Abstract
The members of the Sir2 family, or sirtuins, are major regulators of the response to different types of stress. The members of the family have adapted to increasing complexities throughout evolution and have become diversified by increasing their number, specificity, and localization and acquiring novel functions. Sirtuins have been consistently implicated in the cross-talk between the genomic information and environment from the prokaryotes onward. Evidence suggests that in the transition to eukaryotes, histones became one of the basic and most conserved targets of the family, to the extent that in yeast and mammals, sirtuins were originally described as NAD+-dependent histone deacetylases and classified as class III histone deacetylases. A growing number of studies have determined that sirtuins also target a wide range of nonhistone proteins. Many of these targets are also directly or indirectly related to chromatin regulation. The number of targets has grown considerably in the last decade but has provoked an ill-founded discussion that neglects the importance of histones as sirtuin targets. In this review, we summarize our knowledge regarding the range of sirtuin targets described to date and discuss the different functional implications of histone and nonhistone targets throughout evolution.
Keywords: sirtuins, deacetylation, SIRT1-SIRT7, epigenetics, stress response, genome stability, histones
Introduction
During the last decade, the members of the Sir2 family, also known as sirtuins, have become firmly established as key regulators of the response to stress of various types, from metabolic to genotoxic stress. Sirtuins have been implicated in the most important human diseases such as cancer, cardiovascular diseases, diabetes and other endocrine pathologies, malaria, and neurodegenerative diseases, among others.1,2
Sirtuins were originally described as NAD+-dependent histone deacetylases3 and were included in the superfamily of histone deacetylase (HDAC) enzymes as class III HDACs. In fact, there are 4 classes of HDACs: class I (HDAC1, 2, 3, and 8), which are closely related to the yeast transcriptional factor RPD3; class II (HDAC4, 5, 6, 7, 9, and 10), which are similar to another yeast deacetylase HDA1; class III, which includes sirtuins; and class IV (HDAC11). In fact, the process of deacetylation differs markedly between sirtuins and all other HDACs. While class I, II, and IV HDACs transfer the final acetyl group to the aqueous solution and are sensitive to the inhibitor trichostatin A (TSA), sirtuins require NAD+ as an enzymatic co-factor, transfer the acetyl group from the substrate to an ADP-ribose molecule, and are insensitive to TSA.4 Interestingly, ADP-ribosyltransferase activity is also known in sirtuins, although our knowledge about this is currently very limited.5,6
The members of the Sir2 family have been present since they evolved in prokaryotes. They have subsequently undergone considerable functional diver- sification during the course of evolution in order to adapt to increased complexities. For instance, mammals harbor 7 different sirtuins (SIRT1-SIRT7) that differ in their cellular localization, substrate specificity, and functions.7 The fact that some sirtuins have common targets may be a sign of sirtuin cooperation or complementation according to different stimuli.
Interestingly, sirtuins also target many nonhistone proteins, which raises questions regarding the significance of histone or nonhistone targets in global sirtuin function. Here, we provide a general overview of the different histone and nonhistone sirtuin substrates and highlight their functional implications and conservation during evolution.
The Members of the Sirtuin Family
Sirtuins seem to have developed in some types of bacteria as regulators of the metabolic adaptation to energetic fluctuations. Although we do not yet fully understand all the implications of sirtuin functions in prokaryotes, their ability to deacetylate proteins may have first appeared as a mechanism to catabolize acetate before adapting specifically to perform regulatory functions. In this sense, one of the best-conserved sirtuin functions is the regulation of the intermediate metabolism through control of the key enzyme acetyl–CoA synthetase (ACS) (discussed below).
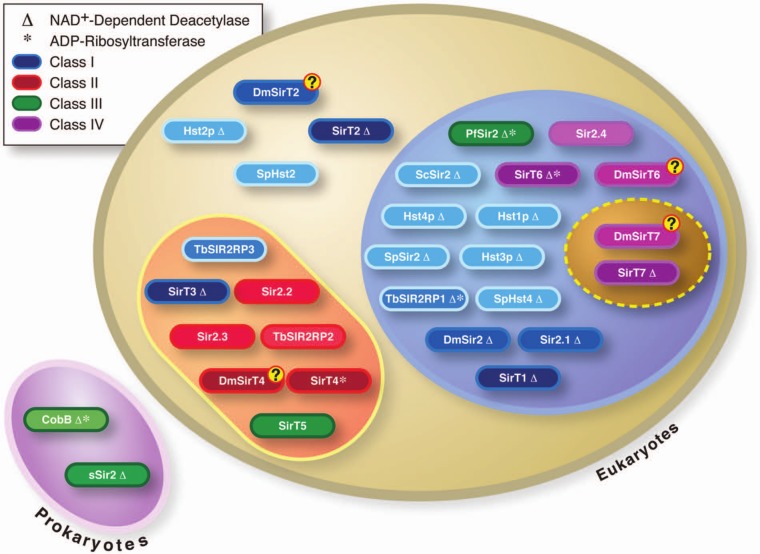
The Sir2 family members encompass all the main phylogenetic domains of living organisms, bacteria and archaea (prokaryotes) and eukaryotes,8 although not all prokaryotes contain sirtuins. Phylogenetic studies have defined 5 lineages or classes of sirtuins: classes I to IV and U.8 Classes II, III, and U are present in prokaryotes, but only classes II and III seem to have been transmitted to eukaryotes (Fig. 1). Consistent with this, the eukaryotic members of classes II and III show mitochondrial localization and target mitochondrial proteins. Interestingly, the 2 eukaryote-specific lineages (I and IV) seem to have appeared in early eukaryotes probably at the same time as chromatin.
Figure 1.
Classification of sirtuin family. The representation includes a list of sirtuins from selected organisms (bacteria to humans) classified phylogenetically according to Frye.8 The schematic shows the general location of each sirtuin. The color each representing a sirtuin belongs to a different class of sirtuins (I-IV), and their color intensity suggests their stage of evolution from light to dark (from lower to higher eukaryotes, respectively). Δ = the current knowledge of their NAD+-dependent deacetylase activity; * = their known ADP-ribosyltransferase activity.
The number of sirtuins per organism appears to have increased during evolution along with complexity, from the presence of 1 member in prokaryotes to 2 in Plasmodium, 4 in Caenorhabditis elegans, 6 in Drosophila, and 7 in mammals. This probably reflects a constant dynamic acquisition of new functions associated with the response of metabolic homeostasis to stress and regulation. Since the evolution of the prokaryotes, sirtuins seem to have been involved in the cross-talk between the genome and environmental changes. For instance, in archaea in which the first examples of the evolution of chromatin-like structures are found, the sirtuin Sir2 is involved in deacetylating another protein, Alba, that appears to control gene expression and genome structure in a manner similar to the way histones act in eukaryotes.9
The functional diversification of Sir2 homologs during evolution is clearly illustrated by their different cellular locations. Three of them (SIRT1, SIRT6, and SIRT7) are clearly localized in the nuclear compartment; in particular, SIRT7 is mostly restricted to the nucleolar region. However, SIRT1 is known to shuttle to the cytoplasm.10,11 Meanwhile, SIRT3 to SIRT5 proteins are mitochondrial proteins with well-known mitochondrial substrates, although full-length SIRT3 is also found in the nucleus under normal conditions.12 SIRT2 is the only mammalian sirtuin localized mainly in the cytoplasm,13 and according to its relationship to the cell cycle, it shuttles to the nucleus during G2/M transition.14
The high degree of conservation among Sir2 family members between bacteria and humans is restricted to their catalytic domain, a region of approximately 250 residues.15 Eukaryotic sirtuins have developed amino (N)– and carboxy (C)–terminal extensions that are divergent among the members of the family16 and that have allowed the acquisition of specific new functions and substrates during evolution. This variety of terminal regions has been proposed as explaining the diversity of sirtuin functions, including the regulation, recruitment, and differential activity of each of the family members.17,18
The Sir2 family structure is based on an NAD+ binding Rossmann-fold domain and a Zn2+ binding domain. The catalytic site is situated inside a hydrophobic channel formed between these 2 binding domains, so that the end of the acetyllysine chain is located close to the nicotinamide ribose of NAD+.19 As expected, mutations in the conserved residues along the NAD+ binding domain disrupt HDAC activity.20
However, in contrast to other classes of HDACs, sirtuins do not use Zn2+ in their catalytic center21 but instead has a structural role. Indeed, the SIRT2 zinc module has a similar topology to the RING finger motif, which in general mediates protein-protein interactions.20 The mechanistic similarities and significant conservation of the catalytic domain between sirtuins and the superfamily of poly (ADP-ribose) polymerases (PARPs) strongly suggest that sirtuins are directly related to these enzymes.
The Different Faces of Sirtuin Activity
One of the most important aspects of sirtuin biology is the dual enzymatic nature of the family. Sirtuins harbor 2 types of related enzymatic activity: deacetylase activity, which in some metabolic contexts can also be defined more generally as a deacylase activity, and mono-ADP-ribosyltransferase (ADPRT) activity. Both appear to derive from the general enzymatic reaction of sirtuins, proposed by Sauve et al.22 in 2001. First, the enzyme binds to NAD+ in the presence of a substrate. Second, it breaks the NAD+ molecule, releasing nicotinamide and retaining the resulting ADP-ribose molecule. Third, in the case of deacetylation, the enzyme transfers the acetyl group from the substrate to the ADP-ribose molecule, releasing O-acetyl-ADP-ribose (OAADPr). Alternatively, the ADPRT is active when, similarly to what occurs with PARPs, instead of transferring the acetyl group from an acetylated-lysine substrate, the enzyme transfers the ADP-ribose molecule to another protein. The OAADPr molecules generated in the deacetylation reaction are themselves a potential second messenger. The exact molecular functions of OAADPr remain elusive, although studies have suggested several targets whose activity may be influenced, such as histone macroH2A,23 cation channel TRPM2,24 and yeast Sir2p-containing complexes themselves.25 Therefore, OAADPr/ADP-ribose promotes pathways that suppress ROS accumulation.
At present, we do not completely understand the nature of this catalytic duality. Our current knowledge suggests that the preeminence of any of these activities or the existence of both in a given sirtuin may be related to specific differences between lineages. For instance, the best-studied class II sirtuin, SIRT4, appears to be mainly an ADP-ribosyltransferase, while the vast majority of class I sirtuins, such as mammalian SIRT1 to SIRT3 or yeast sirtuins, show robust deacetylase activity.6 Evidence suggests that class III and IV sirtuins may generally exhibit both activities (SIRT6, pfSir2A, CobB), depending on the substrate and functional context. However, given our currently limited knowledge of sirtuin substrates outside mammals, these conclusions are not definitive and will require periodic reconsideration. For instance, we cannot rule out the possibility that all sirtuins may harbor both enzymatic activities, using one or the other in different contexts and with the appropriate substrates. Some early studies by Frye15 and Tanny et al.26 suggested that a majority of sirtuins, if not all, may conserve the ability to ADP-ribosylate certain proteins such as histones, BSA, or even themselves, although the activity might be relatively weak. This may have important functional implications, given the involvement of mono-ADP-ribosylation in regulating transcription, apoptosis, and protein translation as well as a role in the signaling of the immune system.27 Other groups have suggested that this general activity is more likely to be an inefficient side effect associated with the deacetylase activity.6 However, the fact that an acetylated residue is not required for ADPRT activity,28 and that certain point mutations in the conserved catalytic domain of SIRT6 can stop its deacetylation activity without altering its ADPRT activity and vice versa,29 suggests that the 2 enzymatic activities are different. This matter is still open to debate.
Some recent studies performed in class III sirtuins have also revealed 2 previously unknown enzymatic activities that recall the original direct involvement of sirtuins in metabolism. First, the mitochondrial SIRT5 was identified as an efficient desuccinylase and demalonylase.30 The second activity, identified in pfSir2A, is an NAD+ glycohydrolase,31 which is surprising since it suggests an alternative use of NAD+ by sirtuins and may represent another regulatory level of metabolic control.
Many questions remain unanswered concerning this matter. How is the dual activity regulated in sirtuins harboring both enzymatic activities? Could a single substrate be the target of both activities? What makes one activity more important than the other for a specific substrate? Future studies should clarify these issues.
The Diversity of Sirtuin Substrates
Sirtuins were originally identified in 1996, as ADP-ribosyltransferases, when the Salmonella typhimurium protein CobB was found to compensate for the absence of CobT in the synthesis of cobalamin (vitamin B12).32,33 In 2000, NAD+-dependent histone deacetylase activity was reported in yeast Sir2p, the founding member of the family, and was shown to be essential for the role of Sir2p in silencing. With the study of the mammalian members of the family, SIRT1 to SIRT7, it soon became clear that sirtuin deacetylase activity was not restricted to histones, encompassing a whole new world of nonhistone substrates. The first of these substrates, identified for mammalian SIRT1, was the tumor suppressor p53.34-36 Since then, the list of nonhistone substrates of the members of the family has grown so long—to include metabolic enzymes, chromatin machinery (enzymes and structure) factors, key transcription factors, cytoskeleton, and many others (Table 1)—that it is difficult to comprehend in its entirety.
Table 1.
Selected Sirtuin Substrates
| HAT(s) | Sirtuin(s) | HAT(s) | Sirtuin(s) | ||
|---|---|---|---|---|---|
| Chromatin related | Metabolism | ||||
| p300 | p300 | SIRT1 | ACS1 | n.d. | SIRT1 |
| SIRT2 | |||||
| MOF | MOF | SIRT1 | ACS2 | n.d. | SIRT3 |
| Suv39h1 | n.d. | SIRT1 | PGC-1α | GCN5 | SIRT1 |
| EZH2 | n.d. | SIRT1 | LXR | n.d. | SIRT1 |
| Tip60 | Tip60 | SIRT1 | FXR | p300 | SIRT1 |
| Stress related | SREBP-1c | CBP/p300 | SIRT1 | ||
| p53 | CBP/p300 | SIRT1 | LKB1 | n.d. | SIRT1 |
| PCAF | SIRT2 | ||||
| Tip60 | SIRT3 | ||||
| FOXO1 | CBP | Sir2α (SIRT1) | PEPCK1 | p300 | SIRT2 |
| FOXO3a | CBP/p300 | SIRT1 | ALDH2 | n.d. | SIRT3 |
| SIRT2 | |||||
| FOXO4 | CBP/p300 | SIRT1 | Ndufa9 | GCN5 | SIRT3 |
| NF-κB | p300 | SIRT1 | SdhA | n.d. | SIRT3 |
| SIRT2 | |||||
| c-Fos | n.d. | SIRT1 | CPS1 | n.d. | SIRT5 |
| c-Jun | p300 | SIRT1 | GDH | n.d. | SIRT3 |
| SIRT4 | |||||
| c-Myc | PCAF | SIRT1 | Other | ||
| Tip60 | SIRT2 | ||||
| p300 | |||||
| HIF-1α | p300 | SIRT1 | CDH1 | n.d. | SIRT2 |
| SIRT6 | |||||
| E2F1 | PCAF | SIRT1 | CDC20 | n.d. | SIRT2 |
| Tip60 | |||||
| p300 | |||||
| DNA repair | Cyclophilin D | n.d. | SIRT3 | ||
| Ku70 | PCAF | SIRT1 | RIP1 | p300 | SIRT1 |
| CBP | SIRT3 | ||||
| XPA | CBP | SIRT1 | α-tubulin | α-TAT | SIRT2 |
| p300 | |||||
| CtIP | n.d. | SIRT6 | Cortactin | PCAF | SIRT1 |
| NBS1 | PCAF | SIRT1 | RAR-β | n.d. | SIRT1 |
| p300 | |||||
| PARP1 | p300/CBP | SIRT1 | Tau | p300 | SIRT1 |
| PCAF | |||||
Note: These are classified into chromatin-related, stress-related, DNA repair, metabolism, and other substrates. The sirtuins in charge of their deacetylation are indicated in the third column. The table also includes the histone acetyltransferases (HATs), also known as lysine acetyltransferases, responsible for the acetylation of each sirtuin substrate.92,96,98,102,103,106,113,114,122,125,152,155,175,182,184,192-201 n.d. = not determined.
A puzzling aspect of sirtuin activity is that there does not seem to be a defined consensus sequence in the regions they target, as a group or individually. Peptide library screening studies that aimed to identify a common pattern for SIRT1 deacetylation substrates concluded that the amino acid sequence around ϵ- Ac-lysine does not have any clear effect on target selection.37 Other enzymatic studies have suggested that sirtuins preferentially recognize acetyllysine in unstructured regions within proteins.38 In the case of ADP-ribosylation, no clear consensus has been reached, although some peptide studies suggest that there may be some loose patterns favoring this modification.5
In higher eukaryotes, sirtuins have acquired important specific roles in different tissues, in many cases related to metabolism and closely linked to the endocrine system. They participate actively in expression programs such as those of development,39,40 differentiation from specific tissues such as skeletal muscle,41 WAT,42 and the nervous system.43
Histone and Nonhistone Chromatin Substrates
Despite its prokaryotic origin, the development of chromatin in eukaryotes appears to have been a milestone in sirtuin history since they underwent major adaptation that enabled them to signal stress conditions to the genome. For this purpose, 2 new lineages intimately related to chromatin, classes I and IV, seem to have arisen in the early stages of eukaryote evolution. Analysis of the available data on the path that sirtuins followed from lower to higher eukaryotes reflects a very important and consistent role for them in the control of different processes through the direct regulation of chromatin structure and expression. This included not only the new sirtuin lineages but also some of the members of the original prokaryote lineages (such as the sirtuin class III Plasmodium pfSir2A).44,45
The functions regulated by this “chromatin adaptation” range from the control of metabolism homeostasis and survival upon stress to the protection of genome stability. Sirtuins perform these chromatin functions through 3 mechanisms.
The main mechanism involves the transcriptional silencing of a particular region, which may encompass a single gene, a defined set of genes, or a whole locus. In most of the cases studied, the silencing established by sirtuins is epigenetic and involves the formation of compacted heterochromatin structures. Two loci have been found to be consistently epigenetically regulated by sirtuins in early eukaryotes onwards: nucleolar rDNA transcription and subtelomeric regions. Both seem to reflect functional adaptations of sirtuins for regulating different types of stress through chromatin. In the first case, it appears to be linked to the response to metabolic and energetic stress in order to control ribosome expression and thereby protein production. This is a very significant regulatory process since it is an energetically expensive process that is key to regulating proliferation.46,47 Other important examples of sirtuin-dependent silencing are the epigenetic silencing of mating-type loci by the yeast Saccharomyces cerevisiae Sir2p and its ortholog Schizosaccharomyces pombe spSir2p48 and the involvement in antigenic variation through silencing of certain sets of var genes of certain sirtuins in protists such as Plasmodium.49,50
The second case, the subtelomeric region, is mainly related to genotoxic stress and is directly associated with a second conserved mechanism of sirtuin in chromatin: the regulation of chromatin structure and organization in order to maintain genome stability. The most obvious cases are the conserved regulation of telomere structure by sirtuins from unicellular protozoa and yeast to humans. Sirtuins have also adapted to regulate the other great structural chromosomal region or constitutive heterochromatin, the pericentromeric area. Constitutive heterochromatin refers to the regions that tend to have a structural role and never decompact, such as centromeres and telomeres, in contrast to facultative heterochromatin, which corresponds to regions that can be compacted in response to certain programs or stimuli, such as development, stress response, or differentiation, and that can decompact when required.51 Thus, in fission yeast S. pombe and mammals, spSir2p and SIRT1 are required for the formation and maintenance of pericentromeric heterochromatin structure.52,53
Another functional aspect closely associated with the role of sirtuins in protecting genome integrity is their conserved role in signaling DNA damage and DNA repair, as we discuss below.54,55 Finally, another interesting aspect of sirtuins in chromatin is associated with the global control of cell cycle progression, as has been shown for mammalian SIRT2 (see below).
Histone substrates
Sirtuin chromatin–associated functions are largely realized through the modulation of epigenetic information by direct deacetylation of specific histone acetylation marks (Fig. 2). In this regard, 2 modifications have been widely conserved during evolution and are functionally relevant to the function of sirtuins: acetylation of histone H4 in lysine 16 (H4K16Ac) and acetylation of histone H3 in lysine 9 (H3K9Ac).
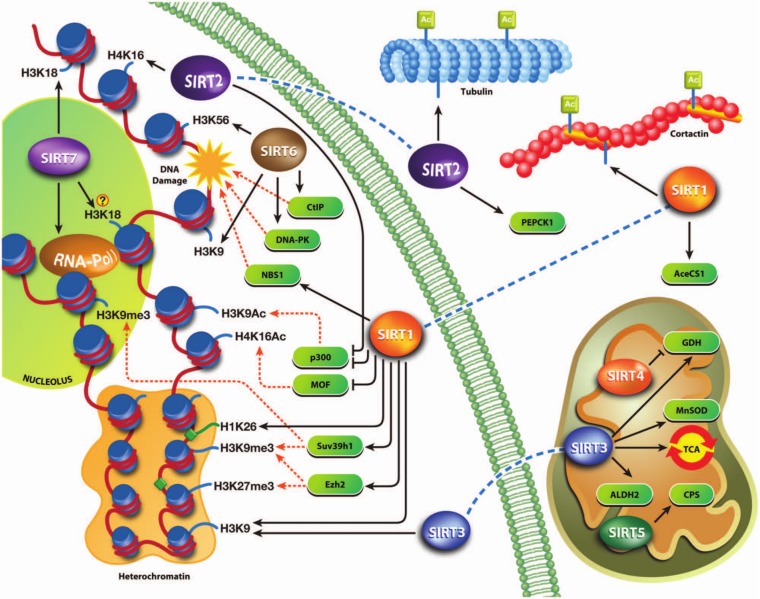
Figure 2.
Sirtuin functions and targets in the cell. The figure mainly shows sirtuin chromatin–related functions that complement each other or differently regulate the same process.
H4K16Ac has exclusive properties due to its unique role in regulating chromatin structure.56 Its presence inhibits the folding of the chromatin fiber in vitro and therefore, as has been suggested, also inhibits the formation of higher orders of chromatin compaction. Acetylation/deacetylation of H4K16 has been associated with epigenetic phenomena throughout evolution, from silencing in S. cerevisiae, through X-chromosome dosage compensation in Drosophila, to silencing in mammals.57 H4K16Ac has also been linked to the regulation of cell cycle progression,58 transcription, DNA repair,59 and DNA replication.60 Moreover, hypoacetylation of H4K16 has been proposed as a hallmark of cancer.61 The functional link between sirtuins and H4K16Ac is mainly restricted to the class I sirtuins, including yeast Sir2p and mammalian SIRT1 to SIRT3.12,56,62
The behavior of the other silencing-related mark, H3K9, is very different from that of H4K16. Deacetylation of H3K9 is a requirement for subsequent methylation in the same residue, H3K9me2/3, a hallmark of higher orders of chromatin compaction or heterochromatin conserved from amoeba to humans.63 Among mammalian sirtuins, class I SIRT1 and class IV SIRT6 are the most functionally important H3K9Ac deacetylases. SIRT6 H3K9Ac deacetylase activity is important for modulating telomere structure and DNA repair of double-strand breaks (DSBs).64
In the case of mammalian SIRT1, deacetylation of H4K16Ac and H3K9Ac is directly associated with the capacity of SIRT1 to coordinate the formation of constitutive and facultative heterochromatin.53,56 Mammalian SIRT3 is mainly a mitochondrial protein that acts as the primary protein deacetylase.65 However, a small SIRT3 subpopulation localizes in the nucleus, where it participates in the repression of key stress-related genes through deacetylation of their promoters in H3K9Ac and H4K16Ac.12,66 It is of particular note that H4K16Ac deacetylation by SIRT2 is related to cell cycle control and not to heterochromatin formation.62 The cytoplasmic protein SIRT2 and its yeast ortholog Hst2p show a very strong preference for H4K16Ac. During G2/M transition, SIRT2 is shuttled to the nucleus, where it deacetylates H4K16Ac globally before entering mitosis.62
Recently, a newly identified modification involved in transcriptional regulation, H3K18Ac, has been linked to another class IV sirtuin, SIRT7.67 SIRT7 deacetylation and the consequent silencing of a specific set of genes were shown to be crucial for maintaining the transformed phenotype in cancer cells.67 Additionally, the enrichment of SIRT7 in nucleoli also underlines the positive function of SIRT7 activity in regulating RNA polymerase I transcription and cell growth.68 Conversely to SIRT1, which silences rDNA by additionally deacetylating H4K16Ac and H3K9Ac,69 SIRT7 binds directly to the RNA polymerase I complex, exerting a positive effect on transcription. However, no deacetylation substrate of the RNA polymerase I complex has been identified.68
Sirtuins have also been linked to DNA damage signaling and DNA repair through the deacetylation of another mark, H3K56Ac. This is involved in DNA damage signaling during S phase70 and is targeted by mammalian SIRT671 and the yeast sirtuins Hst3p and Hst4p.72 Mammalian SIRT1 and SIRT2 are also believed to deacetylate H3K56Ac upon DNA damage.70,73 Histones H3 and H4 are not the only sirtuin substrates among the core histones since Trypanosoma brucei Sir2PP1 shows H2A- and H2B-specific ADP-ribosyltransferase activity.74
Another interesting functional relationship between histones and sirtuins involves the linker histone H1. Mammalian SIRT1, the ortholog of S. cerevisiae Sir2p, has been shown to bind directly and deacetylate the histone H1 isoform H1.4 in lysine 26 (H1K26)56 during the formation of facultative heterochromatin in mammals. One of the contexts in which H1K26Ac deacetylation by SIRT1 may be also important is the formation of heterochromatin by the H3K27me3-specific histone methyltransferase EZH2 during development.39 The SIRT1 ortholog, SIR-2.1, deacetylates H3K9Ac at subtelomeric regions and induces the H3K27 methylation in the germline and modulates the subcellular localization of the linker histone HIS-24 (H1.1) as part of heterochromatin maintenance.75,76 Recently, Li et al.77 described a perfect protein co-localization between SIRT1, histone H1 isoform H1.5 (very similar to H1.4), and H3K9 methylation in the genome of differentiated mammalian cells.
SIRT1 is also responsible for the promotion of 2 other heterochromatin-associated marks: H3K9me3, leading to constitutive and facultative heterochromatin, and H4K20me1, forming facultative heterochromatin.53,56 Other studies have linked SIRT1 and histone deacetylation activity to DNA methylation by Dnmt1 at a number of genomic loci, such as nucleolar rDNA and some tumor suppressor genes.78,79 It should be noted that Dnmt1 activity is enhanced by SIRT1 deacetylation.80
Nonhistone Chromatin Substrates
Histones were the first acetylated protein to be identified,81 and in 1996, histone acetyltransferases (HATs) and HDACs were found to be responsible for their acetylation and deacetylation.82,83 However, 1 year later, lysine modification of a nonhistone protein was confirmed with p53.84 Since then, many nonhistone proteins have been found to be subject to acetylation/deacetylation (Table 1 and Fig. 2).85
One of the most important features of sirtuins in chromatin regulation is that they have adapted to performing their role in chromatin through the coordination of simultaneous events. These events are not only based on direct histone deacetylation but also on its interplay with other chromatin-associated machinery, such as histone-modifying enzymes and structural and transcription factors (discussed below). This interplay implies an interaction with, and in many cases deacetylation of, many of these factors in order to modulate their specific enzymatic activity and function. A good example of this is the formation of facultative heterochromatin by mammalian SIRT1. The formation of compacted chromatin depends not only on the direct deacetylation of H3K9Ac and H4K16Ac and the recruitment and deacetylation of K26 in H1.4 (H1K26)56 but also on the close functional relationship with Suv39h1, the main H3K9me3-specific activity in mammalian cells involved in constitutive and facultative heterochromatin formation.86 SIRT1 promotes Suv39h1 function through a sequence of events. First, SIRT1 binds to Suv39h1, increasing its protein stability.87 Next, SIRT1 recruits Suv39h1 to chromatin. Finally, it promotes Suv39h1 activity through 1) deacetylation of H3K9Ac to allow H3K9me3 methylation by Suv39h1; 2) a conformational change induced by the binding, which increases the specific activity of Suv39h1; and 3) deacetylation of Suv39h1 in the catalytic SET domain residue K266.53 The link between SIRT1 and Suv39h1 is very important for the formation of constitutive (pericentromeric and probably telomeric) and facultative heterochromatin. In this context, a complex, eNoSC, which contains SIRT1, Suv39h1, and nucleomethylin, has been identified.88 The complex forms under stress conditions and contributes to the production of silent chromatin in the rDNA locus.53,88
Another interesting histone methyltransferase linked to SIRT1 is EZH2, a Polycomb factor that is fundamental in development and differentiation. SIRT1 and EZH2 are both components of the PRC4 complex during development in mammals, Drosophila, and possibly C. elegans.39,40,75 Although we have no evidence that EZH2 is a deacetylation target of SIRT1, the high degree of conservation of K266 in the SET domain of EZH2 homologs suggests that this may be the case.
Surprisingly, sirtuins target not only other enzymes that work synergistically with them to promote a function but also very different types of enzymes, including chromatin-related antagonists of sirtuin functions, such as HATs (Fig. 2). These mechanisms probably ensure an efficient fine-tuning response when sirtuins are activated upon stress. An important example is the p300 HAT, which has a preference for H3K9Ac, H3K27Ac, H3K36Ac, H3K37Ac, and H3K56Ac, among others,89,90 and acts as a transcriptional co-activator required for a wide range of important cellular processes, such as those regulated by Forkhead box class O (FOXO) factors, p53, PARP1, and NF-κB. The close control of the p300 function is critical to ensure the activation/repression of many pathways and the maintenance of heterochromatin structure. Therefore, sirtuin family members regulate both p300 histone and nonhistone substrates and at the same time inactivate p300 enzymatic activity through deacetylation. This mechanism has been most thoroughly characterized in mammals with SIRT1 and SIRT2, but it is also known to be conserved in yeast with Hst2p.91-93 Another notable example is the antagonism between some sirtuins and the MYST family of HATs. One member of this family, MOF (also known as MYST1), is the main H4K16Ac HAT in mammals.94,95 As described for p300, SIRT1 deacetylates MOF and represses its enzymatic activity (reducing H4K16Ac even further), protein levels, and chromatin localization.96 MOF is not the only MYST family member whose activity is negatively regulated by SIRT1 deacetylation. Tip60, another p300 substrate, involved in DNA damage response (signaling and repair), is also deacetylated by SIRT1 in order to regulate its protein levels and activity.96-98
A completely different type of interplay is established by sirtuins in the signaling and regulation of DNA damage and DNA repair. To date, the sirtuin known to be most closely involved in DNA repair is the human SIRT6.99 This has been implicated in DSB repair, although it also participates in ssDNA repair pathways, such as base excision repair.99 It is involved in the homologous recombination repair pathway through the deacetylation of CtIP100,101 and in nonhomologous end joining (NHEJ) through the recruitment of DNA-PK to damaged regions, where SIRT6 deacetylates H3K9Ac.64
Another important DNA damage sensor, regulated by sirtuins, is the MRE11-RAD50-NBS1 nuclease complex, which regulates cellular responses to DNA DSBs. In this case, SIRT1 associates with this complex and maintains NBS1 in the hypoacetylated state that is required for its phosphorylation after exposure to ionizing radiation.102 SIRT1 also participates in nucleotide excision repair (NER), the major repair pathway for ultraviolet (UV)–induced DNA damage. In this case, SIRT1 interacts with XPA mainly after UV irradiation and deacetylates it as a requirement for the optimal function of the NER pathway. This deacetylation enhances XPA1 interaction with RPA.103 Consistent with this, SIRT1 downregulation significantly sensitizes cells to UV irradiation.
Sirtuin members can also show functional antagonism. For instance, under stress conditions, SIRT1 deacetylates p300-acetylated PARP1 and blocks its enzymatic activity,104 inhibiting its ability to regulate NF-κB–dependent gene transcription.105,106 Another example of a DNA repair–associated substrate is Ku70. Mammalian SIRT1 and SIRT3 promote survival by DNA repair by deacetylating the DNA repair factor Ku (Ku70 in mammals), which is involved in the NHEJ pathway.107-109 The interaction between yeast Sir2p and the Ku protein (its ortholog in yeast) has also been described in the multiprotein SIR complex, although no deacetylation has been reported.110
Nonhistone/Chromatin-Unrelated Substrates
Sirtuins are involved in a plethora of functions within the 3 phylogenetic domains of organisms (Fig. 2). From prokaryotes through yeast to humans, Sir2p homologs chiefly regulate the efficient adaptation to stress conditions, employing a range of mechanisms: 1) chromatin structure and expression regulation, 2) survival mechanisms, 3) metabolic homeostasis, and 4) maintenance of gene integrity. To achieve this, sirtuins modulate the activity of histone and nonhistone proteins. Among the most representative nonhistone chromatin–unrelated targets are specific transcription factors involved in stress survival, apoptosis inhibition, cell proliferation, metabolism, and many others.
Stress-related transcription factors
One of the most important roles of sirtuins is to promote survival under stress conditions. This conserved role is mainly realized through chromatin regulation but involves several key transcription factors that are directly related to the main survival pathways. Sirtuins not only promote silencing of the gene targets directly regulated by these transcription factors but also selectively modulate, through specific deacetylation, some of their functional features, such as their DNA binding affinity or their localization.
As mentioned above, p53 was the first nonhistone substrate described for sirtuins.34-36 Acetylation at different p53 protein sites is correlated with its stabilization and increases the recruitment of p53 to target promoter regions for gene activation in response to various cellular stresses.111,112 These residues are acetylated by several acetyltransferases, but SIRT1 only targets 2 of these modifications (K381 and K382), which are specifically acetylated by p300.113,114 It is of particular note that the residues targeted by sirtuins are located in the C-terminal regulatory domain, which is not conserved throughout evolution.115 Mammalian SIRT1 deacetylates p53, promoting the cell survival and inhibition of senescence and apoptosis.34,36 Supporting these observations, SIRT1 knockout mice show high levels of apoptosis in thymocytes upon γ-irradiation.116 Interestingly, SIRT2 and SIRT3 also target p53. However, given the different cellular locations of the 3 sirtuins (nuclear for SIRT1, cytoplasmic for SIRT2, and mainly mitochondrial for SIRT3), it is likely that the p53-specific functions of each of them are determined by their location.11,12,14 As it happens with SIRT1, SIRT2 deacetylation activity seems to be necessary to reduce p53 activity and thereby promote cell survival.117 Interestingly, SIRT3 binds to mitochondrially located p53 and deacetylates it, inhibiting p53-dependent growth arrest and senescence.118
The members of the superfamily FOXO of stress-related transcription factors are among the best studied of the nonhistone sirtuin substrates. However, the regulatory effect of some sirtuins in the functional regulation of these factors is still controversial. For instance, SIRT1 deacetylates several of the mammalian FOXO members but in some cases with opposite effects, which suggests that the functional context, such as the cell type or the type of stress, is important for determining the role of the FOXO-related response.119-124 In mammals, SIRT1, SIRT2, and SIRT3 interact and deacetylate the C-terminal domain of FOXO proteins in the nucleus, cytoplasm, and mitochondria, respectively.123,125,126 This functional relationship seems to have been conserved throughout evolution. In fact, the FOXO3a ortholog in yeast (Hcm1p) interacts with Sir2p and shuttles from the cytoplasm to the nucleus during late G1 and early S phase or in response to oxidative stress, although no deacetylation effect has been demonstrated.127 Therefore, Hcm1p nuclear localization depends on the activity of Sir2p. This regulation is actually conserved in higher eukaryotes since the acetylation/deacetylation of FOXOs in mammals regulates, along with other translational modifications, the location and activity of these transcription factors.125,128,129 Similarly to yeast, the ortholog of Sir2p and SIRT1 in C. elegans, SIR-2.1, regulates the FOXO ortholog Daf-16, but no acetylation has been described.130 This may suggest that the conserved link between sirtuins and FOXO does not involve direct deacetylation in organisms other than mammals. However, the fact that the known SIRT1 lysine target K262 in FOXO1 is conserved in other mammalian members of the FOXO family, such as FOXO3a and FOXO4, in yeast Hcm1p, and in C. elegans Daf-16 suggests the opposite.121,130 Further studies are required to resolve this apparent contradiction.
The c-Myc oncogene is another interesting sirtuin substrate. In mammals, SIRT1 and SIRT2 regulate the levels of oncogene proteins. Both proteins increase c-Myc stability, enhancing activation of its transcription targets.131-133 Once again, sirtuin substrates and activities overlap, suggesting a higher level of modulation, possibly as a result of different stimuli.
Some members of the basic leucine zipper (bZIP) family, c-Fos and c-Jun, are also SIRT1 substrates. SIRT1 negatively modulates cell proliferation, differentiation, inflammation, and apoptosis by binding and deacetylating their bZIP domain, repressing AP-1 transcriptional activity.134 NF-κB is another transcription factor that is regulated by acetylation patterns that depend on p300 and sirtuin activity. NF-κB controls the expression of genes that affect important cellular processes, such as cell cycle, angiogenesis, adhesion, and apoptosis.135,136 The p65 subunit is acetylated in several lysine residues, some of which are deacetylated by SIRT1 in the nucleus and SIRT2 in the cytoplasm.137 These lysines are not conserved throughout evolution. This is of particular note since NF-κB has been considered a metazoan-specific transcription factor until the recent discovery of orthologs in cnidarians and the single-celled eukaryote Capsaspora owczarzaki.138
Hypoxia-inducible factors (HIFs) are also important stress-related factors regulated by sirtuins. SIRT1 deacetylates and inactivates HIF-1α, so that during hypoxia, SIRT1 is downregulated, allowing the acetylation and activation of HIF-1α.139 SIRT6 also represses HIF-1α transcriptional activity, in this case by deacetylating H3K9 at HIF-1α target gene promoters. In this case, SIRT6-dependent HIF-1α regulation maintains the efficient flow of glucose into the TCA cycle under normal nutrient conditions.140 Therefore, once again, different sirtuins regulate the same substrates in response to different stimuli.
The last of the representative stress-related transcription factors is E2F1, which is important for regulating cell cycle progression and apoptosis. In the absence of p53, E2F1 is a significant mediator of the apoptotic response to DNA of p53 damage, and its deacetylation by SIRT1 negatively influences its transcription activity.141 In the C. elegans germline, it has been reported that the retinoblastoma gene homolog lin-35 and the E2F-like transcription factor components efl-2 and dpl-1 are required for irradiation-induced germ cell apoptosis,142 but no link to SIR-2.1 has been described.
Metabolism
Since the pioneer studies in 1996 that linked prokaryotic sirtuins with metabolism,33 the number of sirtuin targets has been increasing markedly.54,101,143 Eukaryote sirtuins have a degree of redundancy as several members regulate the activities that the single sirtuin found in prokaryotic cells is claimed to govern in bacteria.144,145 With the evolution of eukaryotes, the cell had to adapt to regulate more than one cellular compartment, which gave rise to enzyme diversification with partially overlapping functions.
ACS seems to be the best-conserved and most frequently targeted sirtuin substrate in metabolism, ranging from the bacterial CobB144,145 to the mammalian SIRT1 and SIRT3 deacetylases.146 Although there is no evidence of the existence of nonhistone substrates for yeast Sir2p and its orthologs, it has been hypothesized that Hst3p and Hst4p are required for acetyl-CoA production due to their capacity to grow on acetate or propionate growth media.147 Recently, it has been proposed that the ACS acetylation/deacetylation mechanism controls energy homeostasis when cells are growing on an acetate carbon source.148
SIRT1 is involved in metabolic regulation at different levels. As mentioned elsewhere, an obvious mechanism is that of direct deacetylation of nuclear metabolic enzymes, such as the nuclear ACS isoform (AceCS1).146 However, the main mechanism by which SIRT1 regulates metabolic homeostasis is chromatin regulation, which it achieves by promoting tissue-specific silencing or activation of a different set of metabolism-related genes. Intimately linked to the endocrine system, SIRT1 participates in the expression program of a wide variety of nuclear hormone receptors and other master regulators of metabolism such as PGC-1α or PPAR-γ.42,149,150 These mechanisms involve binding of SIRT1 to the factor and probably deacetylation. In some of these cases, such as PGC-1α, SIRT1 seems to participate directly in the activation of its gene targets,149 while in others such as PPAR-γ, SIRT1 represses its activity by interfering with the binding of the master regulator with its co-repressor complex (such as SMRT or NcoR).42 As an example, SIRT1 coordinates lipid metabolism by 1) deacetylating nuclear receptors; 2) deacetylating transduction signaling enzymes, such as the liver X receptor (LXR) and the farnesoid X receptor (FXR); 3) repressing SREBP-1c in the context of hepatic lipid synthesis; and 4) activating AMPK (via deacetylation of LKB1).146,151-154
SIRT2, the cytoplasmic sirtuin, is also involved in metabolism. This member of the family regulates the gluconeogenesis pathway through another p300 substrate, the enzyme PEPCK1. SIRT2 deacetylates and stabilizes the protein. Indeed, downregulation of SIRT2 expression in the liver of mice decreases blood glucose levels.155
As expected, mitochondrial sirtuins are mainly involved in metabolic regulation. For instance, SIRT3 regulates metabolism processes based on promoting energy production and is the main mitochondrial deacetylase.156 Among the known mitochondrial substrates, some of its most significant deacetylation targets are the mitochondrial ACS isoform (AceCS2), the TCA cycle–related enzyme glutamate dehydrogenase (GDH), mitochondrial ALDH2, the detoxifying MnSOD,157 some subunits of the NADH dehydrogenase (complex I), and the succinate dehydrogenase (complex II) of the electron transport chain.158-162
The other mitochondrial sirtuin with deacetylase activity is SIRT5, which upregulates the catabolism of ammonia in the urea cycle by deacetylating carbamoyl phosphate synthetase 1 (CPS1).163 Although SIRT5 has a weak deacetylase activity against the histone H4 peptide,164 its activity against CPS1 is robust, suggesting that other specific substrates may exist.163 As mentioned above, SIRT5 is also an efficient desuccinylase and demalonylase.165 Lysine malonylation and succinylation occur on several mammalian proteins; indeed, many mitochondrial proteins were found to contain lysine malonylation and succinylation. Interestingly, lysine succinylation of proteins was also found in bacteria,166,167 but protein lysine malonylation had not been reported previously.30
SIRT4 regulates amino acid catabolism through repression of the enzymatic activity of GDH by mono-ADP- ribosylation.92,164,168,169 The effect on this substrate is the opposite to that of SIRT3 activity, suggesting that the 2 activities are antagonistic in some other contexts. Indeed, the SIRT4 knockout mouse phenotype shows the opposite features to those observed in SIRT3 deficiency. Moreover, SIRT4 requires SIRT1 to regulate fatty acid oxidation, respiration, and AMPK phosphorylation,170 which also implies that SIRT4 has a role in the sirtuin-dependent coordinated response in metabolism. There is evidence that SIRT4 can also regulate insulin secretion in response to glucose through the insulin-degrading enzyme and the subunits ANT2 and ANT3 of the ATP/ADP translocase. However, no ADP-ribosylation has been described, despite evidence that they interact.171
Other unrelated targets
Further sirtuin targets are involved in other important functions, such as signal transduction, cellular structure and integrity, and cell cycle regulation, among others. For instance, SIRT2 participates in cell cycle control through H4K16Ac regulation and probably also through its functional relationship with p53.62,172 However, SIRT2 has also been associated with the control of mitotic exit by regulation of the APC/C regulatory complex. SIRT2 deacetylates and activates APC/C co-activators CDH1 and CDC20.173 SIRT1 has also been treated as an antitumorigenic sirtuin due to its inhibitory effect on the β-catenin pathway.174
Other remarkable sirtuin substrates are cyclophilin D and RIP1. Both are involved in necrotic cell death but are activated by the deacetylation activity of SIRT3 and SIRT2, respectively.175,176
Sirtuins are also related to the maintenance of cytoskeleton dynamic structure. North et al.164 were the first to report the relationship between SIRT2 and the α-tubulin acetylation level. SIRT2-mediated α-tubulin deacetylation seems to be important for mitotic entry, mitosis progression,177,178 and axonal degeneration.179 However, there is still some controversy about this function180 since no acetylation/deacetylation processes have been described in yeast in which Sir2p plays an important regulatory role. Indeed, the α-tubulin acetylation pattern was previously related to microtubule destabilization by HDAC6, first reported by Hubbert et al.181 SIRT1 is also involved in cytoskeleton regulation. Indeed, this sirtuin deacetylase inhibits cortactin, a cytoskeletal protein that promotes actin polymerization and stabilizes actin branching.182
SIRT1 functions are involved in several diseases, such as Alzheimer disease, in which SIRT1 deacetylates 2 important neural proteins, the retinoic acid receptor β (RAR-β) and the microtubule-associated protein tau. In the first case, SIRT1 acts to stimulate the RAR-β protein and therefore increases ADAM10 transcription and α-secretase production.183 In the second case, SIRT1 deacetylation of the tau protein prevents the accumulation of phosphorylated tau.184 Both types of regulation have a protective effect on neurons and may act in a complementary way.
Histone Versus Nonhistone Substrates
The study of sirtuins has progressed rapidly in the past decade. From the initial isolated studies in organisms such as yeast, we have now started to assemble an integrated, overall picture of the role of sirtuins based on the study of all the functions associated with the different family members and their trajectories through the course of evolution. The discovery that nonhistone proteins can also be targets of reversible lysine acetylation/deacetylation revealed a whole new regulatory layer to what had been considered for a long time a territory exclusive to histones.
The number of known nonhistone sirtuin substrates in mammals has grown considerably since 1997 and currently numbers more than 50. The contrast between this figure and the limited list of histone targets has given rise to an ill-founded discussion in the field that implies a preeminence of nonhistone over histone substrates in sirtuin-associated functions. It should be stressed that most of our knowledge comes from the best-studied sirtuin to date, SIRT1.
Several lines of evidence indicate the seminal involvement of histones in eukaryotic sirtuin function. A first important issue is the degree of conservation of histones as substrates. If we exclude some metabolic enzymes, histones are the only group of sirtuin substrates completely conserved in all eukaryotes (Fig. 3). Consistent with this, the vast majority of members of the eukaryote-exclusive sirtuin lineages, classes I and IV, appear to be exclusively histone-modifying enzymes,8 suggesting that this was their original basal function. One of the best examples of the chromatin-oriented behavior of sirtuins during evolution is found in the Alba protein of archaea. In this organism, the only sirtuin member regulates chromatin compaction through the deacetylation of the major nonhistone architectural DNA binding protein, Alba.9 Interestingly, archaea histone proteins are organized in octamers, with significantly shorter tails that do not sustain posttranslational modifications.185 It has been suggested that Alba is a structural linker chromatin protein similar to eukaryotic histone H1, but it actually has a strong similarity with the histone H4 sequence around K16 (Fig. 3).185 In fact, although Alba seems to be conserved in all eukaryotes (Fig. 3), its function in archaea is only relatively conserved in some lower eukaryotes, such as protozoa. In contrast, in the rest of the eukaryotes, Alba has become specialized to participate in RNA metabolism.186
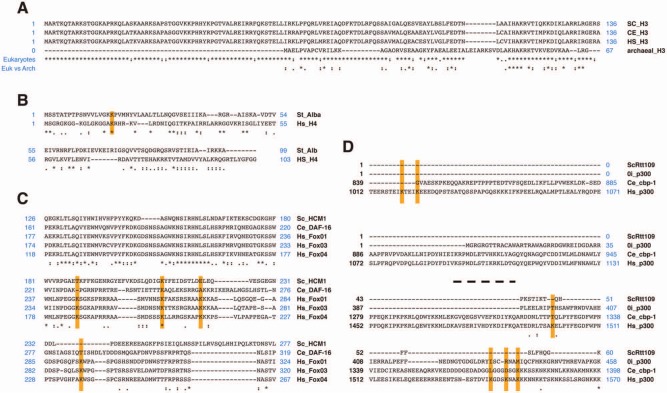
Figure 3.
Protein alignments using ClustalW. (A) Histone H3 alignment: alignment among eukaryotes versus archaea H3. (B) Alignment between archaea Alba protein and human histone H4. As it is shown, the sequence around the K16 residue is conserved in both cases. (C) FOXO protein alignment (fragment) from S. cerevisiae (Hcm1p) and C. elegans (Daf-16) to human homologs. The lysines known to be deacetylated by sirtuins in any of them are highlighted. (D) Alignment of p300 using S. cerevisiae (Rtt109p), Ostreococcus, C. elegans (cpb-1), and human homologs. Alignment of 2 different regions of the p300 homologs containing SIRT1 and SIRT2 lysine targets.
Analysis of all sirtuin substrates identified so far suggests that most of these nonhistone substrates have been acquired relatively recently and probably reflect the increased complexity, cell differentiation, and wide variety of physiological environments found in higher eukaryotes. Two examples of this adaptation are p53 and NF-κB. p53 is conserved from invertebrates, such as Drosophila and C. elegans, to humans. NF-κB also seems to have been acquired in the lower eukaryotes. In both cases, there is no evidence of any acetylation/deacetylation event in nonmammalian orthologs. In fact, the protein sequence of these orthologs is poorly conserved throughout evolution, including the known acetyllysines targeted by the mammalian sirtuins (Fig. 3). On the other hand, members of the FOXO superfamily show a high level of conservation of the acetylated lysines that are targeted by sirtuins in mammals. Although this family’s functions are not conserved among the different orthologs, all FOXO members appear to be regulated by sirtuins. However, no sirtuin-dependent deacetylation has been reported in organisms other than mammals. This may not indicate a conserved role but suggests the possible existence of a conserved target sequence. In other cases, a conserved functional link of sirtuin-dependent deacetylation from lower to higher eukaryotes is clear. This is the case for p300, which nevertheless does not show conservation of the lysine residues targeted by sirtuins in different organisms. Taken together, these observations suggest that many sirtuin- dependent regulatory deacetylation events may have appeared later in evolution. Another interesting example of the recent specialization of sirtuins is the case of mammalian SIRT3 and its role as the main mitochondrial deacetylase.187 SIRT3 and SIRT2 share an ortholog from early eukaryotes to Drosophila. However, mammalian SIRT2 localizes mainly in the cytoplasm, has identical H4K16Ac specificity, and regulates global levels of H4K16Ac during the cell cycle,62 as does its yeast ortholog Hst2p. This suggests that SIRT2 is the functional ortholog of Hst2p. Therefore, mammalian SIRT3 and its mitochondrial functions may be a very recently evolved functional adaptation.
A substantial proportion of nuclear substrates identified for sirtuins are actually chromatin-related factors and are involved in the 3 main roles of sirtuins in chromatin: regulation of compacted heterochromatin, transcriptional silencing, and protection of genome integrity. These substrates include structural proteins such as linker histone H1, chromatin-modifying enzymes such as histone and DNA methyltransferases (Suv39h1, Dnmt1), HATs (p300, MOF, Tip60), repair factors (XPA, NBS1), and specific transcription factors involved in a wide range of functions, such as stress survival, development and differentiation, metabolic homeostasis, and endocrine activity, among others. Remarkably, most of these cases also require previous direct deacetylation of histones by sirtuins, as has been noted for SIRT1 and SIRT6. Together, these observations suggest that histones are at the core of sirtuin functions, to which many different regulatory layers have been added. A likely explanation is that the larger genome size and its packing capacity188 evolved to coordinate chromatin machinery that includes nonhistone proteins.
Finally, it is important to note that this discussion is not restricted to sirtuins exclusively but represents a complete change in the paradigm that has been accepted for more than 40 years and that has regarded these enzymes basically as histone enzymes and not more generally as protein enzymes. Today, we know that more than 2,000 proteins are acetylated in mammalian cells189,190 and therefore that acetylation is a much more significant and frequent regulatory mechanism than we previously appreciated. For instance, the number of targets of the other HDACs is probably as important as that of the sirtuins, at least among nuclear proteins. The importance of histone methylation may be about to receive due recognition, albeit probably not on such a large scale, thanks to the growing list of proteins that are known to be methylated.191
Final Remarks
In the last decade, extraordinary progress has been made in the field of sirtuin research towards defining the basic mechanisms that account for the conservation of sirtuins throughout evolution. Some of the most important matters regarding this family of enzymes remain unexplained, such as the nature of the enzymatic duality and the targets and functional implications of ADP-ribosylation activity. Considerable effort will be required in the coming years to complete our integrated and global picture of sirtuin biology.
Acknowledgments
The authors apologize to many colleagues whose work could not be cited in this review due to space limitations. The authors thank members of the Vaquero group for fruitful discussions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: The chromatin biology group is supported by the Spanish Ministry of Science and Innovation (grant SAF2011-25860), the Catalonian government agency Agència de Gestió d’Adjuts Universitaris I de Recerca (AGAUR) (2009SGR914), and a grant from the Association Française Ataxie de Friedreich.
References
- 1. Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287(51):42444-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483-8 [DOI] [PubMed] [Google Scholar]
- 3. Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol. 2000;65:297-302 [DOI] [PubMed] [Google Scholar]
- 4. Yuan H, Marmorstein R. Structural basis for sirtuin activity and inhibition. J Biol Chem. 2012;287(51):42428-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hawse WF, Wolberger C. Structure-based mechanism of ADP-ribosylation by sirtuins. J Biol Chem. 2009;284(48):33654-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du J, Jiang H, Lin H. Investigating the ADP- ribosyltransferase activity of sirtuins with NAD analogues and 32P-NAD. Biochemistry. 2009;48(13):2878-90 [DOI] [PubMed] [Google Scholar]
- 7. North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5(5):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273(2): 793-8 [DOI] [PubMed] [Google Scholar]
- 9. Bell SD, Botting CH, Wardleworth BN, Jackson SP, White MF. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science. 2002;296(5565):148-51 [DOI] [PubMed] [Google Scholar]
- 10. Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213(1):88-97 [DOI] [PubMed] [Google Scholar]
- 11. Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282(9):6823-32 [DOI] [PubMed] [Google Scholar]
- 12. Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21(8):920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16(10):4623-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. North BJ, Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS One. 2007;2(8):e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260(1):273-9 [DOI] [PubMed] [Google Scholar]
- 16. Mead J, McCord R, Youngster L, Sharma M, Gartenberg MR, Vershon AK. Swapping the gene-specific and regional silencing specificities of the Hst1 and Sir2 histone deacetylases. Mol Cell Biol. 2007;27(7):2466-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao K, Chai X, Clements A, Marmorstein R. Structure and autoregulation of the yeast Hst2 homolog of Sir2. Nat Struct Biol. 2003; 10(10):864-71 [DOI] [PubMed] [Google Scholar]
- 18. Flick F, Luscher B. Regulation of sirtuin function by posttranslational modifications. Front Pharmacol. 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greiss S, Gartner A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol Cells. 2009;28(5):407-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finnin MS, Donigian JR, Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8(7):621-5 [DOI] [PubMed] [Google Scholar]
- 21. Holbert MA, Marmorstein R. Structure and activity of enzymes that remove histone modifications. Curr Opin Struct Biol. 2005;15(6):673-80 [DOI] [PubMed] [Google Scholar]
- 22. Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL. Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry. 2001;40(51):15456-63 [DOI] [PubMed] [Google Scholar]
- 23. Kustatscher G, Hothorn M, Pugieux C, Scheffzek K, Ladurner AG. Splicing regulates NAD metabolite binding to histone macroH2A. Nat Struct Mol Biol. 2005;12(7):624-5 [DOI] [PubMed] [Google Scholar]
- 24. Grubisha O, Rafty LA, Takanishi CL, et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem. 2006;281(20):14057-65 [DOI] [PubMed] [Google Scholar]
- 25. Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell. 2005;121(4):515-27 [DOI] [PubMed] [Google Scholar]
- 26. Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99(7):735-45 [DOI] [PubMed] [Google Scholar]
- 27. Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70(3):789-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fahie K, Hu P, Swatkoski S, Cotter RJ, Zhang Y, Wolberger C. Side chain specificity of ADP-ribosylation by a sirtuin. FEBS J. 2009;276(23):7159-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci U S A. 2012;109(29):11800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2012;334(6057):806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. French JB, Cen Y, Sauve AA. Plasmodium falciparum Sir2 is an NAD+-dependent deacetylase and an acetyllysine-dependent and acetyllysine-independent NAD+ glycohydrolase. Biochemistry. 2008;47(38):10227-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide: 5,6-dimethylbenzimidazole phosphoribosyltransferase activity in CobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J Biol Chem. 1998;273(48):31788-94 [DOI] [PubMed] [Google Scholar]
- 33. Tsang AW, Escalante-Semerena JC. CobB function is required for catabolism of propionate in Salmonella typhimurium LT2: evidence for existence of a substitute function for CobB within the 1,2-propanediol utilization (pdu) operon. J Bacteriol. 1996;178(23):7016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149-59 [DOI] [PubMed] [Google Scholar]
- 35. Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21(10):2383-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137-48 [DOI] [PubMed] [Google Scholar]
- 37. Blander G, Olejnik J, Krzymanska-Olejnik E, et al. SIRT1 shows no substrate specificity in vitro. J Biol Chem. 2005;280(11):9780-5 [DOI] [PubMed] [Google Scholar]
- 38. Khan AN, Lewis PN. Unstructured conformations are a substrate requirement for the Sir2 family of NAD-dependent protein deacetylases. J Biol Chem. 2005;280(43):36073-8 [DOI] [PubMed] [Google Scholar]
- 39. Kuzmichev A, Margueron R, Vaquero A, et al. Composition and histone substrates of Polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102(6):1859-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furuyama T, Kitayama K, Shimoda Y, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279(33):34741-9 [DOI] [PubMed] [Google Scholar]
- 41. Fulco M, Schiltz RL, Iezzi S, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12(1):51-62 [DOI] [PubMed] [Google Scholar]
- 42. Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429(6993): 771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang F, Wang S, Gan L, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mancio-Silva L, Lopez-Rubio JJ, Claes A, Scherf A. Sir2a regulates rDNA transcription and multiplication rate in the human malaria parasite Plasmodium falciparum. Nat Commun. 2012;4:1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goyal M, Alam A, Iqbal MS, et al. Identification and molecular characterization of an Alba-family protein from human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 2012;40(3):1174-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cockell MM, Perrod S, Gasser SM. Analysis of Sir2p domains required for rDNA and telomeric silencing in Saccharomyces cerevisiae. Genetics. 2000;154(3):1069-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freeman-Cook LL, Gomez EB, Spedale EJ, et al. Conserved locus-specific silencing functions of Schizosaccharomyces pombe sir2+. Genetics. 2005;169(3):1243-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kyes SA, Kraemer SM, Smith JD. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot Cell. 2007;6(9):1511-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tonkin CJ, Carret CK, Duraisingh MT, et al. Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum. PLoS Biol. 2009;7(4):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trojer P, Reinberg D. Facultative heterochromatin: is there a distinctive molecular signature? Mol Cell. 2007;28(1):1-13 [DOI] [PubMed] [Google Scholar]
- 52. Shankaranarayana GD, Motamedi MR, Moazed D, Grewal SI. Sir2 regulates histone H3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr Biol. 2003;13(14):1240-6 [DOI] [PubMed] [Google Scholar]
- 53. Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450(7168):440-4 [DOI] [PubMed] [Google Scholar]
- 54. Bosch-Presegue L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2(6):648-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kruszewski M, Szumiel I. Sirtuins (histone deacetylases III) in the cellular response to DNA damage: facts and hypotheses. DNA Repair (Amst). 2005;4(11):1306-13 [DOI] [PubMed] [Google Scholar]
- 56. Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16(1):93-105 [DOI] [PubMed] [Google Scholar]
- 57. Vaquero A. The conserved role of sirtuins in chromatin regulation. Int J Dev Biol. 2009;53(2-3):303-22 [DOI] [PubMed] [Google Scholar]
- 58. Megee PC, Morgan BA, Mittman BA, Smith MM. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science. 1990;247(4944):841-5 [DOI] [PubMed] [Google Scholar]
- 59. Bird AW, Yu DY, Pray-Grant MG, et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419(6905):411-5 [DOI] [PubMed] [Google Scholar]
- 60. Schwaiger M, Stadler MB, Bell O, Kohler H, Oakeley EJ, Schubeler D. Chromatin state marks cell-type- and gender-specific replication of the Drosophila genome. Genes Dev. 2009;23(5):589-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391-400 [DOI] [PubMed] [Google Scholar]
- 62. Vaquero A, Scher MB, Lee DH, et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20(10):1256-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krauss V. Glimpses of evolution: heterochromatic histone H3K9 methyltransferases left its marks behind. Genetica. 2008;133(1):93-106 [DOI] [PubMed] [Google Scholar]
- 64. McCord RA, Michishita E, Hong T, et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY). 2009;1(1):109-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab. 2012;23(9):467-76 [DOI] [PubMed] [Google Scholar]
- 66. Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol. 2012;32(24):5022-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barber MF, Michishita-Kioi E, Xi Y, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487(7405):114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20(9):1075-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Armstrong CM, Kaeberlein M, Imai SI, Guarente L. Mutations in Saccharomyces cerevisiae gene SIR2 can have differential effects on in vivo silencing phenotypes and in vitro histone deacetylation activity. Mol Biol Cell. 2002;13(4):1427-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yuan J, Pu M, Zhang Z, Lou Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle. 2009;8(11):1747-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Michishita E, McCord RA, Boxer LD, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 2009;8(16):2664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Celic I, Masumoto H, Griffith WP, et al. The sirtuins Hst3 and Hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr Biol. 2006;16(13):1280-9 [DOI] [PubMed] [Google Scholar]
- 73. Vempati RK, Jayani RS, Notani D, Sengupta A, Galande S, Haldar D. p300-mediated acetylation of histone H3 lysine 56 functions in DNA damage response in mammals. J Biol Chem. 2010;285(37):28553-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Garcia-Salcedo JA, Gijon P, Nolan DP, Tebabi P, Pays E. A chromosomal SIR2 homologue with both histone NAD-dependent ADP-ribosyltransferase and deacetylase activities is involved in DNA repair in Trypanosoma brucei. EMBO J. 2003;22(21):5851-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wirth M, Paap F, Fischle W, et al. HIS-24 linker histone and SIR-2.1 deacetylase induce H3K27me3 in the Caenorhabditis elegans germ line. Mol Cell Biol. 2009;29(13):3700-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jedrusik MA, Schulze E. Linker histone HIS-24 (H1.1) cytoplasmic retention promotes germ line development and influences histone H3 methylation in Caenorhabditis elegans. Mol Cell Biol. 2007;27(6):2229-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li JY, Patterson M, Mikkola HK, Lowry WE, Kurdistani SK. Dynamic distribution of linker histone H1.5 in cellular differentiation. PLoS Genet. 2012;8(8):e1002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pruitt K, Zinn RL, Ohm JE, et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2(3):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Espada J, Ballestar E, Santoro R, et al. Epigenetic disruption of ribosomal RNA genes and nucleolar architecture in DNA methyltransferase 1 (Dnmt1) deficient cells. Nucleic Acids Res. 2007;35(7):2191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng L, Yuan Z, Ling H, et al. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol Cell Biol. 2011;31(23):4720-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964;51:786-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brownell JE, Allis CD. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6(2):176-84 [DOI] [PubMed] [Google Scholar]
- 83. Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272(5260): 408-11 [DOI] [PubMed] [Google Scholar]
- 84. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595-606 [DOI] [PubMed] [Google Scholar]
- 85. Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15-23 [DOI] [PubMed] [Google Scholar]
- 86. Garcia-Cao M, O’Sullivan R, Peters AH, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet. 2004;36(1):94-9 [DOI] [PubMed] [Google Scholar]
- 87. Bosch-Presegue L, Raurell-Vila H, Marazuela-Duque A, et al. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell. 2010;42(2):210-23 [DOI] [PubMed] [Google Scholar]
- 88. Murayama A, Ohmori K, Fujimura A, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133(4):627-39 [DOI] [PubMed] [Google Scholar]
- 89. Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459(7243):113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Szerlong HJ, Prenni JE, Nyborg JK, Hansen JC. Activator-dependent p300 acetylation of chromatin in vitro: enhancement of transcription by disruption of repressive nucleosome-nucleosome interactions. J Biol Chem. 2010;285(42):31954-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bouras T, Fu M, Sauve AA, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280(11):10264-76 [DOI] [PubMed] [Google Scholar]
- 92. Black JC, Mosley A, Kitada T, Washburn M, Carey M. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell. 2008;32(3):449-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Albaugh BN, Arnold KM, Lee S, Denu JM. Autoacetylation of the histone acetyltransferase Rtt109. J Biol Chem. 2011;286(28):24694-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gu W, Szauter P, Lucchesi JC. Targeting of MOF, a putative histone acetyl transferase, to the X chromosome of Drosophila melanogaster. Dev Genet. 1998;22(1):56-64 [DOI] [PubMed] [Google Scholar]
- 95. Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5(2):367-75 [DOI] [PubMed] [Google Scholar]
- 96. Lu L, Li L, Lv X, Wu XS, Liu DP, Liang CC. Modulations of hMOF autoacetylation by SIRT1 regulate hMOF recruitment and activities on the chromatin. Cell Res. 2011;21(8):1182-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yamagata K, Kitabayashi I. Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem Biophys Res Commun. 2009;390(4):1355-60 [DOI] [PubMed] [Google Scholar]
- 98. Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem. 2010;285(15):11458-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315-29 [DOI] [PubMed] [Google Scholar]
- 100. Kaidi A, Weinert BT, Choudhary C, Jackson SP. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329(5997):1348-53 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101. Religa AA, Waters AP. Sirtuins of parasitic protozoa: in search of function(s). Mol Biochem Parasitol. 2012;185(2):71-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yuan Z, Seto E. A functional link between SIRT1 deacetylase and NBS1 in DNA damage response. Cell Cycle. 2007;6(23):2869-71 [DOI] [PubMed] [Google Scholar]
- 103. Fan W, Luo J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell. 2010;39(2):247-58 [DOI] [PubMed] [Google Scholar]
- 104. Rajamohan SB, Pillai VB, Gupta M, et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29(15):4116-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO. Transcriptional coactivation of nuclear factor-kappaB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem. 2003;278(46):45145-53 [DOI] [PubMed] [Google Scholar]
- 106. Hassa PO, Haenni SS, Buerki C, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280(49):40450-64 [DOI] [PubMed] [Google Scholar]
- 107. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390-2 [DOI] [PubMed] [Google Scholar]
- 108. Jeong J, Juhn K, Lee H, et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39(1):8-13 [DOI] [PubMed] [Google Scholar]
- 109. Thrower DA, Bloom K. Dicentric chromosome stretching during anaphase reveals roles of Sir2/Ku in chromatin compaction in budding yeast. Mol Biol Cell. 2001;12(9):2800-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97(5):621-33 [DOI] [PubMed] [Google Scholar]
- 111. Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804(8):1684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu L, Scolnick DM, Trievel RC, et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19(2):1202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang YH, Tsay YG, Tan BC, Lo WY, Lee SC. Identification and characterization of a novel p300-mediated p53 acetylation site, lysine 305. J Biol Chem. 2003;278(28):25568-76 [DOI] [PubMed] [Google Scholar]
- 115. D’Erchia AM, Tullo A, Lefkimmiatis K, Saccone C, Sbisa E. The fatty acid synthase gene is a conserved p53 family target from worm to human. Cell Cycle. 2006;5(7):750-8 [DOI] [PubMed] [Google Scholar]
- 116. Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Peck B, Chen CY, Ho KK, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9(4):844-55 [DOI] [PubMed] [Google Scholar]
- 118. Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One. 2010;5(5):e10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007; 6(4):505-14 [DOI] [PubMed] [Google Scholar]
- 120. Liu L, Arun A, Ellis L, Peritore C, Donmez G. Sirtuin 2 (SIRT2) enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via deacetylating forkhead box O3a (Foxo3a) and activating Bim protein. J Biol Chem. 2012;287(39):32307-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121. Daitoku H, Hatta M, Matsuzaki H, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101(27):10042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem. 2004;279(28):28873-9 [DOI] [PubMed] [Google Scholar]
- 123. Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551-63 [DOI] [PubMed] [Google Scholar]
- 124. Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24(5):1021-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6(2):105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jacobs KM, Pennington JD, Bisht KS, et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4(5):291-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rodriguez-Colman MJ, Reverter-Branchat G, Sorolla MA, Tamarit J, Ros J, Cabiscol E. The forkhead transcription factor Hcm1 promotes mitochondrial biogenesis and stress resistance in yeast. J Biol Chem. 2010;285(47):37092-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280(21):20589-95 [DOI] [PubMed] [Google Scholar]
- 129. Peserico A, Chiacchiera F, Grossi V, et al. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci. Epub 2013. January 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rizki G, Iwata TN, Li J, et al. The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011;7(9):e1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Menssen A, Hydbring P, Kapelle K, et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci U S A. 2012;109(4):E187-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marshall GM, Liu PY, Gherardi S, et al. SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability. PLoS Genet. 2011;7(6):e1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu PY, Xu N, Malyukova A, et al. The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ. 2013;20(3):503-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang R, Chen HZ, Liu JJ, et al. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem. 2010;285(10):7097-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470(2):M55-62 [DOI] [PubMed] [Google Scholar]
- 136. Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221-7 [DOI] [PubMed] [Google Scholar]
- 137. Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 2010;123(Pt 24):4251-8 [DOI] [PubMed] [Google Scholar]
- 138. Rothgiesser KM, Fey M, Hottiger MO. Acetylation of p65 at lysine 314 is important for late NF-kappaB-dependent gene expression. BMC Genomics. 2010;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38(6):864-78 [DOI] [PubMed] [Google Scholar]
- 140. Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2009;140(2):280-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang C, Chen L, Hou X, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8(9):1025-31 [DOI] [PubMed] [Google Scholar]
- 142. Schertel C, Conradt B. C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development. 2007;134(20):3691-701 [DOI] [PubMed] [Google Scholar]
- 143. de Oliveira RM, Pais TF, Outeiro TF. Sirtuins: common targets in aging and in neurodegeneration. Curr Drug Targets. 2010;11(10):1270-80 [DOI] [PubMed] [Google Scholar]
- 144. Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298(5602):2390-2 [DOI] [PubMed] [Google Scholar]
- 145. Gardner JG, Escalante-Semerena JC. In Bacillus subtilis, the sirtuin protein deacetylase, encoded by the srtN gene (formerly yhdZ), and functions encoded by the acuABC genes control the activity of acetyl coenzyme A synthetase. J Bacteriol. 2009;191(6):1749-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E. SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1,2 and HMGCS1,2. Aging (Albany NY). 2010;3(6):635-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Starai VJ, Takahashi H, Boeke JD, Escalante-Semerena JC. Short-chain fatty acid activation by acyl-coenzyme A synthetases requires SIR2 protein function in Salmonella enterica and Saccharomyces cerevisiae. Genetics. 2003;163(2):545-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chan CH, Garrity J, Crosby HA, Escalante-Semerena JC. In Salmonella enterica, the sirtuin-dependent protein acylation/deacylation system (SDPADS) maintains energy homeostasis during growth on low concentrations of acetate. Mol Microbiol. 2011;80(1):168-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gurd BJ. Deacetylation of PGC-1alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):589-97 [DOI] [PubMed] [Google Scholar]
- 150. Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582(1):46-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28(1):91-106 [DOI] [PubMed] [Google Scholar]
- 152. Kemper JK, Xiao Z, Ponugoti B, et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10(5):392-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr. 2008;138(3):497-501 [DOI] [PubMed] [Google Scholar]
- 154. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283(41):27628-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jiang W, Wang S, Xiao M, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43(1):33-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444(1):1-10 [DOI] [PubMed] [Google Scholar]
- 157. Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103(27):10230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lombard DB, Alt FW, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49(2):304-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lu Z, Bourdi M, Li JH, et al. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity. EMBO Rep. 2011;12(8):840-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137(3):560-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437-44 [DOI] [PubMed] [Google Scholar]
- 165. Du J, Zhou Y, Su X, et al. Sirt5 is a NAD- dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Peng C, Lu Z, Xie Z, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12):M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ahuja N, Schwer B, Carobbio S, et al. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282(46):33583-92 [DOI] [PubMed] [Google Scholar]
- 169. Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941-54 [DOI] [PubMed] [Google Scholar]
- 170. Nasrin N, Wu X, Fortier E, et al. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285(42):31995-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Fakhrai-Rad H, Nikoshkov A, Kamel A, et al. Insulin-degrading enzyme identified as a candidate diabetes susceptibility gene in GK rats. Hum Mol Genet. 2000;9(14):2149-58 [DOI] [PubMed] [Google Scholar]
- 172. Li Y, Matsumori H, Nakayama Y, et al. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells. 2011;16(1):34-45 [DOI] [PubMed] [Google Scholar]
- 173. Kim HS, Vassilopoulos A, Wang RH, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20(4):487-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Firestein R, Blander G, Michan S, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3(4):e2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Narayan N, Lee IH, Borenstein R, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492(7428):199-204 [DOI] [PubMed] [Google Scholar]
- 176. Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123(Pt 6):894-902 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177. Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23(9):3173-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Inoue T, Hiratsuka M, Osaki M, et al. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. 2007;26(7):945- 57 [DOI] [PubMed] [Google Scholar]
- 179. Suzuki K, Koike T. Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience. 2007;147(3):599-612 [DOI] [PubMed] [Google Scholar]
- 180. Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington’s disease phenotypes in vivo. PLoS One. 2012;7(4):e34805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455-8 [DOI] [PubMed] [Google Scholar]
- 182. Zhang Y, Zhang M, Dong H, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28(3):445-60 [DOI] [PubMed] [Google Scholar]
- 183. Bonda DJ, Lee HG, Camins A, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10(3):275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Luijsterburg MS, White MF, van Driel R, Dame RT. The major architects of chromatin: architectural proteins in bacteria, archaea and eukaryotes. Crit Rev Biochem Mol Biol. 2008;43(6):393-418 [DOI] [PubMed] [Google Scholar]
- 186. Aravind L, Iyer LM, Anantharaman V. The two faces of Alba: the evolutionary connection between proteins participating in chromatin structure and RNA metabolism. Genome Biol. 2003;4(10):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35(12):669-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ammar R, Torti D, Tsui K, et al. Chromatin is an ancient innovation conserved between Archaea and Eukarya. Elife. 2012;1:e00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2010;36(2):108-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lundby A, Lage K, Weinert BT, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2(2):419-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29(33):4617-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Giandomenico V, Simonsson M, Gronroos E, Ericsson J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol Cell Biol. 2003;23(7):2587-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Scott I, Webster BR, Li JH, Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem J. 2012;443(3):655-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Al-Bassam J, Corbett KD. alpha-tubulin acetylation from the inside out. Proc Natl Acad Sci U S A. 2012;109(48):19515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Patel JH, Du Y, Ard PG, et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24(24):10826-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Faiola F, Liu X, Lo S, et al. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol Cell Biol. 2005;25(23):10220-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Van Den Broeck A, Nissou D, Brambilla E, Eymin B, Gazzeri S. Activation of a Tip60/E2F1/ERCC1 network in human lung adenocarcinoma cells exposed to cisplatin. Carcinogenesis. 2011;33(2):320-5 [DOI] [PubMed] [Google Scholar]
- 199. Vries RG, Prudenziati M, Zwartjes C, Verlaan M, Kalkhoven E, Zantema A. A specific lysine in c-Jun is required for transcriptional repression by E1A and is acetylated by p300. EMBO J. 2001;20(21):6095-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Geng H, Liu Q, Xue C, et al. HIF1alpha protein stability is increased by acetylation at lysine 709. J Biol Chem. 2012;287(42):35496-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cohen HY, Lavu S, Bitterman KJ, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13(5):627-38 [DOI] [PubMed] [Google Scholar]