Abstract
Sirtuins (SIRT1-SIRT7), the mammalian homologs of the silent information regulator 2 (Sir2) in Saccharomyces cerevisiae, have been a major focus of study in the scientific community this past decade because of their emerging role in cancer biology and other age-related diseases. Emerging functions for this unique family of enzymes include roles in genomic stability, angiogenesis, metabolism, and anoikis. Here, we review recent developments on the role of sirtuins in cancer with a particular focus on SIRT3 and its role in the hallmarks of cancer and as a potential drug target for cancer treatment.
Keywords: sirtuin-3, SIRT3, sirtuins, cancer, tumor promoter, tumor suppressor, anoikis
Introduction
Cancer is one of the leading causes of death worldwide.1 Despite advances in treatment, outcomes have not improved significantly. This highlights the importance of exploring new areas of investigation to effect change and curtail this devastating disease.
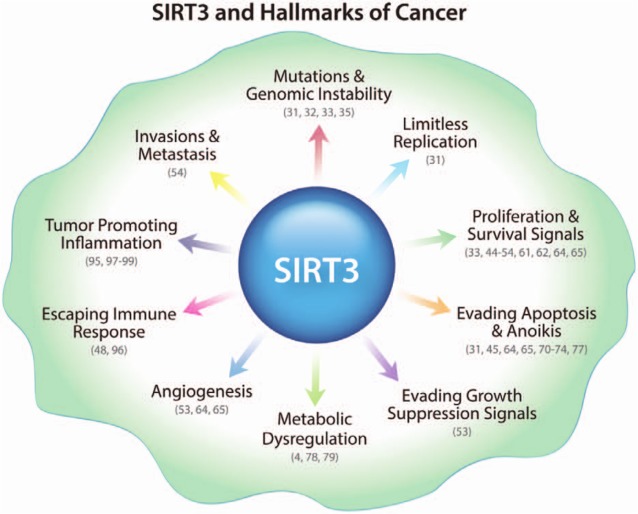
Sirtuins (SIRT1-SIRT7), the mammalian orthologs of the silent information regulator 2 (Sir2) in Saccharomyces cerevisiae, have been a major focus of study in the scientific community this past decade in part because of their emerging role in cancer biology.2-4 This evolutionarily conserved family of proteins works as NAD-dependent deacetylases or ADP-ribosyltransferases.5,6 Different sirtuins are located in different subcellular compartments and can thereby modulate different targets in the cell, which may explain their important role in tumorigenesis.3,7 An important member of this family that appears to be a critical modulator of tumorigenesis is sirtuin-3 (SIRT3).7 In a previous review,7 we discussed the dual role of SIRT3 in cancer, where we highlighted its role in cell survival, apoptosis, and metabolism in regards to cancer biology.7 The aim of the current review is to highlight recent developments in the role of sirtuins in cancer biology with a particular focus on SIRT3 and its role in the hallmarks of cancer (Figure 1) and as a potential drug target for cancer treatment.
Figure 1.
Mutations and genomic instability: Genomic instability and mutations may result in the amplification of oncogenes or inactivation of tumor suppressors, which drive tumor progression. SIRT3 promotes mutagenesis and increases genomic stability through ROS and deacetylation of H4K16ac and H3K9ac.31-33,35 Limitless replication: Normal cells have a limited life span, whereas cancer cells possess infinite replicative potential, also called cell immortalization. SIRT3 is involved in the immortalization process in the presence of Myc or Ras.31 Proliferation and survival signals: Cancer cells become self-sufficient in growth signals and therefore acquire uncontrolled proliferative capacity. SIRT3 plays a role in proliferative and survival signaling pathways in normal and cancer cells (tumor promoter) via maintaining ROS thresholds, activation of a FOXO3-dependent antioxidant mechanism and NF-κB, and its modulation through MAPK/ERK, PI3K/Akt, and AMP kinase pathways.33,44-54,61,62,64,65 Evading apoptosis and anoikis: Apoptosis, a form of programmed cell death, is a genetically regulated cell-suicidal mechanism involved in the regulation of normal tissue homeostasis. However, cancer cells are able to bypass this mechanism. In addition to regulating cell proliferation and survival, SIRT3 plays a proapoptotic role (tumor suppressor) in normal and cancer cell types.31,64,65,70-74,77 Anoikis resistance or anchorage-independent growth contributes to cancer development and progression. SIRT3 plays a role in mediating anoikis resistance and tumorigenesis.45 Evading growth suppression signals: Cancer cells negatively regulate cell proliferation, and many of these programs depend on the actions of tumor suppressor genes. p53-induced growth arrest is regulated by the mitochondrial SIRT3 deacetylase.53 Metabolic dysregulation: Cancer cells have developed the ability to reprogram their cellular metabolism to sustain growth and tumor progression. SIRT3 modulates cellular metabolism, the Warburg effect, and tumorigenesis.4,78,79 Angiogenesis: To sustain the growth of tumors, cancer cells chronically activate angiogenesis and an unbalanced mix of proangiogenic signals. SIRT3 is indirectly associated with angiogenesis through HIF-1α and p53.53,64,65 Escaping immune response: Cancer cells acquire the ability to evade immunological destruction, especially by T and B lymphocytes, macrophages, and natural killer cells. SIRT3 is involved in inflammation and autoimmune dysfunction through activation of the renin-angiotensin system.48,96 Tumor-promoting inflammation: Innate immune cells, which are designed to fight infections and heal wounds, inadvertently support these cancer hallmarks, thereby leading to tumor promotion. SIRT3 is involved in proinflammatory responses through the modulation of ROS.95,97-99 Invasion and metastasis: Cancer cells invade local tissue, spread to distant sites, and develop new tumors at secondary sites. Increased levels of SIRT3 transcription are associated with lymph node metastasis in breast cancer.54
Sirtuins and Cancer: An Overview
Cancerous cells possess 6 hallmarks that include self-sufficiency in growth signals, insensitivity to antigrowth signals, apoptosis evasion, sustained angiogenesis, limitless replicative potential, and tissue invasion and metastasis.8 Additional characteristics that have recently emerged include dysregulation of cellular energy, avoidance of immune distraction, genomic instability, and tumor-promoting inflammation. These features define the myriad of dysregulated cancerous cell attributes.9 Interestingly, growing evidence supports an important role for sirtuins in these cancer hallmarks. However, it is important to keep in mind that several sirtuins exhibit a dual role in tumorigenesis.7 Understanding the mechanisms that regulate this duality is critical to developing sirtuin-based anticancer therapeutics.
In this review, we discuss the different molecular and cellular transitions that cancerous cells undergo and highlight the current evidence that sirtuins, and specifically SIRT3, can modulate these processes and thereby affect the outcome of tumorigenesis.
SIRT3 and Genomic Instability
The 6 hallmarks of cancer8 are acquired directly or indirectly via several “hits” or mutations on the DNA that lead to genomic instability.9-12 These multiple “hits,” as stated by Knudson’s13 hypothesis or the mutator phenotype hypothesis,14,15 can result from chronic exposure to carcinogens, such as tobacco, alcohol, or viruses such as HPV.16 These mutations may result in the amplification of oncogenes or inactivation of tumor suppressors.15,16 In addition, the multistep model of cancer also demonstrates that normal cells must undergo several genetic and cellular changes to become cancerous cells.11
Direct links between sirtuins and genomic stability have been demonstrated, primarily via a genetic approach. Mice lacking SIRT1 exhibit genomic instability, impaired DNA damage response, and tumorigenesis.17 Similarly, SIRT2 functions as a G2 checkpoint mitotic regulator, thus preventing chromosomal instability, and it might also function as a tumor suppressor.18-20 SIRT6-deficient mice also show signs of genomic instability and an aging-like phenotype.21 In the case of SIRT3, direct evidence for its role in genomic instability has not been as forthcoming, especially given that most studies support the concept that SIRT3 is primarily mitochondrial in its localization.22-29 SIRT3-null mice appear to have normal development and fertility.23,30 However, recently, Kim et al.31 demonstrated that mouse embryonic fibroblasts (MEFs) from SIRT3 knockout mice exhibit abnormal mitochondrial physiology, increased stress-induced superoxide levels, and genomic instability. Additionally, these mice develop ER/PR-positive mammary tumors over a 24-month observation period, suggesting a tumor suppressor role for SIRT3 in this cancer type.31 Mechanistically, SIRT3-mediated regulation of reactive oxygen species (ROS) may be the path regulating genomic instability. Since SIRT3 seems to regulate ROS through Mn-SOD and SOD2 modulation,32,33 and because increased ROS levels have been associated with mutagenesis promotion and genomic instability,34 these studies suggest an indirect role for SIRT3 in controlling genomic stability.4 Additional studies that support nuclear localization for SIRT3 upon cellular stress suggest that the histone modifications H4K16Ac and H3K9Ac are targeted by SIRT3,35 thus introducing the possibility that SIRT3 might also play a direct regulatory role in controlling genomic stability.
SIRT3 Controls the Limitless Replicative Potential of Cancer Cells
Normal cells become senescent after a defined number of cell doublings because of their limited capacity for replication.36,37 Cancerous cells, in contrast, can perpetually maintain their telomere lengths during replication, possess limitless replication potential, and are known to be immortalized.37 Telomeres are essential for chromosomal protection and function and shorten after each cell division. The enzyme telomerase maintains telomeric length during cell replication.38 Human telomerase reverse transcriptase (hTERT) creates single-stranded DNA using single-stranded RNA as a template. Upregulated levels of hTERT are a common feature of all tumors.39,40
Evidence supports a role for both SIRT1 and SIRT6 in telomere function in part because of their nuclear localization.41-43 Inhibition of SIRT1 is associated with enhanced telomerase activity in human cells, such as HeLa cells.43 Whether this can be demonstrated in other cell types or whether this contributes to the immortalization of tumors has yet to be determined. However, there seems to be a direct link between SIRT1, telomerase function, and tumorigenesis. SIRT6 also seems to be important in maintaining telomeric function. Downregulation of SIRT6 activity results in telomere dysfunction and end-to-end chromosomal fusions.42 In addition, SIRT6-deficient cells show an increased susceptibility to genotoxic DNA damage, resulting in the accumulation of chromosomal abnormalities and genomic instability.21 Because most reports support mitochondrial localization for SIRT3, no information has yet been reported that links SIRT3 to telomeres and telomeric functions. However, the data by Kim et al.31 support that SIRT3 knockout in MEFs creates a transformation- permissive phenotype that allows immortalization in the presence of oncogenic hits with Myc or Ras. Whether this event is linked to telomeric function is a question that needs to be explored.
SIRT3 Controls Proliferative and Survival Signaling
Cell proliferation is controlled in normal cells by the production and release of proliferative and antiproliferative signals. Therefore, maintaining the balance between both proliferative and antiproliferative signaling processes is essential. However, this balance is tipped toward the production and release of uncontrolled proliferative signals that cannot be regulated by the antiproliferative signals in cancer cells.9 Several studies highlight a prosurvival role for SIRT3 in both normal and cancer cells/tissues under different cellular conditions,33,44-54 thus supporting a tumor promoter role for SIRT3 in cancer. Hence, SIRT3 seems to control proliferative and survival pathways in certain cell/tissue types, including oral cancer, breast cancer, cardiomyocytes, HEK293 cells, and neurons, although the exact mechanisms by which SIRT3 exerts these roles are still not fully understood.
SIRT3 contributes to cell proliferation and survival by different mechanisms. The role of ROS in cancer cell biology is well established. ROS plays an important role in transformation to a tumorigenic phenotype.55,56 However, normal cells also require low levels of ROS for normal physiological functions. Thus, normal cells possess lower ROS levels compared to cancer cells.57,58 ROS can modulate both cell survival and apoptotic pathways.59,60 SIRT3 regulates ROS levels in normal and cancer cells. In the subset of cancers in which SIRT3 is overexpressed, such as head and neck squamous cell carcinoma (HNSCC),44 SIRT3 maintains ROS levels at the appropriate level for maintaining a proliferative and aggressive phenotype, thus promoting tumorigenesis and preventing apoptosis. In HeLa cells, SIRT3 deacetylates Ku70, which augments Ku70-Bax interactions and prevents Bax translocation to the mitochondria, thus protecting these cells from genotoxic and oxidative stress-mediated cell death and promoting cell survival.46 In cardiomyocytes, SIRT3 suppresses ROS via the activation of a FOXO3a-dependent antioxidant mechanism via modulation of the MAPK/ERK and PI3K/Akt proliferation signaling pathways49 and via activation of NF-κB.61 Furthermore, SIRT3 exerts a neuroprotective role in Huntington disease by modulating the AMP kinase pathway.62 Hypoxia-inducible factors (HIFs) mediate cellular responses to hypoxia and directly activate the transcription of genes involved in tumorigenesis, including vascular endothelial growth factor (VEGF) and transforming growth factor α (TGF-α).63 Under hypoxic conditions, SIRT3 overexpression inhibits ROS production, glycolysis, proliferation, and HIF-1α stabilization and its downstream transcriptional activity, thus decreasing tumorigenesis.64,65 In summary, these pathways are commonly dysregulated in several cancer types. Given that SIRT3 controls these multiple pathways supports the concept that SIRT3 is positioned to regulate tumorigenesis.63,66-69
SIRT3, Apoptosis, Cell Death, and Growth Suppression
A proapoptotic role has also been advanced for SIRT3 in some normal and cancer cell types.31,64,65,70-74 This dual role for SIRT3 in normal and cancer cells has been discussed in detail in our previous review.7 Briefly, we reported that SIRT3 promotes survival and protects several cell types from cellular damage by maintaining mitochondrial integrity and functions and by enhancing their resistance to stress-mediated cell death, where ROS regulation is central to these processes. Apoptotic cell death and growth suppression pathways seem to be regulated by additional signaling pathways that include JNK, Bax, HIF-1α, but also ROS.
Recent evidence continues to support this dual role for SIRT3. The tumor suppressor p53 is commonly mutated in most cancer types.75,76 In bladder cancer, SIRT3 deacetylates p53, thus abrogating growth arrest and cellular senescence and supporting a prosurvival role for SIRT3.53 In contrast, in hepatocellular carcinoma, SIRT3 levels are downregulated. Thus, SIRT3 seems to prevent p53 degradation by reducing the activity of the negative regulator Mdm-2 on p53.74 Interestingly, a recent report supports that SIRT3 as well as SIRT2, SIRT6, and HDAC3 are all correlated with the overall survival of chronic lymphocytic leukemia patients. However, a poor prognosis was associated with the overexpression of HDAC7 and HDAC10 but the underexpression of HDAC6 and SIRT3.77 Furthermore, SIRT3 enhances growth arrest and apoptosis in several osteosarcoma and colorectal carcinoma cells and in noncancer human cell lines such as lung and retinal epithelial cells.70 This action is mediated, at least in part, by SIRT3 modulation of the JNK2 signaling pathway in these cell lines.70
SIRT3, Metabolism, and Cancer
The role of SIRT3 in metabolism and tumorigenesis has been extensively discussed.4,78,79 Furthermore, it is important to highlight that altered tumor metabolism is one of the emerging hallmarks of tumorigenesis.9 In recent work, SIRT3 was found to be an important regulator of the Warburg effect. SIRT3 mediates metabolic reprogramming by destabilizing HIF-1α, which regulates glycolytic gene expression. Specifically, loss of SIRT3 increases ROS production, and this leads to HIF-1α stabilization. In breast cancers, SIRT3 expression is reduced, and its loss is correlated with the upregulation of HIF-1α target genes. Furthermore, SIRT3 overexpression suppresses glycolysis and proliferation in breast cancer cells, suggesting a metabolic mechanism for tumorigenesis.78,79 In another report, SIRT3 was found to suppress HIF-1α and tumor growth by inhibiting ROS production. Thus, since metabolism and the mitochondria play a major role in tumorigenesis, and SIRT3 regulates metabolism within the mitochondria, SIRT3 is pivotally located to balance the metabolic targets that control several age-related diseases including cancer. This implicates SIRT3 as a potential target to prevent and treat these diseases.
SIRT3 and Angiogenesis
Angiogenesis facilitates cancer development and progression.80 The association between sirtuins and angiogenesis has been well established for SIRT1. SIRT1 is highly expressed during blood vessel formation.81 Downregulation of SIRT1 function blocks sprouting angiogenesis and branching morphogenesis of endothelial cells, thus resulting in the downregulation of genes, such as FOXO1, that are involved in blood vessel development and vascular remodeling.81 In addition, endothelial cell–specific deletion of SIRT1 in mice blunts ischemia-induced neovascularization in the hindlimb.81 In agreement with this report, activation of SIRT1 by resveratrol in vivo resulted in increased capillary density via VEGF and nitric oxide synthase upregulation 3 weeks after myocardial infarction.82 Another important angiogenic factor is HIF-1. HIF-1 seems to activate SIRT1 under hypoxic conditions, and SIRT1 selectively augments HIF-2 signaling during hypoxia, indicating a link between HIF and SIRT1 signaling during hypoxia.83 Moreover, SIRT1 deacetylates and inactivates p53, a proapoptotic and antiangiogenic factor, thereby mediating a cardioprotective role and suggesting that SIRT1 modulation of p53 may be a mechanism by which SIRT1 controls angiogenesis.84,85
To our knowledge, there are no reports demonstrating a direct role for SIRT3 in the regulation of angiogenesis. However, as mentioned above, an association between SIRT3 and both HIF-1α and p53 has been reported.53,64,65 SIRT3 partially abrogates p53 activity to enact growth arrest and senescence in a bladder carcinoma cell environment.53 Loss of SIRT3 also increases ROS production and HIF-1α stabilization. Thus, when SIRT3 expression is reduced, such as in human breast cancers, its loss is associated with the upregulation of HIF-1α target genes.64 Similar effects are also noted in human colon carcinoma and osteosarcoma cells.65 Thus, SIRT3 stable knockdown results in enhanced tumorigenesis in a xenograft model and augmented HIF-1α protein stability and transcriptional activity.65 Although these reports did not investigate angiogenesis in these cancer types, their results support an opposite effect on HIF-1α by SIRT3 compared to SIRT1 and, by extension, an opposing role in angiogenesis. In contrast, the role of SIRT3 on p53 seems consistent with that of SIRT1 since both can deacetylate and inactivate p53 transcripts, thus promoting cell survival. Therefore, one may expect a proangiogenic role for SIRT3, at least in bladder carcinoma,53 but not in breast and colon carcinoma and osteosarcoma,64,65 based on the current reports. Additional studies are needed to establish a clear understanding of the role of SIRT3 in angiogenesis.
SIRT3, Inflammation, and the Immune Response
Growing evidence indicates that inflammation contributes to tumor formation by modulating the tumor microenvironment.9,86,87 Several sirtuin members, including SIRT1, SIRT6, and SIRT7, are involved in inflammation.88,89 Most of these studies support an inhibitory effect of these sirtuins on inflammation. For instance, SIRT1 deacetylates the RelA/p56 subunit of NF-κB, a transcription factor involved in inflammation, and thereby attenuates its downstream signaling cascades and inflammation.90 Similarly, SIRT6 attenuates NF-κB signaling at the transcriptional level.91 Both SIRT1- and SIRT7-null mice demonstrate autoimmune-like conditions and the development of inflammatory cardiomyopathy.92 In addition, SIRT6 appears to play a role in inflammation by enhancing the proinflammatory cytokine expression of TNF-α and IL-6 in innate and adaptive immune cells by modulating NAD cellular levels via Nampt enzymatic activity.93
The link between mitochondrial functions, inflammation, and cancer has been well documented in lung and colorectal cancers.94 SIRT3 positively regulates antioxidant gene expression, thus decreasing ROS accumulation in tubulointerstitial lesions in proteinuric kidney disease, thereby leading to decreased inflammation.95 In addition, activation of the renin-angiotensin system, ACE/Ang II/AT1R, is associated with downregulation of the prosurvival genes Nampt and Sirt3, increase in ROS production, and proinflammatory cytokine and chemokine release, leading to cell senescence, inflammation, and the development of autoimmune dysfunctions.48,96 Moreover, SIRT3 is a key effector of neutrophil and lymphocyte immune responses after exercise. Thus, a regulatory role for SIRT3 in inflammatory pathways appears plausible.97,98 In aggregate, although direct evidence for an association between SIRT3, inflammation, and cancer does not yet exist, there is supporting evidence suggesting that these events may work in concert; this is an area that remains to be explored.
SIRT3, Invasion, and Metastasis
The multistep model of cancer states that the accumulation of genetic alterations precedes the onset of cancer. These mutations become phenotypically translated at the cellular level to ultimately reach an end point of invasion and metastasis. This final step involves the loss of cell-to-cell and cell–to–extracellular matrix (ECM) adhesion, loss of basement membrane adhesion, and survival of tumor cells in the bloodstream to reach and colonize new metastatic sites.9 The role of several sirtuins has been demonstrated in invasion and metastasis. SIRT1 expression levels correlate with tumor stage, lymph node metastasis, tumor invasion, and shorter overall survival in gastric carcinoma patients.99 In addition, SIRT1 downregulation attenuates prostate cancer cell migration and invasion, suggesting that SIRT1 may be a promising target to prevent the metastasis of prostate cancer.100 Ashraf et al.54 reported that SIRT3 transcription levels are associated with lymph node–positive metastatic breast cancer. These studies indicate an association between SIRT1 and SIRT3 levels and the aggressiveness and progression of these cancer types.
SIRT3 and Anoikis
Apoptosis resulting from the loss of cell adhesion or inappropriate adhesion to the ECM is defined as anoikis.101 Resistance to anoikis contributes to the development and progression of cancer. Anoikis resistance and anchorage independency allow tumor cells to invade adjacent tissues and organs as well as metastasize to lymph nodes and other distant sites. Thus, understanding the mechanisms that regulate anoikis resistance is important for counteracting tumor progression and preventing metastasis.
We reported that anoikis activates the CD95/Fas-mediated signaling pathway that is regulated by receptor-interacting protein (RIP), a kinase that shuttles between Fas-mediated cell death and integrin/focal adhesion kinase (FAK)–mediated survival pathways.102 RIP seems to play a dual role in these processes since it can bind to FAK in human tumor cells, providing a prosurvival signal, and it interacts with Fas in cell death pathways to initiate anoikis.102-104 Therefore, RIP participates in both prosurvival and cell death pathways and thus constitutes a possible target for modulating anoikis. Because SIRT3 also regulates cell survival, apoptosis, and tumorigenesis,7,44 the authors hypothesized that SIRT3 might engage in cross-talk with Fas/RIP/FAK death-survival pathways in cancer cells. Indeed, we showed that SIRT3 mediates anoikis resistance in oral cancer and its negative relation with RIP expression.45 We further demonstrated that RIP likely negatively regulates SIRT3 in anoikis resistance, and an anoikis-resistant orasphere phenotype defined by higher SIRT3 and low RIP expression contributes to a more aggressive phenotype in oral squamous cell carcinoma (OSCC) development. Multiple studies have supported the concept that spheroid formation promotes cancer cell survival and tumorigenesis.105-107 In addition, SIRT3 localizes to the mitochondrial matrix, and RIP can localize to the mitochondrial outer membrane,108,109 suggesting that these 2 molecules may interact indirectly through other molecules in the context of anoikis resistance. One putative molecule would be cyclophilin D. RIP appears to negatively regulate cyclophilin D, thus inducing mitochondrial-mediated cell death.108 In addition, SIRT3 interacts with and deacetylates the regulatory component of the mitochondrial permeability transition pore, cyclophilin D.50 These data support the idea that RIP and SIRT3 play opposite roles in regulating cell death and survival through a common third molecule, such as cyclophilin D. Furthermore, evidence suggests that RIP regulates ROS-mediated signaling,110,111 and RIP and SIRT3 may be functionally related through ROS regulation pathways in the context of anoikis resistance.
SIRT3’s Dual Role in Cancer
Several sirtuin family members play a dual role in tumorigenesis. This duality primarily relates to SIRT1 and SIRT37,112-114 and may result from tissue and tumor type–specific function. In addition, the cellular microenvironment, whether under normal or cellular stress conditions, may play an important role in determining this dual function. More studies are needed to explore this complex role of SIRT3 in different cancer types and under different cellular conditions to clearly determine under which conditions SIRT3 functions as a tumor promoter versus a tumor suppressor.
SIRT3 as a Potential Therapeutic Target for Cancer: What Does the Future Hold?
Sirtuins are linked to age-related diseases including diabetes, hearing loss, and cancer. Hence, it is surmised that these diseases usually share common pathways. An advantage to developing novel and specific modulators of sirtuins might allow the treatment of several age-related diseases using a common drug. However, the mechanism of action of such drugs and their off-target effects would have to be clearly identified. For instance, in the case of a proposed tumor suppressor effect for SIRT3 in human colon carcinoma and osteosarcoma,65 the SIRT3 activator would enhance the tumor suppressor effect of SIRT3 in this cancer type and would reduce ROS production. However, because of the reported dual role of sirtuins, including that for SIRT1 and SIRT3, such therapies could be met with potential unwanted side effects. A big concern would be the potential promotion of other different cancer types, given the opposing roles of SIRT3 in breast and head and neck cancer. However, with the discovery of unique biomarkers for each cancer type, the development of effective personalized cancer therapy with proper and novel targeted therapies, and delivery systems to the specific sites of interest, this issue or concern could be minimized. Thus, personalized therapy may allow us to better screen patients for the best modality of treatment to achieve better outcomes.
Several inhibitors of sirtuins have been developed and tested in vitro,44,115-118 but few have been tested in vivo for the treatment of age-related diseases, including cancer.119,120 However, a SIRT1 activator, resveratrol, has been tested in ongoing clinical trials for treating different age-related diseases.121,122 To our knowledge, there are no reported clinical trials on modulating sirtuin activity for cancer therapy.
In summary, the use of sirtuin activators/inhibitors is still in an early stage of development. Whether sirtuin modulators will deliver as novel anticancer agents is yet to be determined. However, the data accumulated thus far suggest some interesting leads and opportunities in this rapidly developing field.
Concluding Remarks
Sirtuins are emerging as a unique family of enzymes playing critical regulatory roles in genomic stability, metabolism, angiogenesis, and anoikis. The dual role of sirtuins in cancer development highlights the importance of understanding their biological mechanisms of action in each tumor to determine whether inhibition or activation might be of therapeutic value.
Acknowledgments
The authors thank Ken Riger and Claire Jones for their assistance with the design of the figure.
Footnotes
Declaration of Conflicting Interests: Eric Verdin is a member of the scientific advisory board of SIRTRIS/GSK. The remaining authors declared no potential conflicts of interest, with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health grant R56DE014429 to Y.L.K. and funding from King Abdulaziz University (Jeddah, Saudi Arabia) to T.Y.A.
References
- 1. Hoyert Donna L., Xu Jiaquan. 2012. Deaths: Preliminary Data for 2011. National Vital Statistics Reports, Vol. 61, No 6, October 10, 2012. Available from: http://www.cdc.gov/nchs/fastats/lcod.htm [PubMed] [Google Scholar]
- 2. McGuinness D, McGuinness DH, McCaul JA, Shiels PG. Sirtuins, bioageing, and cancer. J Aging Res. 2011;2011:235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489-504 [DOI] [PubMed] [Google Scholar]
- 4. Martinez-Pastor B, Mostoslavsky R. Sirtuins, metabolism, and cancer. Front Pharmacol. 2012;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haigis MC, Guarente LP. Mammalian sirtuins: emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913-21 [DOI] [PubMed] [Google Scholar]
- 6. Fischle W, Kiermer V, Dequiedt F, Verdin E. The emerging role of class II histone deacetylases. Biochem Cell Biol. 2001;79:337-48 [PubMed] [Google Scholar]
- 7. Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. SIRT3 and cancer: tumor promoter or suppressor? Biochim Biophys Acta. 2011;1816:80-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70 [DOI] [PubMed] [Google Scholar]
- 9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74 [DOI] [PubMed] [Google Scholar]
- 10. Bakhoum SF, Compton DA. Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest. 2012;122:1138-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Floor SL, Dumont JE, Maenhaut C, Raspe E. Hallmarks of cancer: of all cancer cells, all the time? Trends Mol Med. 2012;18:509-15 [DOI] [PubMed] [Google Scholar]
- 12. Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability: an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220-8 [DOI] [PubMed] [Google Scholar]
- 13. Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci U S A. 2003;100:776-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer. 2011;11:450-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14-32 [DOI] [PubMed] [Google Scholar]
- 17. Wang RH, Sengupta K, Li C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiratsuka M, Inoue T, Toda T, et al. Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys Res Commun. 2003;309:558-66 [DOI] [PubMed] [Google Scholar]
- 19. Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inoue T, Hiratsuka M, Osaki M, et al. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. 2007;26:945-57 [DOI] [PubMed] [Google Scholar]
- 21. Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315-29 [DOI] [PubMed] [Google Scholar]
- 22. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lombard DB, Alt FW, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper HM, Spelbrink JN. The human Sirt3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279-85 [DOI] [PubMed] [Google Scholar]
- 26. Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560-7 [DOI] [PubMed] [Google Scholar]
- 28. Jin L, Galonek H, Israelian K, et al. Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci. 2009;18:514-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao J, Lu Z, Joseph JJ, et al. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J Cell Biochem. 2010;110:238-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HS, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662-7 [DOI] [PubMed] [Google Scholar]
- 34. Tudek B, Winczura A, Janik J, et al. Involvement of oxidatively damaged DNA and repair in cancer development and aging. Am J Transl Res. 2010;2:254-84 [PMC free article] [PubMed] [Google Scholar]
- 35. Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-60 [DOI] [PubMed] [Google Scholar]
- 37. Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10:399-406 [DOI] [PubMed] [Google Scholar]
- 38. Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov. 2006;5:577-84 [DOI] [PubMed] [Google Scholar]
- 39. Mao L, El-Naggar AK, Fan YH, et al. Telomerase activity in head and neck squamous cell carcinoma and adjacent tissues. Cancer Res. 1996;56:5600-4 [PubMed] [Google Scholar]
- 40. Chen HH, Yu CH, Wang JT, et al. Expression of human telomerase reverse transcriptase (hTERT) protein is significantly associated with the progression, recurrence and prognosis of oral squamous cell carcinoma in Taiwan. Oral Oncol. 2007;43:122-9 [DOI] [PubMed] [Google Scholar]
- 41. Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michishita E, McCord RA, Boxer LD, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 2009;8:2664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narala SR, Allsopp RC, Wells TB, et al. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell. 2008;19:1210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alhazzazi TY, Kamarajan P, Joo N, et al. Sirtuin-3 (SIRT3), a novel potential therapeutic target for oral cancer. Cancer. 2011;117:1670-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamarajan P, Alhazzazi TY, Danciu T, et al. Receptor-interacting protein (RIP) and sirtuin-3 (SIRT3) are on opposite sides of anoikis and tumorigenesis. Cancer. 2012;118:5800-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku-70. Mol Cell Biol. 2008;28:6384-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD(+) levels dictate cell survival. Cell. 2007;130:1095-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sundaresan NR, Gupta M, Kim G, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hafner AV, Dai J, Gomes AP, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY). 2010;2:914-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pillai VB, Sundaresan NR, Kim G, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim SH, Lu HF, Alano CC. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One. 2011;6:e14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li S, Banck M, Mujtaba S, et al. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One. 2010;5:e10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ashraf N, Zino S, Macintyre A, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer. 2006;95:1056-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695-705 [DOI] [PubMed] [Google Scholar]
- 56. Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441-4 [DOI] [PubMed] [Google Scholar]
- 57. Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794-8 [PubMed] [Google Scholar]
- 58. Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1-3 [DOI] [PubMed] [Google Scholar]
- 59. Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131-41 [DOI] [PubMed] [Google Scholar]
- 60. Gamaley IA, Klyubin IV. Roles of reactive oxygen species: signaling and regulation of cellular functions. Int Rev Cytol. 1999;188:203-55 [DOI] [PubMed] [Google Scholar]
- 61. Chen CJ, Fu YC, Yu W, Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun. 2013;430:798-803 [DOI] [PubMed] [Google Scholar]
- 62. Fu J, Jin J, Cichewicz RH, et al. Trans-(-)-epsilon-viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. J Biol Chem. 2012;287:24460-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Finley LW, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30: 2986-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han SS, Yun H, Son DJ, et al. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol Cancer. 2010;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle. 2007;6:2669-77 [DOI] [PubMed] [Google Scholar]
- 71. Pfister JA, Ma C, Morrison BE, D’Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One. 2008;3:e4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marfe G, Tafani M, Indelicato M, et al. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J Cell Biochem. 2009;106:643-50 [DOI] [PubMed] [Google Scholar]
- 73. Grubisha O, Rafty LA, Takanishi CL, et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem. 2006;281:14057-65 [DOI] [PubMed] [Google Scholar]
- 74. Zhang YY, Zhou LM. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem Biophys Res Commun. 2012;423:26-31 [DOI] [PubMed] [Google Scholar]
- 75. Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453-6 [DOI] [PubMed] [Google Scholar]
- 76. Caamano J, Zhang SY, Rosvold EA, Bauer B, Klein-Szanto AJ. p53 alterations in human squamous cell carcinomas and carcinoma cell lines. Am J Pathol. 1993;142:1131-9 [PMC free article] [PubMed] [Google Scholar]
- 77. Van Damme M, Crompot E, Meuleman N, et al. HDAC isoenzyme expression is deregulated in chronic lymphocytic leukemia B-cells and has a complex prognostic significance. Epigenetics. 2012;7:1403-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Finley LW, Haigis MC. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med. 2012;18:516-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haigis MC, Deng CX, Finley LW, Kim HS, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72:2468-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu J, Huang J, Yao WY, et al. The origins of vacularization in tumors. Front Biosci. 2012;17:2559-65 [DOI] [PubMed] [Google Scholar]
- 81. Potente M, Ghaeni L, Baldessari D., et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fukuda S, Kaga S, Zhan L, et al. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44:43-9 [DOI] [PubMed] [Google Scholar]
- 83. Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971-80 [DOI] [PubMed] [Google Scholar]
- 85. Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444-8 [DOI] [PubMed] [Google Scholar]
- 86. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Galli M, Van Gool F, Leo O. Sirtuins and inflammation: friends or foes? Biochem Pharmacol. 2011;81:569-76 [DOI] [PubMed] [Google Scholar]
- 89. Horio Y, Hayashi T, Kuno A, Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clin Sci (Lond). 2011;121:191-203 [DOI] [PubMed] [Google Scholar]
- 90. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vakhrusheva O, Smolka C, Gajawada P, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703-10 [DOI] [PubMed] [Google Scholar]
- 93. Van Gool F, Galli M, Gueydan C, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park). 2011;25:400-10, 13 [PubMed] [Google Scholar]
- 95. Koyama T, Kume S, Koya D, et al. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med. 2011;51:1258-67 [DOI] [PubMed] [Google Scholar]
- 96. Capettini LS, Montecucco F, Mach F, et al. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18:963-70 [DOI] [PubMed] [Google Scholar]
- 97. Ferrer MD, Tauler P, Sureda A, Tur JA, Pons A. Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci. 2009;27:49-58 [DOI] [PubMed] [Google Scholar]
- 98. Mestre-Alfaro A, Ferrer MD, Banquells M, et al. Body temperature modulates the antioxidant and acute immune responses to exercise. Free Radic Res. 2012;46:799-808 [DOI] [PubMed] [Google Scholar]
- 99. Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453-9 [DOI] [PubMed] [Google Scholar]
- 100. Nakane K, Fujita Y, Terazawa R, et al. Inhibition of cortactin and SIRT1 expression attenuates migration and invasion of prostate cancer DU145 cells. Int J Urol. 2012;19:71-9 [DOI] [PubMed] [Google Scholar]
- 101. Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kamarajan P, Bunek J, Lin Y, Nunez G, Kapila YL. Receptor-interacting protein shuttles between cell death and survival signaling pathways. Mol Biol Cell. 2010;21:481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kurenova E, Xu LH, Yang X, et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24:4361-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151-9 [DOI] [PubMed] [Google Scholar]
- 105. Grimshaw MJ, Cooper L, Papazisis K, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sakuma Y, Takeuchi T, Nakamura Y, et al. Lung adenocarcinoma cells floating in lymphatic vessels resist anoikis by expressing phosphorylated Src. J Pathol. 2010;220:574-85 [DOI] [PubMed] [Google Scholar]
- 107. Iwanicki MP, Davidowitz RA, Ng MR, et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1:144-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285-91 [DOI] [PubMed] [Google Scholar]
- 110. Shen HM, Lin Y, Choksi S, et al. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lin Y, Choksi S, Shen HM, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822-8 [DOI] [PubMed] [Google Scholar]
- 112. Bosch-Presegue L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2:648-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lim CS. SIRT1: tumor promoter or tumor suppressor? Med Hypotheses. 2006;67:341-4 [DOI] [PubMed] [Google Scholar]
- 114. Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Galli U, Mesenzani O, Coppo C, et al. Identification of a sirtuin 3 inhibitor that displays selectivity over sirtuin 1 and 2. Eur J Med Chem. 2012;55:58-66 [DOI] [PubMed] [Google Scholar]
- 116. Ota H, Tokunaga E, Chang K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176-85 [DOI] [PubMed] [Google Scholar]
- 117. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137-48 [DOI] [PubMed] [Google Scholar]
- 118. Heltweg B, Gatbonton T, Schuler AD, et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006;66:4368-77 [DOI] [PubMed] [Google Scholar]
- 119. Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: the road ahead. Front Pharmacol. 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Camins A, Sureda FX, Junyent F, et al. Sirtuin activators: designing molecules to extend life span. Biochim Biophys Acta. 2010;1799:740-9 [DOI] [PubMed] [Google Scholar]
- 122. Patel KR, Scott E, Brown VA, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161-9 [DOI] [PubMed] [Google Scholar]