Abstract
The onset of reproduction is preceded by a host of organismal adjustments and transformations, involving morphological, physiological, and behavioral changes. In highly social mammals, including humans and most nonhuman primates, the timing and nature of maturational processes is affected by the animal’s social milieu as well as its ecology. Here, we review a diverse set of findings on how maturation unfolds in wild baboons in the Amboseli basin of southern Kenya, and we place these findings in the context of other reports of maturational processes in primates and other mammals. First, we describe the series of events and processes that signal maturation in female and male baboons. Sex differences in age at both sexual maturity and first reproduction documented for this species are consistent with expectations of life history theory; males mature later than females and exhibit an adolescent growth spurt that is absent or minimal in females. Second, we summarize what we know about sources of variance in the timing of maturational processes including natal dispersal. In Amboseli, individuals in a food-enhanced group mature earlier than their wild-feeding counterparts, and offspring of high-ranking females mature earlier than offspring of low-ranking females. We also report on how genetic admixture, which occurs in Amboseli between two closely related baboon taxa, affects individual maturation schedules.
Keywords: puberty, maturation, baboons, growth, reproductive hormones, sex differences, ecological differences, socio-demographic differences, genetic differences
Introduction
The onset of reproduction is preceded by a host of organismal adjustments and transformations, involving morphological, physiological, and behavioral changes. The timing of these changes, and thus the onset of reproduction, is influenced by several factors including ecological conditions such as access to nutritional sources, differences in socio-demographic factors, and genetic differences between individuals.
Individuals that develop under different nutritional conditions often mature at different rates; this is widely true of animals ranging from invertebrates to mammals, and has been documented in several primates including humans (e.g., see reviews in Ellison 2001; Day and Rowe, 2002; Plaistow et al., 2004; Alberts 2012;Pusey 2012). Observations of this phenomenon generally support the idea that, in vertebrates, a minimum body size is required for maturation, but this body size can be attained at variable ages. In cattle, for example, puberty occurs after an individual has attained about 60% of adult body weight (Freetly et al., 2011), but individuals vary in the age at which they attain the threshold proportion of adult body size. Animals growing under poor nutritional conditions generally mature later than those experiencing better nutrition (see reviews of the literature for primates in Alberts 2012; Pusey 2012).
Such variability in access to food may arise between populations living in different habitats (e.g., Altmann and Alberts, 2003; Emery Thompson et al., 2007) or in environments that vary across time, or the differences may arise as a result of social stratification within a group. Social stratification – expressed as dominance hierarchies in animal societies – may give rise to differences among individuals in access to food particularly when food is available in limited amounts or when its distribution is clustered in space. In hierarchically structured social systems, for example, high-ranking individuals often limit the extent to which their low-ranking counterparts access resources (reviewed in Silk, 1987). If as a result, high-ranking individuals can differentially provision their young, then parental dominance rank will exert a parental effect, i.e., a parental genotype or phenotype that affects offspring phenotype in the absence of genetic transmission (Kirkpatrick and Lande, 1989; Bernado, 1996; Wolf et al., 1998).
Parental effects may influence offspring phenotypes either through the behavior of the parents or through other epigenetic effects (reviewed in Wells and Stock, 2011). A number of compelling examples of parental effects come from studies of primates. In humans, for instance, the effects of maternal socio-economic status on offspring birth weight, on other components of fitness, and on health and disease outcomes, are well known (e.g. Barker et al., 1993; Emanuel et al., 2004). In non-human primates, too, a number of instances of maternal effects - including effects on growth and maturation -have been reported (reviewed in Maestripieri, 2009). For example, offspring of high-ranking mothers in several non-human primate species reach age of sexual maturity earlier than offspring of low-ranking mothers (e.g., baboons: Altmann et al., 1988; Bercovitch and Strum 1993; Johnson 2003; macaques: Mori, 1979; van Noordwijk and van Schaik 1999; mandrills: Setchell et al., 2006; see Maestripieri 2009 pp. 265 for additional references).
Genetic effects, too, are known to influence age at puberty or age at first reproduction. In western human populations, estimates of heritability for age at maturational events range from 0.5 to 0.9 (Anderson et al., 2007; van den Berg and Boomsma 2007; Silventoinen et al., 2008). In nonhuman primates, the range is greater (from 0.2 for age at first birth in semi-captive and captive rhesus monkeys to 0.87 for age at first birth in captive baboons; Blomquist, 2009; Gagliardi et al., 2010; Williams-Blangero and Blangero, 1995). Overall, the preponderance of evidence in humans (reviewed in Elks et al., 2010), in nonhuman primates (e.g., Blomquist, 2009; Gagliardi et al., 2010; Williams-Blangero and Blangero, 1995), and in other mammals (e.g., Reale et al., 2000) suggests a strong genetic contribution to the timing of sexual maturity.
Amboseli baboons
In this review, we summarize findings on maturation in savanna baboons in the Amboseli basin, a semi-arid short grass savanna in southern Kenya with a large complement of mammalian herbivores and carnivores. Baboons are sexually dimorphic Old World monkeys that live in multi-male multi-female societies. Group size ranges from 10 to ~200 individuals (Altmann, 1980). Birth occurs after approximately 6 months of gestation and is followed by a one- to two-year period of infancy in which the offspring’s dependence on its mother declines (Altmann, 1980; Altmann, 1998). As is characteristic of anthropoids, infancy is followed by a long period of immaturity (e.g., Plant, 1994; Gonzalez-Lagos et al., 2010). Unlike females, who live in their natal group throughout their lives, males disperse around the onset of adulthood, moving to one or a series of other groups over the course of their lives (Pusey and Packer, 1987).
For the past 40 years, the baboons of the Amboseli basin of southern Kenya have been the subjects of continuous, individual-based studies of life history, behavior, ecology, and, more recently, steroid hormones and genetics. The long-term data and natural setting of the Amboseli baboon study are helpful not only for understanding the pace of physical and social maturation, they are also vital for understanding the importance of dispersal, which only occurs in natural or semi-natural settings. In addition, while the Amboseli baboon population is primarily composed of yellow baboons (Papio cynocephalus cynocephalus), the population has experienced some admixture with anubis baboons (Papio cynocephalus anubis) (Alberts and Altmann, 2001; Tung et al., 2008;Charpentier et al., 2012). The genetically admixed nature of the population has enabled us to investigate the effects of genetic background on various aspects of life history including maturation patterns. These characteristics make the Amboseli baboons a good comparison and complement for laboratory studies. Our goals in this review are to describe maturational events, including growth, changes in reproductive hormones, attainment of adult dominance rank, and natal dispersal, and then to summarize the sources of variation in age at maturational stages in both male and female baboons in Amboseli.
Maturation in savanna baboons
Among mammals, primates are characterized by a relatively long period of growth and development, and they mature later than other similar sized mammals (e.g., Plant, 1994). The protracted period of immaturity in primates is postulated to allow for growth of the large brain in this taxon (Allman et al., 1993; Sol, 2009). Furthermore, intraspecific studies of growth and maturation in many anthropoids reveal sex differences in age at both sexual maturity and at first reproduction (e.g., Leutenegger and Kelly, 1977; Leigh, 1992; Leigh and Terranova, 1998). In savanna baboons, for example, the two sexes follow the same physical growth trajectory until shortly after average age of menarche, beyond which males diverge from females in their growth patterns and in age at both sexual maturity and first reproduction. This divergence between males and females in growth rate, growth duration, and age at sexual maturity and first reproduction, termed sexual bimaturism, is common among anthropoids (Leutenegger and Kelly, 1977; Leigh, 1992; Leigh and Terranova, 1998). In addition to differences in physical maturation, males and females differ in patterns of social maturation as described below.
Females
Menarche is marked by the first sexual swelling (Altmann et al., 1977,1988; Gesquiere et al., 2007), and occurs at a median age of 4.5 years in wild-foraging Amboseli females (Fig. 1a). At this stage, female body weight is about 9 kg, which represents about 75% of full adult weight. Females more or less maintain their pre-menarche rate of growth until they reach full adult size (Altmann and Alberts, 2005). The first several estrous cycles after menarche are non-fertile and are characterized by sexual swellings that are small compared to those of late adolescent and parous females (Altmann, 1980; Altmann et al., 1981; Gesquiere et al., 2007). Median age at first live birth is 5.97 years (Fig. 1a).
Figure 1.
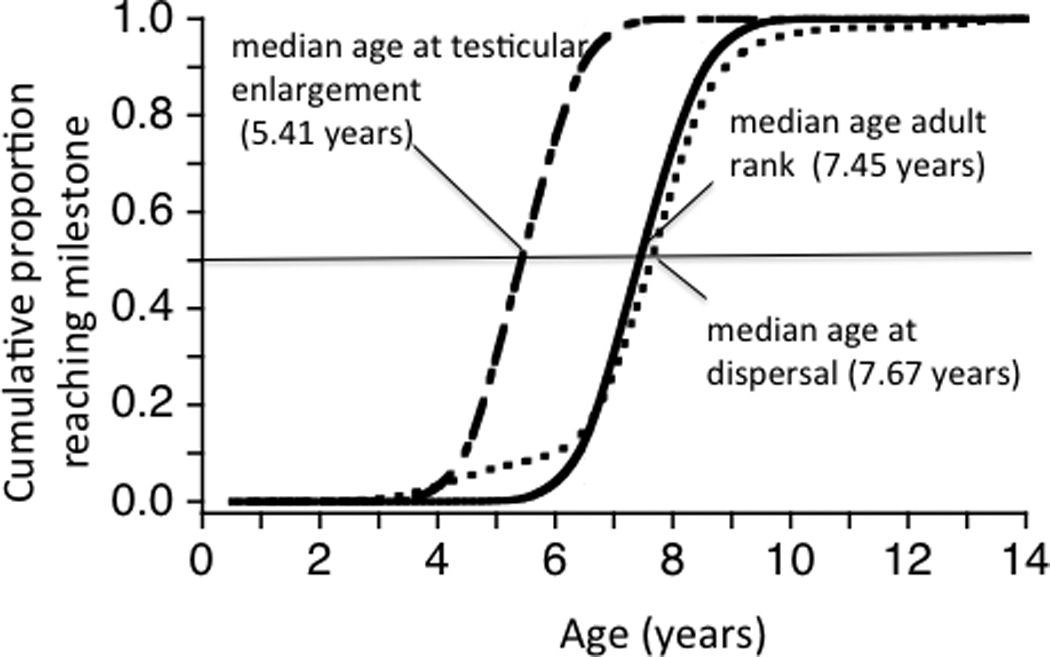
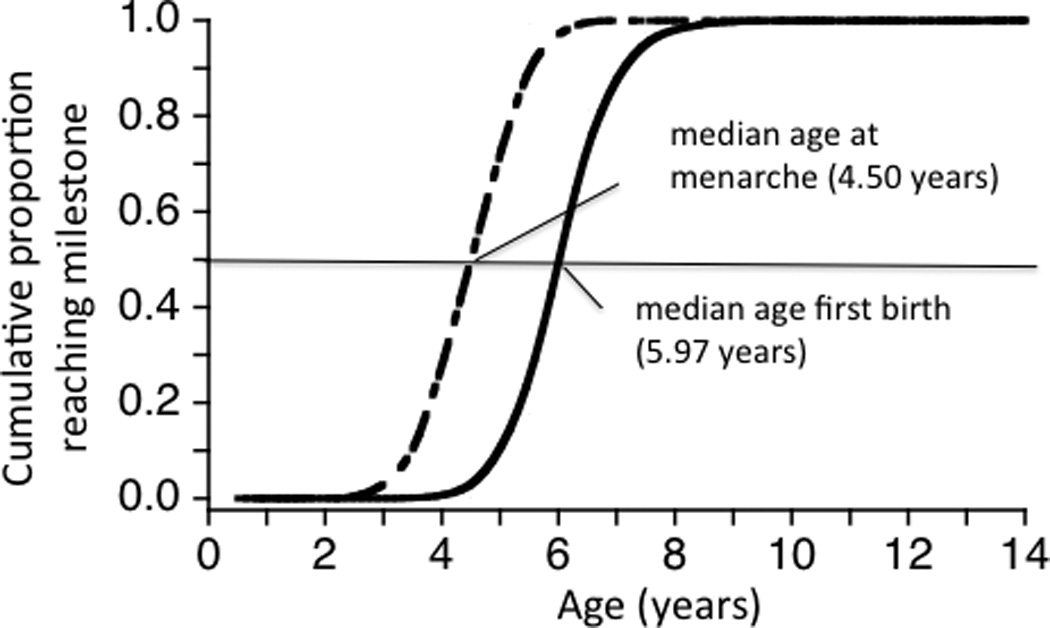
Cumulative proportion of baboons attaining maturational milestones as a function of age in Amboseli. a) Age at menarche (n = 241) and first live birth (n = 183) for females; b) age at testicular enlargement (onset of adolescence) (n = 155), adult rank attainment (onset of adulthood) (n = 81), and natal dispersal (n = 113) for males. Six individuals dispersed before they experienced testicular enlargement, but the large majority of males dispersed after testicular enlargement. Horizontal lines indicate median age at each milestone.
Physical maturation is accompanied by changes in the nature of social relationships. Female dominance rank relations in savanna baboons, as in many other cercopithecine primates, can be described as nepotistic. By menarche, and therefore before females have attained adult body size, daughters typically take the dominance rank immediately below that of their mothers, and younger daughters tend to rank above older daughters unless their mother herself has dropped in rank below the older daughters (Combes and Altmann 2001). This system of dominance rank ‘inheritance’ results in relatively stable dominance ranks across generations (Melnick and Pearl, 1987; Walters and Seyfarth, 1987). Nonetheless, dynamic changes occur during the juvenile years as individuals compete with others to achieve their adult rank. In other words, adult rank is generally predictable but requires competitive interactions to achieve. As one might expect, the nature of female dominance rank acquisition is such that as juvenile females begin to challenge adult females, these challenges first focus on adult females in low-ranking matrilines (Pereira, 1988a). The success of these challenges increases with the maturing female’s age during her juvenility and adolescence (Pereira, 1988a).
In addition to experiencing changes in the nature of agonistic interactions and experiencing dominance rank ascendancy, largely shaped by aggressive interactions, juvenile and adolescent females also participate in affiliative interactions, which are particularly strong with their mothers and with lactating females (Pereira, 1988b). These affiliative interactions may enable juvenile and adolescent females to acquire social skills and experience with infant caretaking (Pereira, 1988b; Fairbanks, 1990). In captive vervet monkeys, for example, experience with infant handling enhances offspring survival among primipares (Fairbanks, 1990). More generally, however, strong affiliative bonds are a central feature of the lives of female baboons. The social relationships of Amboseli females are often quite stable over many years (Silk et al., 2006a, 2006b, 2012), and these bonds confer direct fitness benefits in the form of enhanced infant survival (Silk et al., 2003). Further, female baboons in Moremi, Botswana, that maintain stronger and more stable social bonds live longer than females with weaker and more ephemeral bonds (Silk et al., 2010). These lines of evidence indicate that the affiliative bonds pursued by juvenile and adolescent female baboons lay the foundations for adult social bonds that are critically important for wellbeing, survival, and successful reproduction.
Males
Males experience testicular enlargement at a median age of 5.41 years among the wild-feeding baboons in Amboseli (Fig. 1b; testicular enlargement is the maturational milestone in male baboons that is equivalent to menarche in females). Testicular enlargement signals physiological maturity and the ability to produce viable sperm (e.g., Bercovitch and Goy, 1990; Plant, 1994). However, unlike females, who conceive their first offspring about 1 year after menarche, males experience a 2 year period of reproductive quiescence after testicular enlargement – an adolescent phase that is generally termed subadulthood. Adolescence involves a growth spurt that, by the age of 7–8 years, results in male body size approximately double that of adult females (Altmann and Alberts (2005)). Once they attain this large body size, they are able to agonistically challenge adult males and take a place in the adult male dominance hierarchy; this occurs at a median age of 7.45 years in Amboseli (Fig. 1b).
During the transitional period of adolescence, males experience social distancing, whereby adolescent males participate in few social interactions and tend to keep to the periphery of the group (Pereira, 1988b). The few social interactions males engage in at this stage tend to involve cycling females as well as adolescent and adult males, suggesting that they bias their social interactions to maximize opportunities for learning behaviors that may enhance their socio-sexual skills and competitive ability (Pereira, 1988b). Similar patterns of social distancing by maturing males have been reported for wild chimpanzees (Kraemer et al., 1982), captive mandrills (Setchell, 2003), captive Barbary macaques, (Keuster and Paul, 1996) and wild blue monkeys (Ekernas and Cords, 2007).
Another difference between male and female maturation in baboons lies in the fact that males disperse from their natal group, and many do so before they begin to reproduce. In Amboseli baboons, natal dispersal occurs at a median age of 7.67 years (Fig. 1b). Dispersers face risks of predation during dispersal itself, and they risk aggression from same-sex conspecifics in the new group when they attempt to immigrate (Alberts and Altmann, 1995b). These risks are sometimes also associated with physiological changes, such as rises in testosterone and cortisol (e.g., Alberts et al., 1992), which may in turn suppress the immune system if maintained at chronically elevated levels (e.g., Sapolsky et al., 2000).
The risks associated with dispersal may favor males that disperse after attaining a large enough body size that they are able to both successfully navigate the challenges of travelling alone between groups in savannah environments with appreciable exposure to large predators, and to defend themselves against other adult males and gain access to reproductive opportunities in the new group. In many other primate species, males experience much earlier natal dispersal, often as juveniles, and as pre-reproductive individuals they seem to avoid being treated by adult males as rivals (reviewed in Pusey and Packer, 1987). However, this strategy of juvenile dispersal may not be feasible in species such as baboons, in which males typically disperse singly, in environments with large predators.
The precise timing of natal dispersal is also influenced by reproductive aspects of demography. More specifically, males disperse in response to the availability of adult females – and thus breeding opportunities – in neighboring groups (Alberts and Altmann, 1995b; Altmann, 2000). Similar patterns have been reported in other primate species (reviewed in Pusey and Packer, 1987; Pope, 2000; van Noordwijk and van Schaik, 2004). In other words, the timing of dispersal is shaped not only by its risks, but also by the opportunities to be found in new demographic environments.
Steroid hormone concentrations during maturation
As in humans and other mammalian groups, gonadarche in nonhuman primates is governed by neuroendocrine processes that involve the hypothalamus, the pituitary, and the gonads. At puberty, the hypothalamus secretes gonadotropin-releasing hormone, which stimulates the pituitary gonadotropes to secrete luteinizing hormone and follicle stimulating hormone; this in turn stimulates the testes in males and the ovaries in females to secrete testosterone (T) and estradiol respectively (Plant, 1994), 2006).
Much of our knowledge of physiological processes during puberty in primates has come from experimental studies. In wild mammal populations, detailed analyses of pubertal changes are challenging because studies of physiological processes require access to body fluids or tissues that have not been easy to obtain from wild animals. Consequently, large gaps exist in our knowledge about the endocrinology of immature non-human primates in the wild. However, recent methodological advances have enabled field studies to use noninvasive methods for measuring concentrations of steroid hormone metabolites (Whitten, 1998; Palme, 2005) including those associated with changes during puberty. Taking advantage of commercially available radioimmunoassay (RIA) kits, we have validated the measurement of four steroid hormone metabolites in baboon fecal samples: glucocorticoid, estrogens, progesterone and testosterone (for more detail see Khan et al., 2002; Lynch et al. 2003; Beehner et al. 2009).
Longitudinal studies such as ours provide the opportunity to examine both age-based averages of hormone concentrations (e.g., Fig. 2a, b) and trajectories of hormone changes standardized for the timing of maturation (e.g., Fig 2c). In Amboseli, our age-based profile of female baboon fecal estrogens (fE) indicates that average fE levels in Amboseli females remain constant between 2 and 4 years of age, and then begin to rise (Independent t-test: fE<4y vs. fE≥4y: t697=6.498, p<0.001; Fig. 2a). This increase in average fE across females begins prior to the median age at menarche in the population, reflecting the fact that some females reach puberty before the median age, and others do so after (Fig. 2b). Further, if we examine changes in hormone concentrations as a function of timing relative to menarche, we see that fE concentrations in the 1.5 year-period before menarche remain constant (F2,340=0.049, p=0.952) and only increase after females reach menarche (F2,307=41.929, p<0.001; Fig. 2c). The increase in fE coincides with the ovarian secretion of this hormone as females begin cycling and getting pregnant (usually about 1 year after reaching menarche). Finally, our comparison of fE in females that mature earlier (EM) and those that mature later (LM) than the median age at maturity of the population indicates that EM females have consistently higher levels of fE for age throughout the juvenile period than LM females (Independent t-test: t479=2.551, p=0.011; Fig 2d). Similar data have been reported by Apter and Vikho (1985) in girls, and these authors suggest that girls with early menarche have a decreased sensitivity of their hypothalamus-pituitary axis to the feedback mechanism of circulatory hormones.
Figure 2.
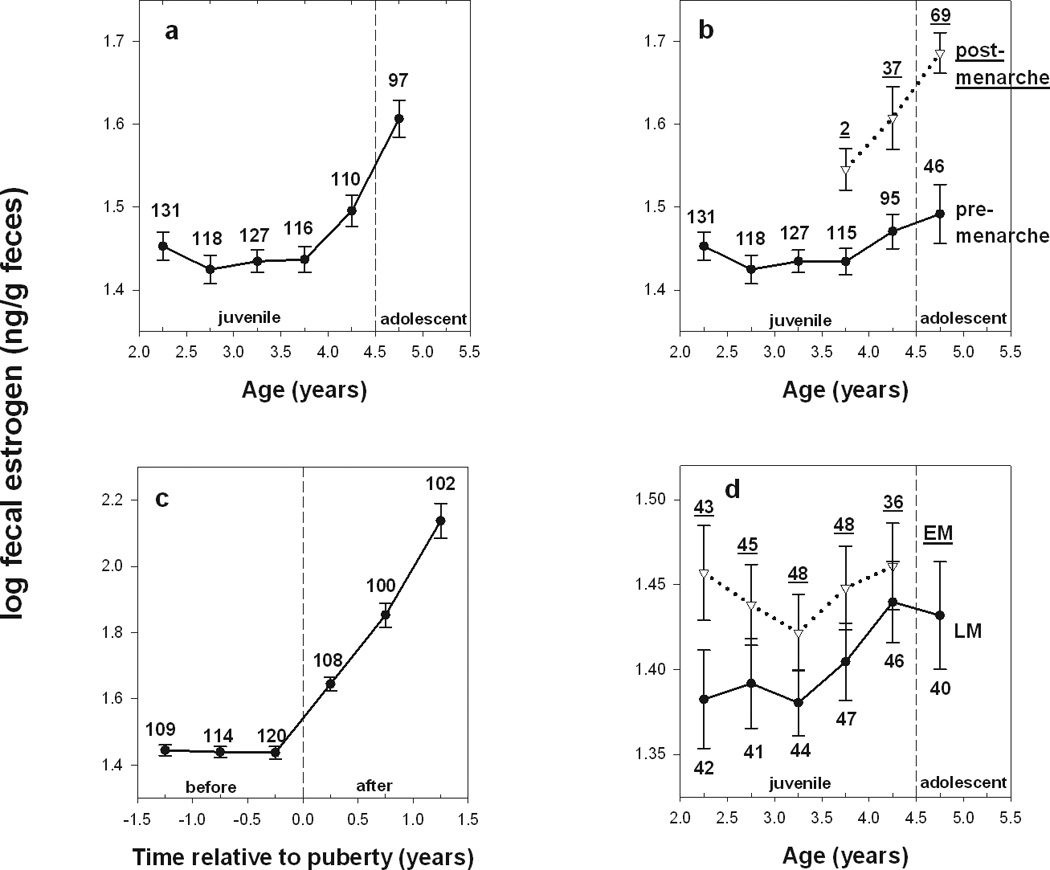
a) Average fE as a function of age in Amboseli females. Each value represents the mean ± SE across females of the log transformed fE concentration (ng/g feces) for a 6-month period. N’s represent the number of females sampled for each age period. The dashed line represents the median age at menarche for the population. b) Average fE as a function of age in female baboons, separating females who did or did not reach menarche in each age period (indicated as separate lines for pre-menarche and post-menarche females). Note that a given female can have 2 values (a pre- and a post- menarche) in the age period in which she reaches menarche. The dashed line represents the median age at menarche for the population. c) Average fE as a function of time before or after menarche; time 0 indicates the attainment of menarche. d) Average fE as a function of age for females that mature early (EM) or late (LM) relative to the population median age at menarche.
In males, our age-based profile of fecal testosterone (fT) levels indicates that average fT is relatively stable between 2 and 5 years of age, and a notable increase occurs at 5 years (Independent t-test: ft<5y vs. ft≥5y, t830=3.214, p=0.001; Fig. 3a). This increase in fT at 5 years is evident in both pre- and post- pubescent males (Fig. 3b), and this is different from the case in females, where elevated fE was attributable to post-menarcheal females (Fig 2b). Further, a marked increase in fT is noticeable 6 month prior to puberty in males (Fig 3c). This sex difference in patterns of fE and fT relative to maturational markers, and the fact that fT increases before males reach testicular enlargement, may be explained by the fact that testicular enlargement occurs in a more gradual fashion than menarche, with the exact timing of sexual maturity more difficult to discern in males than in females (see also discussion in Gesquiere et al. 2005 for more details). Like females, males that mature earlier have consistently higher levels of fT than males that mature later (Independent t-test: t416=2.081, p=0.038; Fig 3d).
Figure 3.
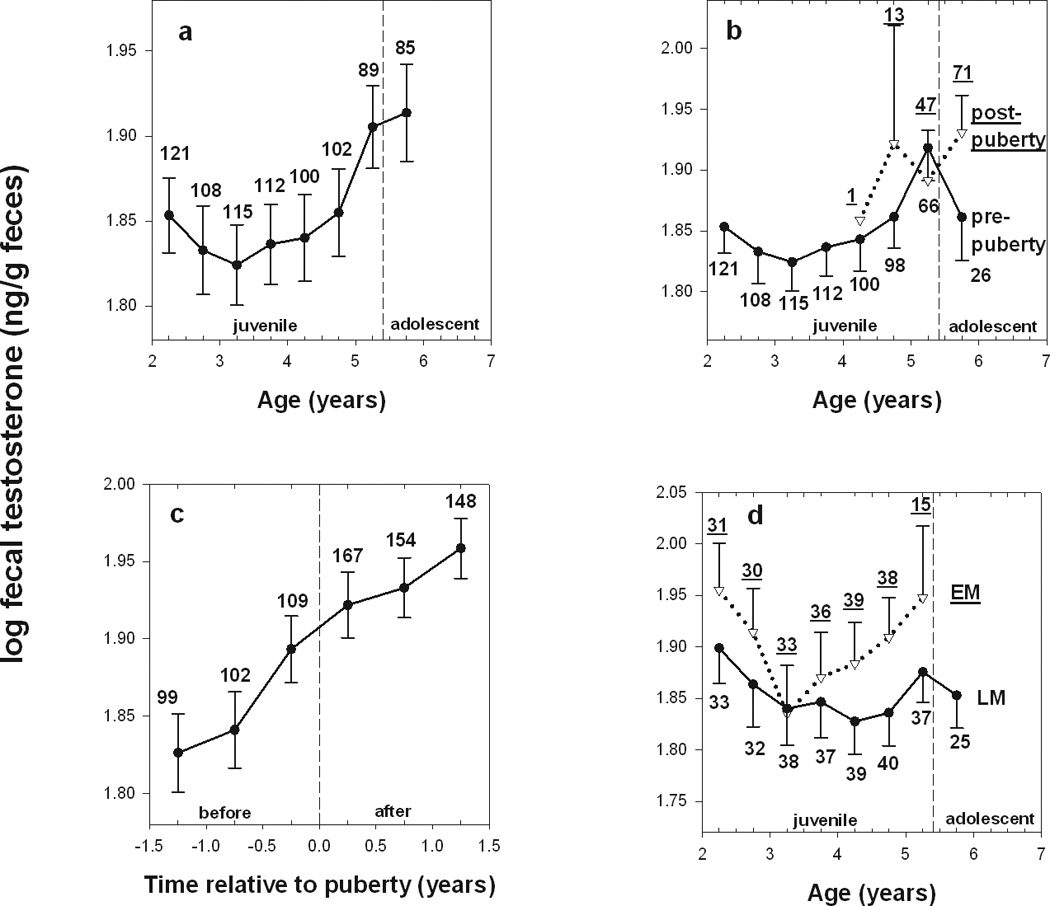
a) Average fT as a function of age in male baboons. Each value represents the mean ± SE across males of the log transformed fT concentration (ng/g feces) for a 6-month period. N’s represent the number of males sampled for each age period. The dashed line represents the median age at testes enlargement for the population. b) Average fT as a function of age in male baboons, separating males who did or did not reach testes enlargement in each age period (indicated as separate lines for pre-pubertal and post-pubertal males). Note that a given male can contribute to both pre- and post-pubertal measures in the age period in which he reaches puberty. c) Average fT values as a function of time before or after testes enlargement; time 0 indicates the attainment of testes enlargement. d) Average fT as a function of age for males that mature early (EM) or late (LM) relative to the population median at testicular enlargement.
Sources of variance in age at sexual maturity and first reproduction
Age at maturity is a key component of Darwinian fitness; all else being equal, organisms that mature earlier will have higher fitness than those that mature later. We have identified and characterized several sources of variance in the timing of maturational processes in the Amboseli baboons, including ecological, socio-demographic (including parental), and genetic effects.
Ecology
Nutritional - or more generally, energetic - differences that result from ecological variability between populations or groups can dramatically influence growth and the timing of puberty. Indeed, plasticity in age at maturity is a common primate response to changes in ecological circumstances and consequent changes in food availability (reviewed in Pusey, 2012). We have studied nutritional differences between animals living in social groups that fed entirely on wild foods (i.e., “wild-feeding groups”) and those that fed part-time at a refuse pit associated with a tourist lodge (i.e., a “food enhanced group”) in Amboseli. Those in the food-enhanced group not only grew at a faster rate but also attained sexual maturity earlier than their wild feeding counterparts (Altmann and Alberts, 2005). Specifically, both males and females in the food-enhanced group were large-for-age, even during the first year of life, and they grew at a faster rate throughout the juvenile period compared to individuals in fully wild foraging groups. Growth rate in the food-enhanced group was 8.8g/day for juvenile males and 24.0g/day for adolescent males. In contrast, growth rates were 5.5g/day and 12.7g/day, respectively, for their wild-feeding counterparts. Female growth rate was relatively constant throughout the growth period and was 8.7g/day in the food-enhanced group and 4.9g/day in the wild-feeding groups (Fig. 4; Altmann and Alberts 2005). As these rates indicate, males experienced a growth spurt of greater magnitude in the food-enhanced condition, and food-enhanced males gained weight at twice the rate that wild-feeding males did during the growth spurt. Further, both males and females matured more than a full year earlier in the food-enhanced group compared to their wild-feeding counterparts. Effects of habitat quality on female maturation patterns have been documented in other primate populations (reviewed in Pusey 2012), but to our knowledge ours is the only study to document a similar effect on male maturation patterns.
Figure 4.
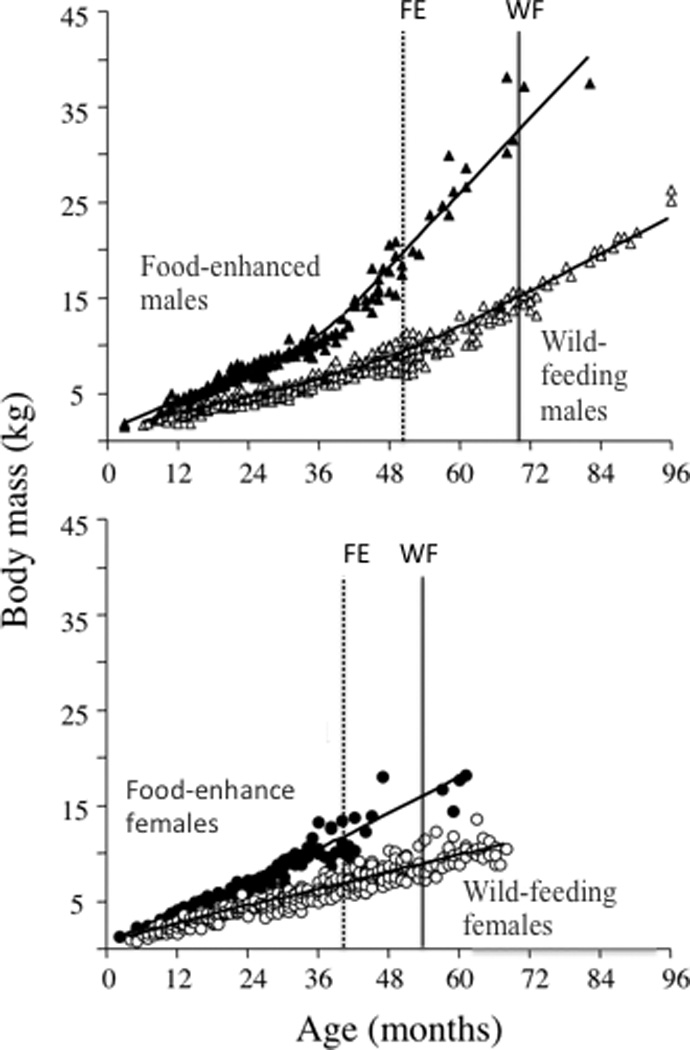
Body mass as a function of age for male (top panel) and female (bottom panel) Amboseli baboons, showing trajectories for both wild feeding animals and those that fed part-time at a human food source (i.e., were food-enhanced). The dotted line represents mean age at testicular enlargement for males (52.5±1.7 months) and mean age at menarche for females (41.2±1.1 months) in the food-enhanced (FE) group; the solid line represents mean age testicular enlargement in males (68.9±0.8 months) and at menarche in females (56.0±0.7 months) in the wild-feeding (WF) group. After Altmann and Alberts (2005).
The effect of nutrition on various components of baboon life history are so profound that entire baboon groups sometimes respond to declines in food availability by shifting their home range (Bronikowski and Altmann, 1996; Alberts et al., 2005). One such case of a home-range shift to better foraging areas resulted in a 40% increase in infant survival and a 5-month decrease in age at female maturity (Altmann and Alberts, 2003). The dramatic consequences of this home-range shift reveal that naturally occurring variation in food quantity or quality (e.g., caused by habitat change, rainfall, or drought), can affect primates profoundly, even if the magnitude of the effect is typically not as great as that of anthropogenic food enhancement.
Socio-demographic and parental effects
In complex and differentiated social systems, offspring are born into and are influenced by the social world of their parents in ways that often lead to intragroup variability in growth and maturation (e.g., Mori, 1979; Altmann et al., 1988; Bercovitch and Strum 1993; van Noordwijk and van Schaik 1999; Johnson 2003; Kleindorfer and Wasser 2004; Setchell et al., 2006). In savanna baboons, a mother’s dominance rank predicts the timing of her offspring’s key life history milestones. For example, within each foraging condition (wild-feeding and food-enhanced) in Amboseli, high-ranking females have large-for-age offspring (Fig. 5; Altmann and Alberts, 2005). Similar effects have been reported for wild chacma baboon females but not males (Johnson, 2003). However, in captive anubis baboons, Garcia et al. (2009) documented a sex-by-rank interaction in which high ranking females produce large-for-age sons but small-for-age daughters. These variations on the theme of maternal effects on offspring maturation patterns highlight both the pervasiveness of such patterns (at least in species where it has been examined) and their flexibility.
Figure 5.

Offspring of high-ranking mothers in Amboseli are larger-for-age than offspring of low-ranking mothers; males and females are pooled in the analysis. After Altmann and Alberts (2005).
In Amboseli, offspring of high-ranking females also experience testicular enlargement or menarche earlier than offspring of low-ranking females (Altmann et al., 1988; Altmann and Alberts, 2005; Charpentier et al., 2008a). As with the effects of rich habitats on maturation, this pattern of accelerated maturation for offspring of high-ranking mothers is consistent with studies in a range of other primate species (reviewed in Pusey 2012). Effects of maternal dominance rank on offspring growth and maturation are likely to arise from within-group differences in access to food between high and low-ranking mothers. High-ranking mothers appear to have disproportionately high access to food and are as a result better able to provision their developing fetus and young during pregnancy and the postnatal period respectively (reviewed in Silk, 2007; Johnson, 2003, 2006). In addition, some of the effects of maternal dominance rank may arise from greater psychosocial stress experienced by low-ranking individuals as a consequence of harassment by higher-ranking individuals (e.g. wild baboons: Kleindorfer and Wasser, 2004; Nguyen et al., 2009). Such sources of psychosocial stress may be sufficiently intense to negatively affect growth and maturation among offspring of low-ranking mothers, as suggested for infant growth in captive anubis baboons (Garcia et al., 2006, 2009) and juvenile growth in wild chacma baboons (Johnson, 2003), but evidence for psychosocial rather than energetic stress as a source of variance in growth is not yet available for wild populations (reviewed in Sapolsky, 1992).
We have also detected effects of maternal dominance rank on steroid hormone concentrations during development. Specifically, we found that adolescent males whose mothers had been low ranking when the males were born had higher fecal glucocorticoid levels than males whose mothers had been high-ranking (Onyango et al., 2008). Impressively, this was true when the glucocorticoid levels were measured 6 to 8 years after the males’ births, 4 to 6 years after males were independent of their mothers, and in many cases several years after the mothers had died. This represents a striking long-term effect of maternal dominance rank on offspring physiology.
Demographic effects also influence the onset of maturation (reviewed in Pusey 2012; Alberts, 2012). For example, in Amboseli, females experience menarche earlier in smaller groups, where they experience lower social density (Altmann and Alberts, 2003). This reflects an effect of competition on maturation processes, and competition may explain why Amboseli females that have living adult maternal sisters – who presumably represent allies more than competitors – are accelerated in their maturation relative to females without sisters (Charpentier et al., 2008b).
In addition to maternal dominance rank, maternal parity affects maturation schedules, such that multiparous mothers tend to have large-for-age offspring while first-time mothers tend to have small-for-age offspring, although these differences are not reflected in age at sexual maturity (Altmann and Alberts 2005). Similar effects of maternal age or parity on offspring size and/or growth have been reported for other non-human primates (e.g., chacma baboons, Johnson 2003; mandrills, Setchell et al., 2001) and ungulates (e.g., sheep, Hyatt et al., 2007; rhinos, Pluhacek et al., 2007). The effect of maternal parity may arise from a positive relationship between parity and reproductive experience. For example, several studies of primates have shown that offspring of first-time mothers have low survival probabilities (reviewed in Maestripieri, 2009), which may derive from primipare’s inexperience in caretaking. The effects of parity on growth of offspring may also arise from variation in physical maturation of the mothers because in some primate species, females begin to reproduce before reaching full adult size and face the need to balance energy allocation to both growth and reproduction (Dixson, 2012). The need to allocate limited energy to both reproduction and growth may result in negative outcomes for offspring. In humans, for example, age is a good predictor of maternal pelvic size, and small pelvic size in adolescent pregnancies has been linked to several complications including dystocia, stillbirth, premature birth, and low birth weight (e.g., Malabarey et al., 2012).
Although maternal effects are expected to influence offspring phenotypes in matrilineal social systems such as that of savanna baboons, paternal effects are less expected and consequently have received less attention. However, in recent years we have demonstrated that baboon fathers in Amboseli differentiate their own offspring from the offspring of other males and intervene in agonistic conflicts on their behalf (Buchan et al., 2003). Fathers also provide a safe buffer zone for mothers and their neonates (Altmann, 1980; Stein, 1984; Nguyen et al., 2009), thereby potentially reducing psychosocial stress in addition to enabling immatures to access food more efficiently (Stein, 1984). Functionally, the extent of father-offspring co-residence between daughters and their fathers during the immature period is negatively associated with age at menarche. That is, when fathers remain in groups with their daughters for longer periods during the daughter’s juvenility, those daughters tend to attain menarche earlier than females whose fathers leave their daughters’ groups (Charpentier et al., 2008b). For sons, the father’s presence also results in early maturation, but only if the father is high-ranking (Charpentier et al., 2008b). These positive paternal effects probably arise because fathers provide a safe zone and intervene favorably on behalf of their offspring during agonistic interactions (Buchan et al., 2003), both of which may protect their offspring from psychosocial stress and injury. Although to our knowledge no comparable data are available from other populations, the Amboseli findings thus far give us confidence that similar investigations will be rewarded by a rich vein of knowledge and that much more remains to be revealed about paternal effects in the Amboseli baboons as well.
Genetic effects
The Amboseli baboon population is part of a large extended population of baboons in southern Kenya and northern Tanzania. Amboseli is situated on the boundary between the ranges of yellow and anubis baboons, two allotaxa (either species or subspecies; see discussions in Jolly, 1993; Newman et al., 2004) that have morphological and behavioral similarities but are readily distinguishable in the field. Yellow baboons are prevalent roughly to the south and east and anubis baboons to the north and west of Amboseli (Jolly, 1993; Newman et al., 2004;Charpentier et al., 2012). As noted above, the Amboseli baboon population consists primarily of yellow baboons, but hybrids between yellow and anubis baboons are found in Amboseli because of the occasional immigration of anubis baboons from outside the basin; hybrids have resulted not only from anubis immigrations and subsequent mating, but also from the movement and successful reproduction of hybrid males between and within study and non-study groups (Samuels and Altmann, 1991; Alberts and Altmann, 2001; Tung et al., 2008;Charpentier et al., 2012). Consequently, hybrids in Amboseli result from yellow-anubis, yellow-hybrid, anubis-hybrid, and hybrid-hybrid matings, culminating in great variation in genetic hybrid scores.
To evaluate the extent of anubis admixture in Amboseli individuals, we employ microsatellite genotyping to generate a “genetic hybrid score” that estimates the proportion of a baboon’s genome that is attributable to anubis ancestry (Tung et al., 2008). This genetic hybrid score varies from 0 (only yellow ancestry detectable) to 1 (only anubis ancestry detectable), with 0.5 representing the equivalent of an F1 hybrid offspring.
In Amboseli, age at maturation in both males and females declines with increasing proportion of anubis ancestry; that is, animals with more anubis ancestry mature earlier than those with more yellow ancestry (Charpentier et al., 2008b). Hybrid intermediacy is the most parsimonious explanation for this effect of genetic background. Unfortunately, although we have maturation data on both yellow and hybrid individuals in Amboseli, the few anubis individuals in the basin are immigrants rather than natal individuals. We consequently have no comparable maturation data for them and cannot test our hybrid intermediacy hypothesis directly. However, hybrids within the genus Papio exhibit intermediacy in most traits that have been examined and in all known hybrid zones in the wild (e.g., Sugawara, 1988; Jolly et al., 1997; Bergman and Beehner, 2004; Ackerman et al., 2006), supporting the notion that Amboseli hybrids are intermediate between anubis and yellow baboons in most traits.
The effects of anubis ancestry are marked in our study population; a male’s degree of anubis ancestry (reflected in his genetic hybrid score) predicts age at testicular enlargement, at adult rank attainment, and at natal dispersal (Charpentier et al., 2008b). An obvious potential source of this admixture-related variation is differences in growth rates between yellow and hybrid individuals, but we lack body mass data for a sufficient number of admixed juveniles to test this hypothesis. The effect of genetic hybrid score on age at natal dispersal is particularly striking. An earlier study in Amboseli (Alberts and Altmann, 1995a), conducted during a period when hybrids were at low frequency in the population, showed that age at natal dispersal was predicted by maternal death and maternal age, such that males whose mothers had died were more likely to disperse early and so were sons of older mothers. However, a decade and half later, after more extensive hybridization had occurred in the population, the genetic hybrid scores of the study subjects were the only significant source of variance in age at natal dispersal, accounting for nearly 22% of the variance in the timing of this maturational milestone and male life history (Fig. 6; Charpentier et al., 2008b). Apparently the strong effects of genetic background on age at natal dispersal in the later study swamped the maternal effects that were detectable during the period when males exhibited relatively little heterogeneity in ancestry.
Figure 6.
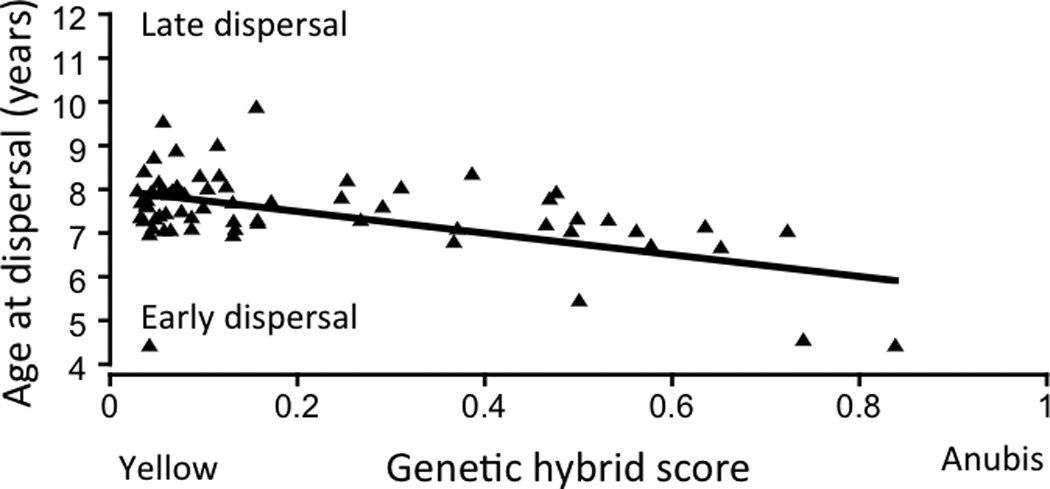
Males with a greater degree of anubis ancestry (a higher “genetic hybrid score”) tend to disperse at an earlier age than males with less anubis ancestry. After Charpentier et al. (2008)
In comparison to males, the effects of anubis ancestry in females, although detectable, are more muted. Only one maturational milestone – age at menarche – reveals an effect of hybridity, and this effect is small, accounting for only ~4% of the variance in age at menarche. Further, not only does genetic hybrid score have a smaller impact on female maturation, but maternal effects have a concomitantly greater impact: the presence versus absence of the mother, the mother’s dominance rank, and the presence of maternal sisters, have significant effects on the timing of female maturation. This difference between males and females – in which maternal effects appear to mitigate genetic effects in females to a greater extent than in males – reflects an interesting gene-by-sex interaction, a type of interaction rarely documented in wild mammal populations (e.g., baboons: Charpentier et al., 2008b; red deer: Foerster et al., 2007).
Characterizing genetic effects on age at maturity in Amboseli baboons represents an interesting new frontier for us. Measuring norms of reaction for anubis-like versus yellow-like hybrid baboons is a tantalizing possibility as sample sizes of hybrids accumulate; doing so would provide unprecedented insight into the role of genetic variation in the timing of maturation in wild mammals, especially in combination with quantitative genetic analyses, or with studies of gene expression associated with development (Babbitt et al., 2011; Tung et al., 2011).
Conclusion
In savanna baboons as in many other sexually dimorphic mammals, males mature later than females (Shea, 1986; Bolter and Zihlman, 2003). Within each sex, however, variation in age at sexual maturity and age at first reproduction are mediated by external factors, including ecology and socio-demography, as well as by genetic effects.
Combined insights from long-term field projects and laboratory studies in the past several decades have greatly improved our understanding of maturational processes in nonhuman primates. However, key questions on the subject still remain unanswered. Normative descriptions of maturational processes – especially hormonal and other physiological processes – are still relatively rare for wild primates. Studies of sources of variance in maturation are even more rare for such populations. But an increasing number of primate field studies are accumulating long-term data that will allow such processes to be studied (Kappeler and Watts, 2012). Indeed, such long term studies of primates and other animals offer our best opportunity for novel and innovative research on topics such as life history (Clutton-Brock and Sheldon, 2010), especially if they can combine phenotypic and environmental data with genetic data on the same individuals (Tung et al., 2010). The future is bright for such studies, which are poised to greatly enhance our understanding of the evolution of puberty and adolescence in humans and their relatives.
Acknowledgements
We gratefully acknowledge the support of the National Science Foundation for the majority of the data represented here; in the past decade in particular we acknowledge support from IBN 9985910, IBN 0322613, IBN 0322781, BCS 0323553, BCS 0323596, DEB 0846286, DEB 0846532 and IOS 0919200. We are also very grateful for support from the National Institute of Aging (R01AG034513-01 and P01AG031719, and the Princeton Center for the Demography of Aging (P30AG024361). We also thank the Chicago Zoological Society, the Max Planck Institute for Demography, the L.S.B. Leakey Foundation and the National Geographic Society for support at various times over the years. We thank the Kenya Wildlife Services, Institute of Primate Research, National Museums of Kenya, and members of the Amboseli-Longido pastoralist communities for their cooperation and assistance in Kenya. A number of people have contributed to the long- term data collection over the years; particular thanks go to the Amboseli Baboon Project long-term field team (R.S. Mututua, S. Sayialel, and J.K. Warutere), and to V. Somen and T. Wango for their assistance in Nairobi, and to K. Pinc for his expertise in the design and management of the Baboon Project database, Babase. We also thank the database technicians who have provided assistance with Babase over the years, particularly D. Onderdonk, C. Markham, T. Fenn, N. Learn, and L. Maryott Roerish. This research was approved by the IACUC at Princeton University and at Duke University and adhered to all the laws and guidelines of Kenya.
References
- Ackermann RR, Rogers J, Cheverud JM. Identifying the morphological signatures of hybridization in primate and human evolution. J. Hum. Evol. 2006;51:632–645. doi: 10.1016/j.jhevol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Alberts SC. Magnitude and sources of variation in male reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Chicago: University of Chicago Press; 2012. pp. 412–431. [Google Scholar]
- Alberts SC, Altmann J. Preparation and activation: determinants of reproductive maturity in male baboons. Behav. Ecol. Sociobiol. 1995a;36:397–406. [Google Scholar]
- Alberts SC, Altmann J. Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 1995b;145:279–306. [Google Scholar]
- Alberts SC, Altmann J. Immigration and hybridization patterns of yellow and olive baboons in Amboseli, Kenya. Am. J. Primatol. 2001;53:139–154. doi: 10.1002/ajp.1. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Hollister-Smith JA, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long-term change in a savanna environment. In: Brockman DK, van Schaik CP, editors. Seasonality in Primates: Studies of Living and Extinct Human and Non-human Primates. Cambridge: Cambridge University Press; 2005. pp. 157–196. [Google Scholar]
- Alberts SC, Sapolsky RM, Altmann J. Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Horm. Behav. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- Allman JM, McLaughlin T, Hakeem A. Brain weight and life-span in primate species. Proc. Natl. Acad. Sci. USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J. Baboon Mothers and Infants. Harvard University Press; 1980. [Google Scholar]
- Altmann J, Altmann SA, Hausfater G. Physical maturation and age estimates of yellow baboons, Papio cynocephalus, in Amboseli National Park, Kenya. Am. J. Primatol. 1981;1:389–399. doi: 10.1002/ajp.1350010404. [DOI] [PubMed] [Google Scholar]
- Altmann J. Models of outcome and process: predicting the number of males in primate groups. In: Kappeler PM, editor. Primate males: Causes and consequences of variation in group composition. Cambridge: Cambridge University Press; 2000. pp. 236–247. [Google Scholar]
- Altmann J, Alberts SC. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA, editors. Offspring: The Biodemography of Fertility and Family behavior. Washington, DC: National Academy Press; 2003. pp. 140–169. [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav. Ecol. Sociobiol. 2005;57:490–501. [Google Scholar]
- Altmann J, Altmann SA, Hausfater G, McCuskey SA. Life history of yellow baboons: physical development, reproductive parameters and infant mortality. Primates. 1977;18:315–330. [Google Scholar]
- Altmann J, Hausfater G, Altmann SA. Lifetime Reproductive Success in savannah baboons. In: Clutton-Brock TH, editor. Lifetime Reproductive Success. Chicago: University of Chicago Press; 1988. pp. 403–418. [Google Scholar]
- Altmann SA. Foraging for survival: yearling baboons in Africa. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Anderson CA, Duffy DL, Martin NG, Visscher PM. Estimation of variance components for age at menarche in twin families. Behav. Genet. 2007;37:668–677. doi: 10.1007/s10519-007-9163-2. [DOI] [PubMed] [Google Scholar]
- Apter D, Vihko R. Premenarcheal endorcrine changes in relation to age at menarche. Clin. Endocrinol. 1985;22:753–760. doi: 10.1111/j.1365-2265.1985.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Babbit CC, Tung J, Wray GA, Alberts SC. Changes in gene expression associated with reproductive maturation in wild female baboons. Genome Biol. Evol. 2011;4:102–109. doi: 10.1093/gbe/evr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Beehner J, Gesquiere L, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. Testosterone related to age and life-history stages in male baboons and geladas. Horm. Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch FB, Goy RW. The socioendocrinology of reproductive development and reproductive success in macaques. In: Ziegler TE, Bercovitch FB, editors. Socioendocrinology of Primate Reproduction. New York: Wilet-Liss; 1990. pp. 59–93. [Google Scholar]
- Bercovitch FB, Strum SC. Dominance rank, resource availability and reproductive maturation in female savanna baboons. Behav. Ecol. Sociobiol. 1993;33:313–318. [Google Scholar]
- Bergman TH, Beehner JC. Social system of a hybrid baboon group (Papio anubis x P-hamadryas) Intl. J. Primatol. 2004;25:1313–1330. [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. Am. Zool. 1996;36:83–105. [Google Scholar]
- Blomquist GE. Environmental and genetic causes of maturational differences among rhesus macaque matrillines. Behav. Ecol. Sociobiol. 2009;63:1345–1352. [Google Scholar]
- Bolter DR, Zihlman AL. Morphometric analysis of growth and development in wild- collected vervet monkeys (Cercopithecus aethiops), with implications for growth patterns in Old World monkeys, apes and humans. J. Zool. (London) 2003;260:99–110. [Google Scholar]
- Bronikowski A, Altmann J. Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behav. Ecol. Sociobiol. 1996;39:11–25. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Fontaine MC, Cherel E, Renoult JP, Jenkins T, Benoit L, Barthes N, Alberts SC, Tung J. Genetic structure in a dynamic baboon hybrid zone corroborates behavioral observations in a hybrid population. Mol. Ecol. 2012;21:715–731. doi: 10.1111/j.1365-294X.2011.05302.x. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Tung J, Altmann J, Alberts SC. Age at maturity in wild baboons: genetic, environmental and demographic influences. Mol. Ecol. 2008a;17:2026–2040. doi: 10.1111/j.1365-294X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multi-male primate society. Proc. Natl.Acad. Sci., USA. 2008b;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Sheldon BC. Individual and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 2010;25:562–573. doi: 10.1016/j.tree.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Combes SL, Altmann J. Status Change During Adulthood: life-history byproduct or kin-selection based on reproductive value? Proc. Roy. Soc. B-Biol. Sci. 2001;268:1367–1373. doi: 10.1098/rspb.2001.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T, Rowe L. Developmental thresholds and evolution of reaction norms for age and size at life-history transitions. Am. Nat. 2002;159:338–350. doi: 10.1086/338989. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Primate sexuality: Comparative studies of the prosimians, monkeys, apes, and humans. Oxford, New York: Oxford University Press; 2012. [Google Scholar]
- Ekernas LS, Cords M. Social and environmental factors influencing natal dispersal in blue monkeys, Cercopithecus mitis stuhlmanni. Anim. Behav. 2007;73:1009–1020. [Google Scholar]
- Elks CE, Perry JRB, Sulem P, Chasman DI, Franceschini N, He CY, Lunetta KL, Visser JA, Byrne EM, Cousminer DL, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010;42 doi: 10.1038/ng.714. 1077-U1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT. On Fertile Ground. Cambridge MA: Harvard University Press; 2001. [Google Scholar]
- Emanuel I, Kimpo C, Moceri V. The association of maternal growth and socio-economic measures with infant birthweight in four ethnic groups. Int. J. Epidemiol. 2004;33:1236–1242. doi: 10.1093/ije/dyh269. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim. Behav. 2007;73:501–512. [Google Scholar]
- Fairbanks LA. Reciprocal benefits of allomothering for female vervet monkeys. Anim. Behav. 1990;40:553–562. [Google Scholar]
- Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB. Nature. 447:1107–1110. doi: 10.1038/nature05912. [DOI] [PubMed] [Google Scholar]
- Freetly HC, Kuehn LA, Cundiff LV. Growth curves of crossbred cows sired by Herefold, Angus, Belgian Blue, Brahman, Boran, and Tuli bulls, and the fraction of mature body weight and height at puberty. J. Anim. Sci. 2011;89:2373–2379. doi: 10.2527/jas.2011-3847. [DOI] [PubMed] [Google Scholar]
- Gagliardi C, Falkenstein KP, Franke DE, Kubisch HM. Estimates of heritability for reproductive traits in captive rhesus macaque females. Am. J. Primatol. 2010;72:811–819. doi: 10.1002/ajp.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Lee PC, Rosetta L. Dominance and reproductive rates in captive female olive baboons, Papio anubis. Am. J. Phys. Anthropol. 2006;131:64–72. doi: 10.1002/ajpa.20405. [DOI] [PubMed] [Google Scholar]
- Garcia C, Lee PC, Rosetta L. Growth in colony living anubis baboon infants and its relationship with maternal energetics and reproductive status. Am. J. Phys. Anthropol. 2009;138:123–134. doi: 10.1002/ajpa.20909. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm. Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lagos C, Sol D, Readers SM. Large-brained animals live longer. J. Evol. Biol. 2010;23:1064–1074. doi: 10.1111/j.1420-9101.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- Hyatt MA, Budge H, Walker D, Stephenson T, Symonds ME. Am. J. Physiol. Integr. Comp. Physiol. 2007;292:R1934–R1942. doi: 10.1152/ajpregu.00802.2006. [DOI] [PubMed] [Google Scholar]
- Johnson SE. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am. J. Phys. Anthropol. 2003;120:83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Johnson SE. Maternal characteristics and offspring growth in chacma baboons. In: Swedell L, Leigh SR, editors. Reproduction and fitness in baboons. New York: Springer; 2006. pp. 177–197. [Google Scholar]
- Jolly CJ. Species, subspecies and baboon systematics. In: Kimbel W, Martin L, editors. Species, species concepts and primate evolution. New York: Wiley; 1993. pp. 67–107. [Google Scholar]
- Jolly CJ, Woolley-Barker T, Beyene S, Disotell TR, Phillips-Conroy JE. Intergeneric Hybrid Baboons. Intl. J. Primatol. 1997;18:597–627. [Google Scholar]
- Kappeler PM, Watts DP, editors. Long-Term Field Studies of Primates. Springer; 2012. [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen. Comp. Endocr. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Kleindorfer S, Wasser SK. Infant handling and mortality in yellow baboons (Papio cynocephalus): evidence for female reproductive competition. Behav. Ecol. Sociobiol. 2004;56:328–337. [Google Scholar]
- Kraemer HC, Horvat JR, Doering C, McGinnis PR. Male chimpanzee development focusing on adolescence: Integration of behavioral with physiological changes. Primates. 1982;23:393–405. [Google Scholar]
- Keuster J, Paul A. Female-female competition and male mate choice in Barbary macaques (Macaca sylvanus) Behaviour. 1996;133:763–790. [Google Scholar]
- Leigh SR. Patterns of variation in the ontogeny of primate body size dimorphism. J. Hum. Evol. 1992;23:27–50. [Google Scholar]
- Leigh SR, Terranova CJ. Comparative perspectives on bimaturism, ontogeny, and dimorphism in lemurid primates. Intl. J. Primatol. 1998;19:723–749. [Google Scholar]
- Leuttenegger W, Kelly JT. Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates. Primates. 1977;8:117–136. [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: medium-term storage conditions and consequences for data interpretation. Gen. Comp. Endocr. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal influences on offspring growth, reproduction, and behavior in Primates. In: Maestripieri D, Mateo JM, editors. Maternal Effects in Mammals. Chicago: University of Chicago Press; 2009. pp. 256–291. [Google Scholar]
- Malabarey OT, Balayla J, Klam SL, Shrim A, Abenhaim HA. Pregnacies in young adolescent mothers: A population-based study on 37 million births. J. Pediatr. and Adolesc Gynecol. 2012;25:98–102. doi: 10.1016/j.jpag.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Pearl MC. Cercopithecines in multimale group. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 121–134. [Google Scholar]
- Mori A. Analysis of population changes by measurement of body weight in the Koshima troop of Japanese monkeys. Primates. 1979;20:371–397. [Google Scholar]
- Newman TK, Jolly CJ, Rogers J. Mitochondrial phylogeny and systematics of baboons (Papio) Am. J. Phys. Anthropol. 2004;124:17–27. doi: 10.1002/ajpa.10340. [DOI] [PubMed] [Google Scholar]
- Nguyen N, van Horn RC, Alberts SC, Altmann J. “Friendships” between new mothers and adult males: adaptive benefits and determinants in wild baboons (Papio cynocephalus) Behav. Ecol. Sociobiol. 2009;63:1331–1344. doi: 10.1007/s00265-009-0786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Persistence of maternal effects in baboons: Mother’s dominance rank at son’s conception predicts stress hormone levels in subadult males. Horm Behav. 2008;54:319–324. doi: 10.1016/j.yhbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme R. Measuring fecal steroids: Guidelines for practical application. Ann. N.Y. Acad. Sci. 2005;1046:75–80. doi: 10.1196/annals.1343.007. [DOI] [PubMed] [Google Scholar]
- Pereira ME. Agonistic interactions of juvenile Savannah baboons. I. Fundamental Features. Ethology. 1988a;79:195–217. [Google Scholar]
- Pereira ME. Effects of age and sex on intra-group spacing behaviour in juvenile savannah baboons (Papio cynocephalus) Anim. Behav. 1988b;36:184–204. [Google Scholar]
- Plaistow SJ, Lapsley CT, Beckerman AP, Benton TG. Age and size at maturity: sex, environmental variability and developmental thresholds. Proc. R. Soc. Lond. B. 2004;271:919–924. doi: 10.1098/rspb.2004.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope TR. The evolution of male philopatry in neotropical monkeys. In: Kappeler PM, editor. Primate males: Causes and consequences of variation in group composition. Cambridge: Cambridge University Press; 2000. pp. 219–235. [Google Scholar]
- Plant TM. Puberty in primates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd ed. New York: Raven Press; 1994. pp. 453–485. [Google Scholar]
- Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol. Cell. Endocrinol. 2006;254:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Pluhacek J, Priya S, Bartos L, Sipek P. Parity as a major factor affecting infant mortality of highly endangered Indian rhinoceros: Evidence from zoos and Dudhwa National Park, India. Conserv. Biol. 2007;139:457–461. [Google Scholar]
- Pusey AE. Magnitude and sources of variation in female reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Chicago: University of Chicago Press; 2012. pp. 343–363. [Google Scholar]
- Pusey AE, Packer C. Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 250–266. [Google Scholar]
- Reale D, Festa-Bianchet M. Quantitative genetics of life-history traits in a long-lived wild mammal. Heredity. 2000;85:593–603. doi: 10.1046/j.1365-2540.2000.00795.x. [DOI] [PubMed] [Google Scholar]
- Samuels A, Altmann J. Baboons of the Amboseli basin: demographic stability and change. Intl. J. Primatol. 1991;12:1–9. [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress-response. In: Becker JB, editor. Behavioral endocrinology. Cambridge, MA: MIT Press; 1992. pp. 287–324. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influencestress responses? Integrating permissive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Setchell JM. Behavioural Development in Male Mandrills (Mandrillus sphinx): Puberty to Adulthood. Behaviour. 2003;140:1053–1089. [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx) Am. J. Phys. Anthropol. 2001;115:349–360. doi: 10.1002/ajpa.1091. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ, Knapp LA. Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank, and group association. Am. J.Phys. Anthropol. 2006;131:498–510. doi: 10.1002/ajpa.20478. [DOI] [PubMed] [Google Scholar]
- Shea BT. Ontogenetic approaches to sexual dimorphism in anthropoids. Hum. Evol. 1986;1:97–110. [Google Scholar]
- Silk JB. Social behavior in evolutionary perspective. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 318–329. [Google Scholar]
- Silk JB. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social relationships among adult female baboons (Papio cynocephalus): II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 2006a;61:197–204. [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus): I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 2006b;61:183–195. [Google Scholar]
- Silk JB, Altmann J, Alberts SC, Cheny DL, Seyfarth RM. Stability of partner choice among female baboons. Anim. Behav. 2012;83:1511–1518. doi: 10.1016/j.anbehav.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Haukka J, Dunkel L, Tynelius P, Rasmussen F. Genetics of pubertal timing and its associations with relative weight in childhood and adult height: The Swedish young male twins study. Pediatrics. 2008;121:E885–E891. doi: 10.1542/peds.2007-1615. [DOI] [PubMed] [Google Scholar]
- Sol D. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol Lett. 2009;5:130–133. doi: 10.1098/rsbl.2008.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DM. The sociobiology of infant and adult male baboons. Norwood, New Jersey: Ablex Publishing Corporation; 1984. [Google Scholar]
- Sugawara K. Ethological Study of the Social Behavior of Hybrid Baboons Between Papio anubis and P., hamadryas in Free-ranging Groups. Primates. 1988;29:429–444. [Google Scholar]
- Tung J, Alberts SC, Wray GA. Evolutionary genetics in wild primates: combining genetic approaches with field studies of natural populations. Trends Genet. 2010;26:353–362. doi: 10.1016/j.tig.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Akinyi MY, Mutura S, Altmann J, Wray GA, Alberts SC. Allele-specific gene expression in wild nonhuman primates. Mol. Ecol. 2011;20:725–739. doi: 10.1111/j.1365-294X.2010.04970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Charpentier MJE, Garfield DA, Altmann J, Alberts SC. Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Mol. Ecol. 2008;17:1998–2011. doi: 10.1111/j.1365-294X.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg SM, Boomsma DI. The familial clustering of age at menarche in extended twin families. Behav. Genet. 2007;37:662–667. doi: 10.1007/s10519-007-9161-4. [DOI] [PubMed] [Google Scholar]
- Van Noordwijk MA, van Schaik CP. The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascularis. Primates. 1999;40:105–130. doi: 10.1007/BF02557705. [DOI] [PubMed] [Google Scholar]
- Van Noordwijk MA, van Schaik CP. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In: Kappeler P, van Schaik C, editors. Sexual selection in primates: New and comparative perspectives. Cambridge: Cambridge University Press; 2004. pp. 208–229. [Google Scholar]
- Walters JR, Seyfarth RM. Conflict and cooperation. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 306–317. [Google Scholar]
- Wells JCK, Stock JT. Re-examining heritability: genetics, life history and plasticity. Trends Endocrinol. Metab. 2011;22:421–428. doi: 10.1016/j.tem.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Brockman DK, Stavisky RC. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Yr. Bk. Phys. Anthropol. 1998;41:1–23. doi: 10.1002/(sici)1096-8644(1998)107:27+<1::aid-ajpa2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Williamblangero S, Blangero J. Heritability of age at first birth in captive olive baboons. Am. J. Primatol. 1995;37:233–239. doi: 10.1002/ajp.1350370305. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Brodie III ED, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]


