Abstract
According to the traditional view, atherosclerosis results from a passive buildup of cholesterol in the artery wall. Yet, burgeoning evidence implicates inflammation and immune effector mechanisms in the pathogenesis of this disease. Both innate and adaptive immunity operate during atherogenesis and link many traditional risk factors to altered arterial functions. Inflammatory pathways have become targets in the quest for novel preventive and therapeutic strategies against cardiovascular disease, a growing contributor to morbidity and mortality worldwide. Here we review current experimental and clinical knowledge of the pathogenesis of atherosclerosis through an immunological lens and how host defense mechanisms essential for survival of the species actually contribute to this chronic disease but also present new opportunities for its mitigation.
Introduction
Cardiovascular disease (CVD) has become the most frequent cause of death globally (Murray et al., 2012). An estimated 17.3 million people die from CVD every year, equivalent to 30% of all deaths worldwide. Low- and middle-income countries currently suffer disproportionally, with more than 80% of all CVD deaths occurring in such countries. In Western Europe and North America, CVD mortality has declined due—at least in part—to successful prevention and therapy, but it still remains the most common cause of deaths in developed countries. Although many diseases affect the cardiovascular system, myocardial infarction (MI) and ischemic stroke caused by atherosclerosis dominate death and disability statistics for all regions of the world.
Atherosclerosis affects large and medium-sized arteries. By causing thrombosis or severe stenosis in arteries, it leads to MI, ischemic stroke, ischemic damage to kidneys and intestines, and many other life-threatening clinical manifestations (Libby, 2013). Before becoming clinically evident, the atherosclerotic process progresses silently for years. Medical scientists therefore must not only address clinically manifest atherosclerotic cardiovascular disease but also identify targets for prevention and early treatment in apparently healthy individuals incubating this insidious disease. Both these tasks require an understanding of the pathogenesis of atherosclerosis.
According to the traditional view, atherosclerosis results from a passive buildup of cholesterol in the artery wall. Accumulating evidence implicates inflammation in the pathogenesis of atherosclerosis, subject to major modulation by immune effector mechanisms (Libby et al., 2011). A century ago, landmark experiments by the Russian pathologist Nikolai Anitschkow showed that cholesterol causes atherosclerosis in rabbits. Epidemiological studies later identified an association between plasma cholesterol concentrations and ischemic heart disease in humans. Further research on cholesterol metabolism enabled the preventive programs that now avert thousands of deaths worldwide (Libby et al., 2000). In particular, the introduction of statins, a class of lipid-lowering drugs, has helped reduce the incidence of MI by about a third (Mihaylova et al., 2012). Emerging clinical evidence casts considerable doubt on interventions that target lipid fractions other than low-density lipoprotein (LDL), notable high-density lipoprotein (HDL, “good” cholesterol), or triglycerides. Hence, pathogenic components beyond the lipid profile have assumed major interest in therapeutic attempts to address the substantial residual risk that persists in the “statin era.”
Inflammation, encompassing both innate and adaptive immunity, characterizes atherogenesis and links many traditional risk factors to altered function of arteries. Inflammatory pathways have become the focus of intense research in the quest for novel preventive and therapeutic strategies against CVD. Technological breakthroughs have offered excellent opportunities for experimental studies with genetically engineered mice that develop hypercholesterolemia and atherosclerosis, such as mouse strains deficient in apolipoprotein E (Apoe−/−) or the LDL receptor (Ldlr−/−). Technical advances have also permitted identification of genes contributing to atherosclerotic cardiovascular disease in humans, most recently by using genome-wide association studies (GWASs) (Deloukas et al., 2013). Large clinical trials and studies of population cohorts have also permitted the evaluation of biomarkers and pathogenic factors in human disease (Emerging Risk Factors Collaboration, 2012).
We review here current knowledge of the pathogenesis of atherosclerosis through an immunological lens—first providing insights from mice and then highlighting the gap between the elegant experimental work in this species and the translation to human disease. We then consider how host defense mechanisms essential for survival actually contribute to chronic diseases that threaten longevity such as atherosclerosis, and we discuss the therapeutic perplex this paradox presents.
Macrophages, Pattern Recognition, and Cholesterol Accumulation
Collections of lipid-laden macrophages characterize the atherosclerotic plaque. When plasma concentrations of cholesterol rise in experimental animals, cholesterol-containing lipoprotein particles infiltrate the artery wall and tarry there as a result of interactions with proteoglycans of the extracellular matrix. Local cellular responses prompt expression of leukocyte adhesion molecules on the endothelial lining of the artery, as well as expression of chemokines by the vascular cells (Mestas and Ley, 2008; Weber and Noels, 2011). These molecular stimuli recruit circulating monocytes to enter the innermost layer of the artery (the tunica intima), where they differentiate into macrophages upon stimulation by mediators such as monocyte-colony stimulating factor (M-CSF) produced locally (Clinton et al., 1992). The accumulation of mononuclear phagocytes in the atherosclerotic lesion depends not only on their influx, mediated by adhesion molecules and chemoattractants, but also on retention within the plaque. Recent work shows that netrin-1, a molecule implicated in neuronal guidance produced in plaque macrophages, acts in a paracrine or autocrine manner to retain inflammatory cells in the atheroma, acting by ligation of its receptor, UNC5b (Swirski et al., 2012; van Gils et al., 2012).
Macrophage differentiation associates with increases in scavenger receptors (SRs) on the cells. These pattern recognition receptors (PRRs) include the transmembrane proteins SR-A (CD204), CD36, MARCO, and LOX-1 (OLR-1), which mediate internalization of a broad range of molecules and particles such as endotoxins, apoptotic bodies, parasite components, and LDL particles. SRs can bind lipoprotein particles after they undergo oxidative modification locally in the arterial wall. Mononuclear phagocytes can also take up cholesterol by micropinocytosis of native LDL and phagocytosis of aggregated LDL (Kruth, 1997, 2002). Eventually, cholesterol entry can overwhelm the cell’s capacity for eliminating these molecules and cholesterol starts to accumulate as cholesteryl ester droplets in the cytosol. With time, the macrophages become overloaded with cholesteryl ester droplets, taking on the characteristic “foam cell” appearance. Many of these lipid-engorged phagocytes eventually die, leading to accumulation of apoptotic bodies and necrotic debris in the lesion, forming a “necrotic” core in the evolving atheromatous lesion. Components of this detritus can promote plaque progression and are prothrombotic. Impaired clearance of such apoptotic debris (impaired efferocytosis) may thus contribute to growth and thrombotic complications of atherosclerotic lesions (Moore and Tabas, 2011).
LDL Oxidation and Innate Immunity in Atherosclerosis
As oxidation confers on component LDL the ability to bind to PRRs and favors foam-cell formation, much interest has focused on LDL oxidation as a therapeutic target. Reactive oxygen species (ROS) primarily attack double bonds in unsaturated fatty-acid residues of triglycerides, phospholipids, and cholesteryl esters. This process produces reactive molecular species that can form adducts on polypeptide chains (e.g., malondialdehyde) that typically generate crosslinks between free amino groups of Lys and Arg residues. Such posttranslational changes of proteins alter their functional and immunogenic properties (Miller et al., 2011; Lichtman et al., 2013).
Some of the truncated lipids remaining after release of such aldehydes have substantial biological activity. Thus, phosphorylcholine species generated after oxidative attack on phosphatidylcholine can activate endothelial cells to express leukocyte adhesion molecules (Prescott et al., 2002). In addition, both phosphorylcholine species and adduct-containing peptide sequences may elicit antibody responses (see below).
Several enzymes can catalyze LDL oxidation. Blood activities of some of these enzymes, including myeloperoxidase and extracellular types of phospholipase A2 (sPLA2, Lp-PLA2), associate with coronary artery disease (CAD) in some studies (Quehenberger and Dennis, 2011). Pharmacologic inhibition or genetic targeting of these enzymes reduces atherosclerosis in mice. Yet, these enzymes have multiple effects, complicating the interpretation of such experiments. For example, PLA2 can exert platelet-activating factor acetylhydrolase activity that degrades this prothrombotic and proinflammatory substance.
Nonenzymatic oxidants such as copper ions can also oxidize LDL. Yet, endogenous antioxidants and buffering mechanisms for transition metal ions render such reactions unlikely to occur in blood. Attempts at preventing CAD events by antioxidant administration have proven disappointing. Although some animal experiments and small-scale clinical trials showed promise, large clinical trials with vitamin C and/or vitamin E, folate, carotenoids, and a small molecule antioxidant drug have not confirmed these benefits (Tardif et al., 2008). Although LDL oxidation likely occurs in the atherosclerotic plaque, these processes have not to date proven suitable as therapeutic targets.
Cholesterol Accumulation Activates the Inflammasome
When cholesterol accumulates in macrophages, crystals can form. Cholesterol crystal formation in macrophages depends on uptake, the intracellular balance between free cholesterol and cholesterol ester, and cholesterol efflux to extracellular acceptors such as apolipoproteins E or AI (Kellner-Weibel et al., 1999). Such intracellular microcrystals can activate a member of the cytosolic-nucleotide binding domain and leucine-rich repeat gene family (NLRP3), activating its associated inflammasome, and promote processing of the proform of interleukin-1β (IL-1β) into a bioactive cytokine (Duewell et al., 2010; Rajamäki et al., 2010). Just as uric acid crystals in joints can cause gout, cholesterol crystals in plaques can incite inflammation in the artery wall. Genetic targeting of either IL-1β or a component in the NLRP3 inflammasome reduces atherosclerosis in mice, supporting a pathogenic role for this pathway of inflammatory activation (Duewell et al., 2010). Thus, inflammasome activation provides a possible pathogenic pathway that links cholesterol accumulation to the chronic inflammatory process of atherosclerosis.
Pattern-Recognition Receptor Activation in Atherosclerosis
Several types of PRRs may participate in atherogenesis. Beyond cytosolic NOD-like receptors, components of inflammasomes, cells in human atheromata express Toll-like receptor (TLR) family members. Several experiments that use gene-targeted mice have addressed the role of TLRs in atherogenesis, yielding rather complex results (Curtiss and Tobias, 2009). TLR2 and TLR4 appear proatherogenic, whereas TLR3, TLR7, and TLR9 may protect against atherosclerosis in mice. TLR2 presents a particularly complex situation because its expression on vascular endothelium appears to promote atherosclerosis, whereas it has protective effects when expressed by myeloid cells (Mullick et al., 2008). Similarly, it is unclear why cell surface exposed TLR2 and TLR4 are proatherogenic, whereas TLR3, TLR7, and TLR9—all of which reside on intracellular membranes—protect against experimental atherosclerosis (Salagianni et al., 2012). MyD88 functions as an adaptor protein for all TLRs (except TLR3) and of the IL1 signaling receptor. Activation of MyD88 via TLR ligation ultimately unleashes the NF-κB pathway that orchestrates the expression of many inflammatory agonists implicated in atherogenesis. Targeted deletion of MyD88 shows a much more pronounced effect of MyD88 on atherosclerosis than interruption of TLR4 itself (Michelsen et al., 2004). This result implies that TLR4-independent pathways for MyD88 activation also contribute substantially to atherogenesis. Furthermore, selective MyD88 deletion in dendritic cells (DCs) enhanced atherosclerosis, reflecting a requirement or TLR signaling in these antigen-presenting cells (APCs) to induce atheroprotection mediated by regulatory T cells (Subramanian et al., 2013).
The distinct effects of different TLRs may relate to their roles in host defense. Several molecules and particles present in the extracellular space may ligate TLR4, including oxidized LDL particles and heat shock protein 60 (Hsp60) (Bae et al., 2009; Cohen-Sfady et al., 2005). The ligands of intracellular TLRs include endogenous and pathogen-derived nucleic acids, and certain lipids implicated in atherogenesis, including free fatty acids.
Genetic studies have addressed directly the role of TLR4 in human cardiovascular disease. Point mutations at Asp299Gly and Thr399***Ile may render TLR4 less effective at binding lipopolysaccharide (LPS), in turn causing hyporesponsiveness to endotoxins. Initial reports linked these mutations to less cardiovascular disease, suggesting that TLR4 signaling promotes atherosclerosis in humans. Subsequent studies, however, did not show that these variants protect against MI, and some observations even suggested a disease-promoting effect of a hyporesponsive TLR4 allele, as summarized in a recent metaanalysis (Zhang et al., 2012). Thus, although mouse experiments point to the operation of PRRs in atherosclerosis, their importance in human disease remains unclear.
Heterogeneity of Mononuclear Phagocytes in Atherosclerosis
The foregoing discussion presumes that all mononuclear phagocytes serve similar functions. Recent results, however, provide strong evidence to the contrary. The phosphatidylinositol-linked cell surface protein known as Ly6c or Gr-1 distinguishes two major subpopulations of mouse monocytes (Table 1). Normal mice, and those that bear mutations rendering susceptibility to diet-induced atherosclerosis, have under basal conditions relatively few circulating monocytes, approximately evenly distributed between the Ly6chi and Ly6clo populations. After initiating an atherogenic diet, the blood of hypercholesterolemic mice shows a dramatic increase in Ly6chi monocytes (Swirski et al., 2007) (Tacke et al., 2007). This monocyte subpopulation exhibits a series of functions that would render them particularly pathogenic in the context of a chronic inflammatory disease such as atherosclerosis. For example, these cells bind with high avidity to activated endothelial monolayers. They express higher amounts of proinflammatory cytokines and proteases implicated in the pathogenesis of atherosclerosis than their Ly6clo counterparts.
Table 1.
Roles of Selected Cells, Mediators, and Biomarkers of Immune Mechanisms in Atherosclerosis
| Role in Mouse Atherosclerosis | Role in Human Atherosclerosis | Comments | |
|---|---|---|---|
| Cell Type | |||
| CD4+ Helper T Cells | IFN-γ-producing Th1
cells predominate in lesions and enhance lesion development |
IFN-γ-producing cells and
dual IFN-γ+ IL-17-producing cells frequent in lesions |
Polarized Th cell subsets less distinct in humans; effect of Th2 and Th17 cells is unclear |
| CD8+ Cytotoxic T Lymphocytes | Relatively rare | Present but less frequent
than CD4+ Th cells |
May increase when T cell inhibitory pathways are impaired; overall proatherogenic |
| Regulatory T (Treg) cells | CD25+ Foxp3+ Treg
cells atheroprotective; present but less frequent as lesions progress |
CD25+ Foxp3+ Treg cells
present but rare in advanced lesions |
CD25 relation to Foxp3 less constant in humans than in mice |
| B1 Lymphocytes | Make natural antibodies specific for Ox-LDL, which may be atheroprotective |
? | |
| B2 Lymphocytes | Found in adventitia of atherosclerotic arteries |
? | Can be proatherogenic,
perhaps independent of antibody secretion |
| Monocytes | Proinflammatory
Ly6Chi Gr-1+ cells home to developing lesions and presumably furnish lesional macrophages |
Human CD14hi
CD16− proinflammatory monocyte presumed source of lesinal macrophages |
Human and mouse subsets not clearly comparable; precursors of M1 macrophages not clear |
| Macrophages | Major inflammatory cell in lesions; mainly M1 slanted |
Major inflammatory cell in lesions; mainly M1 slanted |
M1-M2 dichotomy not always distinct, especially in humans |
| Granulocytes | May be involved very early in lesion development |
No clear evidence of presence in or role in development of lesions |
Can accumulate in thrombi |
| Mast Cells | Contribute to lesion development |
Participation in human plaques less clear than in mice |
|
|
| |||
| Biomarker | |||
| High-sensitivity CRP (hsCRP) | Expressed slightly, or not at all, in mice |
High hsCRP is a risk marker
for clinical disease |
Basal blood CRP levels
reflect integrated overall inflammatory burden; CRP likely not directly involved in atherogenesis or lesion complications |
| Anti-oxLDL antibodies | Levels correlate with degree
of hypercholesterolemia and lesion burden |
Levels correlate with degree of hypercholesterolemia and clinical disease |
Some anti-oxLDL antibodies may be atheroprotective |
| Anti-oxHsp60 antibodies | Levels correlate with degree
of hypercholesterolemia and lesion burden |
Levels correlate with degree of hypercholesterolemia and clinical disease |
|
Cells derived from this proinflammatory population of monocytes appear to home to nascent atheromata in mice in a manner that depends on the chemokine receptor CCR2 (CD192)—the major ligand of which, monocyte chemoattractant protein-1 (MCP-1), abounds in human and murine atheromata. Hypercholesterolemia induces a Ly6chi monocytosis not only in peripheral blood but also in the spleen. Indeed, hypercholesterolemic mice have an expanded population of Ly6chi monocytes in a subcapsular pool in the splenic red pulp (Swirski et al., 2009). These proinflammatory monocytes arise from extramedullary hematopoiesis in this locale. IL-3 and granulocyte monocyte-CSF (GM-CSF) appear to drive expansion of bonemarrow-derived macrophage DC precursors in the spleen (Robbins et al., 2012). Endoplasmic reticulum stress related to intracellular lipid accumulation in leukocytes may also contribute to extramedullary hematopoiesis in the spleen of hypercholesterolemic mice (Westerterp et al., 2012). Proinflammatory monocytes of splenic origin comprise up to a quarter of the mononuclear phagocytes in mouse atheromata (Robbins et al., 2012).
Systemic inflammation or “stress” mediated by the sympathetic nervous system (in particular, beta-3 adrenergic stimulation) can mobilize bone marrow progenitor cells to the spleen, where they propagate to form a pool of proinflammatory monocytes. This observation has particular relevance to acute MI, a condition characterized by adrenergic activation due to pain and anxiety. Cells from this splenic population home to sites of acute injury, such as infarcts of the heart or brain, as well as to sites of chronic inflammation, such as atheroma in mice (Dutta et al., 2012). The human monocyte population that corresponds to the Ly6chi population in mice remains controversial, but the CD14+ CD16− population in humans may subserve this function.
A body of literature identifies a predominance of the M1 subpopulation of macrophages in atheromata (Johnson and Newby, 2009; Stöger et al., 2012). These classically activated macrophages can arise from monocytes in response to the cytokine interferon-γ (IFN-γ) combined with a TLR stimulus and elaborate mediators associated with the progression and complication of atherosclerosis. Their less inflammatory counterparts, the M2 macrophages, may in contrast elaborate mediators that mitigate atherosclerosis. Whether the Ly6chi population of monocytes preferentially gives rise to M1 macrophages remains incompletely substantiated. The polarization between monocyte and macrophage subtypes in humans appears much hazier in humans than in mice, raising a note of caution in facile extrapolations from murine atherosclerosis to the human disease (Table 1; Raes et al., 2005).
Mast Cells in Atherosclerosis
Mast cells do accumulate in human atheromata, particularly at sites of plaque rupture. These cells, classically involved in allergy and defense against parasites, also exhibit functions that could contribute to atherogenesis in vitro (Kovanen, 2007). The conjectures regarding their causal role in aspects of atherogenesis remained unsubstantiated until recently. Both pharmacologic and genetic approaches currently support a contribution of mast cells to experimental atherosclerosis in mice (Sun et al., 2007; Bot et al., 2007). In mice with genetic deficiency of mast cells, adoptive transfer experiments that reconstituted mast cell populations from mice with deficiencies in particular cytokines point to proatherogenic roles of mast-cell-derived IFN-γ and IL-6 in the pathogenesis of atherosclerosis. The signature proteinases of mast cells including chymase and tryptase may also participate in aspects of atherogenesis.
The Participation of Granulocytes in Atherosclerosis
Observations in human atheromata show relatively few polymorphonuclear leukocytes in undisrupted lesions. After plaques rupture or erode, the thrombi that form in consequence can entrap granulocytes and may form neutrophil extracellular traps (NETS) (Megens et al., 2012). These cells release very high amounts of ROS and the pro-oxidant enzyme myeloperoxidase. Thus, the arrival of granulocytes on the scene of a disrupted plaque could aggravate local oxidative stress and amplify plaque progression. Some mouse experiments provide support for earlier involvement of polymorphonuclear leukocytes in murine atherosclerosis (Drechsler et al., 2010; Weber et al., 2008). The degree to which these mouse experiments apply to human atherogenesis remains uncertain.
Platelets as a Source of Inflammatory Mediators
Conventional concepts relegate platelets to a role in thrombus formation. Ample experimental and clinical data substantiate platelet participation in thrombosis. Yet, these particles also contain abundant preformed proinflammatory mediators including CD40 ligand (CD154), RANTES, and IL-6. Thus, platelets constitute small “cluster bombs” of mediators of innate immunity (Croce and Libby, 2007). Upon activation—for example, by thrombin—platelets spew forth these proinflammatory proteins in copious amounts, also amplifying local inflammation at sites of thrombus formation (Lievens and von Hundelshausen, 2011). In addition, platelets modulate T cell activation and may therefore also impact adaptive immunity in atherosclerotic lesions (Gerdes et al., 2011).
Complement Causes Controversy
Complement activation occurs in the atherosclerotic plaque. Cleavage fragments of the complement cascade localize in lesions, and terminal complement complexes form at sites of lipid accumulation even in very early stages of disease (Seifert et al., 1989). These findings prompted the question of whether complement activation contributes to disease development. Experimental studies have yielded contradictory results, however, with some suggesting a surprising, protective role for complement activation through the classical and lectin pathways (Torzewski and Bhakdi, 2013). Interestingly, the C3 cleavage fragment C3a-desArg has important metabolic effects in adipose tissue, where it promotes triglyceride synthesis. C3 activation may therefore affect atherosclerosis by modulating lipid metabolism, as well as vascular inflammation (Lewis et al., 2010).
GWASs have identified the complement factor C1q receptor C1qRp (CD93) as a genetic risk factor for human cardiovascular disease, and smaller genetic studies have associated deficiencies in mannose-binding lectin (MBL) with increased coronary and carotid artery disease. In prospective biomarker studies, circulating concentrations of C1qRp, C3, C4, and C5a predicted risk for future cardiovascular events. Thus, at least some of the consequences of complement activation in plaque may be atheroprotective rather than proatherosclerotic. The mechanisms involved remain unclear.
Dendritic Cells Link Innate and Adaptive Immune Responses
DCs derived from bone marrow precursors of the myeloid lineage express many different PRRs, and populate most tissues where they serve as sentinels of infection and injury. The intima and adventitia of normal mouse and human arteries contain DCs, and their numbers increase in atherosclerotic lesions (Bobryshev, 2010; Cybulsky and Jongstra-Bilen, 2010; Randolph and Potteaux, 2010). DCs can internalize oxLDL and become foam cells in vivo, and thus these cells likely participate in inflammatory responses in early lesions (Packard et al., 2008). DCs activated as part of an innate immune response in tissues increase expression of the chemokine receptor CCR7, as well as major histocompatibility complex (MHC) and costimulatory molecules—changes that favor their migration into lymphoid tissues and presentation of peptide antigens to naive T cells. Therefore, recognition of PRR ligands primes DCs to initiate T cell responses, a condition that likely pertains to proatherogenic T cell responses (see below). In the absence of innate stimuli, so-called “immature” tissue DCs likely migrate constitutively to lymphoid tissues and present healthy tissue antigens for recognition by naive recirculating self-antigen-specific T cells. The encounter of naive T cells with these immature DCs, which express few costimulatory molecules, leads to T cell death, anergy, or skewed differentiation toward a regulatory program—all consequences that promote T cell tolerance to self. Given the potential catastrophic consequences of active T cell responses to antigens (self or foreign) in the arterial wall, vascular DCs likely normally function to maintain tolerance to vascularwall antigens. This tolerogenic function of DCs provides a path to immunotherapy of atherosclerosis, discussed later.
Clinical Observations Support a Role for Innate Immunity in Human Atherosclerosis
The study of inflammation in human atherosclerosis has lagged behind animal work due to obvious barriers. Human studies do not permit the experimental manipulations that have enabled routine exploration of mechanisms in mice—hence the need to turn to indirect approaches to test hypotheses regarding the role of inflammation in human atherosclerosis. The application of biomarkers of inflammation has proven enormously informative in this regard (Table 1). In particular, the pentraxin C-reactive protein (CRP), an acute phase reactant, has proven particularly useful as a biomarker of inflammation in clinical studies (Libby et al., 2011). Classic inflammatory diseases such as acute infections and autoimmune diseases such as rheumatoid arthritis associate with elevated concentrations of this primarily hepatocyte-derived protein.
A wealth of studies have now established that more modest increases in CRP, measured with a high-sensitivity assay (denoted hsCRP), predict first and recurrent cardiovascular events in a way that adds prognostic information to conventional risk biomarkers (Emerging Risk Factors Collaboration, 2012;Kaptoge et al., 2010; Wennberg et al., 2012). Hepatocytes synthesize CRP in response to stimulation by IL-6. Although probably not a causal risk factor for atherosclerosis like LDL, CRP serves as a useful biomarker of inflammatory status. CRP lacks diurnal variation, has a relatively long half-life, and has an inexpensive and reliable assay. Thus, this integrated marker of inflammation has furnished an informative window on the relationship between inflammation and cardiovascular events, even though it may not itself mediate disease.
CRP elevations above the median not only foretell future cardiovascular events such as MI or stroke, but also identify a group of individuals who benefit from statin therapy despite having average or below average blood concentrations of LDL cholesterol (Ridker et al., 2008). The use of CRP provided clinical validation of the concept, which emerged from laboratory investigations that statins exhibit direct anti-inflammatory effects, independent of their LDL-lowering properties (Ridker et al., 2009). Considerable evidence supports anti-inflammatory actions of the statin class of drugs attributable to impaired prenylation of several cellular targets, including small G proteins such as Ras, Rac, or Rho (Bu et al., 2011). Statins also augment the expression of transcription factors such as Krüppel-like factor-2, which promotes the transcription of a cassette of genes that can exert antiatherosclerotic and antithrombotic properties (Sen-Banerjee et al., 2005).
Large clinical trials currently underway will test the proposition that direct anti-inflammatory interventions that do not affect LDL concentrations can prevent recurrent events in individuals who have sustained a MI. The interventions currently under evaluation include methotrexate in low weekly doses and a monoclonal antibody that neutralizes the proinflammatory cytokine IL-1β (Everett et al., 2013; Ridker et al., 2011). IL-1 markedly stimulates IL-6 gene expression, the driver of hepatic CRP production. In addition, inflammasome activation by cholesterol crystals within plaques provides a stimulus to local production of active IL-1β, providing additional rationale for this therapeutic intervention. Two other large clinical trials will test whether administration of an inhibitor of lipoprotein-associated phospholipase A2 (LpPLA2), a potential generator of proinflammatory lipids in plaques, will improve outcomes of patients with CAD (O’Donoghue et al., 2011; White et al., 2010). While this LpPLA2 inhibitor attenuated inflammation in pig atheromata (Wilensky et al., 2008), the agent did not lower CRP in a phase II trial, raising questions regarding its anti-inflammatory action in humans (Serruys et al., 2008). A smaller clinical trial targeting a soluble phospholipase apparently did not meet its primary endpoint (Nicholls et al., 2012).
Atherosclerosis as a T Cell–Driven Disease
Several lines of evidence point to the operation of adaptive immunity in human atherosclerosis. Plaques contain T cells, including some that bear markers of activation (Hansson and Jonasson, 2009; Jonasson et al., 1985, 1986), and autoantibodies to LDL circulate in atherosclerotic patients (Hartvigsen et al., 2008). The rapid occurrence of arteriopathy in human hearts transplanted across major histocompatibility barriers, sometimes in the absence of classical risk factors for atherosclerosis, underscores the potential of alloimmune responses to produce intimal disease in humans that recapitulates some features of atherosclerosis (Libby, 2012; Salomon et al., 1991). Such observations led to the hypothesis that components of adaptive immunity aggravate and modulate disease development. Subsequent studies in animals, in particular the gene-targeted mouse, support this hypothesis and offer insights into an intricate and nuanced network of immune regulation in atherogenesis (Mallat et al., 2008).
The advanced human atherosclerotic plaque contains T cells, largely effector-memory cells of which a substantial proportion display signs of activation. About two-thirds of human plaque T cells bear CD4 and the balance CD8, whereas CD4+ T cells dominate in atherosclerotic mice. T cells of human lesions exhibit a T helper-1 (Th1) cell-associated cytokine secretion pattern, including IFN-γ and tumor necrosis factor (TNF), and mouse experiments have demonstrated important proatherogenic effects of Th1 cells and their cytokines (Frostegård et al., 1999). As in other conditions, T cells of human atherosclerotic plaques do not show the same degree of polarization as those of inbred mouse strains, but Th1 cytokines do prevail and arise from a larger number of T cells in human plaques than do Th2 cell effector cytokines (in mice, typically IL-4, IL-5, and IL-13) (Table 1).
The signature Th1 cytokine, IFN-γ, promotes atherogenesis in mice. Thus, targeted deletion of IFN-γ or its receptor leads to reduced disease, whereas administration of recombinant cytokine protein aggravates atherosclerosis in hypercholesterolemic mice (Gupta et al., 1997; Whitman et al., 2000). Interference with IFN-γ signaling also blocks allograft arteriopathy in mice (Nagano et al., 1997). Several molecular targets may contribute to such an effect of IFN-γ. It inhibits proliferation and collagen production by vascular smooth muscle cells (SMCs), thus impairing repair processes in the vessel wall and possibly reducing plaque stability (Amento et al., 1991). Furthermore, IFN-γ hampers cholesterol efflux from macrophages and affects scavenger receptor expression. This cytokine also promotes hyperglycemia and increases fat inflammation, conditions thought to enhance the risk of atherosclerotic cardiovascular disease (Rocha et al., 2008).
The role of Th17 cells and their cytokines in atherosclerosis has undergone intense study, yielding mixed results (Cheng et al., 2011; Pober, 2011; Smith et al., 2010). Th17 T cells seem to lack decisive influence on mouse atherosclerosis, in contrast to Th1 cells, although they may modulate lesion formation and composition. In contrast, studies of Th2 cells do not support a major role for this subset in atherosclerosis. Indeed, Th2 cells and their characteristic cytokines may promote formation of arterial aneurysms rather than intimal proliferative lesions characteristic of atherosclerosis (Shimizu et al., 2004).
In humans, a CD28 null subset of CD4+ T cells expands in the setting of chronic inflammatory diseases, cytomegalovirus infection, or advanced age. These cells elaborate many proinflammatory cytokines and exhibit cytotoxicity. CD28 null CD4+ T cells localize in unstable human lesions and, after MI, these cells increase in peripheral blood (Liuzzo et al., 2007). The CD28 null T cells in CAD patients exhibit an oligoclonal pattern of T cell receptor (TCR) gene usage, and many react with HSP60, suggesting they represent a population of effector cells expanded by one or a few antigens. Furthermore, after isolation from blood, these cells resist regulatory T (Treg) cell suppression in vitro. Members of the TNF family signaling system (e.g., OX40) may activate CD28 null T cells (Dumitriu et al., 2012). Yet, evidence of the causal participation of CD28 null T cells in human atherosclerosis remains speculative. Mice do not have an equivalent population of T cells.
Natural killer T (NKT) cells have particular interest for atherosclerosis, as they recognize lipid antigen and localize in lesions both in mice and in humans (albeit at lower abundance). The invariant NKT cell undergoes activation when lipid antigens bound to CD1d ligate its Vbeta14+ TCR. Human atheromata contain cells that display CD1 (Melián et al., 1999). Administration of a model antigen presented via CD1, α-galactosylceramide, significantly accelerates early atherosclerosis in hypercholesterolemic mice. Production of a set of proinflammatory cytokines that include IFN-γ and TNF (Tupin et al., 2004) and expression of MHC molecules and leukocyte adhesion molecules in lesions accompany this aggravation of atherosclerosis. Indeed, CD1d deficiency results in reduced disease in atherosclerosis-prone mice. In accordance with these findings, mice with CD1d-deficient leukocytes display reduced atherosclerosis—suggesting that endogenous lipid antigens, or possibly those derived from the host microbiome, promote atherosclerosis via recognition by NKT cells (VanderLaan et al., 2007). Several ongoing studies aim to identify such molecules.
Counterbalancing Factors in Adaptive Immune Responses in Atherosclerosis
Adaptive immunity can operate during atherogenesis as a “double-edged sword,” exerting both exacerbating and inhibitory influences on lesion development. Two anti-inflammatory cytokines, IL-10 and transforming growth factor-beta (TGF-β), modulate atherogenesis in hypercholesterolemic mice (Mallat et al., 1999; Lutgens et al., 2002). Eliminating TGF-β signaling to T cells dramatically accelerated experimental atherosclerosis (Robertson et al., 2003). These findings pointed to the subset of regulatory T cells (Treg), which act at least in part by controlling effector T cells, an important atheroprotective cell population (Taleb et al., 2008). In support of this notion, transfer of isolated CD4+CD25+ cells containing natural Treg cells reduced atherosclerosis and counteracted the proatherosclerotic effect of Treg cell deficiency (Ait-Oufella et al., 2006). Elimination of FoxP3+ Treg cells with a diphtheria toxin receptor expression strategy in hypercholesterolemic mice buttressed this concept (Klingenberg et al., 2013).
Subsequent work pointed to Treg cells as important mediators of atheroprotective effects of vaccines (Maron et al., 2002). Treg cells may not only affect the artery wall because Treg cell elimination also yields delayed clearance of cholesterol-rich lipoproteins and elevated plasma cholesterol concentrations (Klingenberg et al., 2013). Elimination of Treg cells unmasks the capacity of Th1 effector cells to suppress lipoprotein receptors such as sortilin-1 on hepatocytes (Klingenberg et al., 2013). These findings highlight the complex roles of Th1 cells in CVD because Th1 cells can exert functions that either promote or attenuate atherosclerosis.
As for other T cell subsets, evidence supporting Treg cell actions in atherogenesis in humans has lagged that in mice. Furthermore, effector T cell subsets may not be as distinct or phenotypically stable in humans (Nussenblatt et al., 2010). Indeed, many T cells isolated from human atherosclerotic coronary arteries produce both IL-17 and IFN-γ, as occurs in other human and mouse tissues affected by autoimmune disease (Eid et al., 2009). Overall, the limited data available for human atherosclerosis support a proatherosclerotic role for Th1 cells and support the operation of atheroprotective adaptive immune mechanisms, but we lack detailed knowledge in this regard.
Costimulatory and coinhibitory pathways profoundly influence T cell responses to proteins by enhancing or inhibiting antigen-driven T cell activation (Lichtman et al., 2013). The most thoroughly studied of these pathways involve members of the B7 family of costimulators, expressed on APCs, which bind to the CD28 family of signaling receptors on T cells. Therapeutics currently in use for treatment of autoimmune disease, allograft rejections, and cancer target these molecules. The same pathways influence atherosclerosis in mice. For example, deletion of the genes encoding B7-1 (CD80) and B7-2 (CD86), the costimulatory ligands of CD28, enhances lesion formation in Ldlr−/− mice (Buono et al., 2004). Conversely, deletion of the genes encoding the coinhibitory molecules PD-L1 (CD274) and PD-L2 (CD273) or their receptor PD-1 (CD269) enhances lesion formation and T cell infiltration in Ldlr−/− mice. Targeted deletions of TNF-TNR superfamily costimulatory pathways, including OX40 Ligand-OX40 (CD134) and CD137L-CD137, also reduce murine atherosclerosis (Jeon et al., 2010; Nakano et al., 2010).
The development of natural and probably induced Treg cells also requires costimulation. Bone marrow chimeric Ldlr−/− mice deficient in the B7:CD28 or ICOS Ligand-ICOS pathways have reduced Treg cells and develop more atherosclerosis and more lesion inflammation than control mice (Afek et al., 2005; Gotsman et al., 2006). Although these chimeric mice have proven instructive in demonstrating the atheroprotective role of Treg cells, there is no evidence that clinical costimulatory blockade, discussed below, reduces Treg cell mediated suppression of or promotes T cell-mediated aggravation of atherosclerosis.
Targeting costimulatory pathways to treat human disease has proven feasible, as exemplified by the use of CTLA-4-Ig, a B7-1-and B7-2-blocking drug used clinically to inhibit T cell responses in patients with autoimmunity or renal allograft rejection. (Lichtman et al., 2013) Costimulatory blockade can increase the frequency of infections, which may limit this approach for CVD to short-term interventions, such as patients with acute coronary syndromes. An antibody blocker of the CD28 family coinhibitor CTLA-4 has received approval to enhance antitumor T cell responses, and blockers of PD1 to treat cancer or chronic viral infections are in clinical trials. These drugs that inhibit the T cell inhibitors increase the risk of autoimmunity and would be predicted to aggravate atherosclerosis, as does anti-PD-1 in Ldlr−/− mice. Clinically useful agonists of the coinhibitory pathways await development.
The Antigen-Presenting Capacity of Arterial-Wall Cells and Immunoprivilege of the Tunica Media
In addition to bone-marrow-derived “professional” APCs, intrinsic vascular-wall cells themselves may participate in antigen presentation in human vascular diseases (Pober and Tellides, 2012). In particular, endothelial cells, which form the crucial interface between the vessel wall and the blood compartment, can present antigens effectively to T lymphocytes. Curiously, human SMCs present antigens to T cells poorly, if at all (Murray et al., 1995). SMCs lack the costimulatory molecule OX40L, one contributor to their inefficacy as APCs (Zhang et al., 2010). The ability of SMCs to produce indoleamine dioxygenase (IDO) likely also contributes to the differential capacity of human endothelial cells and SMCs to act as APCs. Heightened catabolism of tryptophan by IDO can impair local T cell responses (Cuffy et al., 2007). This finding has provided mechanistic basis for the “immunoprivilege” of the middle layer of the artery wall known as the tunica media, a locale populated primarily by SMCs (Dal Canto et al., 2001). IFN-γ, a product of activated T cells, can augment IDO activity in SMCs, furnishing another level of control that may limit the propagation of adaptive immune responses to intimal antigens beyond the vessel wall (Sakash et al., 2002). The clinical significance of these interesting biological observations awaits full explication.
Antigens Implicated in Instigating Adaptive Immunity in Atherosclerosis
The operation of adaptive immune responses, both cellular and humoral, implies an antigenic stimulus as a driver of atherosclerosis. Indeed, two candidate autoantigens—LDL and Hsp60—have emerged based on human studies and animal experiments (Stemme et al., 1995; Xu et al., 1992).
The cytosolic chaperonin Hsp60 has a high degree of homology to similar proteins in prokaryotic pathogens. CVD patients and hypercholesterolemic animals have antibodies and T cell responses to Hsp60. Endothelial cells exposed to hemodynamic or metabolic stress express Hsp60 in a manner that may expose it to the immune system, leading to autoimmune attack on the vessel wall. Early experiments in rabbits and mice reported that immunization against Hsp60 or homologous mycobacterial Hsp65 aggravates atherosclerosis (Xu et al., 1992). More recent studies in gene-targeted mice show that immunization against this antigen may lead to a protective immune response with increased Treg cell numbers, as well as antibody responses concomitant with reduced disease (Afek et al., 2000; Klingenberg et al., 2012; Maron et al., 2002). Therefore, an immune response to Hsp60 likely participates in the atherosclerotic process, in humans as well as in animals. Yet, the details of Hsp involvement remain enigmatic.
LDL itself is another putative autoantigen of atherosclerosis (Figure 1). As the major vehicle for cholesterol transport and a dominant risk factor for CVD, LDL participates causally in the disease process. The LDL particle consists of a hydrophobic core of esterified cholesterol and triglycerides covered by a shell of polar phospholipids, free cholesterol, and a single copy of its large signature apoprotein, the 550 kDa apolipoprotein B100 (apoB100).
Figure 1. A Potential Role for Low-Density Lipoprotein as an Antigen that Promotes Atherosclerosis.
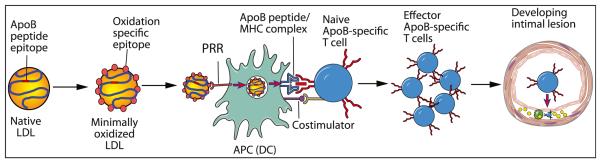
Native LDL can undergo slight oxidative modification that permits its uptake by PRRs on APCs (which may include DCs) but does not alter amino acid side chains in ApoB sufficiently to interfere with immunogenicity. The ApoB-derived proteins, presented in the context of self MHC, with appropriate costimulation, stimulate proliferation of the antigen-specific T cell clone and aggravation of atherogenesis.
Antibodies reactive to LDL components include natural immunoglobulin M (IgM) antibodies to its phospholipid components but also high-affinity IgG antibodies to protein and lipid epitopes (Miller et al., 2011). Antibodies reactive with the phosphorylcholine head of phosphatidylcholine, a prevalent phospholipid that becomes exposed in oxidized, but not native LDL, constitute a large proportion of all natural antibodies. Although the antigen-binding sites of these antibodies have little diversity and low affinity, exposure to an antigen can raise the titer of antiphospholipid antibodies, as observed in patients infected with T. pallidum. Similarly, antiphospholipid antibody titers increase when hypercholesterolemic mice develop atherosclerosis.
B cell hydridomas established from nonimmunized, hypercholesterolemic Apoe−/− mice showed identity of some of these spontaneous autoantibodies with certain natural antibodies (Binder et al., 2008). Thus, the E06 anti-oxLDL antibody of Apoe−/− mice was identical to a natural IgA antibody called T15. This anti-phosphorylcholine antibody binds not only to oxLDL but also to phospholipids exposed on the surface of apoptotic cells and to the cell wall of S. pneumoniae. This observation implies that molecular mimicry operates between autoimmune reactions to LDL and the immune response to pneumococci. Immunization with heat-killed pneumococci reduced the size of atherosclerotic lesions in hypercholesterolemic Ldlr−/− mice; therefore, immunity directed against pneumococcal phospholipids conveys partial protection against atherosclerosis by molecular mimicry (Binder et al., 2003). This finding agrees with previous observations that spleen B cells carry atheroprotective immunity and that splenectomized humans show increased cardiovascular morbidity (Robinette and Fraumeni, 1977).
LDL evokes expansion not only of B1 cells producing natural antibodies but also of B2 cells producing high-affinity IgG antibodies. This response involves T cell help. Indeed, analysis of lesions from atherosclerotic patients and hypercholesterolemic mice has identified T cells that recognize LDL epitopes. Interestingly, T cells recognize “native” oligopeptide motifs of the ApoB100 protein rather than oxidized ones, and extensive LDL oxidation extinguishes T cell reactivity (Figure 1; Hermansson et al., 2010). Paradoxically, oxidation enhances uptake of LDL into APCs through scavenger receptors. Therefore, an optimal window of oxidation may generate autoimmunity to LDL, whereby an LDL particle that has undergone minimal oxidation allows uptake by APCs, but immunodominant T cell epitopes remain unoxidized, allowing T cells to recognize ApoB100 peptide-MHC complexes on the APC surface (Figure 1). In support of this conjecture, administration of antibodies to the Vβ domain of ApoB100 binding TCRs prevents anti-LDL immunity by blocking the immunological synapse (Hermansson et al., 2010). This intervention modulates not only cellular and humoral anti-LDL responses but also atherosclerotic lesion development.
The B Cell Balance in Atherogenesis
Accumulating data, primarily derived from atherosclerotic mice, support a role for B cells in modulating atherogenesis. Initial studies showed that hyperlipidemic mice have increased atherosclerosis following splenectomy (Caligiuri et al., 2002). Adoptive transfer of B cells from splenectomized mice mutes the accelerated atherogenesis. Nonselective B cell depletion achieved by administration of an anti-CD20 antibody, however, reduces murine atherosclerosis (Ait-Oufella et al., 2010). Subsequent studies that use interruption of B cell activating factor (BAFF) signaling point to a role for the B2 subpopulation of lymphocytes in promoting atherogenesis in mice (Kyaw et al., 2013; Sage et al., 2012). Current experimental evidence supports the view that natural antibody derived from B1 lymphocytes mutes experimental atherogenesis (see below), whereas B2 cells aggravate this process (Table 1). The B2 population appears to aggravate atherogenesis through an antibody-independent mechanism that augments the action of proinflammatory cytokines (Kyaw et al., 2012). Thus, as in the case of T lymphocytes, distinct subpopulations of B lymphocytes exert opposite effects on experimental atherosclerosis. These observations suggest several novel therapeutic avenues including manipulation of the cell subpopulations with biological therapy and evoking natural antibody responses by vaccination, as discussed below (Nilsson et al., 2013; Townsend et al., 2010). Although human atheromata do contain some B lymphocytes, the applicability of the burgeoning results on B cell biology in mouse lesions to the human situation remains uncertain.
Vaccination against Atherosclerosis: Science Fiction or Future Reality?
The identification of autoantigens led to the proposal that immunization against them might alleviate disease (Amir et al., 2012; Binder et al., 2007; Nilsson et al., 2013). The first immunization study with oxLDL showed a remarkable, protective effect against atherosclerosis, reducing lesion size in hypercholesterolemic rabbits by approximately 30% (Palinski et al., 1995). Several other studies of rabbits and mice replicated this finding, with reductions of lesions up to 60%. As discussed above, these studies have implicated both cell-mediated and humoral effects in the observed atheroprotection.
The LDL particle contains multiple lipid species and may bear other apoproteins besides ApoB. In addition, LDL can transport other hydrophobic or amphipathic molecules, such as endotoxins. Identification of the key antigens associated with the particle could spur the development of vaccination strategies. A screen of LDL-derived peptides for immunogenicity has identified a set of peptides that were used for immunization (Pierides et al., 2013). Some display protective effects and currently serve to develop experimental vaccines. Studies are evaluating different adjuvants and administration routes, such as mucosal immunization with or without conjugation to the B subunit of cholera toxin, subcutaneous administration with alum adjuvant, and DC-based immunization (Hermansson et al., 2011; Klingenberg et al., 2010; Wigren et al., 2009). The lack of human leukocyte antigen (HLA) restriction for atherosclerotic CVD in humans presents a further conceptual challenge because T cell epitopes may vary depending on the HLA alleles in each individual. Therefore, an antiatherosclerosis vaccine may have to contain a combination of immunogenic peptides in a suitable adjuvant. A recent small clinical study of an anti-oxidized LDL antibody monitoring glucose uptake in human atherosclerotic plaques did not meet its primary endpoint (GLACIER, Goal of Oxidized Ldl and Activated Macrophage Inhibition by Exposure to a Recombinant Antibody, http://www.clinicaltrials.gov/ct2/show/NCT01258907?term=GLACIER&rank=1). The results of this negative study await publication.
Ultimate proof of efficacy of novel therapies for atherosclerosis requires large clinical trials that monitor endpoints such as MI or ischemic stroke, not biomarkers such as glucose uptake in plaque. Such outcome studies require daunting resources, a consideration that limits the number of strategies that can undergo such evaluation. Current progress regarding in vivo imaging of atherosclerosis may open new opportunities for monitoring human disease (Quillard and Libby, 2012). Such technologies would inform the design of clinical endpoint studies immensely, both by guiding a rational choice of dosing regimen and by providing biomarkers of efficacy in smaller populations and shorter observation periods than required in clinical endpoint trials. Imaging technology may therefore catalyze progress in clinical applications of vascular immunology, not only with regard to vaccines, but also to other therapeutic modalities.
Concluding Remarks
The evidence summarized above supports the operation of both innate and adaptive immunity in atherosclerosis. Within the realm of innate immunity, mononuclear phagocytes, as well as mast cells, platelets, granulocytes, and the complement cascade, may all contribute to this disease. In the adaptive, cellular immune response, T cells of several subtypes, antigen-presenting DCs, and both B1 and B2 B cells and humoral immunity exert modulatory effects on atherosclerosis.
Yet, the case for participation of inflammation and immunity in human atherogenesis remains much less well substantiated than in the elegant mouse experiments replete in the literature (Table 1). Many of the convenient classifications of subsets of cells with contrasting functions appear much more clear cut in mice than in humans. Inbreeding of mice provides a degree of genetic homogeneity unattainable in human studies. Moreover, the laboratory environment may alter the microbiome in ways that impact immune functions. The “hygiene hypothesis” highlights substantial differences between field mice, exposed to multiple environmental stimuli, and the inbred strains of mice we use experimentally, studiously protected from such conditions (Devalapalli et al., 2006; Rook, 2010). Thus, humans exhibit not only an enormous genetic diversity compared to mice but also may experience environmental stimuli that could modulate both adaptive and innate immunity in ways that differ from our preferred species for laboratory experimentation. Recent work suggests that the patterns of inflammatory gene responses in inflammation differ drastically between humans and mice, with concordance at random levels (Seok et al., 2013). As in the field of immunology in general, the particular case of atherosclerosis research requires a judicious interpretation of the experimental literature when extrapolating its results to human disease.
The application of contemporary genetic tools may help close the loop of causality in humans. GWASs and newer deep-sequencing data have begun to identify associations between variants in determinants of immune and inflammatory function and atherosclerotic events in humans. The chemokine CXCL-12 emerged in first-generation GWAS as associated with human MI. The more recent CARDIoGRAMplusC4D Consortium GWAS provided a network analysis that implicated inflammation as one of the four pathways most strongly associated with human MI (Deloukas et al., 2013). In particular, recent genetic studies provide robust data linking the IL-6 receptor to coronary heart disease (Sarwar et al., 2012).
Finally, application of the newfound appreciation of inflammation and immunity in atherosclerosis presents a clinical challenge. Global interference with these important host-defense mechanisms can yield an immunosuppressed individual with impaired tumor surveillance and increased susceptibility to opportunistic infections. The splendid redundancy of immune and inflammatory responses presents the challenge of identifying particular mediators of these host-defense mechanisms susceptible to manipulation in a manner that can mitigate atherosclerosis without undue suppression of essential host-defense mechanisms. Despite considerable progress in the understanding of inflammation and immunity in this disease, harvesting its fruits in terms of limiting human disease remains an aspiration and important goal of future research.
ACKNOWLEDGMENTS
P.L. was supported by grants from the National Heart, Lung, and Blood Institute (HL80472; HL48743; HL80731). A.H.L. was supported by a grant from the National Institutes of Health (HL87282). G.K.H. was supported by grants from the Swedish Research Council (grants 6816 and 8703), the Swedish Heart-Lung Foundation, the Foundation for Strategic Research (SSF), the Stockholm County Council, and the European Commission. P.L. and G.K.H. were both supported by a grant from Fondation Leducq. P.L. is an unpaid consultant and involved in clinical trials for Novartis.
REFERENCES
- Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- Afek A, Harats D, Roth A, Keren G, George J. A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis. 2005;183:57–63. doi: 10.1016/j.atherosclerosis.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate intersitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Amir S, Hartvigsen K, Gonen A, Leibundgut G, Que X, Jensen-Jarolim E, Wagner O, Tsimikas S, Witztum JL, Binder CJ. Peptide mimotopes of malondialdehyde epitopes for clinical applications in cardiovascular disease. J. Lipid Res. 2012;53:1316–1326. doi: 10.1194/jlr.M025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Hartvigsen K, Witztum JL. Promise of immune modulation to inhibit atherogenesis. J. Am. Coll. Cardiol. 2007;50:547–550. doi: 10.1016/j.jacc.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Chou MY, Fogelstrand L, Hartvigsen K, Shaw PX, Boullier A, Witztum JL. Natural antibodies in murine atherosclerosis. Curr. Drug Targets. 2008;9:190–195. doi: 10.2174/138945008783755520. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab. Invest. 2010;90:970–984. doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 2011;22:165–170. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J. Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, Lakkis FG, Fuchs D, Pober JS, Tellides G. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J. Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. Suppl. 2009;50:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Jongstra-Bilen J. Resident intimal dendritic cells and the initiation of atherosclerosis. Curr. Opin. Lipidol. 2010;21:397–403. doi: 10.1097/MOL.0b013e32833ded96. [DOI] [PubMed] [Google Scholar]
- Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J. Clin. Invest. 2001;107:R15–R22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. CARDIoGRAMplusC4D Consortium. DIAGRAM Consortium. CARDIOGENICS Consortium. MuTHER Consortium. Wellcome Trust Case Control Consortium Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalapalli AP, Lesher A, Shieh K, Solow JS, Everett ML, Edala AS, Whitt P, Long RR, Newton N, Parker W. Increased levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scand. J. Immunol. 2006;64:125–136. doi: 10.1111/j.1365-3083.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Finlayson CJ, Loftus IM, Antunes RF, Lim P, Bunce N, Kaski JC. High levels of costimulatory receptors OX40 and 4-1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ. Res. 2012;110:857–869. doi: 10.1161/CIRCRESAHA.111.261933. [DOI] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett BM, Pradhan AD, Solomon DH, Paynter N, MacFadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AAK, et al. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013 doi: 10.1016/j.ahj.2013.03.018. [Epub before print] Published online May 6, 2013. http://dx.doi.org/10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, Hansson GK, Li N. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb. Haemost. 2011;106:353–362. doi: 10.1160/TH11-01-0020. [DOI] [PubMed] [Google Scholar]
- Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- Gupta S, Pablo AM, Jiang Xc., Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J. Lipid Res. 2008;50:S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr. Opin. Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J. Clin. Invest. 1985;76:125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J, Emerging Risk Factors Collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC, Rothblat GH. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:1891–1898. doi: 10.1161/01.atv.19.8.1891. [DOI] [PubMed] [Google Scholar]
- Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- Klingenberg R, Ketelhuth DF, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe−/− mice. Immunobiology. 2012;217:540–547. doi: 10.1016/j.imbio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol. Rev. 2007;217:105–122. doi: 10.1111/j.1600-065X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Kruth HS. The fate of lipoprotein cholesterol entering the arterial wall. Curr. Opin. Lipidol. 1997;8:246–252. doi: 10.1097/00041433-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Kruth HS. Sequestration of aggregated low-density lipoproteins by macrophages. Curr. Opin. Lipidol. 2002;13:483–488. doi: 10.1097/00041433-200210000-00003. [DOI] [PubMed] [Google Scholar]
- Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS ONE. 2012;7:e29371. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh BH. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(−/−) mice. PLoS ONE. 2013;8:e60430. doi: 10.1371/journal.pone.0060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RD, Jackson CL, Morgan BP, Hughes TR. The membrane attack complex of complement drives the progression of atherosclerosis in apolipoprotein E knockout mice. Mol. Immunol. 2010;47:1098–1105. doi: 10.1016/j.molimm.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M, Schönbeck U. Cholesterol and atherosclerosis. Biochim. Biophys. Acta. 2000;1529:299–309. doi: 10.1016/s1388-1981(00)00161-x. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J. Clin. Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb. Haemost. 2011;106:827–838. doi: 10.1160/TH11-08-0592. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, Rizzello V, Rebuzzi AG, Rumi C, Maseri A, Crea F. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 2007;50:1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 2002;22:975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 2008;50:S364–S369. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- Megens RT, Vijayan S, Lievens D, Döring Y, van Zandvoort MA, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012;107:597–598. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- Melián A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 1999;155:775–786. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C, Cholesterol Treatment Trialists’ (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AG, Libby P, Pober JS. Human vascular smooth muscle cells poorly co-stimulate and actively inhibit allogeneic CD4+ T cell proliferation in vitro. J. Immunol. 1995;154:151–161. [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J. Clin. Invest. 1997;100:550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Fukumoto Y, Satoh K, Ito Y, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. OX40 ligand plays an important role in the development of atherosclerosis through vasa vasorum neovascularization. Cardiovasc. Res. 2010;88:539–546. doi: 10.1093/cvr/cvq211. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Cavender MA, Kastelein JJ, Schwartz G, Waters DD, Rosenson RS, Bash D, Hislop C. Inhibition of secretory phospholipase A(2) in patients with acute coronary syndromes: rationale and design of the vascular inflammation suppression to treat acute coronary syndrome for 16 weeks (VISTA-16) trial. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2012;26:71–75. doi: 10.1007/s10557-011-6358-9. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert Rev. Vaccines. 2013;12:311–321. doi: 10.1586/erv.13.4. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Bielekova B, Childs R, Krensky A, Strober W, Trinchieri G, Center for Human Immunology, Autoimmunity and Inflammation, National Institutes of Health National Institutes of Health Center for Human Immunology Conference, September 2009. Ann. N Y Acad. Sci. 2010;1200(Suppl 1):E1–E23. doi: 10.1111/j.1749-6632.2010.05682.x. [DOI] [PubMed] [Google Scholar]
- O’Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, Maggioni AP, Bode C, Weaver D, Johnson JL, et al. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619. e611. doi: 10.1016/j.ahj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Packard RRS, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierides C, Bermudez-Fajardo A, Fredrikson GN, Nilsson J, Oviedo-Orta E. Immune responses elicited by apoB-100-derived peptides in mice. Immunol. Res. 2013;56:96–108. doi: 10.1007/s12026-013-8383-1. [DOI] [PubMed] [Google Scholar]
- Pober JS. Interleukin-17 and atherosclerotic vascular disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:1465–1466. doi: 10.1161/ATVBAHA.111.228338. [DOI] [PubMed] [Google Scholar]
- Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler. Thromb. Vasc. Biol. 2002;22:727–733. doi: 10.1161/01.atv.0000016153.47693.b2. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Dennis EA. The human plasma lipidome. N. Engl. J. Med. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ. Res. 2012;111:231–244. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani A, Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. author reply 6561–6562. [DOI] [PubMed] [Google Scholar]
- Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Potteaux S. Vascular dendritic cells as gatekeepers of lipid accumulation within nascent atherosclerotic plaques. Circ. Res. 2010;106:227–229. doi: 10.1161/CIRCRESAHA.109.212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr., Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, et al. JUPITER Trial Study Group Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am. Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo J-L, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette CD, Fraumeni JF., Jr. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin. Exp. Immunol. 2010;160:70–79. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice—brief report. Arterioscler. Thromb. Vasc. Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- Sakash JB, Byrne GI, Lichtman A, Libby P. Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-asociated cells: implications for persistent Chlamydophila pneumoniae infection. Infect. Immun. 2002;70:3959–3961. doi: 10.1128/IAI.70.7.3959-3961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salagianni M, Galani IE, Lundberg AM, Davos CH, Varela A, Gavriil A, Lyytikäinen LP, Lehtimäki T, Sigala F, Folkersen L, et al. Toll-like receptor 7 protects from atherosclerosis by constraining “inflammatory” macrophage activation. Circulation. 2012;126:952–962. doi: 10.1161/CIRCULATIONAHA.111.067678. [DOI] [PubMed] [Google Scholar]
- Salomon RN, Hughes CCW, Schoen FJ, Payne DD, Pober JS, Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am. J. Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, et al. IL6R Genetics Consortium Emerging Risk Factors Collaboration Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert PS, Hugo F, Hansson GK, Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab. Invest. 1989;60:747–754. [PubMed] [Google Scholar]
- Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serruys PW, García-García HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Bøtker HE, et al. Integrated Biomarker and Imaging Study-2 Investigators Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J. Clin. Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, Mac-Farlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat. Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Libby P. The ins and outs of inflammatory cells in atheromata. Cell Metab. 2012;15:135–136. doi: 10.1016/j.cmet.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S, Tedgui A, Mallat Z. Regulatory T-cell immunity and its relevance to atherosclerosis. J. Intern. Med. 2008;263:489–499. doi: 10.1111/j.1365-2796.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L’Allier PL, Pfeffer MA, Aggressive Reduction of Inflammation Stops Events (ARISE) Trial Investigators Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:1761–1768. doi: 10.1016/S0140-6736(08)60763-1. [DOI] [PubMed] [Google Scholar]
- Torzewski M, Bhakdi S. Complement and atherosclerosis-united to the point of no return? Clin. Biochem. 2013;46:20–25. doi: 10.1016/j.clinbiochem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol. Rev. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren H-G, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 2007;170:1100–1107. doi: 10.2353/ajpath.2007.060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- Wennberg P, Wensley F, Di Angelantonio E, Johansson L, Boman K, Rumley A, Lowe G, Hallmans G, Danesh J, Jansson JH. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb. Res. 2012;129:68–73. doi: 10.1016/j.thromres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug-Gourley S, Wittes J, et al. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am. Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigren M, Bengtsson D, Dunér P, Olofsson K, Björkbacka H, Bengtsson E, Fredrikson GN, Nilsson J. Atheroprotective effects of Alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ. Res. 2009;104:e62–e70. doi: 10.1161/CIRCRESAHA.109.196667. [DOI] [PubMed] [Google Scholar]
- Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler. Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- Zhang P, Manes TD, Pober JS, Tellides G. Human vascular smooth muscle cells lack essential costimulatory molecules to activate allogeneic memory T cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:1795–1801. doi: 10.1161/ATVBAHA.109.200758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhang L, Zhou B, Wang Y, Song Y, Rao L, Zhang L. Lack of association between TLR4 Asp299Gly polymorphism and atherosclerosis: evidence from meta-analysis. Thromb. Res. 2012;130:e203–e208. doi: 10.1016/j.thromres.2012.07.008. [DOI] [PubMed] [Google Scholar]


