Abstract
The photocytotoxicity of 16 polycyclic aromatic hydrocarbons (PAHs) on the priority pollutant list of the United States Environmental Protection Agency (US EPA) were tested in human skin HaCaT keratinocytes. A selected PAH was mixed with HaCaT cells and irradiated with a solar simulator lamp for a dose equivalent to 5 min of outdoor sunlight and the cell viability was determined immediately and also after 24 h of incubation. For the cells without incubation after the treatments, it is found that all PAHs with three rings or less, except anthracene, are not photocytotoxic, while the four or five-ring PAHs (except chrysene), benz[a]anthracene, dibenzo[a,h]anthracene, benzo[ghi]perylene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, benzo[b]fluorenthene, fluorenthene, and pyrene, are photocytotoxic to the human skin HaCaT keratinocytes. If the cells were incubated for 24 h after the treatments, the photocytotoxic effect of the PAHs was greatly amplified in comparison to the nonincubated cells. For the 24 h incubated cells, all PAHs except naphthalene exhibit photocytotoxicity to some extent. Exposure to 5 μM of the 4- and 5-ring PAHs (except chrysene) and 3-ring anthracene more than 80% of the cells lose viability. The photocytotoxicity of the PAHs correlates well with several of their excited state properties: light absorption, excited singlet-state energy, excited triplet-state energy, and HOMO-LUMO energy gap. All the photocytotoxic PAHs absorb light at >300 nm, in the solar UVB and UVA region. There is a threshold for each of the three excited state descriptors of a photocytotoxic PAH: singlet energy <355 kJ/mol (corresponding to 337 nm light), triplet energy <230 kJ/mol (corresponding to 520 nm light), HOMO-LUMO gap <3.6 eV (corresponding to 344 nm light) obtained at the Density Functional Theory B3LYP/6-31G(d) level.
Keywords: polycyclic aromatic hydrocarbons (PAHs), phototoxicity, human skin keratinocytes, light absorption, excited state energy, HOMO–LUMO gap, DFT
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a class of environmental contaminants that absorb sunlight in the UVA range (320–400 nm). Some of the PAHs with 4 or more rings also absorb visible light (>400 nm). It has been reported that PAHs are generally more toxic when they are exposed to light than kept in the dark (Landrum et al., 1987; Arfsten et al., 1994; Huang et al., 1995; Betowski et al., 2002; Yu, 2002; Yan et al., 2004). Absorption of light energy can promote PAHs to their higher electronic states. The excited state energy can be released by emitting light or heat, or transferred to molecular oxygen, solvent molecules, or biological molecules in the cell to generate reactive oxygen species (ROS), reactive intermediates, free radicals, or photo-modified PAH products that can damage cellular constituents, resulting in toxicity such as genotoxicity (Foote, 1976; Huang et al., 1995; Betowski et al., 2002; Yu, 2002). Human contamination with PAHs and their photoproducts is mainly through skin absorption, inhalation, or food consumption (Baum, 1978; Pitts, 1979; Connell et al., 1997). The United States Environmental Protection Agency (US EPA) has classified some of the PAHs as probable human carcinogens (USEPA, 1982; National Toxicology Program, 1998). PAHs can induce cancer tumor, primarily in the skin, lungs, and bladder (Conney, 1982; Dipple, 1985; Connell et al., 1997; National Toxicology Program, 1998; Warshawsky, 1999). Naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, benzo[ghi]perylene, indeno[1,2,3-cd]pyrene are listed as priority pollutants by the US EPA (USEPA, 1982).
It has been demonstrated that PAHs are photocytotoxic in fish (Schirmer et al., 1998), photocytotoxic and photomutagenic in bacteria (Yan et al., 2004). The question is whether these results are relevant to humans. The use of a human skin cell line for toxicity test would provide some direct evidences. It has been shown that human keratinocytes are suitable for the study of human biology and disease processes involving the skin (Barlow and Pye, 1997). Keratinocytes are the predominant cell type in the epidermis, and HaCaT is the first permanent epithelial cell line from adult human skin that exhibits normal differentiation (Boukamp et al., 1988). It can be cultured using highly specialized media, providing direct testing of photobiological effects by light (Leigh et al., 1996). Therefore, the use of human HaCaT keratinocytes to investigate the in vitro cytotoxic and photocytotoxic effects of PAHs brings a direct link to the in vivo situation in the human skin. In this study, we report the cytotoxicity and photocytotoxicity of the 16 priority PAHs Figure 1 on HaCaT keratinocytes and a relationship between photocytotoxicity and PAH excited state properties, such as excited triplet- and singlet-state energy, light absorptivity, and HOMO–LUMO energy ap, will be established.
Fig. 1.
Chemical Structure and ring-numbering system for the 16 priority PAHs.
MATERIALS AND METHODS
Material
HaCaT keratinocytes, a transformed human epidermal cell line (Boukamp et al., 1988), were obtained from Dr. Norbert Fusenig of the German Cancer Research Centre (Heidelberg, Germany). Fetal bovine serum (FBS), Dulbecco’s minimum essential medium (DMEM) and trypsin EDTA solution were purchased from American Type Cell Culture (Manassas, VA). Naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, benzo[k]fluoranthene, chrysene, benzo[a]pyrene, dibenzo[a,h]anthracene and benzo[ghi]perylene were from Sigma-Aldrich (St. Louis, MO). Benzo[b]flouranthene and indeno[1,2,3-cd]pyrene were from Ultra Scientific (North Kingstown, RI). Penicillin/streptomyocin, dimethyl sulfoxide (DMSO), and phosphate buffered saline (PBS) were from Fisher Scientific (Houston, TX).
Cell Culture and Treatment
The HaCaT cells were grown in DMEM with 10% FBS and 1% penicillin/streptomyocin. The cells in 25 cm2 culture flasks were incubated with 5% CO2 at 37°C in a humidified incubator. After the HaCaT cells grew to the expected concentration, they were harvested by trypsinizing the cell with 0.25% trypsin/EDTA and incubated at 37°C for 5 min to obtain the complete cell detachment. After the cell suspension was centrifuged at 2000 rpm for 3 min, cell pellets were resuspended, and washed twice with 1× PBS. The cell suspension in 1× PBS was counted and adjusted to 1× 105/mL.
Cytotoxicity Test
A HaCaT keratinocyte suspension (100 μL in 1× PBS with a cell concentration of 1 × 105 cells/mL) and a PAH solution (100 μL in 1× PBS with 4% DMSO) were combined in each well of two 96-well plates. PAH solutions with a desired concentration were prepared by serial dilutions of the DMSO stock solutions with 1× PBS. On the basis of a previous study (Yan et al., 2004), the minimum PAH concentrations that can lead to photomutagenicity are different for each PAH and can be divided into two general groups: one that causes photomutagenicity at lower concentrations (<25 μM) and the other at higher concentrations (>25 μM). For the current photocytotoxicity test, the doses for the strong photomutagenic PAHs in Salmonella typhimurium TA 102 (Yan et al., 2004), anthracene, benz[a]anthracene, benzo[a]pyrene, benzo[ghi]perylene, indeno[1,2,3-cd]pyrene, and pyrene, were 0, 0.008, 0.04, 0.2, 1, and 5 nmol/ well (or 0, 0.04, 0.2, 1, 5, and 25 μM) and doses for the weaker or nonphotomutagenic PAHs, acenaphthene, acenaphthylene, benzo[k]fluoranthene, chrysene, fluorene, benzo[b]fluoranthene, fluoranthene, dibenzo[a,h]anthracene, naphthalene and phenanthrene, were 0, 0.2, 1, 5, 25, 125 nmol/ well (or 0, 1, 5, 25, 125, and 625 μM), respectively.
There were two six-well sets for each concentration of a PAH for cytotoxicity test, one set was the control without light irradiation and the other was irradiated by a 300-W Xe lamp from ORIEL Instruments (Stratford, CT) that produces a simulated solar radiation. Irradiation time of 5 min was chosen to achieve a light dose of 1.1 J/cm2 of UVA and 2.1 J/cm2 of visible light, which are equivalent to 5 min sunlight irradiation at 11 a.m. in a clear day during March to July in Jackson, Mississippi (UVA: 0.95–1.5 J/cm2, Visible: 3.2–4.5 J/cm2), based on measurements of outdoor sunlight intensity. After light irradiation, the treated cells were either immediately tested for cell viability or incubated for 24 h before the test. Each well was added with fluorescein diacetate (FDA, 100 μL, 10 ng/mL) and incubated for 35 min at 37°C in a 5% CO2 incubator. FDA is a nonpolar compound which readily diffuses into cell where intracellular esterase hydrolyzes the dye to produce fluorescein (Rotman and Papermaster, 1996). Fluorescence intensity of FDA, which is proportional to the number of viable cells, is read using a Biosystem Fluoroskan II microplate reader (Helsinki, Finland) with filters set at 538 nm (emission) and 485 nm (excitation). Differences between the light exposed and the control groups without light irradiation were performed by one-way analysis of variance (ANOVA; The SAS System for windows, version 8, SAS Institute, Gary, NC, USA). Means were separated by Tukey’s test. Differences at P < 0.05 were considered significant.
Light Absorptivity of the PAHs
Relative light absorptivity of the 16 PAHs was calculated by multiplying the emission spectra of the lamp and the PAH’s absorption spectra at each wavelength in the range of 300–700 nm, followed by integrating the area under the resulting curve (Krylov et al., 1997). The emission spectrum of the 300-W Xe lamp was recorded on a Fluoromax-2 spectrofluorometer from Instruments S.A. Inc. (Trenton, NJ) in the range of 300–700 nm. A Pyrex glass filter was used to mimic the irradiation condition. The absorption spectrum of each PAH was recorded on a CARY 300 UV-VIS spectrophotometer from Varian Inc. (Houston, TX). After integration, the absorptivity of each PAH was normalized to that of indeno[1,2,3-cd]pyrene.
Computation of Excited State Singlet and Triplet Energies, HOMO–LUMO Gaps
Density functional (DFT) B3LYP method has been demonstrated to predict excellent geometries and energies (Bauschlicher et al., 1995; El-Azhary and Suter, 1996). Therefore, the ground- and triplet-state species of PAHs were studied using the B3LYP nonlocal density functional approximation (Lee et al., 1988; Miehlich et al., 1989; Becke, 1993; Stephens et al., 1994). The geometry of the ground- and triplet-state structures was optimized by means of the Berny approach, a modified Schlegel method (Schlegel, 1982). Vibration frequency calculations were performed to confirm that the obtained geometries represent minimum energy structures. The solvent effect was included by the polarized continuum model using the integral equation formalism (Cances et al., 1997). In this model, the liquid is represented by a dielectric continuum, characterized by its dielectric constant ε (ε = 78.39 for water). The solute is placed in a cavity created in the continuum. The distribution of electronic density of the solute polarizes the continuum and generates an electric field inside the cavity, which in turn affects the geometry and electronic structure.
The singlet- and triplet-state energies were obtained using the TD-DFT method. TD-DFT has been recently shown to yield relatively accurate excitation energies for large molecules (Straman et al., 1998; Sheng et al., 2004), and in some cases the TD-DFT results are qualitatively comparable to those of CAS-PT2 calculations, although at substantially lower computational costs. Therefore, the excitation energy calculations were carried out at the TD-DFT level employing the 6-31G(d) basis set with the geometries having been optimized at the B3LYP/6-31G(d) level. The HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) energy levels were also obtained at the B3LYP/6-1G(d) level. All the calculations were performed using the Gaussian 03 quantum chemistry package (Frisch et al., 2004).
RESULTS
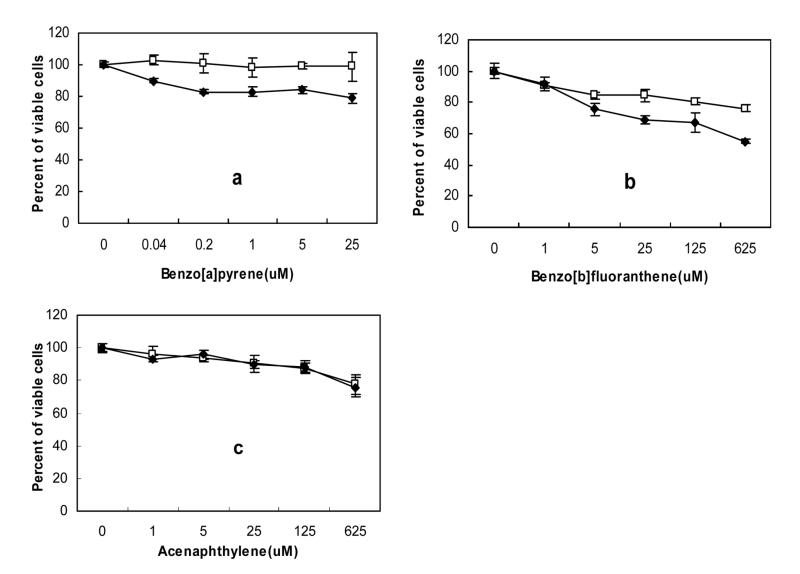
Figure 2 shows the viability of human skin HaCaT keratinocytes exposed to several concentrations of a PAH in PBS with 5 min irradiation using the solar simulator lamp along with the control without light irradiation. Based on the immediate cell viability data with or without light irradiation shown in Figure 2(a–c) and the cell viability percentage listed in Table I, the 16 PAHs are divided into three groups. For the first group of PAHs, shown in Figure 2(a) for benzo[a]pyrene as an example, the percent of viable HaCaT cells decreases due to the exposure to light irradiation and the increasing concentration of the PAH in the range of 0–5 μM (P < 0.05 at 5 μM), while there is no cytotoxicity if without light irradiation. All these PAHs are clearly hotocytotoxic to HaCaT keratinocytes. This group includes seven PAHs: anthracene, benzo[a]pyrene, benzo[ghi]perylene, dibenzo[a,h]anthracene, fluoranthene, indeno[1,2,3-cd]pyrene, and pyrene. For the second group of PAHs, shown in Figure 2(b) for benzo[b]fluoranthene as an example, the percent of viable HaCaT cells decreases with the increase of the concentration of the PAHs, with or without light irradiation. However, it is obvious that the cell viability decrease is much more for the light irradiated samples. Therefore, these PAHs are both photocytotoxic and chemical cytotoxic. This group includes two PAHs: benz[a]anthracene and benzo[b]fluoranthene. The third group of PAHs, shown in Figure 2(c) for anthenaphthylene as an example, is generally not photocytotoxic or chemical cytotoxic. They are only weakly photocytotoxic at high concentrations of >125 μM. This group includes the remaining sevel PAHs: acenaphthene, acenaphthylene, benzo[k]fluoranthane, chrysene, fluorene, naphthalene, and phenanthrene.
Fig. 2.
Viability of HaCaT cells due to the exposure to a PAH with (◆) or without (□) light radiation. The cell viability was measured without further incubation following treatment. The light dose was 1.1 J/cm2 UVA + 2.1 J/cm2 visible, equivalent to 5 min under the outdoor sunlight. The results were expressed as a percentage of the reading in control wells. Error bars were the mean of six culture wells.
TABLE I.
Comparison of relative photocytotoxicity of the 16 PAHs with their excited state properties
| PAH Name | Viable Cells/nmola
Without Incubation -Light + Light Normalizedb (%) |
Viable Cells/nmola
with 24 Incubation -Light + Light Normalizedb (%) |
Light Absorptivityc (J) |
Triplet Energyd (kJ/mol) |
Singlet Energyd (kJ/mol) |
HOMO– LUMOd (eV) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Naphthalene | 97 ± 2 | 97 ± 1 | 100 | 76 ± 8 | 74 ± 4 | 97 | 0.051 | 262 | 427 | 4.29 |
| Acenaphthene | 95 ± 4 | 99 ± 9 | 104 | 99 ± 5 | 77 ± 8 | 74 | 0.021 | 261 | 414 | 4.19 |
| Acenaphthylene | 93 ± 2 | 96 ± 3 | 103 | 103 ± 3 | 76 ± 7 | 78 | 0.17 | 210 | 301 | 3.50 |
| Fluorene | 98 ± 5 | 95 ± 4 | 97 | 88 ± 6 | 69 ± 8 | 78 | 0.036 | 293 | 447 | 4.49 |
| Phenanthrene | 92 ± 2 | 91 ± 4 | 99 | 102 ± 7 | 65 ± 2 | 64 | 0.071 | 265 | 388 | 4.21 |
| Anthracene | 99 ± 5 | 55 ± 2 | 56 | 82 ± 9 | 7 ± 0 | 8.5 | 0.36 | 175 | 313 | 3.20 |
| Fluoranthene | 102 ± 8 | 71 ± 2 | 70 | 72 ± 8 | 2 ± 0 | 2.8 | 0.19 | 226 | 329 | 3.57 |
| Pyrene | 94 ± 6 | 62 ± 3 | 66 | 57 ± 8 | 4 ± 1 | 7.0 | 0.12 | 203 | 353 | 3.42 |
| Benz[a]anthracene | 84 ± 4 | 47 ± 3 | 56 | 51 ± 6 | 6 ± 2 | 12 | 0.12 | 201 | 326 | 3.36 |
| Chrysene | 93 ± 5 | 88 ± 3 | 95 | 75 ± 4 | 59 ± 4 | 79 | 0.054 | 224 | 364 | 3.77 |
| Benzo[b]fluoranthene | 84 ± 4 | 69 ± 3 | 82 | 75 ± 6 | 7 ± 1 | 9.3 | 0.25 | 227 | 335 | 3.55 |
| Benzo[k]fluoranthene | 93 ± 5 | 91 ± 3 | 98 | 61 ± 9 | 19 ± 2 | 31 | 0.39 | 217 | 312 | 3.27 |
| Benzo[a]pyrene | 101 ± 1 | 71 ± 3 | 70 | 33 ± 7 | 3 ±0 | 9.1 | 0.66 | 173 | 302 | 2.99 |
| Dibenz[a,h]anthracene | 101 ± 4 | 88 ± 6 | 87 | 65 ± 11 | 25 ± 8 | 38 | 0.44 | 217 | 329 | 3.46 |
| Benzo[ghi]perylene | 92 ± 1 | 74 ± 2 | 80 | 81 ± 4 | 12 ± 2 | 15 | 0.12 | 197 | 315 | 3.15 |
| Indeno[1,2,3-cd]pyrene | 97 ± 3 | 82 ± 4 | 84 | 75 ±9 | 4 ± 1 | 5.3 | 1.00 | 184 | 281 | 2.97 |
Percent of viable HaCaT cells determined either immediately or after 24 h incubation after exposure to 1 nanomole (or 5 μM) of a PAH and 1.1 J/cm2 UVA + 2.1 J/cm2 visible light irradiation. The results were expressed as a percentage of the readings of the control wells. Each data point was the mean of six wells.
The cell viability of the light irradiated group was normalized to the control group without light irradiation.
J(norm): Normalized light absorptivity of the 300 W lamp emission by the PAHs. All data were normalized to that for Indeno[1,2,3-cd]pyrene.
The DFT computed excited state properties at the B3LYP/6-31G(d) level. Solvent effect was included using the polarized continuum model.
If the cells were incubated for 24 h after the treatment with 5 min irradiation in the presence of 5 μM of a PAH, all PAHs, except naphthalene, showed some degree of photocytotoxicity in comparison to the control without light irradiation (P < 0.05). These data are also listed in Table I. The cytotoxicity data for the 24 h incubated cells parallels the data without incubation, but is greatly amplified. In general, the normalized cell viability for the nonincubated cells treated with the photocytotoxic PAHs ranged from 56–87%, while the viability for 24 h incubated cells for the same PAHs was <15% (except for dibenz[a,h]anthracene which is 38%). The nonphotocytotoxic PAHs determined without incubation showed to be photocytotoxic after 24 h incubation, with the normalized cell viability in the range of 64–78%. Benzo[k]fluoranthene even decreased to 31%. Therefore, it clearly showed that the 24 h incubation data were more sensitive to the test than the data without incubation. We also carried out the 48 h incubation and the resultant cell viability was about the same as the 24 h incubation results (data not shown).
As shown in Table I, the relative light absorptivity of the 16 PAHs can be divided into three general groups: (i) strong absorbing PAHs (0.36–1.0): anthracene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, indeno[1,2,3-cd]pyrene; (ii) medium absorbing PAHs (0.12–0.25): acenaphthylene, fluoranthene, pyrene, benz[a]anthracene, benzo[a]fluoranthene, and benzo[ghi]perylene; and (iii) weak absorbing PAHs (0.02–0.07): naphthalene, acenaphthene, fluorene, phenanthrene, and chrysene. Looking across the table, it is easy to see that all the photocytotoxic PAHs (in bold face) absorb light in the lamp’s emission range of >300 nm.
The computed singlet- and triplet-excited state energies and the HOMO-LUMO gaps are also listed in Table I. The singlet excited state energies range from 281 to 447 kJ/mol and the triplet excited state energies range from 173 to 293 kJ/mol for the 16 tested PAHs. Anthracene, acenaphthylene, benzo[a]pyrene, benzo[ghi]perylene, and indeno[1,2,3-cd]pyrene have the lowest excited singlet as well as the excited triplet state energies. These same PAHs also have the smallest HOMO-LUMO gaps.
DISCUSSION
PAHs have been known to be phototoxic for a long time (Landrum et al., 1987; Arfsten, 1994; Yu, 2002). Reports of PAH phototoxicity on various cells, plants, microorganisms, and animals have been published (Kagan and Kagan, 1986; Huang et al., 1993; Utesch et al., 1996; Pelletier et al., 1997; Swartz et al., 1997; Schirmer et al., 1998). Structure and phototoxicity relationships have also been reported in plant species and fish (Huang et al., 1993; Arfsten et al., 1996; Schirmer et al., 1998). However, this is the first study on human skin cells using the most representative PAHs. Although human beings can get contaminated with PAHs through food consumption and respiration, contamination through absorption onto the skin is inevitable. Humans contaminated with PAHs are also likely to be exposed to the sunlight irradiation. Thus, the study of the photocytotoxic effect of PAHs in skin cells is relevant to human health.
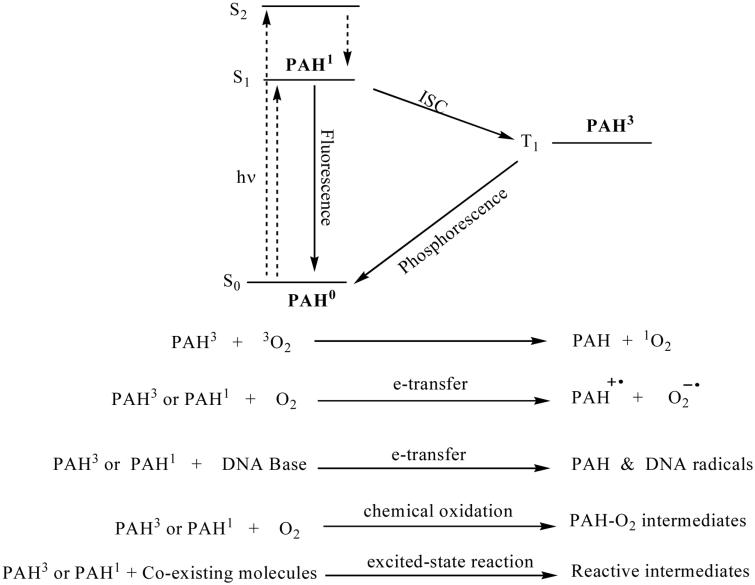
A typical phototoxic compound either absorbs light or can be sensitized by coexisting light-absorbing molecules. Upon receiving light energy directly or from another molecule through sensitization, the phototoxic molecules usually go through a series of excited state reactions to exert toxicity. PAHs mostly absorb the sunlight directly in the UVA/UVB and some in the visible region (Dabestani and Ivanov, 1999). Upon light absorption, PAHs will be promoted to the excited singlet state and a series of excited state transitions and/or reactions may occur as shown in Figure 3. In addition to fluorescence and nonradiative decay of the singlet state by internal conversion, the singlet state molecules can intersystem cross to its triplet state. Both the singlet and triplet excited state molecules can react with the surrounding molecules to generate reactive species such as singlet oxygen, superoxide, free radicals of PAH or biological molecules, and reactive intermediate products. Singlet oxygen and free radicals have been suggested to be some of the reactive intermediates leading to DNA cleavage or formation of DNA-PAH covalent adducts (Dong et al., 2000; Dong et al., 2002; Yu, 2002), oxidation of guanine (Liu et al., 1998), or lipid peroxidation (Yu et al., 2006; Yan et al., 2006). The main photochemical reaction for PAHs is the reaction with molecular oxygen leading to more water-soluble oxygenated compounds (Yu, 2002). Photoreaction of PAHs generally increases the toxicity by formation of new photoproducts with toxicological properties distinct from the parent PAHs (Huang et al., 1993; Krylov et al., 1997).
Fig. 3.
Excited state reactions of PAHs leading to the formation of reactive intermediates
Theoretically, the larger the conjugated π-orbital system, the greater is the absorption at longer wavelengths. The light absorptivity of the four- or more-ring PAHs are greater than that of the two- or three-ring PAHs. There is no acute photocytotoxicity for the two- or three-ring PAHs except anthracene, while all the four- or more-ring PAHs are acutely photocytotoxic, except chrysene and benzo[k]fluoranthene which are not photocytotoxic at the concentration of 0–5 μM. The light absorptivity of anthracene, due to its linear arrangement of the three benzene rings, is much greater than the other three-ring PAHs. Therefore, anthracene is also much more photocytotoxic than the other three-ring PAHs. After 24 h incubation, the five-ring benzo[k]fluoranthene also becames photocytotoxic while chrysene was still the weakest photocytotoxic among the four-ring PAHs. The reason that chrysene is not strongly phototoxic is because chrysene absorbs the >300 nm light very weakly (light absorptivity of 0.054 in Table I). It was shown before that chrysene photolyzes very slowly in pure water irradiated by UVA light (Xu et al., 2004). Therefore, we can conclude that most of the photocytotoxic PAHs in the human skin HaCaT keratinocytes absorb light in the strong or medium range.
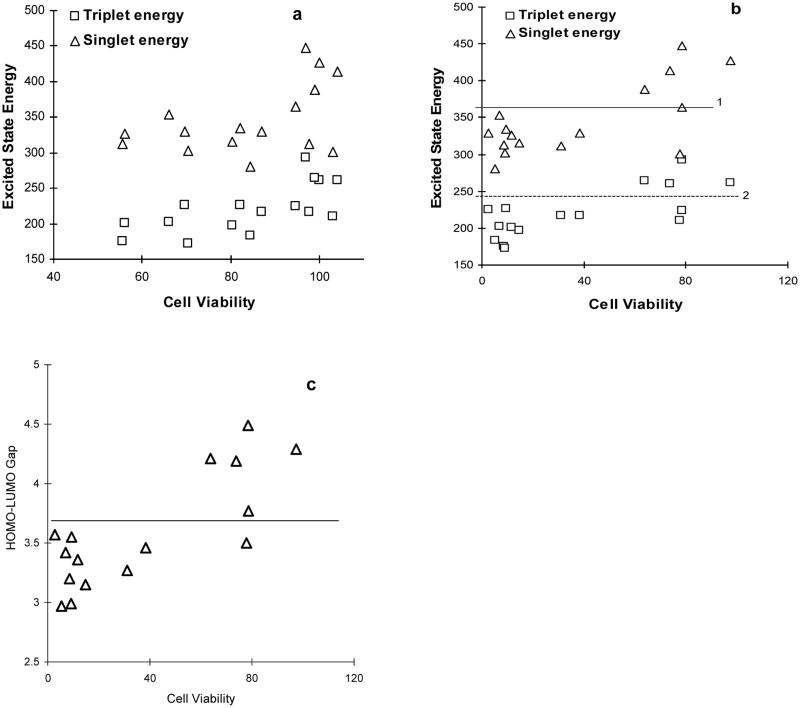
The DFT-computed triplet and singlet excited state energies are listed in Table I. The relationship between the excited state energy and cell viability is presented in Figure 4. The cell viabilities were normalized to the control (without light irradiation). The results for the toxicity test without incubation are very scattered and the general trend is that the lower the excited state energy, the stronger is the photocytotoxicity [Fig. 4(a)]. However, the 24 h incubation data clearly points to a threshold excited state energy and the cell viability [Fig. 4(b)]. It can be seen that the most photocytotoxic PAHs tended to have the singlet excited state energy of <355 kJ/mol (under line 1) and the triplet excited state energy of <230 kJ/mol (under line 2). There are two exceptions, acenaphthylene and chrysene. Since chrysene does not absorb light at >300 nm used for these experiments (Table I) as discussed earlier, it is logical to believe that only very small amounts of the chrysene molecules get to the excited state to cause photocytotoxicity to the HaCaT cells. As for acenaphthylene, we do not have a good explanation. Nonetheless, 15 out of 16 PAHs follow the pattern that certain excited state energy level is necessary for the molecule to achieve the photocytotoxicity.
Fig. 4.
Relationship between the exited state energy and cell viabilities after 5 min irradiation in the presence of 5 μM of a PAH without (a) or with (b) and (c) 24 h incubation.
There is also a threshold level for the HOMO-LUMO energy gap as it is for the excited singlet and triplet state energies. All the photocytotoxic PAHs have a HOMO-LUMO gap of <3.6 eV [Fig. 4(c)]. It was demonstrated that the HOMO-LUMO gap was one of the indexes of molecular electronic structure related to light absorption and chemical reaction of the PAHs (Mekenyan et al., 1994; Chen et al., 1996; de Lima Ribeiro and Ferreira, 2005). Newsted et al. (1987) reported that chemicals with a HOMO-LUMO gap of less than 7.1 ± 0.4 eV calculated at the semiempirical AM1 level were considered phototoxic. This HOMO-LUMO gap values of about 7.1 eV were too high compared to the experimental data, which is in the range of 3–4 eV as summarized by Dabestani and Ivanov (1999). Betowski et al. (2002) reported the excited singlet state energy at the CIS/6-31G and CIS/6-311G(d,p) level. However, the CIS excitation energies, as well as the energy obtained through the semiempirical and ab initio Hartree-Fock methods, are always higher than the experimental values (Kubicki et al., 1999). The TD–DFT excitation energies have been proved to be in close agreement with the experimental values for other molecules (Sheng and Leszczynski, 2004). Therefore, since the excitation energies obtained at the TD-DFT B3LYP/6-31G(d) level in this work are in good agreement with the experimental values, the HOMO-LUMO gap value critical for phototoxicity in the skin cells should be <3.6 eV, instead of the previous reported 7.1 eV (Newsted and Giesy, 1987). Again acenaphthylene is the one exception. The lower absolute hardness (HOMO-LUMO gap) is associated with lower stability of aromatic molecules (Gutman et al., 1977 a,b). This clearly indicates that the <3.6 eV HOMO-LUMO gap is critical for PAH’s photocytotoxicity in keratinocytes.
CONCLUDING REMARKS
In conclusion, 9 of the 16 EPA priority PAHs are strongly photocytotoxic to human skin HaCaT keratinocytes under our test conditions. All of them, except naphthalene, exhibit photocytotoxicity to different extents if the cells were incubated for 24 h after irradiation. The photocytotoxicity clearly depends on the size of the conjugated system and the arrangement of the rings of the PAHs. While the 4-ring and 5-ring PAHs, except chrysene which does not absorb the light used for this study, are all strongly photocytotoxic; and only anthracene among the 3- or 2-ring PAHs is strongly photocytotoxic due to its linear ring arrangement that lowers the HOMO-LUMO energy gap or excited state energies that allow anthracene to absorb the light used. The photocytotoxicty strongly depends on the singlet and triplet excited state and the HOMO-LUMO energy gap. Among the 16 tested PAHs, there appears to be a threshold for the excited state energies for the strong photocytotoxic ones: singlet excited state energy of <355 kJ/mol, triplet excited state energy of <230 kJ/mol, and HOMO-LUMO gap of <3.6 eV; and these energies’ corresponding wavelength of light are 337, 520, and 344 nm, respectively. The singlet excited state energy and the HOMO-LUMO energy gap, important indicators for light absorption, match the wavelength of light used in this experiment of >300 nm.
Since human exposure to PAHs in the skin is inevitable, these results provide direct evidence that PAHs can be harmful to humans in the skin when exposed to light. Therefore, phototoxicity of PAHs, in addition to the known toxicity associated with metabolism, needs to be considered for risk assessment for PAHs.
Acknowledgments
NIH SCORE
S06 GM08047
NIH-RCMI
G12RR13459
REFERENCES
- Arfsten DP, Davenport R, Schaeffer DJ. UV-A coexposure enhances the toxicity of aromatic hydrocarbons, munitions, and metals to photobacterium phosphoreum. Biomed Environ Sci. 1994;7:101–108. [PubMed] [Google Scholar]
- Arfsten DP, Schaeffer DJ, Mulveny DC. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: A review. Ecotoxicol Environ Saf. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- Barlow Y, Pye RJ. Ketratinocyte culture. Methods Mol Biol. 1997;75:117–129. doi: 10.1385/0-89603-441-0:117. [DOI] [PubMed] [Google Scholar]
- Baum E, Gelboin H, Ts’O T. Polycyclic Aromatic Hydrocarbons and Cancer. Academic Press; New York: 1978. pp. 45–70. [Google Scholar]
- Bauschlicher CW. A comparison of the accuracy of different functionals. Chem Phys Lett. 1995;246:40. [Google Scholar]
- Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648. [Google Scholar]
- Betowski LD, Enlow M, Riddick L. The phototoxicity of polycyclic aromatic hydrocarbons: a theoretical study of excited states and correlation to experiment. Comput Chem. 2002;26:371–377. doi: 10.1016/s0097-8485(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–767. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cances MT, Mennucci V, Tomasi J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys. 1997;107:3032. [Google Scholar]
- Chen JW, Kong LR, Zhu CM, Huang QG, Wang LS. Correlation between photolysis rate constant of polycyclic aromatic hydrocarbons and frontier molecular orbital energy. Chemosphere. 1996;33:1143–1150. [Google Scholar]
- Connell DW, Hawker DW, Warne MJ, Vowles PP, McCombs K, Starkweather AW. Introduction into Environmental Chemistry. CRC Press LLC; Boca Raton, FL: pp. 1997pp. 205–217. [Google Scholar]
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- Dabestani R, Ivanov IN. Invited Review: A Comparison of Physical, Spectroscopical and Photophysical Properties of Polycyclic Aromatic Hydrocarbons. Photochem Photobiol. 1999;70:10–34. [Google Scholar]
- de Lima Ribeiro FA, Ferreira MMC. QSAR model of the phototoxicity of polycyclic aromatic hydrocarbon. Theochem. 2005;719:191–200. [Google Scholar]
- Dipple A. Polycyclic aromatic hydrocarbons and carcinogenesis. Ameraican Chemical Society; Washington, DC: 1985. [Google Scholar]
- Dong S, Fu PP, Shirsat RN, Hwang H-M, Leszczynski J, Yu H. UVA light-induced DNA cleavage by isomeric methylbenz[a]anthracenes. Chem Res Toxicol. 2002;15:400–409. doi: 10.1021/tx015567n. [DOI] [PubMed] [Google Scholar]
- Dong S, Hwang H-M, Shi X, Holloway L, Yu H. UVA-induced DNA single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem Res Toxicol. 2000;13:585–593. doi: 10.1021/tx990199x. [DOI] [PubMed] [Google Scholar]
- El-Azhary AA, Suter HU. Comparison between Optimized geometries and vibrational frequencies calculated by the DFT methods. Phys Chem. 1996;100:15056. [Google Scholar]
- Foote CS. Free Radical Biology. Academic Press; New York: 1976. pp. 85–133. [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Lyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Wallingford, CT: 2004. Gaussian. [Google Scholar]
- Gutman I, Milun M, Trinajstic N. Graph theory and molecular orbitals, XIX. Non-parametric resonance energies of arbitrary conjugated systems. J Am Chem Soc. 1977a;99:1692–1704. [Google Scholar]
- Gutman I, Milun M, Trinajstic N. Topological resonance energies of annlenes. Croat Chem Acta. 1977b;49:441–452. [Google Scholar]
- Huang X-D, Dixon DG, Greenberg BM. Impact of UV radiation and photomodification on the toxicity of PAHs to the higher plant Lemna gibba (Duckweed) Environ Toxicol Chem. 1993;12:1067–1077. [Google Scholar]
- Huang X-D, Dixon DG, Greenberg BM. Increased polycyclic aromatic hydrocarbon toicity following their photomidification in natural sunlight: Impactss on the Duckweed Lemna gibba L. G-3. Ecotoxicol Environ Safety. 1995;32:194–200. doi: 10.1006/eesa.1995.1102. [DOI] [PubMed] [Google Scholar]
- Kagan J, Kagan E. The toxicity of benzo[a]pyrene and pyrene in the mosquito Aedes aegypti in the dark and in the presence of ultraviolet light. Chemosphere. 1986;15:243–251. [Google Scholar]
- Krylov SN, Huang X-D, Zeiler LF, Dixon DG, Greenberg BM. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons. I. Physical model based on chemical kinetics in a two-compartment system. Environ Toxicol Chem. 1997;16:2283–2295. [Google Scholar]
- Kubicki JD, Blake GA, Apitz SE. Molecular models of benzene and selected polycyclic aromatic hydrocarbons in the aqueous and absorbed states. Environ Toxicol Chem. 1999;18:1956–1662. [Google Scholar]
- Landrum PF, Giesy JP, Oris JT, Allred PM, Vandermeulen JH, Hrudey SE. Symposium on Oil Pollution of Freshwater. Pergamon; Ann Arbor: 1987. pp. 304–318. [Google Scholar]
- Lee C, Yang W, Parr R. Developemnt of the colle-salvetti correlation energy formula into a functional of the electron density. Phys Rev B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- Leigh IM, Newton Bishop JA, Kripke ML. Instruction Skin Cancer. Cancer Surv. 1996;26:1–6. [PubMed] [Google Scholar]
- Liu Z, Lu Y, Rosenstein B, Lebwohl M, Wei H. Benzo[a]pyrene enhances the formation of 8-hydroxy-2′-deoxyguanosine by ultraviolet A radiation in calf thymus DNA and human epidermoid carcinoma. Biochemistry. 1998;37:10307–10312. doi: 10.1021/bi980606o. [DOI] [PubMed] [Google Scholar]
- Mekenyan OG, Ankley GT, Veith GD, Call DJ. QSARs for photoinduced Toxicity. I. Acute lethality of polycyclic aromatic hydrocarbons to Daphnia magna. Chemosphere. 1994;28:567–582. [Google Scholar]
- Miehlich B, Savin A, Stoll H, Preuss H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett. 1989;90:5622. [Google Scholar]
- National Toxicology Program, P. H. S. US Department of Health and Human Services . Integrated Laboratory Systems. Research Triangle Park, NC: 1998. pp. 178–181. [Google Scholar]
- Newsted JL, Giesy JP. Predictive models for photoinduced acute toxicity of polycyclic aromatic hydrocarbons to Daphnia magna, Strauss (Cladocera, Crustacea) Environ Toxicol Chem. 1987;6:445–461. [Google Scholar]
- Pelletier MC, Burgess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrae lavae and juveniles. Environ Toxicol Chem. 1997;16:2190–2199. [Google Scholar]
- Pitts JN. Photochemical and biological implications of the atmosphere reactions of amines and benzo[a]pyrene. Philos Trans R Soc (London) A. 1979;290:551–576. [Google Scholar]
- Rotman B, Papermaster BW. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Nat Acad Sci USA. 1966;55:134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer K, Dixon DG, Greenberg BM, Bols NC. Ability of 16 priority PAHs to be directly cytotoxic to a cell line from the rainbow trout gill. Toxicology. 1998;127:129–141. doi: 10.1016/s0300-483x(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Schirmer K, Herbrick JS, Greenberg BM, Dixon DG, Bols NC. Use of fish gill cells in culture to evaluate the cytotoxicity and photocytotoxicity of intact and photomodified creasote. Environ Toxicol Chem. 1998;18:1277–1288. [Google Scholar]
- Schlegel GB. Optimization of equilibrium geometries and transition struuctures. J Comp Chem. 1982;3:214. [Google Scholar]
- Sheng Y, Leszczynski J. Theoretical study of the substituent and solvent effects on the molecular structures, absorption and emission spectra of open-form spiropyrans. Collect Czechoslovak Chem Comm. 2004;69:47–62. [Google Scholar]
- Sheng Y, Leszczynski J, Garcia AA, Rosario R, Gust D, Springer J. Comprehensive theoretical study of the conversion reactions of spiropyrans: substituent and solvent effects. J Phys Chem. 2004;108:16233. [Google Scholar]
- Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. Phys Chem. 1994;98:11623. [Google Scholar]
- Straman RE, Scuseria GE, Frisch MJ. An efficient implementation of time -dependent density-functional theory for the calculation of excitation energies of large molecules. Chem Phys. 1998;109:8218. [Google Scholar]
- Swartz RC, Ferraro SP, Lamberson JO, Cole FA, Ozretich RJ, Boese BL, Schults DW, Behrenfeld M, Ankley GT. Photoactivation and toxicity of mixtures of polycyclic aromatic hydrocarbon compounds in marine sediment. Environ Toxicol Chem. 1997;16:2151–2157. [Google Scholar]
- USEPA. Office of the Federal Registration (OFR) Appendix A: Priorty pollutants. Fed Reg. 1982;47:52309. [Google Scholar]
- Utesch D, Eray K, Diehl E. Phototoxicity testing of polycyclic aromatic hydrocarbons (PAH) in mammalian cells in vitro. Polycycl Arom Compd. 1996;10:117–121. [Google Scholar]
- Warshawsky D. Polycyclic aromatic hydrocarbons in carcinogenesis. Environ Health Perspect. 1999;107:317–320. doi: 10.1289/ehp.99107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yan J, Wang X, Yu H, Milliken T. Photochemical reaction of chrysene in acetonitrile/water. Polycycl Arom Compd. 2004;24:249–256. [Google Scholar]
- Yan J, Wang L, Fu P, Yu H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pullutant list. Mutat Res. 2004;557:99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Xia Q, Cherng S, Wamer WG, Howard PC, Yu H, Fu PP. Photoirradiation of representative polycyclic aromatic hydrocarbons and twelve methylbenz[a]anthracenes with UVA Light: Formation of lipid peroxidation. Toxicol Ind Health. 2006;22:147–151. doi: 10.1191/0748233706th259oa. [DOI] [PubMed] [Google Scholar]
- Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: Photochemistry and phototoxicity. J Environ Sci Health Part C: Environ Carcinog Ecotoxic Revs. 2002;20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xia Q, Yan J, Herreno-Saenz D, Wu Y-S, Tang I-W, Fu PP. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light-a pathway leading to generation of reactive oxygen species, lipid peroxidation, and DNA damage. J Environ Res Public Health. 2006;3:348–354. doi: 10.3390/ijerph2006030045. [DOI] [PMC free article] [PubMed] [Google Scholar]