Abstract
Cells must continuously respond to stressful insults via the upregulation of cytoprotective pathways. The longevity factor and deacetylase SIRT1 plays a critical role in coordinating this cellular response to stress. SIRT1 activity and levels are regulated by cellular stressors, including metabolic, genotoxic, oxidative, and proteotoxic stress. As a stress sensor, SIRT1 impacts cell survival by deacetylating substrate proteins to drive the cell towards a cytoprotective pathway. Extreme stress conditions, however, can cause SIRT1 to lead cells down an apoptotic pathway instead. SIRT1 is frequently dysregulated in cancer cells and has been characterized to have a dual role as both an oncogene and a tumor suppressor, likely due to its pivotal function in regulating cytoprotection. Recently, the ability of SIRT1 to regulate HSF1-dependent induction of the heat shock response has highlighted another pathway through which SIRT1 can modulate cytoprotection. Activation of HSF1 results in the production of cytoprotective chaperones that can facilitate the transformed phenotype of cancer cells. In this review, we discuss the stress-dependent regulation of SIRT1. We highlight the role of SIRT1 in stress management and cytoprotection and emphasize SIRT1-dependent activation of HSF1 as a potential mechanism for cancer promotion.
Keywords: SIRT1, metabolic stress, stress responses, heat shock response, HSF1, chaperones, cytoprotection, cancer, apoptosis, genotoxic stress
Introduction to Sirtuins
The sirtuin family is evolutionarily conserved from prokaryotes to eukaryotes.1 Silent information regulator 2 (Sir2), the original sirtuin to be characterized, was first observed in yeast during an extensive screen for silencing factors.2 Sir2 is a class III NAD+-dependent histone deacetylase that causes transcriptional silencing due to the removal of histone acetyl groups, resulting in the tighter packaging of chromatin.3 Mammalian cells contain 7 sirtuin family members, SIRT1 to SIRT7.4,5 The mechanism of sirtuin deacetylation is unique in that the SIRT protein binds to a NAD+ molecule, thus cleaving nicotinamide and transferring the acetyl group from a substrate protein onto the NAD+ backbone.6 The result is a 1:1:1 ratio of nicotinamide, O-acetyl-ADP-ribose, and the deacetylated substrate protein as measured by HPLC quantification.6 In the absence of enzyme or acetylation of the substrate protein, no ADP-ribose or nicotinamide is generated from available NAD+, thus establishing the necessity of NAD+ hydrolysis in the catalysis of SIRT1 deacetylation activity.6 Additionally, SIRT3, SIRT4, and SIRT6 possess ADP-ribosyltransferase activity capable of transferring the O-acetyl-ADP-ribose onto acceptor protein residues.5
Interest in sirtuins has grown with their association with longevity. It was found that an extra copy of Sir2 transformed into yeast resulted in a reduction in recombination of ribosomal DNA and a subsequent 30% increase in the life span of the yeast mother cell.7 The Sir2 homolog Sir2.1 was later discovered to extend the life span in Caenorhabditis elegans, suggesting that Sir2 homologs and the NAD+/NADH ratio may also be responsible for extending the life span in higher eukaryotes such as mammals.8 While several studies have supported the role of Sir2 in longevity, recently, the impact of sirtuins in the extension of fly and worm life spans has been questioned due to confounding issues with genetic background.9 However, while new experiments using C. elegans with identical genetic backgrounds show that the original effect on the life span by Sir2 overexpression was overestimated, there is still a 10% to 14% extension in life span observed by increased Sir2 levels, thus continuing to support a role for SIRT1 as a longevity factor.10
As might be expected for a factor that can extend the life span, SIRT1 has been characterized to promote cytoprotection upon exposure to stressful conditions. Here, we review the regulation of SIRT1 activity and levels by stress and the role of SIRT1 in facilitating cytoprotection. We discuss the dual role of SIRT1 as both an oncogene and a tumor suppressor. Finally, we speculate on the role of SIRT1 in the induction of the heat shock response and molecular chaperones as a mechanism for cancer promotion.
Regulation of SIRT1 Activity by Stress
The deacetylase activity of SIRT1 has been well documented in the regulation of several stress-induced transcription factors including p53,11,12 HSF1,13-15 NF-κB,16-18 PGC-1α,19,20 and the FOXO family of transcription factors.21-23 The activation of SIRT1 upon stress may therefore be an evolutionarily conserved process to drive cellular homeostasis by invoking a variety of stress response pathways. As a critical stress sensor, it follows that SIRT1 activity and levels are modulated by multiple cellular stresses, thus allowing for the coordination of the appropriate cellular response.
Regulation of SIRT1 activity by metabolic stress
Sirtuins are linked to metabolism based on their unique ability to break down NAD+ during protein deacetylation, resulting in the formation of nicotinamide and O-acetyl-ADP-ribose.24 Therefore, a prime mode of the regulation of SIRT1 activity is through metabolic stress. SIRT1 depends on NAD+ as a critical co-factor for enzymatic activity, therefore indicating SIRT1 as an energy sensor. Changes in levels of NAD+, NADH, or their ratio, resulting from changes in diet or metabolic status, can lead to changes in SIRT1 activity.
Various enzymes in the NAD+ salvage pathway have also been implicated in the regulation of SIRT1 activity (Fig. 1). Nicotinamide phosphoribosyltransferase (NAMPT) is an enzyme that converts nicotinamide to nicotinamide mononucleotide (NMN), which then reacts with ATP to regenerate NAD+.25 Increased NAMPT activity has been shown to activate SIRT1 activity in human vascular smooth muscle cells.26 Interestingly, NAMPT is induced after some forms of stress including complete serum removal, thus linking metabolic stress pathways with other stress pathways.26 NAMPT levels decrease as cells age, and the resulting decline in NAD+ levels may contribute to the lowered SIRT1 activity observed upon aging.26
Figure 1.

Model of SIRT1-dependent deacetylation of substrate proteins. SIRT1 binds to NAD+ and an acetylated substrate protein to catalyze the transfer of the acetyl group to the NAD backbone, thus resulting in the deacetylated substrate protein, nicotinamide, and O-acetyl-ADP-ribose in a 1:1:1 ratio. Upon triggering of the NAD+ salvage pathway, NAMPT catalyzes the conversion of nicotinamide to NMN. NMN is then converted back to NAD+ by nicotinamide mononucleotide adenylyltransferase 1 to 3. CR has been shown to trigger the NAD+ salvage pathway by activating NAMPT, and AMPK promotes an intracellular increase in NAD+ levels.
In yeast, worms, and flies, nicotinamide is recycled back to NAD+ in 4 steps that compose the NAD+ biogenesis pathway. The first step is nicotinamide catalysis by PNC1 to produce nicotinic acid. PNC1 is upregulated by environmental stressors, leading to an increased stress resistance and life span in worms and flies.27-29 Thus, PNC1 can promote cell survival in response to environmental stress.
Caloric restriction (CR) is a 30% to 40% decrease in dietary intake with maintained nutrition that has been shown to increase longevity and protect against age-related disease.30 The life extension effects of CR were first established as early as 1935 using calorically restricted rats31 and have now been extended to yeast,32,33 nematodes,34,35 flies,36,37 and mice.38 In fasted mice, an increase in NAMPT activity correlates with an increase in NAD+ levels and enhances SIRT1 transcriptional activity.39 Interestingly, it has been reported that a 10-fold change in cellular NAD+ concentration is required to affect Sir2 activity in yeast.40 However, CR studies in yeast have shown that Sir2 activity is regulated by a reduction of NADH, a competitive inhibitor of Sir2.41,42 CR was found to increase the replicative life span of budding yeast by Sir2 activation due to decreasing the NADH levels, resulting in an increase in the NAD+/NADH ratio.41 Furthermore, the deletion of Sir2 blocks the beneficial effects of CR on the yeast life span.43
AMP-activated protein kinase (AMPK), activated in response to increasing amounts of AMP, functions as an energy sensor that responds to cellular metabolic stress including CR.44 SIRT1 and AMPK are therefore 2 evolutionarily conserved energy-sensing molecules that are vital in the regulation of energy homeostasis, with SIRT1 sensing change to the NAD+/NADH ratio and AMPK sensing change to the AMP/ATP ratio.45 AMPK increases the NAD+/NADH ratio in skeletal muscle after exposure to an AMPK activator (AICAR),45 proving AMPK to be an indirect activator of SIRT1. The role of AMPK as an energy sensor and regulator of metabolism led to the investigation of its function in the life extension effects observed in a low glucose environment. A change in AMPK activity coupled with increased NAD+ levels was found 18 to 24 hours and 30 to 36 hours after glucose restriction, respectively, in skeletal muscle. This indicates that AMPK activity precedes the change in NAD+ levels in mammalian cells, resulting in an increase in SIRT1 activity in a low glucose environment.46 Together, AMPK and SIRT1 may lead to improved fitness in C. elegans, as AMPK and SIRT1 homologs are important regulators of the life span.47,48
Not only is SIRT1 regulated in part by metabolism, but SIRT1 is also required for the CR phenotype. CR produces complex behavioral changes in mammals, including increased physical activity and an increase in distance coverage, which is most likely associated with foraging behavior.49 Moreover, wild-type mice and SIRT1 knockout mice demonstrate vastly different phenotypes upon CR, with the SIRT1 knockout mice exhibiting a phenotype very similar to wild-type control mice that are not on a restricted diet.50 Recently, it was shown that CR can synergize with heat shock stress to promote increased thermotolerance and fitness in a sir2.1-dependent manner.51 sir2.1, the mammalian SIRT1 homolog, is also necessary for the cytoprotection conferred by CR and heat shock to preserve movement in a C. elegans polyglutamine neurodegenerative disease model.51 SIRT1, therefore, is an energy sensor that can coordinate the response to metabolic stress at both the cellular and the organismal level.
Regulation of SIRT1 activity by protein modulators
As SIRT1 is a critical coordinator of cellular stresses, including metabolic stress, its activity must be finely regulated. Two proteins that are known to function as SIRT1 modulators are Deleted in Breast Cancer 1 (DBC1) and Active Regulator of SIRT1 (AROS). DBC1 was initially cloned from a region homozygously deleted in 3.5% of breast cancers.52 DBC1 has been found to directly interact with SIRT1 and inhibit deacetylase activity both in vivo and in vitro.53 DBC1-mediated downregulation of SIRT1 deacetylates p53 and inhibits p53 transcriptional activity. The repression of SIRT1 activity by DBC1 leads to an increase in p53 acetylation and therefore an upregulation of p53-dependent apoptotic activity. Likewise, the RNA interference of DBC1 results in an increase of SIRT1-mediated deacetylation, thus inhibiting p53-dependent apoptosis.54 In a similar manner, DBC1 has also been shown to regulate the acetylation status of the SIRT1 targets peroxisome proliferator–activated receptor γ (PPARγ) and HSF1.14,55 Interestingly, the DBC1-SIRT1 interaction increases following DNA damage and oxidative stress. The stress-induced DBC1-SIRT1 interaction requires ATM-dependent phosphorylation of DBC1 at Thr454, thus creating a second binding site for SIRT1.56 Therefore, stressful conditions do not only activate SIRT1 but can also blunt SIRT1 activity, suggesting that the cell has the ability to fine-tune the control of this factor.
AROS, a small ribosomal binding protein, has been shown to interact with SIRT1 and modulate its activity. In contrast to DBC1, AROS increases the deacetylase function of SIRT1. AROS enhances SIRT1-mediated deacetylation of p53, thus inhibiting p53-mediated transcriptional activity.57 Recently, it was shown that AROS, like DBC1, also has an impact on HSF1 acetylation status and the heat shock response.14 The AROS-SIRT1 interaction is likely another way in which the cell can fine-tune stress-induced SIRT1 activity. It will be interesting to investigate which cellular stresses can regulate the AROS-SIRT1 interaction.
Regulation of SIRT1 activity by stress-inducible posttranslational modifications
SIRT1 is highly modified by posttranslational modifications, and these modifications are regulated by cellular stress. Mass spectrometry has identified 13 phosphorylation sites within SIRT1.58 One of the kinases identified to phosphorylate SIRT1 is the c-Jun N-terminal kinase (JNK). JNK inducibly binds to and phosphorylates SIRT1 upon oxidative stress at Ser27, Ser47, and Thr530, resulting in increased nuclear localization of SIRT1.59 JNK belongs to the mitogen-activated protein kinase (MAPK) family and is responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock.60 This could explain, in part, how these various stress stimuli lead to an increase in SIRT1 activity on its nuclear targets.
Mammalian target of rapamycin (mTOR) is another stress-inducible kinase that has been identified to phosphorylate SIRT1. mTOR phosphorylates SIRT1 on Ser47, thus inhibiting SIRT1 activity.61 mTOR is a key cellular sensor of nutrient and energy levels as well as redox status and is activated by increased levels of insulin, growth factors, amino acids, and oxidative stress.62 SIRT1 physically interacts with the mTOR-Raptor complex, and a single amino acid substitution in the TOR signaling motif in SIRT1 prevents Ser47 phosphorylation.61 Under conditions of CR, mTOR activity is inhibited, thus leading to enhanced SIRT1 activity.
Casein kinase II (CK2) is another stress-activated kinase implicated in SIRT1 regulation. Four CK2 phosphorylation sites have been identified in murine SIRT1 at Ser154, Ser649, Ser651, and Ser683.63 Two of these sites are also phosphorylated in human SIRT1 at the corresponding amino acids Ser659 and Ser661.64 Ser659 and Ser661 lie within the Essential for SIRT1 Activity (ESA) region of human SIRT1.65 The ESA region interacts with the catalytic domain of SIRT1, thus activating its catalytic activity and increasing the affinity for SIRT1 substrates. Phosphorylation at Ser659 and Ser661 has been proposed to enhance the interaction of the ESA region with the catalytic core. It is possible that this would affect DBC1 binding to SIRT1, as the binding site for ESA in the catalytic domain is also the binding site for DBC1.53 Therefore, the control of SIRT1 function by its own C-terminal domain and the regulation of this interaction by CK2 may be an important regulatory mechanism for SIRT1 activity.
Aside from phosphorylation, SIRT1 can be modified by sumoylation, methylation, and transnitrosylation. SIRT1 is sumoylated at Lys734, and SUMO1/Sentrin-Specific Peptidase 1 (SENP1), a nuclear desumoylase, removes this modification.66 Sumoylation of SIRT1 increases its catalytic activity as measured by p53 deacetylation, and desumoylation by SENP1 reduces its deacetylase activity.66 Stress-inducing agents, including ultraviolet radiation and hydrogen peroxide, were found to promote the association of SIRT1 with SENP1.66 Therefore, certain stress-inducing agents may counteract the antiapoptotic activity of SIRT1 by recruiting SENP1 to SIRT1, resulting in the desumoylation and inactivation of SIRT1.
SIRT1 is methylated via the methyltransferase Set7/9, which methylates SIRT1 at Lys233, Lys235, Lys236, and Lys238. SIRT1 interacts with Set7/9 both in vitro and in vivo, and the interaction is increased upon DNA damage. SIRT1 methylation may inhibit SIRT1 deacetylation activity, as DNA damage inhibits the interaction of SIRT1 and p53, leading to an increase in p53 acetylation levels.67
SIRT1 has also been found to be transnitrosylated by nitrosylated GAPDH.68 Transnitrosylation of SIRT1 inhibits SIRT1 deacetylase activity, as measured by an increase in levels of acetylation of the SIRT1 target PGC-1α. Cys387 and Cys390 within the catalytic core of SIRT1 are targeted by nitrosylation. These cysteines are involved in the coordination of Zn2+ binding, and nitrosylation of these sites may result in SIRT1 protein misfolding. Multiple types of posttranslational modifications, therefore, are involved in fine-tuning SIRT1 activity in response to stress.
Regulation of SIRT1 Levels by Stress
Stress-responsive regulation of SIRT1 expression levels by transcription factors
In addition to the regulation of SIRT1 at the activity level, SIRT1 can also be regulated at the expression level. SIRT1 expression is altered by various stress-inducible transcription factors including p53, c-Myc, FOXO family members, and certain PPAR family members. p53 is inducible by a myriad of stresses, including DNA damage and oxidative stress. p53 has been shown to regulate SIRT1 expression by binding to the sirt1 promoter at 2 binding sites to repress sirt1 mRNA expression.69 As p53 is a SIRT1 deacetylation target, this provides a feedback loop to regulate SIRT1 expression by modulating the acetylation status and thus the activity state of this protein. Consistent with a negative role for p53 in regulating SIRT1 expression, p53-null mice show increased basal expression of SIRT1 in certain tissues.70 The negative regulation of SIRT1 by p53 could also be an explanation for why SIRT1 levels can be higher in tumors that have lost p53.
The proto-oncogene c-Myc is activated through the MAPK pathway and is responsive to various mitogenic and stress signals. c-Myc induces SIRT1 expression by binding to the sirt1 promoter and activating transcription.71 As SIRT1 deacetylates c-Myc, resulting in decreased c-Myc stability, a c-Myc–SIRT1 feedback loop likely exists that may be relevant in cancer.
FOXO transcription factors are SIRT1 targets that can also increase SIRT1 expression. FOXO1, which can be regulated by insulin levels and is thus under metabolic control, can increase SIRT1 expression by binding to FOXO1 binding sites in the sirt1 promoter.70,72 SIRT1-mediated deacetylation of FOXO1 leads to an increase in the transcriptional activity of FOXO1, thus indicating a positive feedback loop between SIRT1 and FOXO1.72 FOXO3, on the other hand, translocates into the nucleus in the absence of nutrients and interacts with p53, inhibiting its suppressive activity on SIRT1 transcription and thus leading to increased SIRT1 levels.70 FOXO3 is also a deacetylase target of SIRT1, but unlike FOXO1, SIRT1 can either activate or inhibit the transcriptional activity of this factor depending on the circumstances.22
PPARs are nuclear receptors that are also regulated by the metabolic state. The 3 subtypes, PPARα, PPARγ, and PPARβ/δ, are expressed in multiple organs and regulate different physiological functions such as energy metabolism, insulin action, and inflammation. Fasting can induce PPARα activity in mice and induce SIRT1 expression through a PPARα binding site in the sirt1 promoter.39 While PPARα may not be a direct target of SIRT1, SIRT1 can regulate the activity of its co-activator PGC-1α, thus creating a positive feedback loop.73 PPARβ/δ can also induce SIRT1 expression by inducing Sp1 binding to the sirt1 promoter.74 PPARγ, in contrast to PPARα and PPARβ/δ, represses SIRT1 expression through direct interactions with the sirt1 promoter.55 As PPARγ is a deacetylation target of SIRT1, this suggests the presence of a negative feedback loop.
Hypermethylated in Cancer 1 (HIC1) is a transcriptional repressor that can bind to the sirt1 promoter and negatively regulate sirt1 mRNA expression.75 The repressive effect of HIC1 is dependent on the carboxyl terminal binding protein (CtBP), an NAD+/NADH redox sensor.76 Nutrient deprivation decreases the interaction of CtBP with HIC1, thus decreasing the repressor function of HIC1 and increasing SIRT1 expression.76 HIC1 is also a deacetylase target of SIRT1, thus providing another negative feedback loop mechanism.77 Therefore, multiple stress-responsive transcription factors can regulate SIRT1 levels and thus influence the ability of SIRT1 to deacetylate target proteins.
Stress-responsive regulation of SIRT1 expression levels by RNA stability and translation control
Regulation of RNA stability and translation are other ways in which stress can regulate SIRT1 levels. A stress-regulated mRNA binding protein, as well as specific miRNAs, has been demonstrated to regulate SIRT1 expression levels. HuR, a ubiquitously expressed mRNA binding protein, increases SIRT1 levels by binding to the 3′ UTR of p53 mRNA, thus stabilizing it.78 Oxidative stress triggers the dissociation of the HuR-SIRT1 mRNA complex, thereby promoting SIRT1 mRNA degradation, decreasing SIRT1 transcriptional levels, and lowering cell survival. The cell cycle checkpoint kinase Chk2 is implicated in this process, as it is activated by hydrogen peroxide, interacts with HuR, and is predicted to phosphorylate HuR at residues Ser88, Ser100, and Thr118. Mutation of these residues reveals a complex pattern of HuR binding, with Ser100 appearing to be important for HuR dissociation from SIRT1 mRNA after hydrogen peroxide treatment.78
Several stress-regulated miRNAs control SIRT1 expression by binding to sirt1 mRNA and suppressing translation or reducing mRNA stability. The first miRNA described to regulate SIRT1 was miR-34a.79 This miRNA was first discovered as a tumor suppressor in neuroblastoma.80 miR-34a is regulated by metabolism, as its levels were shown to increase in mice fed a high-fat diet.81 miR-195, also regulated by metabolism, has been shown to regulate SIRT1 expression in cardiomyocytes.82 miR-199a, induced by hypoxia, targets both SIRT1 and the hypoxia-inducible factor HIF-1α.83 Therefore, interactions with RNA binding proteins and miRNAs can influence SIRT1 expression levels and thus the cellular response to stress.
SIRT1, Stress, and Cellular Fitness
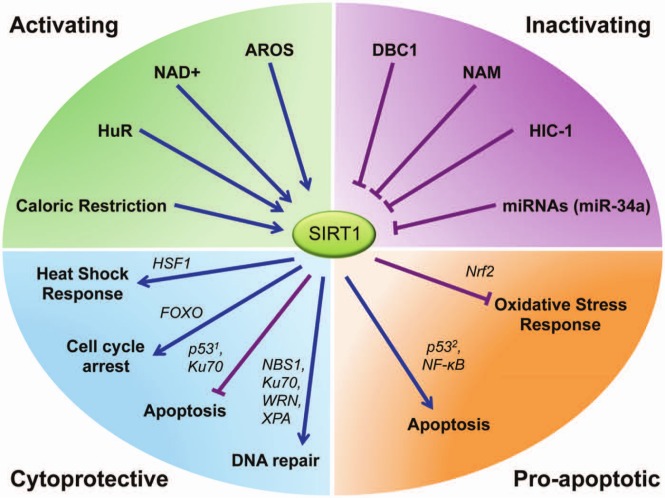
SIRT1 can function in either a cytoprotective manner or a proapoptotic manner by activating stress response pathways through a number of factors including p53,11 FOXO,22 HSF1,13,15 Nuclear Factor Erythroid-Derived 2-Related Factor 2 (Nrf2),84 and various DNA damage repair factors85-91 (Fig. 2). Under moderate stress, the result of modulation of these pathways is to promote metabolic homeostasis, prevent apoptosis, and repair oxidative cellular damage and damaged DNA. Under chronic or extreme stress conditions, however, SIRT1 can promote cell death by inducing apoptosis.
Figure 2.
SIRT1 is activated and inhibited by stress-responsive factors and plays a dynamic role in regulating cytoprotection and apoptosis. SIRT1 levels and activity are mediated by metabolism and interactions with various stress-responsive regulators. SIRT1 activation by CR, HuR, NAD+, and AROS influences the activity of several targeted factors including HSF1, nuclear p53, FOXO, and DNA repair proteins. Deacetylation of these substrate proteins results in a decrease of apoptosis and an increase in stress adaptation. Conversely, SIRT1-mediated deacetylation of Nrf2, NF-κB, and cytoplasmic p53 can lead to a proapoptotic environment due to increased ROS and decreased p53 activity. These dynamic processes of SIRT1 may be inhibited by its interactions with DBC1, nicotinamide, HIC-1, and miRNAs. 1 = nuclear; 2 = cytoplasmic.
SIRT1 promotes cytoprotection upon moderate stress
Under moderate stress conditions, SIRT1 promotes cytoprotection via modulation of a number of cell survival regulators, including p53, FOXO, HSF1, NF-κB, and DNA damage response proteins. p53 is a well-characterized substrate of SIRT1 that is critical in cell cycle checkpoint regulation, apoptosis, and tumor suppression.11,12 p53 is transcriptionally activated by a number of protein kinases including ATM,92 ATR,93 and the MAPK family.94 The activity of p53 is mediated by its acetylation status, where deacetylation reduces activity, thus allowing cells to bypass p53-mediated apoptosis.75,95,96 SIRT1-null mice show that p53 is hyperacetylated when compared to wild-type mice,97 while overexpression of SIRT1 in cell lines suppressed p53-dependent apoptosis and increased cell survival after exposure to oxidative stress.12 In addition, the p53 family member p73 is also deacetylated by SIRT1, resulting in the suppression of apoptosis.98
The FOXO family of transcription factors has proven to be important in many biological functions, implicating it as an essential factor in cellular fitness and protection. These functions include checkpoint regulation99 and detoxification.100 In response to oxidative or genotoxic stress, FOXO proteins translocate to the nucleus and activate target genes involved in cytoprotection and DNA repair.22,101 FOXO interactions with SIRT1 are controlled during stress stimuli. Specifically, FOXO3 and SIRT1 have been shown to form a complex upon stress, allowing for SIRT1-mediated deacetylation of this transcription factor.22 FOXO3 deacetylation potentiates cell cycle arrest and induces the transcription of DNA repair target genes while simultaneously attenuating FOXO-mediated apoptosis, thus allowing the cell to avoid apoptosis and maintain stress resistance. Deacetylation of FOXO family members leads to an increase in DNA binding affinity of this transcription factor, resulting in the transcription of cytoprotective target genes,102,103 thereby allowing for cytoprotection and cell survival.22,104,105
The heat shock response regulator HSF1 has recently been established as a SIRT1 substrate.13,15 The heat shock response is the cell’s cytoprotective response to protein-damaging stress that results in the induction of molecular chaperones to restore proteostasis and promote cell survival. SIRT1 deacetylates HSF1 and thus promotes DNA binding.13 The interaction of SIRT1 with HSF1 has been shown to be induced by heat shock.15 Induction of the heat shock response by SIRT1, therefore, is another way to promote cytoprotection. The regulation of HSF1 by SIRT1 and implications in cancer will be discussed later in this review.
In addition to the regulation of transcription factor activity, SIRT1 has an established role in interactions with DNA damage response proteins. Deacetylation of the DNA repair proteins NBS1, Werner syndrome protein (WRN), xeroderma pigmentosum group A protein (XPA), and Ku70 by SIRT1 have all been shown to control genomic stability.86-91 The MRE11-RAD50-NBS1 complex is a conserved nuclease complex that can sense damaged DNA and regulate the cellular response to DNA double-strand breaks.106 The regulatory subunit of this complex, NBS1, is mutated in the human genetic disease Nijmegen breakage syndrome, a chromosomal instability disorder that results in multiple symptoms including microcephaly, radiation sensitivity, and predisposition to lymphoid malignancy.107 SIRT1 maintains NBS1 in a hypoacetylated state, thus allowing it to be responsive to DNA damage–induced phosphorylation by the ATM kinase.91 Phosphorylation activates NBS1, leading to the initiation of DNA damage repair.91
WRN, a RecQ DNA helicase family member, helps to maintain genome stability and is another target of SIRT1.107 Werner syndrome, an autosomal recessive disorder associated with premature aging and cancer predisposition, is caused by mutations in the WRN gene.108 SIRT1 interacts with WRN in a manner that is enhanced after DNA damage.89 The acetylation of WRN decreases its helicase and exonuclease activities, while deacetylation by SIRT1 reverses this effect.89
SIRT1 also interacts with XPA, a nucleotide excision repair (NER) factor that is essential for the NER process.109 The interaction occurs in an ultraviolet irradiation–inducible fashion.87 Complementation experiments using XPA-null cells with an acetylation mimic of XPA shows that SIRT1- mediated deacetylation of XPA is required for optimal NER.87
Ku70 is another DNA repair factor that can interact with SIRT1. Upon exposure to radiation, SIRT1 enhances both the DNA repair capacity of the cell and the deacetylation of Ku70.88 Overexpression of SIRT1 increases the repair of DNA strand breaks induced by radiation, while SIRT1 siRNA decreases this repair activity.88 SIRT1 forms a complex with Ku70, leading to deacetylation, suggesting that SIRT1 may modulate DNA repair activity through regulating the acetylation status of repair protein Ku70 following DNA damage.88
Interestingly, in addition to its function in DNA repair, Ku70 is one of several proteins that inhibit apoptosis by sequestering the proapoptotic factor BAX, a BCL-2 family member, from the mitochondria.110 SIRT1 has been implicated in promoting cytoprotection through Ku70 deacetylation, thus strengthening its ability to sequester the proapoptotic BAX protein.86 Upon nonstress conditions, BAX is localized in the cytosol complexed with Ku70.85,111 Upon DNA damage–inducing stress, Ku70 becomes acetylated, which breaks the interaction with BAX, allowing it to translocate to the mitochondria.85 SIRT1, through deacetylation of Ku70, can strengthen the interaction of Ku70 with BAX, thus preventing apoptosis and promoting cytoprotection.85 Therefore, SIRT1 can lead to enhanced cytoprotection through deacetylating transcription factors, DNA repair factors, and a factor directly involved in preventing apoptosis.
SIRT1 as a proapoptotic factor
While SIRT1 has been well established as a cytoprotective factor upon moderate stress, in certain cases, SIRT1 can induce apoptosis. High levels of reactive oxygen species (ROS), for instance, can cause SIRT1 to activate apoptosis through a number of different routes including Nrf2, NF-κB, and p53- controlled pathways. The transcription factor Nrf2 is a central regulator of the cellular response to oxidative stress.112 High ROS levels cause Nrf2 to bind to antioxidant response elements (AREs) within promoter regions of phase II detoxification enzymes, allowing for upregulation of superoxide dismutase and glutathione S-transferase genes, among other antioxidant gene targets.113 Acetylation of Nrf2 promotes DNA binding and target gene transcription, while deacetylation of Nrf2 disengages it from the ARE, resulting in transcriptional termination.114 Therefore, SIRT1 is a negative regulator of Nrf2 and consequently downregulates this adaptive response to oxidative stress.84
NF-κB is another transcription factor that can be activated by ROS and other cellular stress conditions. As NF-κB transcriptionally upregulates a number of survival genes, such as the IAP family of apoptotic inhibitors, the overactivation of NF-κB is common in tumorigenesis and chemotherapy resistance.115 SIRT1 has been shown to form a complex with the RelA/p65 subunit of NF-κB, deacetylating Lys310.116 This decreases NF-κB activity and downregulates survival genes, thus resulting in increased apoptosis.116
As a distinct mechanism to induce apoptosis at the mitochondrial level, high ROS can direct cytosolic p53 to induce apoptosis via the SIRT1-induced targeting of p53 to this organelle.117 In this pathway, deacetylated p53 binds to the outer membrane of the mitochondria and outcompetes BAX for binding to BCL-2 proteins. The subsequent activation of BAX then leads to the release of cytochrome c from the mitochondria and the induction of apoptosis.118 SIRT1, therefore, has dual functions of both promoting cytoprotection and apoptosis, depending on the nature and severity of the stress.
Activation of the Heat Shock Response by SIRT1 and Implications in Cancer
As certain conditions can cause SIRT1 to be cytoprotective, while others can cause SIRT1 to induce cell death, SIRT1 has been implicated both as an oncogene and as a tumor suppressor.119,120 Here, a role for SIRT1 as an oncogene through the activation of HSF1 and the cytoprotective heat shock response is explored.
SIRT1-dependent regulation of HSF1
As mentioned earlier, HSF1 has recently been established as a SIRT1 substrate13,15 and is the master regulator of the heat shock response, the cell’s cytoprotective molecular reaction to protein-damaging stress (Fig. 3). While the classic inducer of this adaptive stress response is heat shock, other stressors can also induce this response, including oxidative stress, heavy metals, and various pathophysiological states.121 The heat shock response results in the induction of molecular chaperone genes, called heat shock proteins (HSPs), which function to restore proteostasis and promote cell survival. In nonstressed cells, HSF1 exists in a monomeric form. Upon stress, HSF1 forms trimers that accumulate in the nucleus and bind to heat shock elements in the promoters of target genes.122 Hyperphosphorylation leads to transcriptional activity, and hsp genes are induced.123
Figure 3.
SIRT1-dependent regulation of HSF1 and the heat shock response. HSF1 exists as an inactive monomer in the cytoplasm. Upon induction of the heat shock response by denaturing stress, it trimerizes and translocates to the nucleus where it binds to heat shock elements found in the promoter regions of HSP genes. Transcription of hsp genes is promoted by the hyperphosphorylation of HSF1, while the attenuation of the heat shock response is regulated by a dual mechanism involving negative feedback inhibition from HSPs and acetylation at a critical lysine residue within the DNA binding domain of HSF1, causing loss of affinity for DNA. SIRT1 is a NAD+-dependent HDAC that deacetylates HSF1, thus promoting stress-induced HSF1 DNA binding ability and increasing HSP expression. Protein modulators AROS and DBC1 have recently been shown to impact hsp70 transcription, HSF1 acetylation status, and HSF1 recruitment to the DNA.14
SIRT1 regulates the heat shock response through deacetylation of HSF1.13 HSF1 can be acetylated at multiple sites, and the acetylation of Lys80 within the DNA binding domain inhibits DNA binding ability.13 Activation of SIRT1 leads to the deacetylation of HSF1, resulting in prolonged binding of HSF1 to the hsp70 promoter and induction of the heat shock response. Conversely, downregulation of SIRT1 promotes the attenuation of the heat shock response via increased HSF1 acetylation and decreased HSF1 DNA binding ability. Interestingly, the interaction of SIRT1 with HSF1 has been shown to be regulated by stress, as endogenous SIRT1 co-immunoprecipitates with HSF1 only upon heat shock in mouse embryonic fibroblasts (MEFs).15
The regulation of HSF1 and the heat shock response by SIRT1 have been shown to be biologically significant in a number of ways. As expected for a cytoprotective factor, the overexpression of SIRT1 was found to confer increased tolerance to high-temperature heat shock in 293T cells.13 As HSF1 and SIRT1 are both aging factors, it is interesting to note that WI-38 human fibroblast cells at late passage numbers show a decreased heat shock response and reduced activation of HSF1 DNA binding by heat shock that correlates with a reduced abundance of SIRT1.13
SIRT1 has also been shown to work together with HSF1 to protect against α-synuclein pathology, an aging-related disorder.15 In this study, a transgenic mouse was used bearing the human α-synuclein gene with the A53T mutation, which causes familial early-onset Parkinson disease.15 When the A53T mice were crossed with SIRT1 transgenic mice, SIRT1 was found to prolong the life span of the mice and to decrease α-synuclein aggregates in the mouse brain. SIRT1 was found to deacetylate HSF1 and increase HSP70 levels in the brains of the A53T mice but not in brains of mice not expressing α-synuclein A53T. Therefore, these combined results suggest that SIRT1 deacetylates HSF1 and activates chaperone expression only under stress conditions, including heat shock stress or aggregation-induced stress.
Association of HSPs and HSF1 with cancer
HSPs, the transcriptional targets of HSF1, are elevated in a number of cells and tissues from a variety of cancers including prostate,124 lung,125 pancreas,126 bladder,127 and breast.128 HSPs comprise several distinct classes of molecular chaperones that maintain proper protein function in the cell by facilitating the folding, translocation, and proteolytic turnover of many critical regulators of cell growth and cell death.129-131 Multiple studies have shown the upregulation of HSPs in cancers and its association with increased aggression and lower survival rates.132-135 Consequently, elevated HSP expression is associated with poor prognosis and resistance to chemotherapy136 and is often used as a biomarker for tumor progression and prognosis, although not all cancers (i.e., renal cell carcinoma) present this correlation.137 In particular, overexpression of HSP27, HSP70, and HSP90 seems to play crucial roles in cancer cell survival.138,139 For instance, overexpression of HSP27 in rat colon adenocarcinoma cells injected into syngeneic animals increased tumorigenicity, resulting in an increased tumor size and a delay in tumor regression.140 Overexpression of HSP70 in Rat-1 fibroblasts can lead to reversible oncogenic transformation.141 In tumor cells, knockdown of HSP70 with antisense oligomers led to the induction of apoptosis and the inhibition of tumor cell proliferation.142 The inhibition of HSP90 by small molecule inhibitors has also shown promising anticancer results.143 These combined results suggest that tumors may take advantage of chaperone-based cell survival mechanisms, thus allowing the cancer cells to proliferate.
The manner in which increased expression of HSPs promotes cancer is likely to occur through multiple cytoprotective mechanisms, including effects on maintaining proteostasis, preventing apoptosis, and facilitating growth-promoting signaling pathways. Rapidly proliferating tumor cells have increased dependency on protein folding for survival due in part to an increase in the expression of multiple proteins for oncogenic signaling pathways.144 In addition, the mutagenic nature of tumor cells results in an increase in polymorphic variants of essential proteins that may have folding difficulties, thus requiring increased assistance with protein folding for cell survival.143 HSPs, through their effects of promoting protein folding and maintaining proteostasis, are therefore likely to assist cancer cells in this regard.
In addition to effects on proteostasis, HSPs can act at several points in the apoptotic pathway to inhibit apoptosis, including preventing cytochrome c release, regulating the apoptosome, and preventing caspase activation.139 HSP70 has been widely studied with regard to apoptosis and has been shown to interfere with the assembly of the apoptosome by preventing the recruitment of procaspases 9 and 3.145 The mechanism by which HSP70 is cytoprotective against apoptosis may also be through inhibitory binding and sequestration of proapoptotic proteins.
Facilitating growth-promoting signaling pathways is another key way in which HSPs may aid in tumor progression. HSP90 is highly relevant in this regard due to its oncogenic client proteins, including mutant p53, NF-κB, HER2, B-Raf, and AKT.131 In fact, certain tumors have been found to be dependent on HSP90, and HSP90 inhibitors can selectively target these cancer cells.146 The overexpression of multiple HSPs in cancer cells could therefore help promote cell survival and aid cancer progression through multiple mechanisms.
HSF1, the master regulator of chaperones, has itself been implicated in cancer in a number of experiments. For instance, HSF1 was found to promote Ras-induced transformation, as HSF1-null MEFs produced fewer Ras-induced foci than wild-type MEFS.147 HSF1 is also required for transformation induced by HER2 in MCF-10A cells148 and was shown to cooperate with HER2 to promote mammary tumorigenesis and metastasis.149 In addition, HSF1 promotes lymphomas in p53-deficient mice.150 Oncogenesis has in fact recently been shown to be an inducer of the heat shock response due to the activation of HSF1 through unknown mechanisms.151 It may be that the variety of stresses common to cancer, such as hypoxia, lack of nutrients, ATP depletion, and acidosis, combine to promote HSF1 activity. HSF1 genome occupancy in cancer cells was found to include genes involved in cell cycle regulation, signaling, metabolism, adhesion, and translation as well as known hsp target genes.151 This set of gene targets could all be significant in promoting oncogenesis.
Implications of SIRT1-induced activation of the heat shock response in cancer
It is possible that the upregulation of SIRT1 observed in many cancers may facilitate HSF1 activation that is commonly observed in tumorigenesis. It is interesting that DBC1, the negative regulator of SIRT1, has been found to be homozygously deleted in certain breast cancers. DBC1 has been shown experimentally to inhibit HSF1 activity and the heat shock response.14 Therefore, deleting this inhibitor of SIRT1 may indirectly promote HSF1 activity in cancer as well as promote the deacetylation of p53, thus enabling cancer cell survival. Developing an understanding of how HSF1, SIRT1, and SIRT1 modulators influence cancer may lead to the discovery of new cancer therapies.
Final Thoughts
The role of SIRT1 in the activation and regulation of stress response mechanisms may represent a vital evolutionarily conserved function in cellular homeostasis. A better understanding of the role of SIRT1 in stress may provide insight into diseases marked by a dysfunction in critical adaptive stress responses including cancer.
Acknowledgments
The authors thank the members of the Westerheide laboratory for helpful discussion over the course of this review.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a departmental start-up grant from the Cell Biology, Microbiology and Molecular Biology Department at the University of South Florida to S.D.W.
References
- 1. Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888-902 [DOI] [PubMed] [Google Scholar]
- 2. Rine J, Strathern JN, Hicks JB, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fritze CE, Verschueren K, Strich R, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haigis MC, Guarente LP. Mammalian sirtuins: emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913-21 [DOI] [PubMed] [Google Scholar]
- 5. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landry J, Slama JT, Sternglanz R. Role of NAD(+) in the deacetylase activity of the SIR2-like proteins. Biochem Biophys Res Commun. 2000;278:685-90 [DOI] [PubMed] [Google Scholar]
- 7. Kaeberlein M, McVey M, Guarente L. Using yeast to discover the fountain of youth. Sci Aging Knowledge Environ. 2001;2001:pe1. [DOI] [PubMed] [Google Scholar]
- 8. Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227-30 [DOI] [PubMed] [Google Scholar]
- 9. Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1-2 [DOI] [PubMed] [Google Scholar]
- 11. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149-59 [DOI] [PubMed] [Google Scholar]
- 12. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137-48 [DOI] [PubMed] [Google Scholar]
- 13. Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raynes R, Pombier KM, Nguyen K, Brunquell J, Mendez JE, Westerheide SD. The SIRT1 modulators AROS and DBC1 regulate HSF1 activity and the heat shock response. PLoS One. 2013;8:e54364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J Neurosci. 2012;32:124-32 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83-105 [DOI] [PubMed] [Google Scholar]
- 18. Jung KJ, Lee EK, Kim JY, et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143-50 [DOI] [PubMed] [Google Scholar]
- 19. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434: 113-8 [DOI] [PubMed] [Google Scholar]
- 20. Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605-15 [DOI] [PubMed] [Google Scholar]
- 22. Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011-5 [DOI] [PubMed] [Google Scholar]
- 23. Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551-63 [DOI] [PubMed] [Google Scholar]
- 24. Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41-8 [DOI] [PubMed] [Google Scholar]
- 25. Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683-90 [DOI] [PubMed] [Google Scholar]
- 26. van der Veer E, Ho C, O’Neil C, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282: 10841-5 [DOI] [PubMed] [Google Scholar]
- 27. Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423: 181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallo CM, Smith DL, Jr, Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol. 2004;24:1301-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835-44 [DOI] [PubMed] [Google Scholar]
- 31. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size: 1935. Nutrition. 1989;5:155-71, discussion 72 [PubMed] [Google Scholar]
- 32. Kaeberlein M, Powers RW, 3rd, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193-6 [DOI] [PubMed] [Google Scholar]
- 33. Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288-90 [DOI] [PubMed] [Google Scholar]
- 34. Johnson TE. Caenorhabditis elegans 2007: the premier model for the study of aging. Exp Gerontol. 2008;43:1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C-elegans. Aging Cell. 2009;8:113-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187-98 [DOI] [PubMed] [Google Scholar]
- 37. Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37: 47-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayashida S, Arimoto A, Kuramoto Y, et al. Fasting promotes the expression of SIRT1, an NAD+-dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem. 2010;339:285-92 [DOI] [PubMed] [Google Scholar]
- 40. Anderson RM, Latorre-Esteves M, Neves AR, et al. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302:2124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahat O, Maoz N, Cohen HY. Multiple pathways regulating the calorie restriction response in yeast. J Gerontol A Biol Sci Med Sci. 2011;66:163-9 [DOI] [PubMed] [Google Scholar]
- 43. Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344-8 [DOI] [PubMed] [Google Scholar]
- 44. Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamilton B, Dong Y, Shindo M, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97-103 [DOI] [PubMed] [Google Scholar]
- 50. Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. [DOI] [PubMed] [Google Scholar]
- 51. Raynes R, Leckey BD, Jr, Nguyen K, Westerheide SD. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J Biol Chem. 2012;287:29045-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hamaguchi M, Meth JL, von Klitzing C, et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci U S A. 2002;99:13647-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583-6 [DOI] [PubMed] [Google Scholar]
- 54. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPARg-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010;38:7458-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yuan J, Luo K, Liu T, Lou Z. Regulation of SIRT1 activity by genotoxic stress. Genes Dev. 2012;26:791-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277-90 [DOI] [PubMed] [Google Scholar]
- 58. Sasaki T, Maier B, Koclega KD, et al. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nasrin N, Kaushik VK, Fortier E, et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vlahopoulos S, Zoumpourlis VC. JNK: a key modulator of intracellular signaling. Biochemistry (Mosc). 2004;69:844-54 [DOI] [PubMed] [Google Scholar]
- 61. Back JH, Rezvani HR, Zhu Y, et al. Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent inhibition of sirtuin 1. J Biol Chem. 2011;286:19100-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443-6 [DOI] [PubMed] [Google Scholar]
- 63. Kang H, Jung JW, Kim MK, Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One. 2009;4:e6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zschoernig B, Mahlknecht U. Carboxy-terminal phosphorylation of SIRT1 by protein kinase CK2. Biochem Biophys Res Commun. 2009;381:372-7 [DOI] [PubMed] [Google Scholar]
- 65. Kang H, Suh JY, Jung YS, Jung JW, Kim MK, Chung JH. Peptide switch is essential for Sirt1 deacetylase activity. Mol Cell. 2011;44:203-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang Y, Fu W, Chen J, et al. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu X, Wang D, Zhao Y, et al. Methyltransferase Set7/9 regulates p53 activity by interacting with sirtuin 1 (SIRT1). Proc Natl Acad Sci U S A. 2011;108:1925-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kornberg MD, Sen N, Hara MR, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804:1684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105-8 [DOI] [PubMed] [Google Scholar]
- 71. Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185:203-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okazaki M, Iwasaki Y, Nishiyama M, et al. PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocr J. 2010;57:403- 13 [DOI] [PubMed] [Google Scholar]
- 75. Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437-48 [DOI] [PubMed] [Google Scholar]
- 76. Zhang Q, Wang SY, Fleuriel C, et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104:829-33 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77. Dehennaut V, Loison I, Pinte S, Leprince D. Molecular dissection of the interaction between HIC1 and SIRT1. Biochem Biophys Res Commun. 2012;421:384-8 [DOI] [PubMed] [Google Scholar]
- 78. Abdelmohsen K, Pullmann R, Jr, Lal A, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25: 543-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712-5 [DOI] [PubMed] [Google Scholar]
- 80. Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017-22 [DOI] [PubMed] [Google Scholar]
- 81. Lee J, Padhye A, Sharma A, et al. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75-84 [DOI] [PubMed] [Google Scholar]
- 83. Rane S, He M, Sayed D, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cohen HY, Lavu S, Bitterman KJ, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627-38 [DOI] [PubMed] [Google Scholar]
- 86. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390-2 [DOI] [PubMed] [Google Scholar]
- 87. Fan W, Luo J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell. 2010;39:247-58 [DOI] [PubMed] [Google Scholar]
- 88. Jeong J, Juhn K, Lee H, et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39:8-13 [DOI] [PubMed] [Google Scholar]
- 89. Li K, Casta A, Wang R, et al. Regulation of WRN protein cellular localization and enzymatic activities by SIRT1-mediated deacetylation. J Biol Chem. 2008;283:7590-8 [DOI] [PubMed] [Google Scholar]
- 90. Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol Cell. 2007;27:149-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yuan Z, Seto E. A functional link between SIRT1 deacetylase and NBS1 in DNA damage response. Cell Cycle. 2007;6:2869-71 [DOI] [PubMed] [Google Scholar]
- 92. Talukder KA, Azmi IJ, Ahmed KA, et al. Activation of p53/ATM-dependent DNA damage signaling pathway by shiga toxin in mammalian cells. Microb Pathog. 2012;52:311-7 [DOI] [PubMed] [Google Scholar]
- 93. Tibbetts RS, Brumbaugh KM, Williams JM, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13: 152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brown L, Benchimol S. The involvement of MAPK signaling pathways in determining the cellular response to p53 activation: cell cycle arrest or apoptosis. J Biol Chem. 2006;281:3832-40 [DOI] [PubMed] [Google Scholar]
- 95. Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277-90 [DOI] [PubMed] [Google Scholar]
- 96. Yuan F, Xie Q, Wu J, et al. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J Biol Chem. 2011;286:6940- 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100: 10794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dai JM, Wang ZY, Sun DC, Lin RX, Wang SQ. SIRT1 interacts with p73 and suppresses p73-dependent transcriptional activity. J Cell Physiol. 2007;210:161-6 [DOI] [PubMed] [Google Scholar]
- 99. Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782-7 [DOI] [PubMed] [Google Scholar]
- 100. Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316-21 [DOI] [PubMed] [Google Scholar]
- 101. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410-25 [DOI] [PubMed] [Google Scholar]
- 102. Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589-95 [DOI] [PubMed] [Google Scholar]
- 104. Daitoku H, Hatta M, Matsuzaki H, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem. 2004;279: 28873-9 [DOI] [PubMed] [Google Scholar]
- 106. Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93-6 [DOI] [PubMed] [Google Scholar]
- 107. Digweed M, Sperling K. Nijmegen breakage syndrome: clinical manifestation of defective response to DNA double-strand breaks. DNA Repair (Amst). 2004;3:1207-17 [DOI] [PubMed] [Google Scholar]
- 108. Ozgenc A, Loeb LA. Current advances in unraveling the function of the Werner syndrome protein. Mutat Res. 2005;577:237-51 [DOI] [PubMed] [Google Scholar]
- 109. Li L, Elledge SJ, Peterson CA, Bales ES, Legerski RJ. Specific association between the human DNA repair proteins XPA and ERCC1. Proc Natl Acad Sci U S A. 1994;91:5012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Amsel AD, Rathaus M, Kronman N, Cohen HY. Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc Natl Acad Sci U S A. 2008;105:5117-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol. 2003;5:320-9 [DOI] [PubMed] [Google Scholar]
- 112. Vasdev S, Gill VD, Singal PK. Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Exp Clin Cardiol. 2006;11:206-16 [PMC free article] [PubMed] [Google Scholar]
- 113. Nguyen T, Nioi P, Pickett CB. The Nrf2- antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203-8 [DOI] [PubMed] [Google Scholar]
- 116. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2:241-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17: 631-6 [DOI] [PubMed] [Google Scholar]
- 119. Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012;1271:10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bosch-Presegue L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2:648-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097-100 [DOI] [PubMed] [Google Scholar]
- 122. Anckar J, Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78-88 [DOI] [PubMed] [Google Scholar]
- 123. Kline MP, Morimoto RI. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17:2107-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tang D, Khaleque MA, Jones EL, et al. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10:46-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zimmermann M, Nickl S, Lambers C, et al. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: a multi-institutional case-control study. Clin Chim Acta. 2012;413:1115-20 [DOI] [PubMed] [Google Scholar]
- 126. Gress TM, Muller-Pillasch F, Weber C, et al. Differential expression of heat shock proteins in pancreatic carcinoma. Cancer Res. 1994;54:547-51 [PubMed] [Google Scholar]
- 127. Syrigos KN, Harrington KJ, Karayiannakis AJ, et al. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology. 2003;61:677-80 [DOI] [PubMed] [Google Scholar]
- 128. Cheng Q, Chang JT, Geradts J, et al. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012;14:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bukau B. Molecular chaperones and folding catalysis, regulation, cellular function and mechanism. Amsterdam: Harwood Academic Publishers; 1999 [Google Scholar]
- 130. Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571-9 [DOI] [PubMed] [Google Scholar]
- 131. Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood). 2003;228:111-33 [DOI] [PubMed] [Google Scholar]
- 132. Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. Apmis. 2003;111:523-30 [DOI] [PubMed] [Google Scholar]
- 133. Elstrand MB, Kleinberg L, Kohn EC, Trope CG, Davidson B. Expression and clinical role of antiapoptotic proteins of the bag, heat shock, and Bcl-2 families in effusions, primary tumors, and solid metastases in ovarian carcinoma. Int J Gynecol Pathol. 2009;28:211-21 [DOI] [PubMed] [Google Scholar]
- 134. Langdon SP, Rabiasz GJ, Hirst GL, et al. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin Cancer Res. 1995;1:1603-9 [PubMed] [Google Scholar]
- 135. Piura B, Rabinovich A, Yavelsky V, Wolfson M. [Heat shock proteins and malignancies of the female genital tract]. Harefuah. 2002;141:969-72, 1009, 10 [PubMed] [Google Scholar]
- 136. Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ramp U, Mahotka C, Heikaus S, et al. Expression of heat shock protein 70 in renal cell carcinoma and its relation to tumor progression and prognosis. Histol Histopathol. 2007;22:1099-107 [DOI] [PubMed] [Google Scholar]
- 138. Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164-72 [DOI] [PubMed] [Google Scholar]
- 139. Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907-18 [DOI] [PubMed] [Google Scholar]
- 140. Garrido C, Fromentin A, Bonnotte B, et al. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res. 1998;58:5495-9 [PubMed] [Google Scholar]
- 141. Volloch VZ, Sherman MY. Oncogenic potential of Hsp72. Oncogene. 1999;18:3648-51 [DOI] [PubMed] [Google Scholar]
- 142. Wei YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother. 1995;40:73-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761-72 [DOI] [PubMed] [Google Scholar]
- 144. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916-9 [DOI] [PubMed] [Google Scholar]
- 145. Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469-75 [DOI] [PubMed] [Google Scholar]
- 146. Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407-10 [DOI] [PubMed] [Google Scholar]
- 147. Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Meng L, Gabai VL, Sherman MY. Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene. 2010;29:5204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Xi C, Hu Y, Buckhaults P, Moskophidis D, Mivechi NF. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J Biol Chem. 2012;287:35646-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086-97 [DOI] [PubMed] [Google Scholar]
- 151. Mendillo ML, Santagata S, Koeva M, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549-62 [DOI] [PMC free article] [PubMed] [Google Scholar]