Abstract
Objective
To describe the clinical utility of the nicotinic ganglionic acetylcholine receptor (α3-AChR) autoantibody as a marker of neurological autoimmunity and cancer.
Design
Case-control study.
Setting
Mayo Clinic, Rochester, Minnesota.
Patients
A total of 15 000 patients seen at Mayo Clinic (2005–2007) and evaluated on a service basis for paraneoplastic neurological autoimmunity for whom clinical information was obtained retrospectively by medical record review as well as 457 neurologically asymptomatic patients or control subjects of whom 173 were healthy, 245 had lung cancer, and 39 had systemic lupus erythematosus or Sjögren syndrome.
Outcome Measures
Neurological, oncological, and serological associations of α3-AChR autoantibody seropositivity.
Results
Of 15 000 patients tested on a service basis, 1% were seropositive (median, 0.12 nmol/L; range, 0.03–18.8 nmol/L; normal, ≤0.02 nmol/L), 55% were male, and the median age was 65 years. Cancer was found (new or by history) in 24 of 78 patients evaluated for cancer while at Mayo Clinic (30%); 43% had adenocarcinoma (more patients had breast cancer than prostate, lung, and gastrointestinal cancers; each of the latter groups had about the same number of patients). Of 12 patients with high antibody values (≥1.00 nmol/L), 83% had pandysautonomia. Of 85 patients with medium antibody values (0.10–0.99 nmol/L), neurological presentations were more diverse and included peripheral neuropathies (36%), dysautonomia (20%, usually limited), and encephalopathy (13%). Of 58 patients with low antibody values (0.03–0.09 nmol/L), 54% had a nonautoimmune neurological disorder or no neurological disorder. Of 245 control patients with lung cancer, 7.8% were seropositive. Only 1 of 212 control patients without cancer (0.5%) was seropositive (P<.001).
Conclusion
The detection of α3-AChR autoantibody aids the diagnosis of neurological autoimmunity and cancer.
The nicotinic ganglionic acetylcholine receptor autoantibody (α3-AChR Ab) causes autoimmune dysautonomia that is either subacute or insidious in onset.1–4 The pathogenicity of α3-AChR Ab was demonstrated in rabbits immunized with a recombinant extracellular fragment of the α3-AChR subunit and in mice injected with IgG from high-titered α3-AChR Ab-positive rabbit or human sera. A direct relationship between antibody titer and severity of dysautonomia occurs in both experimental animals and patients.1,2,4 Patients generally develop profound pandysautonomia with high α3-AChR Ab values (>1.0 nmol/L)4 and limited dysautonomia (eg, postural orthostatic tachycardia syndrome5 or gastroparesis6), with lower α3-AChR Ab values. Pandysautonomia is generally severe and includes impaired pupillary light reflex, anhidrosis, orthostatic hypotension, gastrointestinal dysmotility, sicca manifestations, and bladder dysfunction. Limited dysautonomia is confined to 1 or 2 domains and is often mild.
Since incorporating the α3-AChR Ab assay into the standard Mayo Clinic serological evaluation for paraneoplastic autoimmunity in 2005, we have observed that this Ab has broader oncological and neurological associations than originally recognized. Here we describe the frequency of α3-AChR Ab seropositivity in patients at the Mayo Clinic who were evaluated for paraneoplastic autoantibodies on a service basis as well as the frequency and spectrum of oncological, neurological, and serological accompaniments during a 27-month period. We also determined the frequency of α3-AChR Ab in the sera of the following 3 control groups: (1) healthy persons age-and sex-matched to seropositive patients, (2) neurologically asymptomatic patients with lung cancer, and (3) neurologically asymptomatic patients with systemic lupus erythematosus (SLE) or Sjögren syndrome.
METHODS
PATIENTS
The study was approved by the institutional review board of Mayo Clinic, Rochester, Minnesota (IRB 06-005457). From January 1, 2005, to March 31, 2007, service paraneoplastic autoantibody evaluations were performed on the serum samples of approximately 15 000 patients evaluated clinically at Mayo Clinic’s 3 sites (Rochester, Minnesota; Scottsdale, Arizona; and Jacksonville, Florida).
CONTROLS
Serum samples were available for 173 healthy age- and sex-matched residents of Olmsted County, Minnesota (collected in 2005). Additional controls included 245 neurologically asymptomatic patients with lung cancer prior to oncological treatment (identified from the Mayo Clinic Epidemiology and Genetics of Lung Cancer Research registry enrolled from 1999 to 2005; 35 had small cell lung carcinoma, 70 had adenocarcinoma, 70 had squamous carcinoma, and 70 had other lung cancer types), and 39 control patients with Sjögren syndrome or SLE (patients from the Mayo Clinic ascertained serologically in the Mayo Clinical Immunology Laboratory with high titers of extractable nuclear antigen [Sjögren syndrome antigen A or B] or antinuclear antibody [with or without anti–double-stranded DNA]; diagnoses were confirmed through review of medical records). Selected subjects met the clinical criteria for having SLE or Sjögren syndrome7 and lacked neurological symptoms.
CLINICAL EVALUATION
Two of us (A.M. and S.J.P.) retrospectively obtained medical history, examination findings, and laboratory data (imaging, electrophysiological, physiological [autonomic reflex screen and thermoregulatory sweat test], serological, and cerebrospinal fluid) by medical record review of identified seropositive patients. Neurological diagnoses were determined by at least 1 Mayo Clinic staff neurologist and confirmed by one of us (A.M. or S.J.P.). The severity of dysautonomia was determined by the Composite Autonomic Scoring Scale (scores ≤3 indicate mild failure; ≥7, severe failure)8 and percentage of anterior body anhidrosis.9
SEROLOGICAL EVALUATION
Ganglionic AChR Ab was detected by radioimmunoprecipitation using iodine 125–labeled epibatidine complexed to α3-AChR solubilized from a human neuroblastoma.4,10 Results are expressed as nanomoles of AChR complex bound per liter of serum. No patient’s serum bound 125I-epibatidine alone.
Serum was evaluated by standardized immunofluorescence criteria for IgG neural autoantibodies (antineuronal nuclear antibody [ANNA]–1, 2, 3; amphiphysin antibody; Purkinje cell 1, 2, and Tr antibodies; collapsin response-mediator protein [CRMP]–5 IgG; and antiglial or neuronal nuclear antibody).11,12 Serum was also evaluated by radioimmunoprecipitation assays for neuronal voltage-gated cation channel antibodies (P/Q-type, N-type calcium channels, and potassium channel), muscle (α1) nicotinic acetylcholine receptor, and glutamic acid decarboxylase 65-isoform (GAD65)11,13 and by recombinant Western blot for CRMP-5 IgG.14 Striational antibodies were detected by enzyme-linked immunosorbent assay.15
STATISTICAL ANALYSIS
We compared the frequency of seropositivity for α3-AChR Ab among controls with and without cancer using the Fisher exact test. The duration of symptoms from onset to initiation of treatment was compared for patients whose symptoms did and did not improve with treatment (physician reported) using the Wilcoxon rank sum test. Two-sided P <.05 was considered significant.
RESULTS
NORMAL VALUES
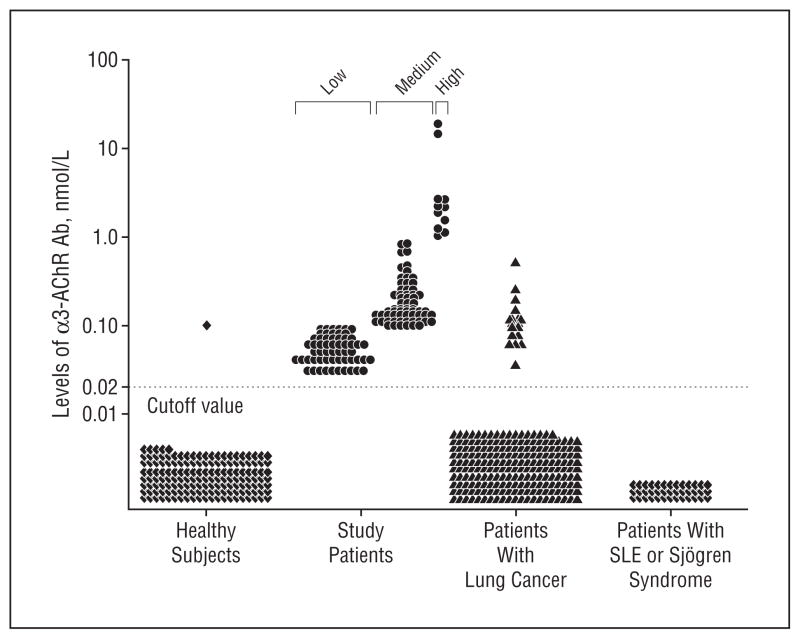
The normal value range for α3-AChR Ab was established by testing the serum of 173 healthy control subjects matched in sex and age to the study patients. Only 1 (0.6%) had a value exceeding 0.02 nmol/L (Figure 1). We therefore analyzed study patients’ data according to the serum α3-AChR Ab value ranges high (≥1.00 nmol/L), medium (0.10–0.99 nmol/L), and low (0.03–0.09 nmol/L).
Figure 1.
Nicotinic ganglionic acetylcholine receptor antibody (α3-AChR Ab) values detected in 155 patients with neurological disease (those referred for paraneoplastic evaluation) and 457 controls (those with cancer only or those without neurological disease or cancer). SLE indicates systemic lupus erythematosus; circles, patients referred for paraneoplastic evaluation; triangles, cancer only; and diamonds, controls without neurological disease or cancer.
PATIENTS
Of 15 000 patients evaluated at the Mayo Clinic for paraneoplastic autoantibodies in a 27-month period, α3-AChR Ab values exceeded 0.02 nmol/L in 155 (1%; median, 0.12 nmol/L; range, 0.03–18.8 nmol/L).
Oncological, neurological, and serological data are summarized in Tables 1, 2, and 3. Six patients (4%) were not white, 86 (55%) were male, and 69 (45%) were female. The median age at neurological symptom onset was 65 years (range, 17–103 years). The median follow-up period was 2 months (range, 0–96 months). Informative follow-up data were available for 70 patients (45%; median, 6 months).
Table 1.
Cancer Associations in 78 α3-AChR Ab–Seropositive Patients Who Underwent Oncological Evaluation by Titera
| No. (%)
|
||||
|---|---|---|---|---|
| Total | High | Medium | Low | |
| Patients | 78 | 9 | 49 | 20 |
| Cancers | 30 | 2 | 20 | 8 |
| Cancer type | ||||
| Adenocarcinoma | 13 (43) | 1 | 11 | 1 |
| Breast | 4 | 0 | 4b | 0 |
| Prostate | 3 | 0 | 2 | 1 |
| Lung | 2 | 1 | 1 | 0 |
| GI tract | 2 | 0 | 2b | 0 |
| Thyroid, papillary | 1 | 0 | 1 | 0 |
| Uterine | 1 | 0 | 1 | 0 |
| Renal cell carcinoma | 2 (6) | 0 | 1 | 1 |
| Lymphoid | 5 (17) | 1 | 3 | 1 |
| B cell lymphoma | 3 | 0 | 2 | 1c |
| CLL | 1 | 1 | 0 | 0 |
| Myeloma | 1 | 0 | 1 | 0 |
| Other | 10 (34) | 0 | 5 | 5 |
| Melanoma | 2 | 0 | 0 | 2b |
| Bladder carcinoma | 2 | 0 | 1 | 1 |
| Small cell carcinoma, lung | 1 | 0 | 0 | 1c,d |
| Lung carcinomad | 1 | 0 | 1 | 0 |
| Thymoma | 1 | 0 | 1b | 0 |
| Thyroid carcinomae | 1 | 0 | 0 | 1 |
| Tonsillar carcinoma, squamous | 1 | 0 | 1 | 0 |
| Ovarian carcinoma | 1 | 0 | 1 | 0 |
Abbreviations: α3-AChR Ab, nicotinic ganglionic acetylcholine receptor autoantibody; CLL, chronic lymphocytic leukemia; GI, gastrointestinal.
A total of 30 cancers were identified in 24 of 78 patients. See “Oncological Associations” subsection of the “Results” section for cancer histories in 77 patients who did not undergo testing for cancer.
Muscle AChR Ab was detected in 2 patients with breast carcinoma and 1 each with GI carcinoma, melanoma, and thymoma; 2 had myasthenia gravis.
Coexisting glutamic acid decarboxylase 65-isoform antibody.
Coexisting antineuronal nuclear antibody 1 (1:30 720) predicted small cell lung carcinoma in a patient with limbic encephalitis, neuropathy, and GI dysmotility.
Unknown histology.
Table 2.
Neurological Associations in 155 α3-AChR Ab–Seropositive Patients by Titer
| Neurological Manifestation | No. (%)
|
|||
|---|---|---|---|---|
| Total | High (n = 12 [8%]) | Medium (n = 85 [55%]) | Low (n = 58 [37%]) | |
| Peripheral nerve, autonomic | 33 (21) | 10 (84)a | 17 (20)b | 6 (10)b |
| Limited dysautonomia | 20 | 3 | 14 | 3 |
| GI dysmotility | 10c | 1 | 8 | 1 |
| Orthostatism | 5 | 1 | 3 | 1 |
| Anhidrosis | 3 | 1 | 1 | 1 |
| Sicca syndrome | 2 | 0 | 2 | 0 |
| Pandysautonomia | 13 | 7 | 3 | 3 |
| Peripheral nerve, somatic | 44 (28) | 0 | 31 (36) | 13 (22) |
| Sensorimotor polyneuropathyd | 28 | 0 | 22 | 6 |
| Small fiber sensory neuropathy | 7 | 0 | 5 | 2 |
| Polyradiculopathy | 4 | 0 | 2 | 2 |
| Cranial neuropathy | 3 | 0 | 1 | 2 |
| Sensory ganglionopathy | 1 | 0 | 0 | 1 |
| Multifocal motor neuropathy | 1 | 0 | 1 | 0 |
| Neuromuscular junction | 4 (3) | 0 | 1 (1) | 3 (5) |
| Myasthenia gravise | 4 (3) | 0 | 1 | 3 |
| Central | 26 (17) | 1 (8) | 20 (24) | 5 (9) |
| Cortical and/or neuropsychiatric | 15 | 1 | 11 | 3 |
| Movement disorderf | 5 | 0 | 5 | 0 |
| Demyelinating CNS disorders | 5 | 0 | 3 | 2g |
| Stiff-man syndrome | 1 | 0 | 1 | 0 |
| Nonautoimmune disorder or noneh | 48 (31) | 1 (8) | 16 (19) | 31 (54) |
Abbreviations: α3-AChR Ab, nicotinic ganglionic acetylcholine receptor autoantibody; CASS, Composite Autonomic Scoring Scale; CNS, central nervous system; GI, gastrointestinal.
Median CASS of 8.0; median anhidrosis of 75%.
Median CASS of 2.0; median anhidrosis 14%.
Abnormal GI motility studies in 2 patients tested.
Axonal neuropathy was more common than demyelinating, which was more common than sensory.
Coexisting muscle AChR-binding Ab was detected in all cases (range, 2.56–28.7 nmol/L; normal value, ≤0.02 nmol/L).
Ataxia, 3 patients; parkinsonism, 2 patients.
One patient had neuromyelitis optica.
Final neurological diagnosis nonautoimmune neurological disorder (eg, noncommunicating hydrocephalus, Parkinson disease, amyotrophic lateral sclerosis, Alzheimer disease, Lewy body disease) or none.
Table 3.
Coexisting Autoantibodies
| Antibody Type Specificity | Patients, No. | Median Values (Range), nmol/L |
|---|---|---|
| Neuronal cation channel | 19 | |
| Voltage-gated calcium channel | 13 | |
| N-type | 8 | 0.06 (0.03–0.15)a |
| P/Q-type | 5 | 0.07 (0.03–13)b |
| Voltage-gated potassium channel | 6 | 0.08 (0.03–0.46)b |
| Neuronal nuclear or cytoplasmic | 14 | |
| GAD65 | 11 | 0.14 (0.03–0.15)b |
| ANNA-1 | 2 | 15 360; 30 720c |
| CRMP-5 | 1 | Western blot positive |
| Skeletal muscle | 13 | |
| AChR | 10 | 2.87 (0.04–38.9)b |
| Striational | 3 | 480; 15 360; 30 720d |
Abbreviations: AChR, muscle acetylcholine receptor; ANNA-1, antineuronal nuclear antibody-1; CRMP-5, collapsin response-mediator protein-5; GAD65, 65kDa isoform of glutamic acid decarboxylase.
Normal values are 0.03 nmol/L or less.
Normal values are 0.02 nmol/L or less.
Normal values are negative at 1:240.
Normal values are negative at 1:60.
ONCOLOGICAL ASSOCIATIONS
Of 78 seropositive patients (50%) who had an evaluation for cancer at Mayo Clinic, 24 (30%) were confirmed to have cancer, either active or by history; 6 had multiple malignant neoplasms. The cancer incidence or type did not correlate with antibody level. Adenocarcinomas accounted for 43% of all identified cancers. Three of 16 with a history of cancer were receiving active treatment when diagnosed with neurological disease and 13 were in apparent remission (2–14 years). Oncological investigation prompted by detection of α3-AChR Ab revealed cancer in another 9 patients (Table 1); 2 had lung carcinomas (1 adenocarcinoma, 1 histology unknown), 2 thyroid carcinomas (1 papillary, 1 histology unknown), 2 bladder carcinomas, 1 renal cell carcinoma, 1 melanoma, and 1 multiple myeloma. Of note, 2 of these 9 patients lacked evidence of neurological autoimmunity. One had genetically confirmed Huntington disease and was found to have bladder carcinoma, and 1 with multifactorial gait disorder was found to have bronchogenic carcinoma. Cancers were detected by whole-body computed tomography (5 patients), whole-body positron emission tomography (3 patients, all with negative computed tomographic scans; Figure 2), and bone marrow aspirate biopsy (1 patient).
Figure 2.

Positron emission tomographic (PET) images of a man presenting at 70 years of age with encephalopathy; nicotinic ganglionic acetylcholine receptor antibody (α3-AChR Ab) value, 0.10 nmol/L. Results of initial evaluation for cancer including computed tomography were normal. A subsequent PET scan revealed a hypermetabolic focus in the superior right lobe of the thyroid gland (arrow). Ultrasound-guided fine-needle aspiration revealed papillary carcinoma.
Of 77 patients who did not undergo oncological evaluation at Mayo Clinic after identification of α3-AChR, 8 had a history of cancer (10%). These cancers were lung, 2; prostate, 2; breast, 1; melanoma, 1; epidermoid brainstem tumor, 1; and multifocal recurrent meningiomas, 1.
NEUROLOGICAL MANIFESTATIONS
Neurological symptoms and signs were multifocal in 29% of patients. Symptom onset was subacute in 46% and insidious in 54%.
Patients With High α3-AChR Ab Values
High autoantibody values were those 1.00 nmol/L or higher. Twelve patients (8%) had a median serum antibody value of 2.03 nmol/L (range, 1.02–18.8 nmol/L). Eleven of those (92%) were considered to have neurological autoimmunity (a neurological disorder with an autoimmune pathogenesis; Table 2). Ten had dysautonomia (84%), 7 severe pandysautonomia, and 3 limited dysautonomia (1 gastrointestinal dysmotility, 1 mild sudomotor impairment, and 1 orthostatism). One patient presented with subacute autoimmune encephalopathy and recovered completely after 5 days of treatment with intravenous methylprednisolone (Table 4). The twelfth patient had orthostatic headache attributed to a dural cerebrospinal fluid leak.
Table 4.
Clinical and Demographic Characteristics and Outcomes for 16 Patients
| Patient No./ Sex/Onset Age, y | Predominant Neurological Disorder | α3-AChR Ab Value, nmol/L | Coexisting Ab Value, nmol/L, titer | Cancer Diagnosis | Therapy | Neurological Improvement | Interval From Onset to Treatment, mo | Interval From Onset to Last Follow-up, mo |
|---|---|---|---|---|---|---|---|---|
| 1/F/35 | Dysautonomia | 18.8 | … | … | Mycophenolate; PLEX | Yes | 9 | 27 |
| 2/F/42 | Dysautonomia | 2.23 | Muscle AChR, 1.17 | … | Azathioprine; IVIg | Yes | 2 | 30 |
| 3/F/44 | Dysautonomia | 5.36 | … | … | Mycophenolate; IVIg | Yes | 11 | 16 |
| 4/M/56 | Dysautonomia | 1.54 | … | … | Mycophenolate; IVIg | No | 200 | 213 |
| 5/F/49 | Dysautonomia | 0.26 | … | … | Prednisone | Yes | 120 | 156 |
| 6/F/64 | Dysautonomia | 0.11 | … | Rectal carcinoma | IVIg | No | 84 | 95 |
| 7/F/83 | Dysautonomia | 0.05 | … | … | IVIg | No | 2 | 5 |
| 8/M/57 | Dysautonomia and MG | 0.30 | Muscle AChR, 7.21; striational, 30 720 | Renal cell carcinoma, metastatic | Azathioprine; prednisone, chemotherapy | Yes | 9 | 21 |
| 9/M/103 | MG | 0.04 | Muscle AChR, 13.4 | Prednisone | Yes | 6 | 12 | |
| 10/M/80 | MG | 0.03 | Muscle AChR, 2.56 | Melanoma, metastatic | Prednisone; surgery | Yes | 12 | 15 |
| 11/F/84 | Encephalopathy | 1.02 | VGKC, 0.04 | … | IVMP | Yes | 2 | 6 |
| 12/M/57 | Frontotemporal syndrome | 0.15 | VGKC, 0.06 | … | Prednisone | Yes | 6 | 8 |
| 13/F/43 | Extrapyramidal disorder | 0.29 | … | … | IVMP; IVIg; rituximab | Yes | 10 | 30 |
| 14/M/69 | Extrapyramidal disorder | 0.26 | … | … | IVMP | Yes | 36 | 42 |
| 15/M/66 | PN | 0.22 | IVIg | Yes | 10 | 22 | ||
| 16/M/33 | PN | 0.14 | ANNA-1, 15 360; CRMP-5a | … | IVIg; prednisone; cyclophosphamide | No | 6 | 18 (died) |
Abbreviations: α3-AChR Ab, nicotinic ganglionic acetylcholine receptor autoantibody; ANNA-1, anti–neuronal nuclear antibody-1; CRMP-5, collapsin response-mediator protein 5; ellipses, zero; IVIg, intravenous immune globulin; IVMP, intravenous methylprednisolone; MG, myasthenia gravis; PLEX, plasma exchange; PN, peripheral neuropathy; VGKC, voltage-gated potassium channel antibody.
Positive by recombinant Western blot.
Patients With Medium α3-AChR Ab Values
Medium values were those between 0.10 and 0.99 nmol/L. The median serum antibody value for these 85 patients (55% of the total) was 0.14 nmol/L. The predominant neurological presentation in 31 patients (36%) was peripheral neuropathy (Table 2). Another 5 patients had neurophysiologic evidence of distal small fiber neuropathy, 2 distal anhidrosis (thermoregulatory sweat test), 2 elevated thresholds for cold sensation, and 1 abnormality in both tests. Seventeen patients (20%) had objective evidence of dysautonomia, 3 pandysautonomia, 14 limited dysautonomia (8 gastrointestinal dysmotility, 3 orthostatism, 2 sicca syndrome [xerostomia and xerophthalmia], and 1 partial anhidrosis), and 6 had coexisting sensorimotor neuropathy.
Eleven patients had acute or subacute onset of cognitive and psychiatric symptoms and signs such as depression, psychosis, executive dysfunction, personality change, and amnestic mild cognitive impairment. One patient (Table 4, patient 12) had a frontotemporal syndrome characterized by depression and dysexecutive symptoms; his condition improved after oral prednisone therapy (Table 4). Two patients had a subacute extrapyramidal syndrome characterized by akinetic rigid parkinsonism. Cerebrospinal fluid was inflammatory in one, and symptoms and signs in the other improved following intravenous methylprednisolone therapy. Three patients had indeterminate ataxia.
The diagnosis in 1 patient was stiffman syndrome (GAD65 Ab, 4.63 nmol/L) and in another was myasthenia gravis (muscle AChR–binding Ab, 28.7 nmol/L); normal values for both antibodies are 0.02 nmol/L or less. The diagnosis in 3 patients was multiple sclerosis and in 16 (19%) was either a non–immune-mediated neurological disorder (11 patients) or a nonneurological disorder (5 patients); 5 patients (30%) had polyclonal hypergammaglobulinemia.
Patients With Low α3-AChR Ab Values
Low values were those between 0.03 and 0.09 nmol/L. The median antibody value in this group of 58 patients (37% of the total) was 0.06 nmol/L. Thirty-one (54%) had either a non–immune-mediated neurological disorder (22 patients) or a nonneurological disorder (9 patients). Twelve (39%) had monoclonal (2 patients) or polyclonal hypergammaglobulinemia (10 patients).
Peripheral neuropathy was the predominant neurological manifestation in 13 patients (22%). Six (10%) had dysautonomia, 3 with pandysautonomia and 3 with limited dysautonomia. Subacute neuropsychiatric presentations were documented in 3 patients, 1 with limbic encephalitis with coexisting ANNA-1 autoantibody and 2 with subacute memory loss and depression. Three patients had myasthenia gravis and were seropositive for muscle AChR–binding Ab (range, 2.56–38.9 nmol/L). Two patients had an inflammatory demyelinating central nervous system disorder, 1 with neuromyelitis optica and 1 with multiple sclerosis.
ACCOMPANYING AUTOANTIBODIES
One or more additional neuronal or muscle autoantibodies were detected in 40 of the α3-AChR Ab–positive patients (26%) in the following descending frequency: GAD65, muscle AChR, neuronal voltage-gated cation channel (N-type calcium channel was more common than potassium channel, which was more common than P/Q-type calcium channel), striational, ANNA-1, and CRMP-5 IgG. Six patients had multiple coexisting antibodies (Table 3).
CEREBROSPINAL FLUID
Abnormalities were detected in 22 of 35 patients tested (63%); 2 had elevated white blood cell counts (100/mL and 9/mL, respectively; normal value, 0–5/mL); 22 increased protein levels (median, 63 mg/dL; range, 48–181 mg/dL; normal range, 14–45 mg/dL); and 4 had 4 or more oligoclonal bands (normal value, <4).
MRI IMAGING
Only 4 of 80 patients tested had remarkable findings. One patient (medium antibody value) presented with an akinetic rigid parkinsonian state and inflammatory cerebrospinal fluid and had bilateral increased signal on T2-weighted and fluid inversion recovery imaging in the putamen and caudate nuclei consistent with basal ganglionitis; CRMP-5 IgG was negative. Three patients had radiologic findings compatible with a diagnosis of multiple sclerosis.
THERAPIES AND OUTCOMES
Table 4 summarizes the outcomes for 16 patients who received immunotherapy; 2 of these patients also received tumor-directed therapy. Neurological improvement was described in 12 patients (75%) who received immunotherapy and in 2 patients who received cancer-directed therapy (chemotherapy in one and tumor resection in the other). The delay from symptom onset to initiation of treatment was not significantly shorter for those reported as improved (P= .76). Follow-up of more than 1 month (median, 9 months; range, 4–20 months) was available for 5 patients with neurological disorders (peripheral neuropathy, 3; cognitive disorder, 1; ataxia, 1) who did not undergo immunotherapy. All but 1 had no change documented; 1 patient with a cognitive disorder continued to decline. Four deaths were reported (mean survival, 28 months after neurological symptom onset; none had a diagnosis of cancer).
CONTROLS
Presence of α3-AChR Ab was more common among neurologically asymptomatic patients with lung cancer (19 of 245) than other controls (1 of 212; 1 of 173 healthy; 0 of 39 with SLE or Sjögren syndrome; P<.001) (Figure 1). Small cell lung carcinoma was not documented in any seropositive patient with lung cancer; 8 had squamous cell carcinoma (median, 0.13 nmol/L; range, 0.07 – 0.53 nmol/ L), 7 had adenocarcinoma (median, 0.11 nmol/L; range, 0.04 – 0.15 nmol/L), and 4 had other lung cancer types (2 non–small cell lung carcinoma not otherwise specified, 1 adenosquamous carcinoma, 1 bronchoalveolar carcinoma; median, 0.20 nmol/L; range, 0.12 – 0.23 nmol/L). No α3-AChR Ab was detected in any control patient with SLE or Sjögren syndrome.
COMMENT
Detection of α3-AChR Ab is common in patients referred for a paraneoplastic serological evaluation (1% vs 0.4% for both ANNA-1 and CRMP-5 IgG) and those with cancer (7.5% in patients with lung cancer vs 0.5% for healthy controls). Studies to date have emphasized the clinical association of α3-AChR Ab with idiopathic or paraneoplastic pandysautonomia (typical values exceed 1.0 nmol/L)4,16 and cancer associations restricted to thymoma and small cell lung carcinoma.4,10 Our present study has identified diverse cancer types, the most common being adenocarcinomas. Serum levels in most patients were lower than 1.0 nmol/L and neurological presentations were more diverse, including peripheral neuropathy and central nervous system disorders.
While 30% of patients who had an oncological evaluation had cancer or a history of cancer, a new cancer was detected in 12% of patients evaluated subsequent to antibody detection. The median duration of follow-up was only 6 months, and only half of patients had an extensive oncologic evaluation. While the rate of cancer detection among seronegative patients during the same follow-up period is unknown, the 15-fold higher prevalence of α3-AChR Ab in controls with cancer than healthy controls indicates the potential of α3-AChR Ab as a marker of cancer. Cancer rates were comparable across groups with low, medium, and high serum autoantibody values.
It is instructive that bladder cancer was detected in a patient with a low serum α3-AChR Ab value whose neurological disorder was confirmed genetically to be Huntington disease and clearly was not immune-mediated.17 Likewise, α3-AChR Ab was detected in 7.8% of neurologically healthy patients with lung cancer. It is also instructive that positron emission tomographic imaging detected cancer in 3 cases with negative computed tomographic images. An unanticipated observation was that of all cancers associated with α3-AChR Ab in the principal study cohort, adenocarcinoma accounted for 43% (breast, lung, prostate, colorectal, and thyroid). By contrast, ANNA-1 is highly predictive of small-cell lung carcinoma in adult patients and neuroblastoma in pediatric patients.
The index of clinical suspicion for an autoimmune etiology as the basis of the neurological presentation was directly proportional to the antibody titer. Patients with high (>1.0 nmol/L) and medium (0.10–0.99 nmol/L) values for α3-AChR Ab were considered to have an auto-immune disorder in 92% and 82% of cases, respectively. Of patients with low α3-AChR Ab values, 54% had non-specific symptoms without a neurological diagnosis and hypergammaglobulinemia was common. In addition to cancer, a monoclonal or polyclonal hypergammaglobulinemia should be considered as a potential cause of falsely positive low antibody values.
It is noteworthy that 21% of seropositive patients had symptoms of dysautonomia. Patients with high α3-AChR Ab values were more likely to have multiple autonomic deficits, and patients with medium or low values were more likely to have limited dyasautonomia. This laboratory previously observed that 14.6% of patients with postural orthostatic tachycardia syndrome had low α3-AChR Ab values.5 Peripheral neuropathy was the most common accompaniment of α3-AChR Ab, documented in 28% of patients. Most had length-dependent sensory or sensorimotor polyneuropathies, but demyelinating neuropathies and disorders of nerve root, dorsal root ganglion, and cranial nerves were also encountered. Central nervous system disorders of subacute onset were encountered in 20% of seropositive patients and mainly affected cortical and subcortical structures. Most of these patients had neuropsychiatric manifestations or extrapyramidal disorders. Immunotherapies were beneficial in 75% of treated patients, but follow-up was limited.
It is unlikely that the AChR α3-subunit is the primary autoantibody target in all neurological presentations described in this article. The α3-AChR–binding Ab that we detected may reflect an antibody primarily directed at 1 of numerous antigenically related AChR α subunits.18 We have described α3-AChR Ab both as a marker of neuronal AChR antibody mediated channelopathy4 and as an accompaniment of a cytotoxic T-cell mediated neuropathology.11 The α3-AChR subunit is not confined to autonomic ganglia. It is found throughout the nervous system including the sensory dorsal root ganglia, trigeminal ganglia, and in rat19,20 and mouse brains.19
We detected 1 or more coexisting antibodies specific for neuronal nuclear or neuronal or muscle cytoplasmic antigens in 26% of seropositive patients. Muscle AChR antibody was frequently encountered, including in 2 patients who had myasthenia gravis in the setting of cancer. Myasthenia gravis coexisted with subacute autonomic failure in 1 patient (in the context of thymoma, as reported previously21), and another patient had dual muscle and neuronal autoimmune channelopathies in the context of metastatic renal carcinoma. The most common autoantibody detected in the company of α3-AChR Ab was GAD65-specific (7% of patients). The GAD65 Ab level in 1 patient approached 20 nmol/L, a value generally distinguishing patients with GAD65 neurologic autoimmunity from patients with uncomplicated type 1 diabetes.13 That patient had stiff-man syndrome without evidence of cancer.
In summary, α3-AChR Ab–seropositive patients have diverse neurological presentations encountered and cancer types detected (most commonly adenocarcinoma). The 30% rate of cancer detected is quite high and should prompt consideration of a cancer search in seropositive patients.
Acknowledgments
Funding/Support: Funding provided by the National Institutes of Health grants R01-DK71209-4 and P01-DK68055.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Study concept and design: McKeon, Lennon, Fealey, and Pittock. Acquisition of data: McKeon, Lachance, Fealey, and Pittock. Analysis and interpretation of data: McKeon, Lachance, Fealey, and Pittock. Drafting of the manuscript: McKeon, Lennon, and Pittock. Critical revision of the manuscript for important intellectual content: Lennon, Lachance, Fealey, and Pittock. Obtained funding: Lennon. Administrative, technical, and material support: Lennon and Pittock. Study supervision: Lennon and Pittock.
References
- 1.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111(6):907–913. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernino S, Low PA, Lennon VA. Experimental autoimmune autonomic neuropathy. J Neurophysiol. 2003;90(3):2053–2059. doi: 10.1152/jn.00408.2003. [DOI] [PubMed] [Google Scholar]
- 3.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037–7042. doi: 10.1523/JNEUROSCI.1485-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 5.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 6.Pasha SF, Lunsford TN, Lennon VA. Autoimmune gastrointestinal dysmotility treated successfully with pyridostigmine. Gastroenterology. 2006;131(5):1592–1596. doi: 10.1053/j.gastro.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Moder KG. Use and interpretation of rheumatologic tests: a guide for clinicians. Mayo Clin Proc. 1996;71(4):391–396. doi: 10.4065/71.4.391. [DOI] [PubMed] [Google Scholar]
- 8.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 9.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64(6):617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 10.Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50(6):1806–1813. doi: 10.1212/wnl.50.6.1806. [DOI] [PubMed] [Google Scholar]
- 11.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56(5):715–719. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 12.Graus F, Vincent A, Pozo-Rosich P, et al. Antiglial nuclear antibody: marker of lung cancer-related paraneoplastic neurological syndromes. J Neuroimmunol. 2005;165(1–2):166–171. doi: 10.1016/j.jneuroim.2005.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase auto-immunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- 14.Keegan BM, Pittock SJ, Lennon VA. Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann Neurol. 2008;63(4):531–534. doi: 10.1002/ana.21324. [DOI] [PubMed] [Google Scholar]
- 15.Griesmann G, Kryzer TJ, Lennon VA. Autoantibody profiles of myasthenia gravis and Lambert-Eaton myasthenic syndrome. In: Rose N, Hamilton RG, Detrick B, editors. Manual of Clinical Laboratory Immunology. Washington, DC: ASM Press; 2002. pp. 1005–1012. [Google Scholar]
- 16.Vernino S, Sandroni P, Singer W, Low PA. Autonomic ganglia: target and novel therapeutic tool [invited article] Neurology. 2008;70(20):1926–1932. doi: 10.1212/01.wnl.0000312280.44805.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gusella JF, Wexler NS, Conneally PM, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 18.Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74 (8):1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Whiteaker P, Peterson CG, Xu W, et al. Involvement of the alpha3 subunit in central nicotinic binding populations. J Neurosci. 2002;22(7):2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Chang GQ, Jiao YQ, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 1998;809(2):238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- 21.Vernino S, Cheshire WP, Lennon VA. Myasthenia gravis with autoimmune autonomic neuropathy. Auton Neurosci. 2001;88(3):187–192. doi: 10.1016/S1566-0702(01)00239-9. [DOI] [PubMed] [Google Scholar]