholog of SIR2, is upregulated in some tissues of mice undergoing fasting or DR but is downregulated in mice on a high fat diet.19-23 Moreover, SIRT1 expression decreases with advanced age.24 SIRT1 is considered a promiscuous deacetylase and has a wide array of targets in the nucleus and cytoplasm. When active, SIRT1 can deacetylate histones and different groups of proteins that are involved in stress response and repair: transcription factors, signaling factors, and DNA repair proteins.25 p53 was the first nonhistone target of SIRT1 to be identified.26-28 SIRT1 also deacetylates many other proteins involved in cellular stress responses, including Ku70, Forkhead box subgroup O (FOXO) proteins, and nuclear factor κ B (NF-κB) among others.25 Through its deacetylation activity, SIRT1 can regulate protein activity and gene expression and rapidly initiate many prosurvival and stress responses under stress conditions.
Adult Stem Cells
In adult animals, tissue homeostasis is maintained by tissue-specific ASCs that can produce all the cell types necessary for tissue function and have the ability to replace those cells when they are lost due to injury or wear and tear.29 In addition to being able to generate all the mature effector cells of the tissue, ASCs also have the ability to self-renew, allowing for replenishment of the ASC pool.30 Because most of the effector cells of tissues are short lived and are turned over regularly throughout life, ASCs must constantly function to replace them to avoid tissue atrophy or aplasia.
ASCs are exposed to the same factors as those that lead to age-related changes in their postmitotic progeny. However, because ASCs are essential for maintaining tissue homeostasis over a lifetime, they must resist the formation and accumulation of damage.31 Many cell extrinsic and intrinsic mechanisms work in concert to protect ASC integrity, including expression of transporter proteins that can efflux toxins, localization to hypoxic niche microenvironments, limitation of reactive oxygen species (ROS) production, reduced metabolic rates, and maintenance in a quiescent cell cycle state.5,32 Despite all these layers of protection, ASCs can still accumulate damage, which manifests as changes in progeny, change in ASC pool size, senescence, and malignant transformation.31,33 Some of these age-associated modifications to ASCs can be reversible and may have implications for therapies.
Conserved regulators of aging are likely to play a role in the preservation of ASCs. DR has been shown to improve the function of aged HSCs, even after transplantation into non-DR recipient mice.34 DR was also found to suppress the development of myeloid leukemia in an irradiation-induced mouse model of leukemia by minimizing the expansion of aberrant HSCs.35 These findings suggest that ASC homeostasis, ASC self-renewal, ASC aging, and tumorigenesis can be modulated by conserved regulators of aging that mediate the DR antiaging effects.1 Supporting this notion, mTOR was found to be upregulated in aged HSCs.36 Rapamycin, the inhibitor of mTOR, limited the age-associated changes in HSCs and improved the transplantation ability of aged HSCs.31 These findings beg the question: do other conserved regulators of aging, such as sirtuins, play a role in the maintenance of tissue homeostasis and regeneration?
SIRT1 in Fetal HSCs
During fetal development, the yolk sac, aorta-gonad-mesonephros, fetal liver, and placenta are all sites of hematopoiesis in mice.37,38 Under homeostatic conditions, fetal hematopoietic stem and progenitor cells (HSPCs) do not require SIRT1 for their function and survival. SIRT1−/− fetal livers have the same frequency of HSPCs defined immunophenotypically as wild-type (WT) fetal livers.39 However, when fetal HSPCs are stressed and pushed to differentiate or self-renew, their ability is limited by the absence of SIRT1. HSPCs isolated from SIRT1−/− yolk sacs had reduced abilities to form colonies in vitro.40 Furthermore, after ex vivo culture, the frequency of immunophenotypic fetal liver HSPCs decreased 20-fold.39 Additionally, HSPCs isolated from SIRT1−/− fetal livers did not perform as well as HSPCs from WT fetal livers after serial replating and serial transplantation.39 These data suggest that SIRT1 is essential for the maintenance of fetal HSPCs under stress.
Mechanistically, SIRT1 protects fetal HSPC self-renewal by reducing oxidative stress. HSPCs isolated from SIRT1−/− fetal livers had increased levels of ROS.39 Treatment with the antioxidant N-acetylcysteine (NAC) reduced cellular ROS levels and limited the increased differentiation seen in SIRT1−/− fetal liver HSPCs. SIRT1 is likely to reduce oxidative stress in HSPCs by regulating its downstream stress resistance genes. Ectopic overexpression of FOXO3a or inhibition of p53 in SIRT1−/− fetal liver HSPCs was also able to restore loss of HSPC maintenance.39 Thus, during fetal murine hematopoiesis, SIRT1 and its downstream targets FOXO3a and p53 regulate a stress management program that is essential for HSPC maintenance under stress conditions (Fig. 1).
SIRT1 in Adult HSCs
In the adult murine hematopoietic system, SIRT1 expression was found to be regulated by proliferation and differentiation. Resting or quiescent HSPCs had the lowest SIRT1 expression, proliferating HSPCs had increased SIRT1 expression, and mature cells had the highest levels of SIRT1 expression.39,41 The changes in expression levels may reflect the differential requirement for SIRT1 in HSPC maintenance under various conditions. Like fetal HSPCs, adult HSPCs do not require SIRT1 for their function and survival under homeostatic conditions. Adult WT and SIRT1−/− mice had similar bone marrow (BM) cellularities.40 There was also no difference in the numbers of HSPCs in SIRT1−/− mice compared to WT mice.41 Furthermore, SIRT1−/− mice do not display any hematopoietic defects during homeostasis.42
However, under stress conditions, adult SIRT1−/− HSPCs respond differently from fetal SIRT1−/− HSPCs. Adult SIRT1−/− HSPCs isolated from young, middle-aged, and old SIRT1−/− mice could transplant recipient mice as well as age-matched WT controls, showing that SIRT1 deficiency does not affect adult HSPCs under the stress of transplantation.42 This is in contrast to fetal HSPCs, which require SIRT1 under transplantation stress.39 This difference may reflect the distinct role of SIRT1 in HSPCs at different developmental stages. Alternatively, this difference may result from the complication of the SIRT1−/− mouse models employed in the studies. SIRT1 deletion in mice results in perinatal and postnatal lethality with high penetrance.21,43-45 The mice employed in these studies are those that survive into their adulthood and are likely to be preselected to adopt compensatory stress resistance programs. To faithfully understand the role of SIRT1 in adult hematopoiesis, the barrier of embryonic lethality must be surpassed. A conditional SIRT1 knockout mouse model specifically deleting SIRT1 from the hematopoietic system will be informative.
Despite being able to perform long-term reconstitution in transplants, adult HSPCs lacking SIRT1 had reduced functionality in vitro. Adult SIRT1−/− HSPCs had reduced clonogenic ability.40 SIRT1 also affects HSPC survival and proliferation in ex vivo cultures, but the findings are controversial. One group found that adult SIRT1−/− HSPCs cycled less than WT HSPCs and did not survive as well when deprived of cytokines and growth factors, while another group found that SIRT1−/− HSPCs were able to proliferate more than WT HSPCs.40,41 Similarly, inhibition of SIRT1 by nicotinamide caused an approximately 4-fold reduction in the percentage of HSPCs found in cultured murine adult WT BM cells.39 While it is unclear whether the effects under the in vitro culture conditions have any physiological relevance, these studies may suggest a role of SIRT1 in adult hematopoietic stress responses. Consistent with this notion, pretreatment with resveratrol, an activator of SIRT1, was shown to reduce the deleterious effects of total body irradiation on the murine hematopoietic system.46
Leukemia and Leukemic Stem Cells
Cancer of the blood, or leukemia, is often associated with a disruption of the normal balance that exists between mature blood cell turnover and their replenishment by HSCs and progenitors. Leukemic cells can be organized hierarchically into fractions that are tumorigenic and nontumorigenic. The tumorigenic portion of leukemic cells comprises the leukemic stem cells (LSCs) that can initiate and maintain the leukemia. They develop from healthy HSCs or from progenitor populations that acquire the ability to self-renew.47,48 LSCs are functionally similar to HSCs with the capacity to self-renew and differentiate, but these processes are aberrantly regulated. LSCs self-renew to generate more LSCs that can initiate and sustain the disease and differentiate to produce the heterogeneous mixture of cells that constitute the nontumorigenic bulk of the tumor.
Chronic myelogenous leukemia (CML), a leukemia that arises from LSCs, is caused by the acquisition of a chromosomal translocation fusion product, BCR-ABL, which encodes an oncoprotein with constitutive tyrosine kinase activity.49-52 CML is characterized by granulopoiesis, splenomegaly, and its triphasic disease course: chronic phase, accelerated phase, and blast crisis phase. CML is considered a stem cell disease because the expression of BCR-ABL is found in mature cells of both arms of the hematopoietic lineage: the myeloid arm (myeloid, erythroid, and megakaryocytic cells) and the lymphoid arm (B cells).53 Additionally, CML can be initiated by the transplantation of either HSCs carrying the BCR-ABL oncogene in the chronic phase or progenitors carrying the BCR-ABL oncogene in the blast crisis stage.54-56
Drugs that specifically target the BCR-ABL fusion protein have been able to control CML. Tyrosine kinase inhibitors (TKIs) such as imatinib, dasatinib, and nilotinib can all manage CML by targeting malignant progenitor cells but appear to have little effect on LSCs.57 CML patients must remain on TKIs for the rest of their lives or risk a relapse.58,59 LSCs can also escape TKI treatment through mutating the kinase domain targeted by TKIs.60,61 In developed countries, most CML patients are diagnosed during the chronic phase of the disease and thus have the opportunity to use TKIs. However, in the developing world, CML patients are often not identified until the accelerated or blast crisis stage of the disease.62 The ability of LSCs to evolve and evade targeted therapy as well as the timing of CML diagnosis worldwide makes it essential that new therapies target all stages of CML disease and remove the LSCs that drive disease progression. It is now becoming clear that the next approach in CML treatment will be to use combination therapy, combining TKIs with the inhibition of targets specifically activated in LSCs but not in healthy HSCs or progenitors. These combination therapy approaches will ideally cure CML patients while sparing them from the burden of a lifetime on pharmacological treatments with the risk of disease relapse.58
Sirtuin 1 and LSCs
Because SIRT1 controls many cellular stress response and repair pathways, but does not seem to be essential for homeostatic regulation of hematopoiesis, it is an optimal target to test in the treatment of CML.62,63 BCR-ABL expression in CML enhances the expression and activity of SIRT1, possibly by downregulating the expression of hypermethylated in cancer 1 (HIC1), an upstream negative regulator of SIRT1.64-66 This increased expression was found to have functional relevance. Knocking down SIRT1 reduced the proliferation, survival, and clonogenic ability of CML LSCs but had a limited effect in normal HSCs, supporting the notion that SIRT1 may be expendable in normal HSCs but essential for CML LSCs.63,64
Inhibition of SIRT1 has the potential to serve in a combination therapy with imatinib in the treatment of CML. Tenovin-6 (TV-6), a small molecule inhibitor of SIRT1, or TV-6 plus imatinib were both found to be more effective than imatinib alone at reducing the survival and clonogenic capability of CML LSCs in vitro.63 In a more physiological setting, TV-6 alone or TV-6 and imatinib together were able to reduce the numbers of CML LSCs, slow disease progression, and enhance survival in a CML mouse model.63,64 These therapeutic strategies also appear to be effective in human CML, as demonstrated in mice transplanted with CML LSCs isolated from a patient in blast crisis who was not responding to imatinib.63 Thus, targeting SIRT1 may be an effective treatment against both chronic and blast crisis stages of CML (Fig. 2).
SIRT1 protects CML LSCs through its downstream target p53. Inhibition of SIRT1 leads to p53 activation in human CML LSCs, and TV-6 treatment was ineffective in CML LSCs in the absence of p53.63 Interestingly, the same stress resistance mechanism is employed by SIRT1 to protect fetal liver HSPCs and CML LSCs.38 These observations, together with the finding that BCR-ABL induces the expression of SIRT1, suggest that LSCs hijack the SIRT1 tissue maintenance pathway for survival (Fig. 1).
In addition to enhanced stress responses and survival, elevated SIRT1 expression in CML may also promote disease evolution and TKI resistance through increased mutagenesis. SIRT1 deacetylates many components of the DNA damage response and DNA repair pathways, including Ku70, Nijmegen breakage syndrome protein (NBS1), and Werner syndrome protein (WRN).45,67,68 SIRT1 can enhance nonhomologous end joining (NHEJ), an efficient yet error-prone DNA repair mechanism.64,68,69 While SIRT1 may help CML LSCs repair DNA damage and escape apoptosis, it may also facilitate the accumulation of new mutations and promote disease evolution and drug resistance.
Conclusion
Evidence is emerging to support a role for SIRT1 in stress resistance in HSPCs at early developmental stages but not during adulthood. However, SIRT1 is essential for the survival of malignant CML LSCs and promotes disease progression. Targeting the Achilles heel of CML LSCs in SIRT1 will allow for the treatment of advanced stages of CML or CML that has become resistant to current TKI therapies while sparing normal HSCs and hematopoiesis.
Currently, little is known about the function of SIRT2 to SIRT7 in HSCs and the hematopoietic system. However, it has been shown that SIRT2, SIRT3, and SIRT7 expression are strongly downregulated in aged HSCs.70 Additionally, SIRT7 is known to localize to a chromosomal region (17q25.3) often lost in leukemia.71 We anticipate that this is just the beginning of understanding the importance of sirtuins in stem cell and CSC biology. Understanding the role that sirtuins play in HSC maintenance and aging may lead to the identification of therapeutic targets for ameliorating the physiological impact of aging on HSCs and thereby limit BM failure, anemia, and leukemia.
Acknowledgments
The authors thank Katharine Brown and Yufei Liu for their insightful comments on the article.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: M.M. is supported by a postdoctoral fellowship from the Siebel Stem Cell Institute. D.C. is supported by the National Institutes of Health (R01AG040990), Searle Scholars Program, Ellison Medical Foundation, American Heart Association, University of California Office of the President Tobacco-Related Disease Research Program, and Hellman Family Faculty Fund.
References
- 1. Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681-96 [DOI] [PubMed] [Google Scholar]
- 2. Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40(2):333-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenyon CJ. The genetics of ageing. Nature. 2010;464:504-12 [DOI] [PubMed] [Google Scholar]
- 5. Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080-6 [DOI] [PubMed] [Google Scholar]
- 6. Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313-21 [DOI] [PubMed] [Google Scholar]
- 7. Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273-9 [DOI] [PubMed] [Google Scholar]
- 8. Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795-800 [DOI] [PubMed] [Google Scholar]
- 9. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126-8 [DOI] [PubMed] [Google Scholar]
- 11. Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344-8 [DOI] [PubMed] [Google Scholar]
- 12. Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998-6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227-30 [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48-56 [DOI] [PubMed] [Google Scholar]
- 17. Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couzin-Frankel J. Aging genes: the sirtuin story unravels. Science. 2011;334:1194-8 [DOI] [PubMed] [Google Scholar]
- 19. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390-2 [DOI] [PubMed] [Google Scholar]
- 20. Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413-22 [DOI] [PubMed] [Google Scholar]
- 24. Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang Y, Nicholl MB. Sirtuin 1 in malignant transformation: friend or foe? Cancer Lett. 2011;306:10-4 [DOI] [PubMed] [Google Scholar]
- 26. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595-606 [DOI] [PubMed] [Google Scholar]
- 27. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137-48 [DOI] [PubMed] [Google Scholar]
- 28. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149-59 [DOI] [PubMed] [Google Scholar]
- 29. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157-68 [DOI] [PubMed] [Google Scholar]
- 30. Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287-98 [DOI] [PubMed] [Google Scholar]
- 31. Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193:257-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakada D, Levi BP, Morrison SJ. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 2011;70:703-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 2011;13:506-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol. 2003;31:1097-103 [DOI] [PubMed] [Google Scholar]
- 35. Yoshida K, Hirabayashi Y, Watanabe F, Sado T, Inoue T. Caloric restriction prevents radiation-induced myeloid leukemia in C3H/HeMs mice and inversely increases incidence of tumor-free death: implications in changes in number of hemopoietic progenitor cells. Exp Hematol. 2006;34:274-83 [DOI] [PubMed] [Google Scholar]
- 36. Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733-44 [DOI] [PubMed] [Google Scholar]
- 38. Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017-31 [DOI] [PubMed] [Google Scholar]
- 39. Matsui K, Ezoe S, Oritani K, et al. NAD-dependent histone deacetylase, SIRT1, plays essential roles in the maintenance of hematopoietic stem cells. Biochem Biophys Res Commun. 2012;418:811-7 [DOI] [PubMed] [Google Scholar]
- 40. Ou X, Chae HD, Wang RH, et al. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narala SR, Allsopp RC, Wells TB, et al. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell. 2008;19:1210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leko V, Varnum-Finney B, Li H, et al. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood. 2012;119:1856-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McBurney MW, Yang X, Jardine K, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang RH, Sengupta K, Li C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang H, Zhai Z, Wang Y, et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2012;54C:40-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Passegué E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100 Suppl 1:11842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85-109 [PubMed] [Google Scholar]
- 50. Rowley JD. Chromosomal patterns in myelocytic leukemia. N Engl J Med. 1973;289:220-1 [DOI] [PubMed] [Google Scholar]
- 51. Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550-4 [DOI] [PubMed] [Google Scholar]
- 52. Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986; 233:212-4 [DOI] [PubMed] [Google Scholar]
- 53. Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330-40 [DOI] [PubMed] [Google Scholar]
- 54. Fialkow PJ, Jacobson RJ, Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977;63:125-30 [DOI] [PubMed] [Google Scholar]
- 55. Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824-30 [DOI] [PubMed] [Google Scholar]
- 56. Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657-67 [DOI] [PubMed] [Google Scholar]
- 57. Becker MW, Jordan CT. Leukemia stem cells in 2010: current understanding and future directions. Blood Rev. 2011;25:75-81 [DOI] [PubMed] [Google Scholar]
- 58. Crews LA, Jamieson CH. Selective elimination of leukemia stem cells: hitting a moving target. Cancer Lett. Epub 2012 Aug 17. [DOI] [PubMed] [Google Scholar]
- 59. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029-35 [DOI] [PubMed] [Google Scholar]
- 60. Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876-80 [DOI] [PubMed] [Google Scholar]
- 61. Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701-7 [DOI] [PubMed] [Google Scholar]
- 62. Ito T, Zimdahl B, Reya T. aSIRTing control over cancer stem cells. Cancer Cell. 2012;21:140-2 [DOI] [PubMed] [Google Scholar]
- 63. Li L, Wang L, Li L, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yuan H, Wang Z, Li L, et al. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119:1904-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437-48 [DOI] [PubMed] [Google Scholar]
- 66. Issa JP, Zehnbauer BA, Kaufmann SH, Biel MA, Baylin SB. HIC1 hypermethylation is a late event in hematopoietic neoplasms. Cancer Res. 1997;57:1678-81 [PubMed] [Google Scholar]
- 67. Oberdoerffer P, Michan S, McVay M, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Z, Yuan H, Roth M, Stark JM, Bhatia R, Chen WY. SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene. Epub 2012. March 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen W. Accelerating cancer evolution: a dark side of SIRT1 in genome maintenance. Oncotarget. 2012;3:363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Voelter-Mahlknecht S, Letzel S, Mahlknecht U. Fluorescence in situ hybridization and chromosomal organization of the human sirtuin 7 gene. Int J Oncol. 2006;28:899-908 [PubMed] [Google Scholar]


