Abstract
Introduction
Whether intestinal dysmotility and proton pump inhibitor (PPI) use either independently or together contributes to small intestinal bacterial overgrowth (SIBO), and/or small intestinal fungal overgrowth (SIFO) is not known.
Aim
Investigate the role of dysmotility and PPI use in patients with persistent gastrointestinal complaints.
Methods
Patients with unexplained gastrointestinal symptoms and negative endoscopy/radiology tests completed a validated symptom questionnaire and underwent 24-hour ambulatory antro-duodeno-jejunal manometry (ADJM). Simultaneously, duodenal aspirate was obtained for aerobic, anaerobic and fungal culture. Dysmotility was diagnosed by (> 2): absent phase III MMC, absent/diminished postprandial response, diminished amplitude of antral/intestinal phasic activity, impaired antro-duodenal coordination. Bacterial growth ≥103 CFU/mL or fungal growth was considered evidence for SIBO/SIFO. PPI use was documented. Correlation of symptoms with presence of SIBO or SIFO were assessed.
Results
150 subjects (M/F=47/103) were evaluated; 94/150 (63%) had overgrowth: 38/94 (40%) had SIBO, 24/94 (26%) had SIFO, and 32/94 (34%) had mixed SIBO/SIFO. SIBO was predominately due to Streptococcus, Enterococcus, Klebsiella, and E. coli. SIFO was due to Candida. 80/150 (53%) patients had dysmotility and 65/150 (43%) used PPI. PPI use (p=.0063) and Dysmotility (p=.0003) were independent significant risk factors (p<0.05) for overgrowth, but together did not pose additional risk. Symptom profiles were similar between those with or without SIBO/SIFO.
Conclusions
Dysmotility and PPI use were independent risk factors for SIBO or SIFO and were present in over 50% of subjects with unexplained gastrointestinal symptoms. Diagnosis of overgrowth requires testing because symptoms were poor predictors of overgrowth.
Keywords: Dysmotility, proton pump inhibitor, Small Intestinal Bacterial Overgrowth, Small Intestinal Fungal Overgrowth, breath testing, antroduodenojejunal manometry
INTRODUCTION
There is growing recognition that the intestinal microbiome could play an important role in the pathogenesis of gastrointestinal symptoms. Additionally previous studies have implicated abnormal small bowel motility in the pathogenesis of small intestinal bacterial overgrowth (SIBO) 1, 2.
The normal motility of the upper gut consists of organized, repetitive migrating movements (Migrating Motor Complexes- MMCs).2,3 If the normal nerve and muscle function of the gut is disrupted, then two major subtypes of small intestinal dysmotility, notably myopathy (predominant muscle dysfunction) or neuropathy (predominant neuronal dysfunction) have been described2,3. Vantrappen et al. reported that the MMC patterns were abnormal in 5/12 patients with bacterial overgrowth2 suggesting a relationship between altered microbiome and gut dysmotility. Furthermore, Husebye et al. reported that abnormal MMC and burst activity were strong predictors of gram negative bacterial growth in the small bowel4.
Gastric acid is another important barrier for the prevention of bacterial colonization of the stomach and proximal small intestine5. By increasing the gastric pH, PPIs may facilitate the survival and colonization of bacteria6. Hypochlorhydria has also been shown to contribute to the proximal migration of more distally located bacteria in the GI tract7. Recently, Lombardo et al. reported that SIBO, as diagnosed by the glucose hydrogen breath test, occurs more frequently in PPI users than in healthy controls (50% vs. 6%), and in PPI non-users (25%) with IBS7. They further showed that the prevalence of SIBO and the severity of GI symptoms increased after one year of PPI use7. Husebye et al. suggested that an increase of one pH unit in the small intestine corresponded to a 13.8% increase in small bowel microbial counts4. These observations suggest that PPI therapy may have an effect on bacterial concentrations in the small bowel. Although PPI use and dysmotility have been suggested to be associated with SIBO, whether these factors independently or together contribute to the pathogenesis of chronic, unexplained GI symptoms and small intestinal bacterial overgrowth has not been systematically evaluated. Also, whether small intestinal fungal overgrowth (SIFO) may play a role in the pathogenesis of GI symptoms has been scarcely examined.
We tested the hypothesis that SIBO and/or SIFO are more likely to be prevalent in symptomatic patients with either small intestinal dysmotility and/or those taking PPIs. Our aim was to investigate the pathophysiologic role of gastrointestinal dysmotility and PPI use in causing SIBO and/or SIFO in patients with chronic, unexplained GI symptoms by performing prolonged 24 hour antro-duodenal-jejunal manometry and culture of duodenal aspirate, and by examining the relationship of symptoms to these factors.
MATERIALS AND METHODS
We evaluated 150 consecutive patients who presented to a single gastroenterologist between the years of 1995–2010. These subjects had unexplained gastrointestinal symptoms. All of these patients had a negative evaluation for routine gastrointestinal pathology including a normal gastroscopy, colonoscopy, CT scan, routine hematology and biochemical profiles, anti-tTG, TSH, right upper quadrant ultrasound, and small bowel follow-through series. Patients with known gastrointestinal problems including previous GI surgeries (except cholecystectomy, hysterectomy and appendectomy), and those who were using medications that potentially affect intestinal motility (opioids, anticholinergics, antidiarrheals) and those with significant co-morbid medical problems or those who were hospitalized were excluded.
The study was approved by University of Iowa Hospitals and Clinics Investigation Review Board.
Symptom Questionnaire
A validated bowel symptom questionnaire was administered to all subjects prior to the study8. It enquired about the presence or absence of the following ten symptoms in the preceding two weeks: abdominal pain, chest pain, belching, bloating, fullness, indigestion, nausea, diarrhea, vomiting, and gas. If present, patients were asked to rate each symptom’s frequency, intensity, and duration on a 0–3 Likert-like scale. Intensity: 0= no symptoms, 1= mild, 2= moderate, 3= severe symptoms. Frequency: 0= None; 1= Less than 1 episode/week, 2= 1 episode/week, 3= More than 1 episode/week. Duration: 0= None, 1= Less than 10 minutes, 2= 10–30 minutes, 3= Greater than 30 minutes. On this scale, the total score for each symptom could range from 0–9. A mean total score for all 10 symptoms was calculated for each patient.
Patients’ medications were documented and additionally the hospital electronic medical record database was used to confirm the use of PPIs during their initial presentation and during evaluation of their GI symptoms.
Antro-duodeno-jejunal Manometry (ADJM)
Manometric System
We used a 250 cm long elastic catheter that was custom-built with 6 solid state pressure transducers (Koningsberg Instruments, Pasadena, CA). The probe was connected to a six-channel portable solid-state digital data-logger (MicroDigitrapper 4 Mb, Medtronics; Minneapolis, MN) with a sampling frequency of 4 Hz, A-D conversion, temporary storage up to 4 Mb. Upon completion of the study, data were downloaded to an IBM-compatible personal computer for analysis (Gastrosoft version 6.3, Multigram, Synectics Medical Inc.).
Study Protocol
Following an overnight fast, all subjects had an upper endoscopy. Next, using sterile precautions, a 2mm Ligory catheter was passed through the biopsy channel of the upper endoscope into the 3rd and 4th portions of the duodenum. Using gentle suction, approximately 3–5mL of duodenal fluid was aspirated, and the specimen was sent to microbiology for aerobic/anaerobic culture and fungal culture. Next, the nares were numbed with 2% lidocaine gel and the 6-sensor solid-state manometry probe was placed under endoscopic and fluoroscopic guidance such that 2 sensors (5 cm apart) were located in the antrum, 2 sensors (15 cm apart) were located in the duodenum, and 2 sensors (15 cm apart) were located in the jejunum.
The ambulatory recorder was placed in a shoulder bag and the patients were free to ambulate throughout the study and slept at home. Six hours after probe placement, all patients ate a 600 kilocalorie standard meal consisting of a chicken sandwich, 6 oz. of milk, a cookie and a banana. The nutrient composition was 52% carbohydrates, 25% protein and 23% fat. The following morning they were instructed to wake up at 6 am. The motility recording was continued until 11 am and thereafter the probe was removed. An event marker was attached to the recorder, and the patients were encouraged to use this and mark the time of events such as eating, walking, and sleeping or to indicate the occurrence of symptoms such as abdominal pain, passing flatus, etc. They were also provided with a diary, in which they described any event(s) or symptom(s), and recorded its time and duration.
Data analysis
After completion of the recording, the data stored in the portable recorder was transferred to a personal computer for visual display and analysis. Tracings were analyzed by visual inspection for motility patterns such as phase III MMCs, and for quantitative assessment of pressure activity such as area under the curve (AUC) of the pressure waves. Pressure waves that occurred simultaneously in several channels with similar amplitude and duration of ≤ 3 seconds were identified as artifacts and excluded from the analysis. Phase III MMCs were defined as propagating clusters of repetitive contractions with a frequency of 3/minute in the antrum and 11–13/minute in the duodenum and with a duration of at least 3 minutes that was followed by a period of motor quiescence. 3, 9–11 The pressure activity data from each of the two sensors located in the antrum, duodenum, and jejunum were averaged and were used as an overall index of motility for each segment.
Based on the manometric findings, we classified our patients as follows: 1) Normal motility (with both normal frequency and intensity of pressure activity and coordination) 2) Neuropathy (with normal frequency and intensity of pressure activity and lack of coordination), 3) Myopathy (With normal frequency, low intensity of pressure activity and normal coordination) and 4) Mixed (features of neuropathy and myopathy).3, 11
Furthermore, each patient was classified as having either dysmotility if they had two or more of the following characteristics: absence of phase III MMC activity (neuropathy), absence/diminished postprandial response (≤ 2 SD of normal), diminished amplitude of antral/intestinal phasic activity (≤ 20 mm Hg), or impaired antro-duodenal coordination10, 11(propagation of peristaltic waves between antrum and duodenal sensors).
Patients were considered to have small intestinal overgrowth if microbiology reported a positive culture for either aerobic, anaerobic, or fungal organisms. Bacterial concentration ≥103 CFU/mL was considered as positive for SIBO. Additionally, we also assessed the prevalence of bacterial concentrations ≥105 CFU/mL (used for jejunal samples12). Because normally there is no fungus or very low concentrations of fungal organisms in the small bowel13, a diagnosis of SIFO was made if the duodenal culture yielded growth of fungal organisms.
Based on culture results, patients were categorized into four groups: SIBO, SIFO, mixed SIBO/SIFO, and negative for proximal small bowel overgrowth. A second set of analysis was done on two groups: overgrowth positive (patients with SIBO and/or SIFO) and overgrowth negative (culture negative for bacteria and fungi).
PPI users consisted of patients that were taking PPIs at the time of their GI evaluation at our center. PPI use was continued throughout their motility testing. The duration of PPI use and patient compliance with PPI treatment regimen, prior to the onset of symptoms could not be accurately assessed from our questionnaire. PPI users and PPI non-users were compared for the presence or absence of overgrowth. In addition to questionnaire documentation of PPI use an independent assessment of the patient’s chart was performed to confirm/refute PPI use.
The principal investigator (SSR) was not involved in the data analysis and interpretation of study results and was blinded to study ID during discussion of study results. The data analyses were performed independently by CJ and EC-A and verified by AA with assistance from JV.
Statistical Analyses
Fisher’s Exact Test was used for analysis. Individual contingency tables were constructed for overgrowth versus dysmotility and overgrowth versus PPI use. Each individual subset of culture data (SIBO, SIFO, and Mixed SIBO/SIFO) versus dysmotility and versus PPI use was similarly analyzed. A p value <0.05 was considered to be significant. Comparative analyses were performed for both the ≥ 103 and >105 CFU/ml bacterial concentration and are described under the results section. However for the key discussion and analysis, the data analyzed at a bacterial concentrations of > 103 CFU/ml was used. Odds ratios were calculated for each significant contingency table. Symptom analysis was done by constructing 2×2 contingency tables. Fisher exact test and odds ratios were obtained with 95% confidence intervals.
RESULTS
Demographics and Symptom Profiles
168 subjects (M/F=54/114; ages 17–82 years; average age 44) were evaluated. Eighteen subjects were excluded; 6 because of incomplete motility studies or patient compliance with manometry protocol; 6 other subjects who had motility studies but either failed to provide information on PPI use or we could not clearly ascertain its use from their medical records; and 6 other subjects because of incomplete culture data or suspected contamination. Thus, 150 subjects (M/F=46/104; ages 17–82 years; average age 43) were examined.
The mean (95% CI) duration of symptoms were not statistically different (p>0.05) between the groups (SIBO= 58, 32–83 mo; SIFO 47, 27–66; Mixed= 56, 30–83; Negative= 53, 35–71). Comorbid conditions in the study population included diabetes (13 subjects) (SIBO 6, Mixed 1, Negative 4 and SIFO 2) and scleroderma (5 subjects) (SIBO 1, Mixed 1, Negative 2 and SIFO 1). BMI was not different among the groups (mean BMI in SIBO= 31; Mixed= 30, Negative= 31 and SIFO= 29 respectively).
Results for the mean baseline symptom scores are shown in Figures 1–2 and in Table 1. The overall mean baseline total symptom scores were similar between those with SIBO and/or SIFO and those with a negative culture (44 versus 42) (Figure 1). Subjects with SIBO and/or SIFO had higher symptom severity scores for chest pain, belching, bloating, indigestion, nausea, diarrhea, and gas, but lower scores for abdominal pain, fullness, and vomiting (Table 1). The baseline mean total symptom scores for each of the four groups are as follows: SIBO = 43; Mixed SIBO/SIFO = 42; SIFO = 48; and No overgrowth = 42, p= >0.05. The mean scores for each symptom and for each group are shown in Figure 1. There was no difference in the prevalence of symptoms between those who had a positive culture for bacterial overgrowth or fungal overgrowth versus those who had a negative culture, except for chest pain (p<0.05), probably inconsequential.
Figure 1.
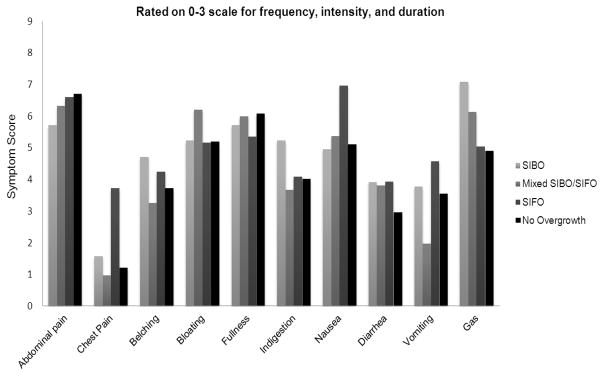
The distribution of the mean symptom severity score for each symptom among the four groups of subjects
Figure 2.
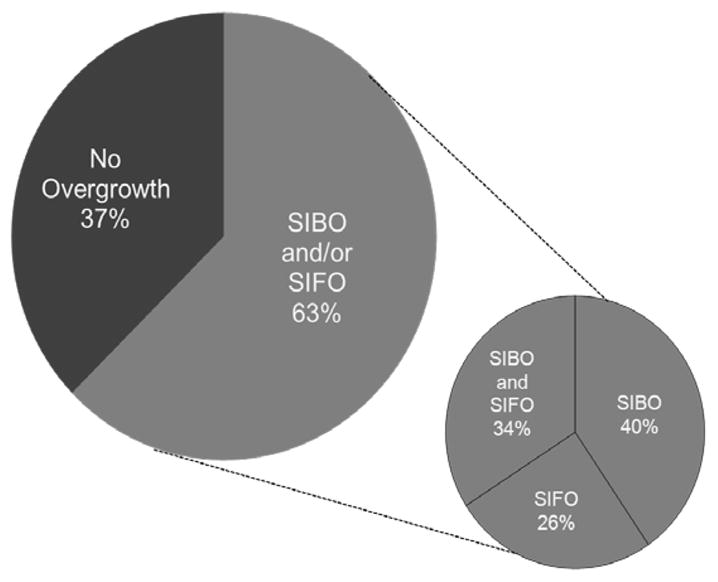
Prevalence of overgrowth in the study population (left) and the distribution of patients with SIBO or SIFO or SIBO/SIFO.
Table 1.
Symptom severity score in patients with and without overgrowth
| Abdominal pain | Chest Pain | Belching | Bloating | Fullness | Indigestion | Nausea | Diarrhea | Vomiting | Gas | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIBO and/or SIFO | 6.21 | 2.09 * | 4.07 | 5.53 | 5.69 | 4.33 | 5.76 | 3.89 | 3.44 | 6.08 | 44.01 |
| No Overgrowth | 6.70 | 1.21 | 3.71 | 5.18 | 6.08 | 4.02 | 5.11 | 2.95 | 3.55 | 4.90 | 42.00 |
p= < 0.05
Prevalence of SIBO/SIFO
Based on a growth of bacterial concentration of ≥103 CFU/mL, we found that 94/150 (62.7%) patients were positive for overgrowth: 38/94 (40%) had SIBO, 24/94 (26%) had SIFO, and 32/94 (34%) had mixed SIBO/SIFO (Figure 2). Based on a growth of bacterial concentration ≥105 CFU/mL, 77/150 (51%) had overgrowth: 20/77 (26%) had SIBO, 40/77 (52%) SIFO, and 16/77 (20%) had mixed SIBO/SIFO (table 2).
Table 2.
Comparison between CFU concentrations in patients with dysmotility and PPI use among groups
| CFU | Factor | Overall group n =150 | SIBO n =38 (25.3%) | SIFO n =24 (16%) | SIBO &SIFO n =32 (21.3%) | No Overgrowth n =56 (37.3%) |
|---|---|---|---|---|---|---|
| 103 | Dysmotility | Yes (n=80) | 23 (60.5%) * | 13 (54.2%) * | 25 (78.1%) * | 19 (33.9%) |
| No (n=70) | 15 (39.5%) | 11 (45.8%) | 7 (21.9%) | 37 (66.1%) | ||
|
| ||||||
| PPI use | Yes (n=65) | 21 (55.3%) * | 11 (45.8%) * | 17 (53.1%) * | 16 (28.6%) | |
| No (n=85) | 17 (44.7%) | 13 (54.2%) | 15 (46.9%) | 40 (71.4%) | ||
|
| ||||||
| Overall group n =150 | SIBO n =20 (13.3%) | SIFO n =40 (26.6%) | SIBO &SIFO n =16 (10.6%) | No Overgrowth n =73 (48.6%) | ||
|
| ||||||
| 105 | Dysmotility | Yes (n=80) | 12 (60.5%) | 22 (55%) | 14 (88%) * | 29 (39.7%) |
| No (n=70) | 8(39.5%) | 18 (45%) | 2 (12%) | 44 (60.3%) | ||
|
| ||||||
| PPI use | Yes (n=65) | 10 (50%) | 21 (52.5%) * | 10 (63%) * | 25 (34%) | |
| No (n=85) | 10 (50%) | 19 (47.5%) | 6 (47%) | 54 (66%) | ||
= < 0.05 versus no overgrowth group
When we compared the two bacterial concentrations ( >103 vs. >105), we found significant difference in the prevalences of positive cultures for SIBO, 70/150 (103 CFU/mL) vs. 37/150 (105 CFU/mL) based on the presence of a positive culture (p=0.0001) as well as for the SIBO group alone (38/94(103 CFU/mL) vs. 20/73 (105 CFU/mL); p=0.007) and Mixed SIBO/SIFO group (32/94 (103 CFU/mL) vs. 17/73 (105 CFU/mL); p=0.01). (Table 2)
The predominant aerobic bacterial organisms that were cultured included: alpha hemolytic Streptococcus species (n= 32); non-hemolytic Streptococcus including Enterococcus and Stomatococcus species (n= 10); Klebsiella species (n= 8); E. coli (n= 6); Neisseria sp (n= 5); Staphlococcus sp (n= 4); and Enterobacter sp (n= 3). Eight patients had positive anaerobic cultures including Veillonella sp (n= 5); Clostridium species (n= 1); Bacteroides (n= 1); and Peptostreptococcus (n= 1). Fungal growth was predominately due to Candida species (albicans or torulopsis).
The Effects of Dysmotility on Overgrowth
>103 Analysis
80/150 (53%) patients with chronic, GI complaints had dysmotility. 61/80 (76%) patients with dysmotility had small bowel bacterial and/or fungal overgrowth based on bacterial growth of >103 CFU/ml. (Figure 3, Table 2). A significant relationship (p= 0.0003) was found between dysmotility and small bowel bacterial overgrowth, even when controlling for PPI use. Patients with dysmotility had an odds ratio of 3.60 of having a small bowel bacterial overgrowth than those with normal motility.
Figure 3.
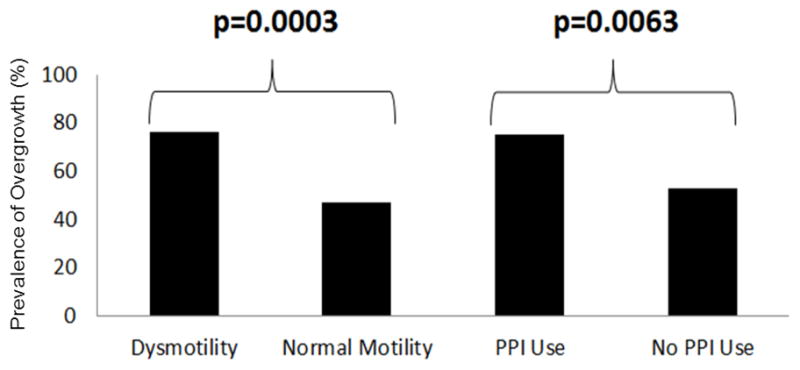
The prevalence of SIBO and/or SIFO in patients with dysmotility and in PPI users
Dysmotility was an independent and significant predictor (p= 0.0003) for small bowel bacterial overgrowth (SIBO and/or SIFO) (Figure 3, Table 2). Analyses done on each separate group (SIBO, SIFO, and Mixed SIBO/SIFO vs. negative for dysmotility) were found to be significant in the SIBO (p= 0.013) and Mixed SIBO/SIFO (p= 0.0001) groups. However, dysmotility was not a significant predictor for SIFO alone (p= 0.14).
>105 Analysis
49/80 (61%) patients with dysmotility had small bowel and/or fungal overgrowth based on bacterial concentration of >105 CFU/ml. A significant relationship (p= 0.005) was found between dysmotility and SIBO at this bacterial concentration threshold, and even when controlling for PPI use (Table 2). Patients with dysmotility had an odds ratio of 2.70 of having SIBO than those with normal motility.
Analyses done on each separate group (SIBO, SIFO, and Mixed SIBO/SIFO vs. negative for dysmotility) were found to be significant in the Mixed SIBO/SIFO (p= 0.0003) but not SIBO (p= 0.13) or SIFO (p= 0.17) groups.
The Effects of PPI Use on Overgrowth
103 Analysis
65/150 (43%) patients with chronic GI complaints were using PPIs. 49/65 (75%) patients on prolonged PPI therapy had small bowel bacterial and/or fungal overgrowth (Figure 3, Table 2). There was a significant relationship (p= 0.0063) between PPI use and small bowel bacterial overgrowth, irrespective of dysmotility. Patients taking PPIs had an odds ratio of 2.72 of having a small bowel bacterial overgrowth than those who were not taking PPIs.
PPI use (p= 0.0063) was an independent and significant predictor for small bowel bacterial overgrowth (SIBO and/or SIFO) (Figure 3, Table 2). Analyses were also performed for each separate group (SIBO, SIFO, and Mixed SIBO/SIFO vs. negative for PPI use) and were found to be significant in the SIBO (p= 0.011) and Mixed SIBO/SIFO (p= 0.038) groups. However, PPI use was not a significant predictor for SIFO alone (p= 0.197).
105 Analysis
40/65 (62%) patients on prolonged PPI therapy had SIBO or SIFO. There was a significant relationship (p= 0.008) between PPI use and SIBO, irrespective of dysmotility. (Table 2) Patients taking PPIs had an odds ratio of 2.46 of having SIBO than those who were not taking PPIs.
Analyses were also performed on each separate group (SIBO, SIFO, and Mixed SIBO/SIFO vs. negative PPI use) and we found that there was a significant difference for the SIFO (p= 0.03) group and Mixed SIBO/SIFO (p= 0.03) group but not for the SIBO (p= 0.19) group.
DISCUSSION
In this study, we evaluated the effects of dysmotility and PPI use on small bowel bacterial and fungal overgrowth by performing a comprehensive assessment in a cohort of patients with chronic gastrointestinal symptoms without clear etiology and whose overlapping symptom profiles could not be categorized based on traditional Rome criteria.
We found evidence for small intestinal bacterial and/or fungal overgrowth as defined by the presence of increased numbers of bacteria or fungi in 63% of this patient population. Furthermore, the majority of patients (76%) with dysmotility had SIBO, SIFO, or Mixed SIBO/SIFO. Similarly, 75% of patients on PPI therapy had cultures that were positive for bacteria and/or fungal organisms.
We found a higher prevalence of overgrowth in PPI users than those recently reported [100/200 (50%)] by Lombardo et al.7 The higher yield in our study is most likely due to the use of small intestinal aspirate and aerobic/anaerobic/fungal culture (generally considered a gold standard, although invasive) for a diagnosis of overgrowth as opposed to the use of a less sensitive glucose hydrogen breath test (sensitivity and specificity ranges are 20–93% and 30–86%, respectively) in the previous studies14–17. Also glucose breath test cannot diagnose SIFO.
Choung et al. found that patients taking PPIs more commonly had an abnormal culture result than patients who were not taking PPIs, although there was no significant relationship at bacterial concentrations ≥105 CFU/mL17. There was, however, a significant association between PPI use and “indeterminate” bacterial concentrations (0 through less than 105 CFU/mL) 17. They suggested that PPI use may be associated with a “low grade” form of SIBO. In the present study, we used the bacterial concentration ≥103 CFU/mL, which is consistent with the findings of Choung et al17. However, unlike their study, we did not find significant differences in the yield of positive test for SIBO between the two bacterial concentrations. Furthermore, the bacterial concentration at which patients may experience symptoms, in the context of SIBO is not known.
Regarding the cut-offs for bacterial counts, we have provided data for both, the conventional ≥ 105 counts and for our proposed ≥ 103 counts. Although we found a significant difference between the two bacterial concentrations for the prevalence of SIBO, even with the higher bacterial concentration (>105CFU/mL), we found a significant association for the presence of SIBO/SIFO with dysmotility and PPI use. This finding further attests to the validity of these proposed risk factors. However, for most of the key data analysis and discussion, we used the lower cut off values since we felt that this count may be more representative of the normal bacterial concentration in the duodenum because of its close proximity to the acid environment in the stomach than the higher cut-off that is typically used for any microbial infection. Furthermore,, we wish to acknowledge that whether otherwise healthy subjects have any bacteria or fungus in this duodenal segment and at count of 103 is not known. We believe that further research regarding bacterial concentrations in different gut segments in healthy subjects is needed.
We hypothesized that the combined presence of two factors such as dysmotility and PPI use may predispose to a higher prevalence of SIBO, but this was not the case, either because the individual prevalence was moderately high or because of the heterogeneity of our patient groups or that these two factors are independent and not related.
One novel finding of our study was the detection of fungal organisms in the 24 patients who only had a positive culture for candida. Unlike SIBO, where there is some recent information, there is virtually no data regarding normal concentrations of fungi in the proximal small bowel. This may in part be due to the slow-growing nature of the fungal organisms and also a lack of knowledge of this possibility. Although the concentration of fungal overgrowth in the proximal bowel is considered to be very low13, we identified that 27% of our patients had positive fungal culture. This observation merits further study and confirmation.
Although candida infections are usually seen in the neonatal, elderly, or immunocompromised13, 18, 19 individuals or those on steroids, or repeated antibiotic use, our findings suggest that fungal organisms are not uncommonly present in patients with chronic GI complaints. In one study, candida was the most common organism identified in nasogastric aspirates from the proximal GI tract of preoperative patients with GI disorders (malignancy, inflammatory bowel disease, and benign conditions).20 Another study identified fungal growth in stool cultures of six patients with diarrhea and abdominal pain.21 Apart from these anecdotal case reports, there has been no systematic study of the prevalence and clinical presentation of small intestinal fungal organisms in patients with chronic, unexplained GI symptoms.
The clinical manifestations of SIBO are non-specific and include symptoms such as gas, bloating, abdominal pain, and diarrhea. More serious manifestations of bacterial overgrowth of the small bowel include malabsorption syndromes, weight loss, malnutrition, vitamin deficiency, and anemia22. Symptoms of SIFO are however not known but based on our study we believe that SIFO shares the same set of symptoms as SIBO. Our study was unable to identify a single symptom or cluster of symptoms that can clinical recognize patients with either SIBO or SIFO. Thus, symptoms were generally poor predictors of bacterial and/or fungal overgrowth. However, SIBO and/or SIFO were prevalent in over 50% of this patient population with dysmotility and chronic use of PPI and should be considered in the differential diagnosis of patients with non-specific chronic GI complaints.
We would advocate screening for SIBO in symptomatic patients who have known dysmotility or taking PPIs. The glucose breath test is widely-used as a non-invasive method of diagnosing SIBO23. Screening for SIBO can start with a non-invasive glucose hydrogen breath test and, if test results are negative and if clinical suspicion remains high, aspiration and culture of small bowel contents may be considered. At present, culture of small bowel aspirate appears to be the only method of identifying fungal organisms in the small bowel.
There are some limitations to our study that should be considered when interpreting these findings. Because our documentation relied on prospective patient questionnaire and medical records, we were unable to accurately determine the duration of PPI treatment in all our subjects. It has been suggested that longer durations of PPI therapy are associated with an increased risk of SIBO7. Another limitation may involve collection and culture of organisms. Although sterile techniques were consistently used, and by a single operator, it is possible that collecting specimens at precisely the same point along the small intestine is difficult. However, the high prevalence of bacteria and candida in duodenal aspirates attests to the presence of these organisms in a significant proportion of this population. The symptom profiles were based solely on the bowel symptom questionnaire and are prone to subject bias, and may account for a lack of difference, especially as these patients had long standing refractory symptoms and were referred to a tertiary care center. Also, other factors such as visceral hypersensitivity and IBS may have been present in these patients leading to reports of greater symptom severity. Also, our results from a selected population presenting to a tertiary care specialist center should be interpreted with caution and may not be generalizable to the population at large.
We conclude that dysmotility and PPI use appear to be important and independent risk factors associated with an overgrowth of small intestinal bacteria and/or fungal organisms. Symptom profiles by themselves are poor predictors of overgrowth in this patient population.
Acknowledgments
Financial Support
Authors’ declaration of personal interests: none
Declaration of Funding interest: this study was partially supported by NIH grant No. 2R01 KD57100-05A2.
Abbreviations
- SIBO
small intestinal bacterial overgrowth
- SIFO
small intestinal fungal overgrowth
- MMC
migrating motor complex
- PPI
proton pump inhibitors
- ADJM
Antro-duodeno-jejunal Manometry
Footnotes
Guarantor: Satish SC Rao, MD, PhD, FRCP, FACG – Study concept and design, study recruitment, manuscript preparation, critical revision, important intellectual content and final approval.
Contributing authors:
Carolyn Jacobs: data collection, data analysis, manuscript writing.
Enrique Coss-Adame, MD: data analysis, statistical analysis, data collection, manuscript preparation.
Ashok Attaluri, MD: Study concept and design, statistical analysis.
Jessica Valestin: Data collection, manuscript preparation.
References
- 1.Hoog CM, Lindberg G, Sjoqvist U. Findings in patients with chronic intestinal dysmotility investigated by capsule endoscopy. BMC Gastroenterol. 2007;7:29. doi: 10.1186/1471-230X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–66. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M, Bharucha AE, Di Lorenzo C, et al. Neurogastroenterology and Motility society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 4.Husebye E, Skar V, Hoverstad T, et al. Abnormal Intestinal Patterns Explain Enteric Colonization with Gram-Negative Bacilli in Late Radiation Enteropathy. Gastroenterology. 1995;109:1078–89. doi: 10.1016/0016-5085(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 5.Laine L, Ahnen D, McClain C, et al. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651–68. doi: 10.1046/j.1365-2036.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanduleanu S, Jonkers D, De Bruine A, et al. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15:379–88. doi: 10.1046/j.1365-2036.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased Incidence of Small Intestinal Bacterial Overgrowth During Proton Pump Inhibitor Therapy. Clin Gastroenterol Hepatol. 2010;8:505–508. doi: 10.1016/j.cgh.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Choi YK, Kraft N, Zimmerman B, et al. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233–8. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 9.Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141–161. doi: 10.1046/j.1365-2982.1999.00147.x. [DOI] [PubMed] [Google Scholar]
- 10.Soffer EE, Thongsawat S, Ellerbroek S. Prolonged ambulatory duodeno-jejunal manometry in humans: normal values and gender effect. Am J Gastroenterol. 1998;93:1318–23. doi: 10.1111/j.1572-0241.1998.441_k.x. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Hasler WL, Parkman HP, et al. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747–762. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 12.Posserud I, Stotzer PO, Björnasson ES, et al. Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome. Neurogastroenterol Motil. 2007;56:802–08. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulze J, Sonnenborn U. Yeast in the Gut: From Commensals to Infectious Agents. Medicine. 2009;106(51–52):837–42. doi: 10.3238/arztebl.2009.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoshini R, Dai SC, Lezcano S, Pimentel M. A Systematic Review of Diagnostic Tests for Small Intestinal Bacterial Overgrowth. Dig Dis Sci. 2001;53:1443–54. doi: 10.1007/s10620-007-0065-1. [DOI] [PubMed] [Google Scholar]
- 15.Compare D, Pica L, Rocco A, et al. Effects of long term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest. 2011;41:380–386. doi: 10.1111/j.1365-2362.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 16.Simrén M, Stotzer P-O. Use and abuse of hydrogen breath test. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choung RS, Ruff KC, Malhotra A, et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059–67. doi: 10.1111/j.1365-2036.2011.04625.x. [DOI] [PubMed] [Google Scholar]
- 18.Calderone RA. Taxonomy and biology of Candida. In: Calderone RA, editor. Candida and Candidiasis. Washington: ASM Press; 2002. pp. 15–27. [Google Scholar]
- 19.Ruhnke M. Epidemiology of Candida albicans infections and role of non-candida-albicans yeast. Curr Drug Targ. 2006;7:495–504. doi: 10.2174/138945006776359421. [DOI] [PubMed] [Google Scholar]
- 20.Reddy BS, Gatt M, Sowdi R, et al. Gastric colonization predisposes to septic morbidity in surgical patients: A prospective study. Nutrition. 2008;24:632–637. doi: 10.1016/j.nut.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Kane JG, Chretien JK, Garagusi VF. Diarrhea caused by Candida. Lancet. 1976;307:335–336. doi: 10.1016/s0140-6736(76)90087-8. [DOI] [PubMed] [Google Scholar]
- 22.Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978–90. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urita Y, Ishihara S, Akimoto T, et al. Seventy-five gram glucose tolerance test to assess carbohydrate malabsorption and small bowel bacterial overgrowth. World J Gastroenterol. 2006;12:3092–3095. doi: 10.3748/wjg.v12.i19.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]


