Abstract
1-Aminopyrene (1-AP) is an environmental mutagen and a metabolite of the mutagenic environmental pollutant, 1-nitropyrene (1-NO2P). Upon light irradiation, 1-AP transforms into oxidation products with a half-life of 7.1 min in 10% methanolic buffer. The presence of free radical/singlet oxygen scavengers DTT, histidine, or NaN3, slows down 1-AP photochemical reaction. The reaction is also slower in the presence of DNA. The photoproducts identified include 1-hydroxyaminopyrene, 1-nitrosopyrene, 1-NO2P, 1-amino-x-hydroxypyrene, and three covalent dimers. The progressive oxidation of the amino group to hydroxyamino, nitroso, and finally nitro is the reverse of the enzymatic reduction of 1-NO2P in living systems. Since it is known that 1-NO2P and 1-nitrosopyrene are genotoxic and 1-hydroxyaminopyrnene can react with DNA to form covalent adducts, the toxicity of 1-AP and its photoproducts and light-induced DNA covalent adduct formation were studied. Using Mutatox® Test, it is found that the lowest effective observable concentrations for 1-AP, 1-AP photoproducts, and 1-NO2P are 1.25, 10, and NA (not applicable) in the direct medium (no S-9) and NA, 5, and 0.625 μM in the S-9 medium, respectively. Therefore, 1-AP photoproducts are more genotoxic than 1-AP itself in the S-9 medium and more mutagenic than 1-NO2P in the direct medium. Thus 1-NO2P alone cannot account for all the mutagenicity of the photoproducts. Irradiation of 1-AP together with DNA leads to covalent DNA adduct formation possibly via the 1-hydroxyaminopyrene intermediate. This suggests that photolysis not only transforms 1-AP into more mutagenic compounds, but also forms DNA covalent adducts.
INTRODUCTION
Aromatic amines are widely used industrial chemicals and wide-spread environmental pollutants (1–6). They are found in crude oils and coal products (1, 4, 7–9). Many of them are carcinogens and mutagens and the carcinogenic risk was realized when urinary bladder cancer was linked to the exposure to aromatic amines (10, 11). The aromatic amines, especially the amino polycyclic aromatic hydrocarbons (PAHs), are also the metabolic products of the mutagenic environmental pollutants, nitro PAHs (12–14). For example, 1-nitropyrene (1-NO2P) is reduced by nitro-reductases to 1-aminopyrene (1-AP) in the living systems (12, 15). The reduction of 1-NO2P to 1-AP is through three progressive two electron reduction processes, forming first 1-nitrosopyrene (1-NOP), then 1-hydroxyaminopyrene (1-HOAP), before it finally becomes 1-AP (12, 16–18). On the other hand, aromatic amines can be oxidized by cytochrome P-450 enzymes to the intermediate hydroxyamino derivatives (11, 19). It is believed that this intermediate can react with DNA to form DNA covalent adducts and subsequently lead to carcinogenesis (13, 20–23). In the case of 1-NO2P, the nitro-reductases reduce 1-NO2P to the intermediate 1-HOAP, which reacts with DNA to form DNA covalent adducts by linking to the guanine bases. The adduct, N-(deoxyguanosin-8-yl)-1-aminopyrene, has been chemically synthesized and incorporated into DNA for structural (24) and mutagenesis studies (25, 26).
Organic pollutants in the natural environment are subjected to transformation/degradation via at least three main routes: (i) bio-transformation by bacteria or other microorganisms, (ii) abiotic reaction of the pollutant with co-existing chemicals, and (iii) transformation by sunlight photolysis. It is known that photolysis could transform some aromatic amines into mutagenic compounds. For example, photo-oxidation of the amino compounds was reported to produce the carcinogenic or mutagenic nitro compounds (21, 27–30). Photolysis of 1-AP was reported to generate 1-NO2P (31). In this article, we report the identification of the photochemical transformation products, study of the photochemical reaction mechanisms, the mutagenicity of the photoproducts, and the formation of DNA covalent adducts through photolysis of 1-AP. The photo-induced genotoxicity of 1-AP was measured with the Mutatox® Test system, which determines the genotoxicity of chemical contaminants by measuring the changes of light emission from a dark strain of the bioluminescent bacteria Vibrio fischeri strain M169. The test has been used as a rapid tool for detecting mutagenicity of DNA-damaging compounds (32–35).
MATERIAL AND MATHODS
Reagents, chemicals, and instrumentation
1-AP and 1-NO2P were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. 1-NOP was synthesized by oxidation of 1-AP with m-chloroperbenzoic acid. 1-HOAP was synthesized by reduction of 1-NO2P with hydrazine in the presence of palladium on carbon as catalyst (36). The product 1-HONP was too unstable to be isolated. Therefore, it was used directly for HPLC and LC-MS analysis to compare with the photo-transformation products of 1-AP. Stock solutions of 1-AP (1 mM) were prepared in methanol and stored in the refrigerator with the exclusion of light. NaCl, MgCl2, L-histidine, NaN3, 1,4-dithiothreitol (DTT) and calf thymus DNA (ct-DNA) were also purchased from Sigma-Aldrich. All solvents used were spectroscopic grade. The water used (18 Ω) was de-ionized by a Barnstead Nanopure Infinity water de-ionization system. UV-Vis spectra were recorded on a CARY 300E UV-Vis Spectrophotometer (Varian, Inc.). The solvent used for all experiments was a 10% methanolic buffer (10 mM, pH 7.1) unless otherwise stated.
Photo-transformation of 1-AP in aqueous solutions in the presence of various chemicals
Two milliliters of 50 μM 1-AP solution in the PBS buffer in the presence or absence of added chemicals were filled in two identical quartz cuvettes (10 mm × 35 mm). The chemicals were NaCl (0.5 M), MgCl2 (54 mM), L-histidine (50 mM), NaN3 (20 mM), DTT (20 mM), and ct-DNA (50 μM in base pairs). Some samples were in a combined solvent of 10% methanol and 40% acetonitrile for better solubility of the photoproducts. The cuvettes were placed on top of a Pyrex glass support and were irradiated by UVA light. The lamp was set beneath the Pyrex glass support and a stream of cool air was blowing toward the bottom of the support during the irradiation period to eliminate heat generated by light. The light source was a 100 W UVA lamp (type B, UVP Inc., Upland, CA) that produces a main emission band at 365 nm. The distance from the surface of the bulb to the Pyrex glass support was 6.5 cm, generating 170 J/cm2 per hour UVA light measured with a Model PMA 2100 radiometer with detector from Solar Light Co. (Philadelphia, PA). After each irradiation period, an absorption spectrum was recorded on the spectrophotometer. Absorption values at 353 nm were used for kinetic analysis. The first-order kinetic process was analyzed by, Ln Ao/At = k t, where Ao and At were the absorption intensity of 1-AP at irradiation time zero and t min, respectively; k is the first-order rate constant. A linear plot was obtained for Ln Ao/At versus t for the initial 30 min of irradiation and a rate constant was calculated from the slope of the plot. The half-life was calculated by t1/2 = 0.693/k.
HPLC analysis of the photoproducts
After each irradiation period, the samples were analyzed with a Waters HPLC system equipped with two Waters 515 pumps, a Waters 717 plus auto-sampler, a Waters 996 photodiode array detector, a pre-column filter (Sigma-Aldrich) and a Lichrospher® 100 RP-8 column (25 cm × 4.0 mm, 5 μm) (Hewlett Packard). A mixture of acetonitrile (A) and water (B) was used as the mobile phase. The following gradient elution was run: 0–10 min: 40 to 90% A; 10–22 min maintained at 90% A; 22–24 min: 90 to 40% A. The flow rate was 1.0 mL/min and the detection wavelength was 254 nm. The temperature of the auto-sampler was controlled at 12°C. Identification of the 1-AP photochemical transformation products was carried out by comparison of the retention time and absorption spectra with that of the standard solutions for 1-NO2P, 1-NOP and 1-HOAP.
LC-MS analysis of the photoproducts
The LC-MS-MS system was a Finnigan LCQDUO mass spectrometer (San Jose, CA). Separation was carried out with the same Lichrospher® 100 RP-8 column and the gradient conditions as for the HPLC analysis. The wavelength of photo-diode array detector was set at 254 nm and the temperature of the auto-sampler was controlled at 12°C. The mass spectra were acquired at 3 scans/s over a m/z range of 50–500. MS-MS was applied when needed. The capillary temperature and APCI vaporizer temperature were 180°C and 450°C, respectively. Nitrogen was used as both a sheath and an auxiliary gas. The mass spectrometer was operated in the positive ion detection mode for 1-AP and in the negative ion detection mode for all the photoproducts.
Light-induced formation of covalent adducts between DNA and 1-AP
A 30 mL solution of 30 μM 1-AP in 10 mM PBS buffer in the presence of 200 μM ct-DNA was filled in a 50 mL Kimax beaker. The beaker was placed on top of the Pyrex glass support and the lamp was set up as described for the above experiments. A 2 mL sample was taken at various irradiation time intervals and all of these samples were divided into two 1 mL portions. One portion was kept in the dark and the other was filled into individual dialysis tubes and dialyzed against 3 × 500 mL buffer for a total of 72 h. The dialysis tubes were Spectra/Por membranes with a MWCO of 12,000. The absorption spectra of the samples after dialysis were recorded and compared with the non-dialyzed samples.
In order to confirm that the above dialysis method efficiently removed all non-covalently bound 1-AP or its photoproducts, the following experiments were conducted as previously described for 1-hydroxypyrene (37). (1) Extraction by chloroform: a 2 mL UVA-irradiated sample was extracted with chloroform (3×5 mL). The organic and aqueous layers were stirred vigorously for 20 min for efficient mixing. The absorbance spectrum of the aqueous layer was recorded. (2) Phenol/chloroform extraction in combination with ethanol precipitation: a 2 mL irradiated sample was extracted with a 1:24 mixture of phenol and chloroform (3×5 mL). After extraction, the remaining aqueous solution was added with 6 mL of 60% ethanol and the mixture was kept in the refrigerator for DNA precipitation. After removing the supernatant, the precipitate was re-dissolved in the PBS buffer to record the absorbance spectrum.
Genotoxicity tests by Mutatox® Test
Stock solutions of 1-AP and 1-NO2P (1 mM) in DMSO were diluted with the PBS buffer (no methanol) and the final DMSO concentration was 4%. A 10 mL of the 1-AP stock solution was added to 150 mL quartz flasks (Quartz Scientific, Fairport Harbor, OH) in triplicates and exposed to October midday sunlight for 1.5 h to obtain the solution containing 1-AP photoproducts. Presence of 1-AP photoproducts were confirmed with HPLC. Mutagenicity of 1-AP, 1-NO2P (both ranged from 0.625 to 10 or 80 μM) and 1-AP photoproduct mixture was determined with Mutatox®Test according to standard procedures listed in the Mutatox® genotoxicity test manual (35). The mutagenic response of the reconstituted bacteria with 2-fold serial dilutions was determined after 16, 20 and 24 h of incubation at 27°C. Activation with S-9 enzymes received 1 h incubation at 35°C. Light emitted from the media control, solvent (4% DMSO) control, positive control (phenol for direct acting and benzo[a]pyrene for S-9 acting) and samples receiving different treatments were measured with Mutatox Analyzer (Azur Environmental). Positive mutagenic agents are defined as those samples which induce a light emission level of Vibrio fischeri strain M169 to at least two times the average control light reading in at least two consecutive sample concentrations. Mutatox®Test was conducted at least twice for the same treatment to assure consistency of the reading.
RESULTS AND DISCUSSION
Photo-transformation of 1-aminopyrene monitored by UV-Vis absorption spectra
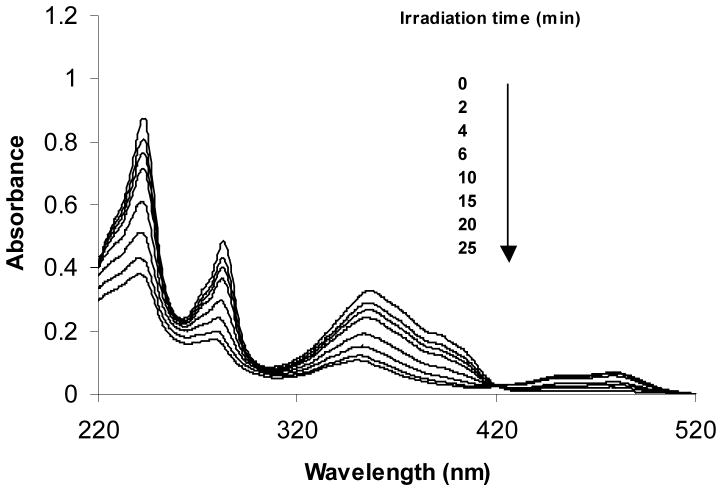
The absorption spectrum of 1-AP has three bands at 353, 283, and 242 nm. Figure 1 shows the absorption spectra of the UVA-irradiated 1-AP (50 μM) recorded after various irradiation periods in 10% methanolic buffer solution containing 40% acetonitrile. The 40% acetonitrile was used for better solubility of the 1-AP photoproducts. As irradiation time increased, the absorption of all three bands decreased and a new band between 420 and 510 nm appeared with an isobestic point near 420 nm. The appearance of an isobestic point signifies that 1-AP was actually transformed into photoproducts that absorb longer wavelength light. Using the intensity at 353 nm to plot versus irradiation time, a first-order kinetic was observed with the reaction rate constant k = 0.069 s−1 and thus the half-life of t1/2 = 10 min. In comparison, the photo-transformation half-life of 1-AP in the 10% methanolic buffer was determined to be 7.1 min.
Figure 1.
UV-Vis spectra for transformation of 1-aminopyrene in 10% methanolic buffer containing 40% acetonitrile monitored by UV-Vis absorption spectra. A solution of 50 μM 1-AP was irradiated with the UVA lamp and samples were taken at desired time intervals for recording of the absorption spectra.
With addition of selected chemicals to the 10% methanolic buffer solution of 1-AP (50 μM), the photo-transformation rate was either speeded up or slowed down. As shown in Table 1, the presence of NaCl (0.5 M) or MgCl2 (54 mM), both of which are present in natural waters, speeded up the photo-transformation process. Presence of NaCl or MgCl2 decreased the photo-transformation half-life of 1-AP from 7.1 min to 2.7 min or 5.1 min, respectively. In this study Na+ and Mg2+ were added at their ambient concentrations in natural waters (38). The presence of DTT, a free radical scavenger, or histidine, a singlet oxygen scavenger, greatly slowed down the photo-transformation of 1-AP. The presence of 20 mM DTT lengthened the half-life of 1-AP 19 times. In the presence of 50 mM histidine, the absorbance of 1-AP at 353 nm did not change. However, the absorbance at both sides of the 353 band increased, indicating that chemical changes occurred either to 1-AP or histidine. The presence of NaN3 (20 mM), both a free radical and a singlet oxygen scavenger, lengthened the photo-transformation half-life of 1-AP from 7.1 min to 17 min. Therefore, it is very possible that both free radical and singlet oxygen are involved in the photo-transformation process of 1-AP. The presence of ct-DNA (50 μM) doubled the photo-transformation half-life of 1-AP. Detailed information is given in the later section containing Figure 4. Presence of a higher percentage of acetonitrile (40%) also slowed down the photo-transformation process due to DNA covalent adduct formation with 1-AP as will be discussed later.
Table 1.
Photo-transformation half-lives (min) for 1-AP (50 μM) irradiated with a 100 W UVA lamp in 10 mM sodium phosphate buffer (pH 7.1) containing 10% methanol in the presence of various chemicals.
| Additional Chemicals | NaCla (0.5 M) | MgCl2a (54 mM) | DTT (20 mM) | Histidine (50 mM) | NaN3 (20 mM) | ct-DNA (50 μM) | CH3CN (40%) | |
|---|---|---|---|---|---|---|---|---|
| Half-life (min) | 7.1 | 2.7 | 5.1 | 136 | NAb | 17 | 14 | 10 |
The pH of NaCl and MgCl2 samples was maintained at 8.3 to simulate that in seawater. A separate experiment showed that the photo-transformation half-life is about the same at pH 7.1.
NA means that there was no decrease in absorbance at 353 nm after more than 60 min of irradiation. However, the absorbance at both sites of the 353 nm band increased some.
Figure 4.
Absorption spectra of DNA covalent adduct of 1-AP. A solution of 30 μM 1-AP in 200 μM calf thymus DNA was irradiated with UVA light for 1 h. Its absorption spectrum (line # 2) is compared with the same solution before UVA irradiation (line #1), and the same solution after dialysis with semi-permeable membrane (MWCO 13,000) for 24×3 hours against the same buffer solution (line #3). Line #4 is the control.
HPLC analysis of photoproducts and characterization by LC-MS
After a period of irradiation of 1-AP solution, a sample was injected in the HPLC for analysis. As indicated in Figure 2, 1-AP itself eluted at 10.9 min (molecular mass 217 in Figure 3). After irradiation, several photoproducts appeared as new peaks at 8.4 (1-AP-OH), 8.6 (1-HOAP), 13.1 (1-NOP), 13.8 (1-NO2P), 15.8, 19.0, and 22.1 min (Dimers), respectively. Among them, the peaks at 8.6, 13.1, and 13.8 min matched the molecular masses for 1-HOAP (MW 233), 1-NOP (MW 231), and 1-NO2P (MW 247), respectively. Positive identification of these photoproducts was accomplished by comparing retention time, absorption and mass spectra with the authentic samples. Since the molecular mass for the peak at 8.4 min is the same as 1-HOAP (WM = 233), it is possible that this peak represents an 1-amino-x-hydroxypyrene with the hydroxy group connecting to one of the pyrene ring carbons. The mass spectra of all the photoproducts are shown in Figure 3. Peaks at 15.8, 19.0, and 22.1 min have molecular ions of 462, 447, and 431, respectively. Based on the molecular masses determined by the negative molecular ions, these compounds must be covalent dimers of 1-AP: Dimer 1 has a negative molecular ion of 462 and thus its molecular mass should be 463. This compound should be two 1-AP molecules combined with two extra oxygen atoms and one less hydrogen atom. The linkage between the 1-AP molecules eliminates two hydrogen atoms. Thus, the mass of the dimer is (1-AP)2 + 2O – 3H = 434 + 32 − 3 = 463. Dimer 2 has a negative molecular ion of 447 and thus its molecular mass should be 448. This compound should be two 1-AP molecules linked together with one extra oxygen. Again the linkage will eliminate two hydrogen atoms. Thus the molecular mass of dimer 2 is (1-AP)2 + O – 2H = 434 +16 − 2 = 448. Dimer 3 has a negative molecular ion of 431 and thus its molecular mass should be 432. This compound should be simply two 1-AP molecules linked together by subtracting two hydrogen atoms for the linkage. Thus the molecular mass is (1-AP)2 – 2H = 434 − 2 = 432. Subtracting of 2Hs for all three compounds is a strong indication that a linkage (C-C, C-O, C-N, or N-N) is formed between two 1-AP molecules. In short, dimer 3 is formed by simple linkage of two 1-AP molecules, dimer 2 is the linkage of two 1-AP molecules with one of them has an extra oxygen atom, and dimer 3 is the linkage of two 1-AP molecules with two extra oxygen atoms. Nevertheless, how the two 1-AP molecules are linked and where the oxygen atoms are in dimers 1 and 2 remain to be determined. Complete identification of these dimers needs detailed NMR and IR data.
Figure 2.
HPLC chromatograms of 1-aminopyrene photo-transformation. A solution of 10 μM 1-aminopyrene in 30% acetonitrile/PBS buffer was irradiated with a UVA lamp. Samples were taken at 0, 5, 10, 20, 40, 80, 160 min and analyzed with HPLC.
Figure 3.
Mass spectra of 1-aminopyrene and its photoproducts detected by LCQDUO LC-MS using the APCI ionization mode. Except for 1-aminopyrene, which was detected in the positive ion mode, all others were detected in the negative ion mode.
Light-induced covalent adduct formation between DNA and 1-aminopyrene
In a previous paper, we reported that irradiation of 1-AP with DNA together could cause DNA single strand cleavage (39). Figure 4 shows that irradiation of 1-AP with DNA together can also lead to the formation of covalent adducts between 1-AP and DNA. The absorption spectrum of 1-AP mixed with DNA in solution is shown by line #1 in Figure 4, which has the 353 nm absorption band of 1-AP and the combined absorption band at below 300 nm for both DNA bases and 1-AP. After dialysis with a semi-permeable membrane (MWCO = 12,000) of the mixture, the absorbance at > 300 nm for 1-AP disappeared completely (line #4), signifying that 1-AP could pass through the membrane and enter the outside solution of the dialysis tube freely while DNA was contained in the membrane tube. After 60 min of UVA irradiation of the 1-AP and DNA mixture, the absorbance for the 353 nm band of 1-AP decreased by 50% and the < 300 nm band decreased somewhat (line #2), indicating that some of the 1-AP was degraded or transformed into weaker or none light absorbing compounds in this wavelength region. After dialysis of this sample the same way as for sample #1, the absorption band at 353 nm did decrease slightly, but did not disappear, indicating that some 1-AP or its photoproducts could not pass through the membrane due to covalent binding with DNA. To confirm this result, the sample solution was extracted with chloroform, chloroform-phenol followed by ethanol precipitation, but in none of the cases, the absorption at 353 nm could be removed (data not shown). Therefore, it is a strong indication that 1-AP can form covalent adduct with DNA upon UVA irradiation. This is similar to our previous report that covalent DNA adducts were formed when 1-hydroxypyrene and DNA were irradiated together in solution (37).
Genotoxicity of 1-aminopyrene and its photoproducts determined by Mutatox® Test
The mutagenicity of 1-AP, its photoproduct mixture, and 1-NO2P were tested with the Mutatox® Test system. Tests were carried out in the direct-acting medium and the medium for pro-mutagens (growth medium containing S-9 enzymes). The test reagents included a dark strain of the bioluminescent bacteria Vibrio fischeri strain M169. The test bacteria would emit light upon exposure to DNA damaging chemicals. The mutagenic response scores positive if the light intensity equals or exceeds twice of that of the media or solvent negative control over at least two consecutive concentrations as suggested by the manufacturer (35). The Lowest Observable Effective Concentration (LOEC) is the first concentration at which a more than doubled light intensity is recorded. As shown in Table 2, 1-AP is not mutagenic at concentrations up to 10 μM in the S-9 medium. However, it exhibits a positive mutagenic response with a LOEC of 1.25 μM in the direct medium. 1-AP was also reported to be mutagenic in the absence of the rat S9 activation system, but was less mutagenic in the presence of the S-9 (40). The weakened response was speculated to occur as the result of 1-AP degradation by the S-9 enzymes. The 1-AP photoproduct mixture is mutagenic in both the S-9 and direct media with a LOEC of 5 μM in the S-9 medium and 10 μM in the direct medium, respectively. Therefore, relative to 1-AP, the 1-AP photoproduct mixture is more mutagenic in the S-9 medium and less mutagenic in the direct medium. 1-NO2P is strongly mutagenic in the S-9 medium with a LOEC of 0.625 μM, but not mutagenic in the direct medium up to 10 μM. This is expected because enzymatic activation is needed for 1-NO2P to become mutagenic (12–14). It was reported that photolysis would convert 1-AP into the more mutagenic 1-NO2P (31). This is in agreement with our test results in the S-9 medium. However, the mutagenicity of the 1-AP photoproduct mixture is much weaker in terms of LOEC than 1-NO2P, indicating that 1-NO2P accounts for a small portion of the 1-AP photoproducts as shown in Figure 2. As for the test in the direct medium, the results indicate that 1-AP photoproduct mixture is more mutagenic than 1-NO2P alone. Therefore, the other photoproducts such as 1-HONP, 1-AP-OH, 1-NOP, or the dimers may actually account for the mutagenicity observed in 1-AP photoproduct mixture.
Table 2.
Mutatox test results for 1-aminopyrene, its photoproduct mixture, and 1-nitropyrene.a
| Light output in the S-9 medium | ||||||
|---|---|---|---|---|---|---|
| Concentration (μM) | Media control | Solvent control | Positive control | 1-AP | 1-NO2P | 1-AP PPb |
| 0.625 | 23 | 11 | 32 | 32 | 135 | 41 |
| 1.25 | 22 | 30 | 38 | 27 | 205 | 30 |
| 2.5 | 19 | 26 | 132 | 31 | 80 | 44 |
| 5 | 13 | 19 | 765 | 33 | 30 | 69 |
| 10 | 10 | 6 | 1698 | 32 | 15 | 1373 |
| Response | − | − | + | − | + | + |
|
| ||||||
| LOEC (μM) | NAc | 0.625 | 5 | |||
|
| ||||||
| Light output in the direct medium | ||||||
|
| ||||||
| 0.625 | 4 | 19 | 19 | 19 | 29 | 6 |
| 1.25 | 7 | 7 | 198 | 62 | 12 | 7 |
| 2.5 | 5 | 16 | 1305 | 173 | 9 | 11 |
| 5 | 2 | 9 | 14850 | 1938 | 8 | 17 |
| 10 | 2 | 14 | 891 | 2500 | 9 | 146 |
| 20 | 3216 | |||||
| 40 | 2299 | |||||
| 80 | 3 | |||||
| Response | − | − | + | + | − | + |
|
| ||||||
| LOEC (μM) | 1.25 | NAc | 10 | |||
Solvent control was with 4% DMSO and positive control was with benzo[a]pyrene for the S-9 medium and phenol for the direct medium.
1-AP PP means 1-aminopyrene photoproduct mixture.
NA means that no mutagenic response was seen at this concentration range.
Photochemical transformation of 1-aminopyrene and its reaction with DNA
Based on the above discussion, the photochemical reaction pathways for 1-AP are derived (Figure 5). The photochemical oxidation of 1-AP in an aqueous solution can occur at both the nitrogen and the ring carbons, resulting in intermediate compounds that are mutagenic. There are several interesting points to note: (1) There are at least three pathways for 1-AP photo-oxidation: (i) oxidation of the amino group, (ii) ring oxidation, and (iii) dimer formation; (2) photo-oxidation of 1-AP is the reverse of the enzymatic reduction of 1-NO2P and both produce a 1-HOAP intermediate molecule. The intermediate 1-HOAP can react with DNA to form covalent adducts; (3) the photochemical reaction occurs via free radical intermediates and participation of singlet oxygen as discussed above; (4) formation of the three dimers also indicates that there might be different reactive intermediates leading to the dimers.
Figure 5.
Photochemical transformation pathways of 1-aminopyrene
CONCLUSIONS
In conclusion, photo-oxidation of 1-AP follows at least three pathways with one of them being a progressive two electron oxidation of the amino group, first to the hydroxyamino, then the nitroso, and finally to the nitro group. Similar oxidation pattern was observed for 2-aminofluorene (27, 29). Photolysis converted 2-aminofluorene to 2-aminofluoren-9-one first, then to the nitroso and nitro derivatives. Mechanistically, it is likely that both singlet oxygen and free radicals are involved in the photo-oxidation of 1-AP because the presence of a free radical scavenger, DTT, or a singlet oxygen scavenger, histidine, effectively inhibited the photolysis of 1-AP. In the presence of NaN3, both a singlet oxygen and a free radical scavenger, slowed down the photo-transformation of 1-AP 2.5 times, but not as significantly as DTT or histidine. The route for dimer formation is not clear, but it is likely through a free radical intermediate. Oyama et. al. (41) reported that 1-AP cation radicals could couple with either another cation radical or a neutral 1-AP to form dimers in solution.
Among the photoproducts, 1-HOAP is proven to be able to form DNA covalent adducts when it is allowed to react with DNA (24, 36, 42). In this report, if 1-AP were irradiated together with ct-DNA, 1-AP-DNA covalent adduct was detected, possibly also through the 1-HOAP intermediate. 1-NOP is found to be a metabolic product of 1-NO2P (17) and both 1-NOP and 1-NO2P are mutagenic (17, 31, 43–45). However, the toxicity of 1-AP-OH or the covalent 1-AP dimers remains to be studied. In the Mutatox® Test, 1-NO2P cannot account for all the mutagenicity observed for the 1-AP photoproduct mixture in the direct medium because the photoproduct mixture is more mutagenic than 1-NO2P. Since 1-NOP is known to be not more mutagenic than 1-NO2P (17, 43), the other chemical species must be the causing factors.
Acknowledgments
This research was supported by grants from the National Institutes of Health: MBRS (SCORE) S06 GM08047 and RCMI 5G12RR12459, and by the US Army Research Office: DAAD 19-01-1-0733 to JSU.
Abbreviations
- PAHs
polycyclic aromatic hydrocarbons
- 1-AP
1-aminopyrene
- 1-NO2P
1-nitropyrene
- 1-HOAP
1-hydroxyaminopyrene
- 1-NOP
1-nitrosopyrene
- 1-AP-OH
an unknown hydroxy substituted 1-AP
- DTT
1,4-dithiothreitol
- PBS
10 mM sodium phosphate buffer, pH 7.1
- LOEC
lowest observable effective concentration
- ct-DNA
calf thymus DNA
References
- 1.Weisburger JH. Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat Res. 2002;506–507:9–20. doi: 10.1016/s0027-5107(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 2.Tomkins BA, Ho CH. Determination of polycyclic aromatic amines in natural and synthetic crudes. Anal Chem. 1982;54:91–96. [Google Scholar]
- 3.Garner RC, Martin CN, Clayson DB. In: Chemical Carcinogens. Searle CE, editor. American Chemical Society; Washington DC: 1984. pp. 175–275. [Google Scholar]
- 4.Haugen DA, Peak MJ, Suhrbler KM. Isolation of mutagenic aromatic amines from a coal conversion oil by cation exchange chromatography. Anal Chem. 1982;54:32–37. [Google Scholar]
- 5.Fujikawa K, Fort FL, Samejima K, Sakamoto Y. Genotoxic Potency in Drosophila melanogaster of Selected Aromatic Amines and Polycyclic Aromatic Hydrocarbons as Assayed in the DNA Repair Test. Mutat Res. 1993;290:175–182. doi: 10.1016/0027-5107(93)90157-b. [DOI] [PubMed] [Google Scholar]
- 6.Parkes HG, Evans AE. In: Chemical Carcinogens. Searle CE, editor. American Chemical Society; Washington DC: 1984. pp. 277–301. [Google Scholar]
- 7.Later DW, Andros TG, Lee ML. Isolation and identification of amino polycyclic aromatic hydrocarbons from coal-derived products. Anal Chem. 1983;55:2126–2132. [Google Scholar]
- 8.Later DW, Lee ML. Selective detection of amino polycyclic aromatic compounds in solvent refined coal. Anal Chem. 1982;54:117–123. [Google Scholar]
- 9.Later DW, Pelroy RA, Stewart DL, McFall T, Booth GM, Lee ML, Tedjamulia M, Castle RN. Microbial mutagenecity of isomeric two-, three-, and four-ring amino polycyclic aromatic hydrocarbons. Environ Mutagenesis. 1984;6:497–515. doi: 10.1002/em.2860060404. [DOI] [PubMed] [Google Scholar]
- 10.Yu MC, Skipper PL, Tannebaum SR, Chan KK, Ross RK. Arylamine exposure and bladder cancer risk. Mutat Res. 2002;506–507:21–28. doi: 10.1016/s0027-5107(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 11.Benigni R, Passerini L. Carcinogenicity of the aromatic amines: from structure-activity relationship to mechanisms of action and risk assessment. Mutat Res. 2002;511:191–206. doi: 10.1016/s1383-5742(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 12.Howard PC, Beland FA, Cerniglia CE. Reduction of the carcinogen 1-nitropyrene to 1-aminopyrene by rat intestinal bacteria. Carcinogenesis. 1983;4:985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- 13.Fu PP. Metabolism of nitropolycyclic aromatic hydrocarbons. Drug Metabolism Rev. 1990;22:209–268. doi: 10.3109/03602539009041085. [DOI] [PubMed] [Google Scholar]
- 14.Silvers KJ, Couch LH, Rorke EA, Howard PC. Role of nitroreductases but not cytochromes P450 in the metabolic activation of 1-nitropyrene in the HepG2 human hepatoblastoma cell line. Biochem Pharmacol. 1997;54:927–936. doi: 10.1016/s0006-2952(97)00268-2. [DOI] [PubMed] [Google Scholar]
- 15.Manning BW, Cerniglia CE, Federle TW. Biotransformation of 1-nitropyrene to 1-aminopyrene and N-formyl-1-aminopyrene by the human intestinal microbiota. J Toxicol Environ Health. 1986;18:339–346. doi: 10.1080/15287398609530875. [DOI] [PubMed] [Google Scholar]
- 16.El-Bayoumy K, Sharma C, MLY, Reddy B, Hecht SS. The Intestinal Microflora in the Metabolic Reduction of 1-Nitropyrene to 1-Aminopyrene in Conventional and Germ-Free Rats and in Humans. Cancer Lett. 1983;19:311–316. doi: 10.1016/0304-3835(83)90100-3. [DOI] [PubMed] [Google Scholar]
- 17.Heflich RH, Howard PC, Beland FA. 1-Nitrosopyrene: An intermediate in the metabolic activation of 1-nitropyrene to a mutagen in Salmonella typhimurium TA1538. Mutat Res. 1985;149:25–32. doi: 10.1016/0027-5107(85)90005-3. [DOI] [PubMed] [Google Scholar]
- 18.Herreno-Saenz D, Evans FE, Beland FA, Fu PP. Identification of two N2-deoxyguanosinyl DNA adducts upon nitroreduction of the environmental mutagen 1-nitropyrene. Chem Res Toxicol. 1995;8:269–277. doi: 10.1021/tx00044a600. [DOI] [PubMed] [Google Scholar]
- 19.Kadlubar FF, Fu PP, Jung H, Shaikh AU, Beland FA. The metabolic N-oxidation of carcinogenic arylamines in relation to nitrogen charge density and oxidation potential. Environ Health Perspect. 1990;87:233–236. doi: 10.1289/ehp.9087233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu PP, Herrero-Saenz D, von Tungeln LS, Hart RW, Lin S-D. DNA adducts and carcinogenicity of nitro-polycyclic aromatic hydrocarbons. Polycyclic Aromatic Compounds. 1994;6:71–78. doi: 10.1289/ehp.94102s6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu PP, Herreno-Saenz D. Nitro-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. Environ Carcin & Ecotox Rev. 1999;C17:1–43. [Google Scholar]
- 22.Fu PP, Qui FY, Jung H, von Tungeln LS, Zhan DJ, Lee MJ, Wu YS, Heflich RH. Metabolism of isomeric nitrobenzo[a]pyrenes leading to DNA adducts and mutagenesis. Mutat Res. 1997;376:43–51. doi: 10.1016/s0027-5107(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 23.Howard PC, Heflich RH, Evans FE, Beland FA. Formation of DNA adducts in vitro and in Salmonella typhimurium upon metabolic reduction of the environmental mutagen 1-nitropyrene. Cancer Res. 1983;43:2052–2058. [PubMed] [Google Scholar]
- 24.Gu Z, Gorin A, Krishnasamy R, Hingerty BE, Basu AK, Broyde S, Patel DJ. Solution structure of the N-(deoxyguanosin-8-yl)-1-aminopyrene ([AP]dG) adduct opposite dA in a DNA duplex. Biochemistry. 1999;38:10843–10854. doi: 10.1021/bi9912138. [DOI] [PubMed] [Google Scholar]
- 25.Malia SA, Vyas RR, Basu AK. Site-specific frame-shift mutagenesis by the 1-nitropyrene-DNA adduct N-(deoxyguanosin-8-yl)-1-aminopyrene located in the (CG)3 sequence: effects of SOS, proofreading, and mismatch repair. Biochemistry. 1996;35:4568–4577. doi: 10.1021/bi9525132. [DOI] [PubMed] [Google Scholar]
- 26.Melchior WB, Marques MM, Beland FA. Mutations induced by aromatic amine DNA adducts in pBR322. Carcinogenesis. 1997;18:1949–54. doi: 10.1093/carcin/15.5.889. [DOI] [PubMed] [Google Scholar]
- 27.Strniste GF, Nickols JW, Okinaka RT. Photochemical oxidation of 2-aminofluorene: Correlation between the induction of direct-acting mutagenicity and the formation of nitro and nitroso aromatics. Mutat Res. 1985;151:15–24. doi: 10.1016/0027-5107(85)90177-0. [DOI] [PubMed] [Google Scholar]
- 28.Okinaka RT, Nickols JW, Strniste GF, Whaley TW. In: Polynuclear Aromatic Hydrocarbons: Chemistry, Characterization and Carcinogenesis. Dennis AJ, editor. Bartelle Press; Columbus: 1986. pp. 717–728. [Google Scholar]
- 29.Okinaka RT, Nickols JW, Whaley TW, Strniste GF. Phototransformation of 2-aminofluorene into N-oxidized mutagens. Carcinogenesis. 1984;5:1741–1743. doi: 10.1093/carcin/5.12.1741. [DOI] [PubMed] [Google Scholar]
- 30.Okinaka RT, Whaley TW, Hollstein U, Nickols JW, Strniste GF. In: Polynuclear Aromatic Hydrocarbons: A Decade of Progress. Dennis AJ, editor. Bartelle; Columbus: 1988. pp. 661–671. [Google Scholar]
- 31.Okinaka RT, Nickols JW, Whaley TW, Strinste GF. 1-Nitropyrene: a mutagenic product induced by the action of near ultraviolet light on 1-aminopyrene. Mutat Res. 1986;173:93–98. doi: 10.1016/0165-7992(86)90083-7. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BT. In: Microscale Testing in Aquatic Toxicology: Advances, Techniques, and Practice. Wells PG, Lee K, Blaise C, editors. CRC Press; Boca Raton, FL: 1998. pp. 201–218. [Google Scholar]
- 33.Hwang H-M, Shi X, Ero I, Jayasinghe A, Dong S, Yu H. Microbial ecotoxicity and mutagenicity of 1-hydroxypyrene and its photoproducts. Chemosphere. 2001;45:445–451. doi: 10.1016/s0045-6535(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 34.Sun TSC, Stahr HM. Evaluation and application of a bioluminescent bacterial genotoxicity test. J AOAC Int. 1993;76:893–898. [PubMed] [Google Scholar]
- 35.Microbics. Microbics Mutatox™, Manual Genotoxicity Test System. Azur Environmental; UK: 1995. [Google Scholar]
- 36.Zhou L, Cho BP. Synthesis, characterization, and comparative conformational analysis of N-(deoxyguanosin-8-yl)-1-aminopyrene adducts derived from the isomeric carcinogens 1-, 2-, and 4-nitropyrene. Chem Res Toxicol. 1998;11:35–43. doi: 10.1021/tx970115p. [DOI] [PubMed] [Google Scholar]
- 37.Dong S, Hwang HM, Shi X, Holloway L, Yu H. UVA-induced DNA single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem Res Toxicol. 2000;13:585–593. doi: 10.1021/tx990199x. [DOI] [PubMed] [Google Scholar]
- 38.Morel FMM, Hering JG. Principles and Applications of Aquatic Chemistry. John Wiley and Sons, Inc; New York: 1993. [Google Scholar]
- 39.Dong S, Hwang HM, Harrison C, Holloway L, Shi X, Yu H. UVA light-induced DNA cleavage by selected polycyclic aromatic hydrocarbons. Bull Environ Contam Toxicol. 2000;64:467–474. doi: 10.1007/s001280000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King LC, Kohan MJ, Ball LM, Lewtas J. Mutagenicity of 1-nitropyrene metabolites from lung S9. Cancer Lett. 1984;22:255–262. doi: 10.1016/0304-3835(84)90160-5. [DOI] [PubMed] [Google Scholar]
- 41.Oyama M, Higuchi T, Okazaki S. Mechanistic discrimination of the reaction of 1-aminopyrene cation radical using an electron transfer stopped-flow method. Decay reaction accelerated by neutral molecules. Electrochem Comm. 2001;3:363–366. [Google Scholar]
- 42.Mao B, Vyas RR, Hingerty BE, Bryode S, Basu AK, Patel DJ. Solution conformation of the N-(deoxyguanosin-8-yl)-1-aminopyrene ([AP]dG) adduct opposite dC in a DNA duplex. Biochemistry. 1996;35:12659–12670. doi: 10.1021/bi961078o. [DOI] [PubMed] [Google Scholar]
- 43.El-Bayoumy K, Revenson A, Johnson B, DiBello J, Little P, Hecht SS. Comparative Tumorigenicity of 1-nitropyrene, 1-nitrosopyrene, and 1-aminopyrene administered by gavage to sprague-dawley rats. Cancer Res. 1988;48:4256–4260. [PubMed] [Google Scholar]
- 44.Lafi A, Parry JM. Chromosome aberrations induced by nitro-, nitroso, and amino-pyrene in cultured Chinese hamster cells. Mutagenesis. 1987;2:23–26. doi: 10.1093/mutage/2.1.23. [DOI] [PubMed] [Google Scholar]
- 45.Heflich RH, Fullerton NF, Beland FA. An examination of the weak mutagenic response of 1-nitropyrene in Chinese hamster ovary cells. Mutat Res. 1986;161:99–108. doi: 10.1016/0027-5107(86)90104-1. [DOI] [PubMed] [Google Scholar]