THE RENIN-ANGIOTENSIN SYSTEM
The renin-angiotensin system (RAS) is a hormonal system that is of vital importance not only in the regulation of arterial blood pressure and salt balance, but also in many physiologic and pathophysiologic mechanisms in almost every organ system.1–3 The system consists mainly of a 2-step enzymatic cascade catalyzed by renin and angiotensin-converting enzyme (ACE), generating angiotensin II (Ang II), a single bioactive peptide. Ang II, the main RAS effector hormone, acts through 2 receptor subtypes, Ang II types 1 and 2 receptors (AT1R and AT2R) (Fig. 1).4,5 Both the receptor types belong to the G protein–coupled receptor family but differ in terms of tissue distribution and cell signaling pathways. Most of the functions of Ang II are carried through AT1R. The role and biologic functions of AT2R are less studied. It has been documented that AT2R inhibits and antagonizes AT1R-mediated functions,6–9 and when stimulated by Ang II, AT2R exerts effects that are the opposite of AT1R, including antiinflammatory,10 antiproliferative,10 and antiapoptotic actions (Table 1).11 Hence, AT2R may play an important role in vascular aging.
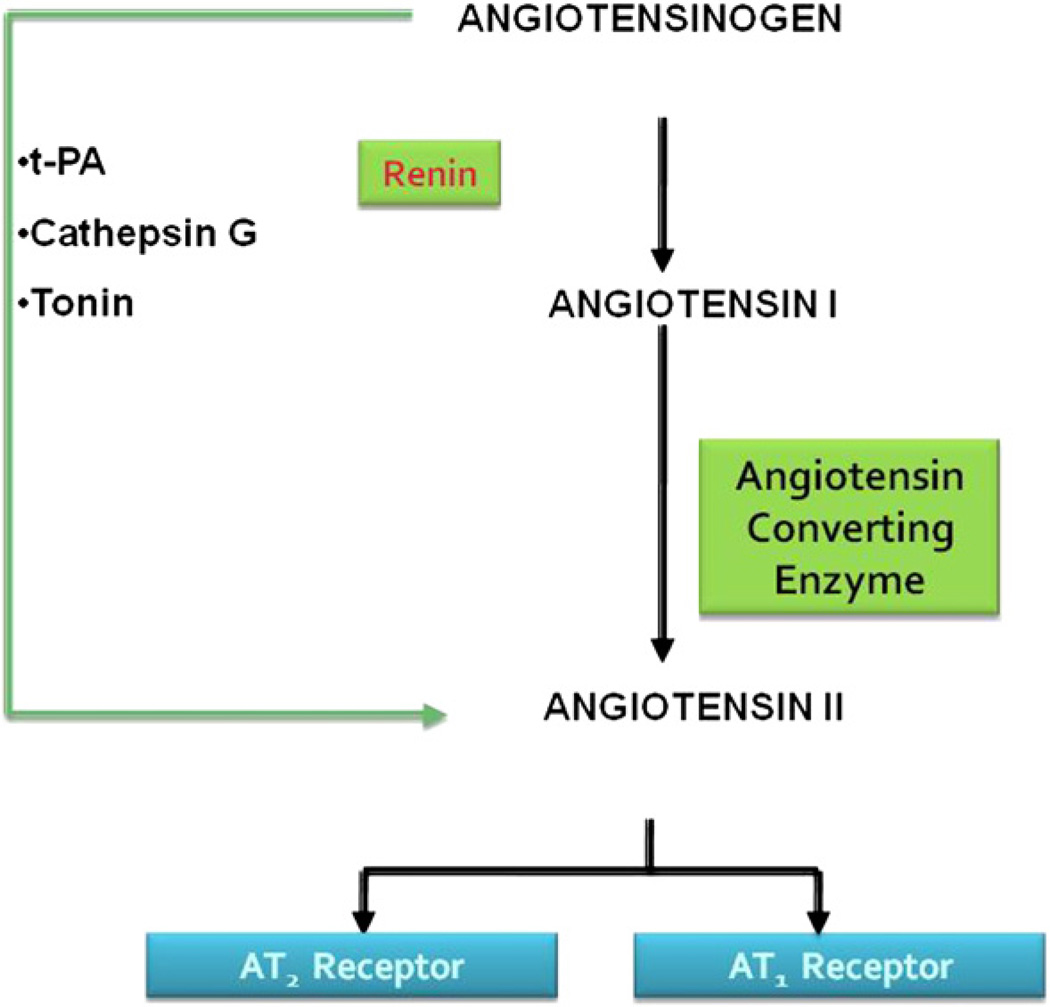
Fig. 1.
The steps in the biochemical pathway that is involved in the formation of the most biologically potent angiotensin peptide Ang II and its interaction with angiotensin receptors. The enzymes renin converts angiotensinogen to angiotensin I, which in turn is converted via angiotensin converting enzyme to Angiotensin II. Other enzymes that facilitate alternative pathways for the formation of Ang II are tPA, cathepsin G, and tonin. tPA, tissue plasminogen activator.
Table 1.
Opposing functions of AT1R and AT2R, which might be linked to aging
| AT1R | AT2R |
|---|---|
| Vasoconstriction | Vasodilatation |
| Cell growth | Antigrowth |
| Cell proliferation | Cell differentiation |
| Antinatriuresis | Natriuresis |
| Production of O2− | Production of nitric oxide |
| Stimulation of fibroblast proliferation and collagen synthesis | Inhibition of fibroblast proliferation |
| Apoptosis | Antiapoptosis |
Evidence suggests that virtually every organ system in the human body possesses a local RAS. The components of RAS are present in peripheral tissues such as vasculature, kidneys, adrenal glands, heart, and immune cells, all of which locally produce Ang II.12–14 These local systems seem to be independently regulated and compartmentalized from the plasma circulation.15
Binding of Ang II to AT1R or AT2R activates various complex signal transduction pathways. Through AT1R, Ang II activates various intracellular protein kinases. These intracellular signaling cascades include receptor- and non-receptor–mediated tyrosine kinases, serine/threonine kinases, mitogen-activated protein kinase (MAPK) family (extracellular signal-regulated kinase, c-Jun N terminal kinase, and p38MAPK), p70 S6 kinase, Akt/PKB (protein kinase B), and various protein kinase C isoforms.16–19 These intracellular signals have been linked to vascular remodeling through induction of hypertrophy, hyperplasia, and migration of vascular smooth muscle cells.16–19 In contrast, AT2R signals through 3 major transduction pathways that seem to oppose the actions of AT1R: (1) activation of various protein phosphatases causing protein dephosphorylation, (2) activation of the nitric oxide/cyclic GMP system, and (3) stimulation of phospholipase A2, with subsequent release of arachidonic acid.20 Of these pathways, MAPK and phosphotyrosine phosphatase (PTP) have been the most studied classic signaling cascade of AT1R and AT2R.21–25 AT1R activates MAPK cascade, whereas AT2R inhibits MAPK and activates PTP.24 The influence of cross talk between AT1R and AT2R on activation of these signaling pathways is still largely unknown.
CHANGES IN RAS WITH AGING
Most of the studies on the effect of aging on RAS have been done in animal models. The effects of aging on RAS have been studied in tissues and in circulation. There seems to be a differential regulation of the circulating and intrarenal RAS during aging.26 On the tissue-specific level, renal Ang II content increases in older animals.27 In contrast, aging is associated with a decline in the concentration of the components of the circulating RAS in animals, including reduction in renal tissue renin messenger RNA levels, juxtaglomerular cell renin content, responsiveness of renin release to various challenges, and plasma renin and Ang II levels.27–33 The decline in the concentration of the components of the circulating RAS during aging may be a consequence of the age-related increase in pressure, because plasma Ang II levels do not decline in rats without increased pressure during aging.26 The reduction in the levels of the circulating RAS components may also have predisposed to the increased renal vasoconstrictor responses to exogenously administered Ang II in older animals.27 Upregulation of AT1R has been observed in both the heart and the vasculature,1,2 suggesting an important role of RAS in senescence. On the other hand, AT2R is expressed in large quantities in fetal tissues but its expression decreases in the neonatal period and reaches a comparatively low level in the adult animal.34 However, the capacity for AT2R reexpression is retained in the adult, because upregulation is a common response to circumstances of cardiovascular tissue damage, such as myocardial infarction, heart failure, and hypertension.27,35–37 The only available studies on microvascular AT2R expression and action in humans demonstrate that AT2R expression can be induced chronically in hypertensive diabetic subjects by AT1R blockade and, under these circumstances, mediates vasodilation.27,37 However, the interpretation of these studies and their applicability in human studies is still an area of debate.
There is evidence that an altered ratio between AT1R and AT2R levels may result in elevated blood pressure and induction of inflammation.38 The contribution of changes in the expression of AT1R and AT2R to the increased production of inflammatory cytokines observed in older individuals is yet to be explored. It also seems that the use of AT1R blockade increases AT2R activity in vivo.39,40 Beneficial actions of AT1R blockers on remodeling and cardiac fibrosis were completely abolished by simultaneous AT2R blockade, suggesting that such beneficial effects are because of AT2R activation rather than AT1R blockade.41–43
How aging might influence RAS is still largely unknown. Genetic and environmental factors may contribute44 but fail to account entirely for any changes with age. There is evidence from human monozygotic twin studies that methylation patterns can change with aging.45 The process of aging and development is accompanied by selective methylation of genes that are not needed for function of the differentiated cell. Evidence from animal and human studies suggests that in utero expression of the angiotensin receptors is regulated by methylation of the angiotensin receptor genes.46,47 However, no studies are available on the effect of aging on the regulation of AT1R and AT2R and their genes in humans. Given the importance of these receptors in performing the major functions of RAS and the gap in knowledge related to how aging triggers and affects these systems, studies as proposed here may have important implications for human health.
RAS AND ITS ROLE IN CHRONIC INFLAMMATION AND FRAILTY IN OLDER ADULTS
Inappropriate, chronic, low-grade inflammation is implicated in the pathogenesis of many common and disabling diseases in older adults. Most of these diseases are slowly progressive and have a clear association with advancing age.48–50 In addition, chronic inflammation is associated with functional decline, frailty, and increased mortality.51,52 The clinical criteria for frailty include weight loss, low levels of activity, muscle weakness, exhaustion, and slow walking speed.51
The causes that trigger chronic inflammatory activation in older adults are likely heterogeneous and include multiple chronic disease states, redox imbalance, senescent cells, and increased body fat.53–57 These triggers act through nuclear factor κB signal transduction, which leads to increased expression of multiple inflammatory mediators including tumor necrosis factor (TNF) α, interleukin (IL) 1b, IL-6, cyclooxygenase 2, and inducible nitric oxide synthase.53–55 The inflammatory cytokine IL-6, total white blood cells, neutrophils, and monocytes have also been identified as significant correlates of frailty in older populations.58,59 Although the cause cannot be proven from these studies, the consistent and reproducible associations between increased expression of markers of inflammation and frailty in older adults suggest that inflammatory pathways are more active in frail older adults than in nonfrail adults and that chronic inflammation worsens disease status, leading to muscle strength decline and stem cell failure.48,60 Hence, chronic inflammation may play an important role in late life decline. Frailty status provides an important in vivo model for chronic inflammation and etiology of inflammation and for RAS change.
Substantial evidence confirms the role of RAS in activation of inflammatory pathways. Most of the functions of Ang II are carried through AT1R. The role and biologic functions of AT2R are less studied. It has been reported that AT2R inhibits and antagonizes AT1R-mediated functions (see Table 1).6–9 The activation of AT1R has a powerful proinflammatory effect.61 AT1R actions include induction of reactive oxygen species,62 hypertrophy and apoptosis,11 and stimulation of fibroblast proliferation and collagen synthesis.63 AT1R antagonists exert cardiovascular protection, in part through their vascular antiinflammatory effects.64 AT1R activation affects cytokine levels by increasing IL-6,65 TNF-α,66–70 and interferon gamma production71 and decreasing nitric oxide and cyclic GMP production.72 AT1R expression seems to be limiting for the effect of Ang II. Upregulation of AT1R expression enhances the action of Ang II in vitro as well as in vivo.73
The molecular mechanisms through which angiotensin receptors manipulate cytokines production and chronic inflammation remain unclear (Fig. 2). Ang II activates the signal transducer and activator of transcription proteins 3 (STAT3).74 STAT3 is a key signal transduction protein that mediates cell differentiation, proliferation, apoptosis, inflammation, and tumor cell evasion of the immune system.75 Binding sites have been identified for STAT3 within the promoter region of TNF-α.76 Mutation of the 3 base pairs of the STAT3 binding site had considerable effects on the promoter activity, demonstrating that STAT3 upregulates TNF-α expression.76
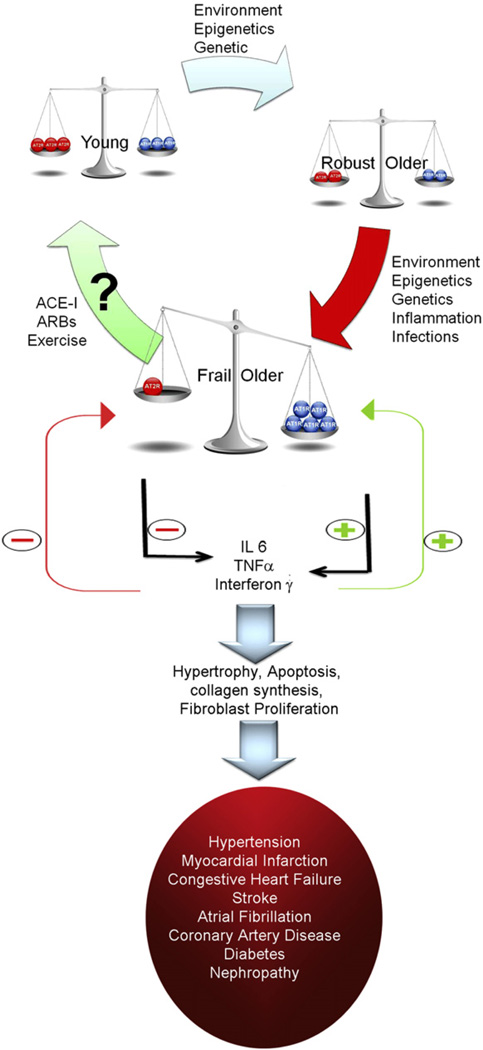
Fig. 2.
A hypothetical model for changes in the angiotensin receptors with aging and/or frailty, resulting in increased production of cytokines, pathologic changes, and development/worsening of diseases. Note that with robust aging, the balance is maintained between the angiotensin receptors despite decrease in both AT1R (blue circles) and AT2R (red circles). With development of frailty that balance is tipped toward more expression of AT1R and less AT2R predisposing to increased cytokine production, which further widens the gap by increasing the expression of AT1R and reducing expression of AT2R. ACEi, ACE inhibitor; ARBs, Ang II receptor blockers.
To date, few have studies examined the influence of increased inflammation on RAS. In animal models IL-6, released locally, contributes substantially to the vascular dysfunction produced by Ang II.77 Treatment of mice with IL-6 for 18 days increased vascular AT1R expression.78 Because the upregulation of AT1R expression in vitro and in vivo is involved in IL-6–induced propagation of oxidative stress and endothelial dysfunction, the interaction of the proinflammatory cytokine IL-6 with RAS may represent an important pathogenetic mechanism in inflammatory diseases in older population.
AGING RAS—DISEASE INTERACTIONS CULMINATING IN THE DEVELOPMENT OF FRAILTY
RAS contributes to the pathogenesis of several human diseases that have a clear association with advanced aging, including hypertension, myocardial infarction, congestive heart failure, stroke, atrial fibrillation, coronary artery disease, diabetes, and nephropathy. Large population studies have clearly demonstrated that both ACE inhibitors and Ang II receptor blockers (ARB) have been shown to be effective in preventing or regressing some of the age-associated effects of these diseases in humans and animals.79–81
Myocardial Infarction
The expression of both AT1R and AT2R is upregulated in cardiac tissue after myocardial infarction. Induction of myocardial infarction in mice lacking AT2Rs caused significant damage to the heart as compared with the wild-type mice,42,82 demonstrating that the beneficial effects of AT1R blockade after myocardial infarction may be partially mediated by the AT2R.83
Left Ventricular Hypertrophy
The extent of left ventricular hypertrophy is aggravated by the activity of RAS,84,85 independent of, and in addition to, the effect of elevated blood pressure.86,87 At similar blood pressure levels, incidence of left ventricular hypertrophy was greater with the ARB losartan than with the β-blocker atenolol throughout a follow-up of 5 years.88–90
Atrial Fibrillation
Treatment with ARB has been shown to reduce the incidence of atrial fibrillation by 21% in hypertensive patients.91–93 The mechanism underlying this protective effect is related to the prevention of left atrial dilation and atrial fibrosis and to the reduction of conduction velocity.81
Stroke
Several clinical trials have demonstrated a prominent effect of ARB treatment on the prevention of stroke.88,94–97 At a similar blood pressure, control ARB had an additional 25% reduction in strokes compared with those on a β-blocker.88 A similar result was also observed in the Study on COgnition and Prognosis in the Elderly (SCOPE).
Atherosclerosis
Activation of RAS through AT1R (1) induces vasoconstriction and formation of extracellular matrix and matrix metalloproteinases, (2) enhances migration and proliferation of vascular smooth muscle cells, (3) increases synthesis of plasminogen activator inhibitor (PAI-1), and (4) stimulates release of proinflammatory cytokines, including IL-6 and TNF-α.98
Diabetes
In a meta-analysis, treatment with ARBs has been shown to reduce the incidence of diabetes mellitus by 23%, regardless of the presence of cardiovascular disease.99–101
Renal Damage
Treatment with ARBs improves renal damage in patients with and without diabetes.102–104
Dementia
Hypertension induces damage to brain microcirculation, which contributes to the development of dementia. However, evidence on the benefit of RAS blockade on cognitive function has been controversial. The role of angiotensin IV on cognitive function has been described.105–107
Muscle Strength
A fully functional RAS exists in the skeletal muscle microvasculature. Studies have also confirmed that skeletal muscles generate Ang II locally.108–110 The polymorphism of the ACE gene is an important factor in determining physical performance.111 However, clinical studies are needed to confirm a role for blockade of RAS in muscle function.
Osteoporosis, Fracture Risk, and Bone Marrow Density
Clinical studies indicate a possible role of RAS in bone metabolism and fracture risk. Patients treated with an ACE inhibitor showed an increased bone mineral density and a reduced fracture risk.112–114 In addition, individuals with decreased ACE activity have a higher bone marrow density than individuals with increased ACE activity.115
SUMMARY
RAS plays a broad role in vascular regulation, inflammation, oxidative stress, and apoptosis. Each of these molecular realms has been hypothesized to influence the aging phenotype. RAS also clearly influences multiple disease states with increasing age, and pharmaceuticals targeting these pathways are now a mainstay of treatment of many older adults. RAS blockade exerts potent antiatherosclerotic, antihypertensive, antiinflammatory, antiproliferative, and oxidative stress–lowering properties. Given the influence of RAS on frailty-related diseases and traits, and the age-related changes in this system that seem to accelerate these conditions, further evaluation on the causes, multisystemic interactions, and intervention development on RAS regulation is indicated.
REFERENCES
- 1.Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41(6):1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 2.Heymes C, Silvestre JS, Llorens-Cortes C, et al. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139(5):2579–2587. doi: 10.1210/endo.139.5.6023. [DOI] [PubMed] [Google Scholar]
- 3.Min LJ, Mogi M, Iwai M, et al. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8(2):113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Chiu AT, McCall DE, Nguyen TT, et al. Discrimination of angiotensin II receptor subtypes by dithiothreitol. Eur J Pharmacol. 1989;170(1–2):117–118. doi: 10.1016/0014-2999(89)90145-3. [DOI] [PubMed] [Google Scholar]
- 5.Chang RS, Lotti VJ. Two distinct angiotensin II receptor binding sites in rat adrenal revealed by new selective nonpeptide ligands. Mol Pharmacol. 1990;37(3):347–351. [PubMed] [Google Scholar]
- 6.Hein L, Barsh GS, Pratt RE, et al. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377(6551):744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 7.Ichiki T, Labosky PA, Shiota C, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377(6551):748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 8.Masaki H, Kurihara T, Yamaki A, et al. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101(3):527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AbdAlla S, Lother H, Abdel-tawab AM, et al. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276(43):39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 10.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83(12):1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 11.Bascands JL, Girolami JP, Troly M, et al. Angiotensin II induces phenotype-dependent apoptosis in vascular smooth muscle cells. Hypertension. 2001;38(6):1294–1299. doi: 10.1161/hy1201.096540. [DOI] [PubMed] [Google Scholar]
- 12.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 13.Nahmod KA, Vermeulen ME, Raiden S, et al. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17(3):491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- 14.Jurewicz M, McDermott DH, Sechler JM, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18(4):1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 15.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5(2):89–100. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 16.Griendling KK, Ushio-Fukai M, Lassegue B, et al. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29(1 Pt 2):366–373. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi S, Frank GD, Mifune M, et al. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem Soc Trans. 2003;31(Pt 6):1198–1202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- 18.Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol. 2003;35(6):780–783. doi: 10.1016/s1357-2725(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Motley ED, Frank GD, et al. Recent progress in signal transduction research of the angiotensin II type-1 receptor: protein kinases, vascular dysfunction and structural requirement. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(4):305–322. doi: 10.2174/156801605774322355. [DOI] [PubMed] [Google Scholar]
- 20.Steckelings UM, Kaschina E, Unger T. The AT2 receptor–a matter of love and hate. Peptides. 2005;26(8):1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Dechend R, Fiebler A, Lindschau C, et al. Modulating angiotensin II-induced inflammation by HMG co-A reductase inhibition. Am J Hypertens. 2001;14(6 Pt 2):55S–61S. doi: 10.1016/s0895-7061(01)02070-2. [DOI] [PubMed] [Google Scholar]
- 22.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639–672. [PubMed] [Google Scholar]
- 23.Kambayashi Y, Bardhan S, Takahashi K, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268(33):24543–24546. [PubMed] [Google Scholar]
- 24.Bedecs K, Elbaz N, Sutren M, et al. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 1997;325(Pt 2):449–454. doi: 10.1042/bj3250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horiuchi M, Hayashida W, Kambe T, et al. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272(30):19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 26.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 27.Thompson MM, Oyama TT, Kelly FJ, et al. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1787–R1794. doi: 10.1152/ajpregu.2000.279.5.R1787. [DOI] [PubMed] [Google Scholar]
- 28.Anderson S. Ageing and the renin-angiotensin system. Nephrol Dial Transplant. 1997;12(6):1093–1094. doi: 10.1093/ndt/12.6.1093. [DOI] [PubMed] [Google Scholar]
- 29.Anderson S, Rennke HG, Zatz R. Glomerular adaptations with normal aging and with long-term converting enzyme inhibition in rats. Am J Physiol. 1994;267(1 Pt 2):F35–F43. doi: 10.1152/ajprenal.1994.267.1.F35. [DOI] [PubMed] [Google Scholar]
- 30.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. 1993;22(6):842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 31.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9(4):699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 32.Masilamani S, Zhang XZ, Baylis C. Blunted pressure natriuretic response in the old rat: participation of the renal nerves. Am J Kidney Dis. 1998;32(4):605–610. doi: 10.1016/s0272-6386(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 33.Reckelhoff JF, Baylis C. Proximal tubular metalloprotease activity is decreased in the senescent rat kidney. Life Sci. 1992;50(13):959–963. doi: 10.1016/0024-3205(92)90174-n. [DOI] [PubMed] [Google Scholar]
- 34.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24(3):261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 35.Jones ES, Vinh A, McCarthy CA, et al. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120(3):292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widdop RE, Vinh A, Henrion D, et al. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008;35(4):386–390. doi: 10.1111/j.1440-1681.2008.04883.x. [DOI] [PubMed] [Google Scholar]
- 37.Savoia C, Touyz RM, Volpe M, et al. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49(2):341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- 38.Warnholtz A, Nickenig G, Schulz E, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99(15):2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 39.Weber MA. Clinical experience with the angiotensin II receptor antagonist losartan. A preliminary report. Am J Hypertens. 1992;5(12 Pt 2):247S–251S. doi: 10.1093/ajh/5.12.247s. [DOI] [PubMed] [Google Scholar]
- 40.Guan H, Cachofeiro V, Pucci ML, et al. Nitric oxide and the depressor response to angiotensin blockade in hypertension. Hypertension. 1996;27(1):19–24. doi: 10.1161/01.hyp.27.1.19. [DOI] [PubMed] [Google Scholar]
- 41.Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35(5):1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 42.Oishi Y, Ozono R, Yoshizumi M, et al. AT2 receptor mediates the cardioprotective effects of AT1 receptor antagonist in post-myocardial infarction remodeling. Life Sci. 2006;80(1):82–88. doi: 10.1016/j.lfs.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 43.Carey RM, Howell NL, Jin XH, et al. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38(6):1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- 44.Staessen JA, Wang J, Bianchi G, et al. Essential hypertension. Lancet. 2003;361(9369):1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 45.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the life-time of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert JS, Lang AL, Nijland MJ. Maternal nutrient restriction and the fetal left ventricle: decreased angiotensin receptor expression. Reprod Biol Endocrinol. 2005;3:27. doi: 10.1186/1477-7827-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogdarina I, Welham S, King PJ, et al. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100(4):520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 49.Fujita J, Tsujinaka T, Ebisui C, et al. Role of interleukin-6 in skeletal muscle protein breakdown and cathepsin activity in vivo. Eur Surg Res. 1996;28(5):361–366. doi: 10.1159/000129477. [DOI] [PubMed] [Google Scholar]
- 50.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 52.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83(5):1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 53.Chung HY, Cheng KQ, Chung GJ. Molecular inflammation in aging process. Nippon Ronen Igakkai Zasshi. 2004;41(4):357–364. doi: 10.3143/geriatrics.41.357. [in Japanese]. [DOI] [PubMed] [Google Scholar]
- 54.Chung HY, Sung B, Jung KJ, et al. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8(3–4):572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Jung KJ, Yu BP, et al. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123(12):1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 56.Ren JL, Pan JS, Lu YP, et al. Inflammatory signaling and cellular senescence. Cell Signal. 2009;21(3):378–383. doi: 10.1016/j.cellsig.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki M, Ikeda H, Sato Y, et al. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res. 2008;42(7):625–632. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 58.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 59.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 60.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki Y, Ruiz-Ortega M, Lorenzo O, et al. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35(6):881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 62.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105(3):393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 63.Cipollone F, Fazia M, Iezzi A, et al. Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2-dependent matrix metalloproteinase activity. Circulation. 2004;109(12):1482–1488. doi: 10.1161/01.CIR.0000121735.52471.AC. [DOI] [PubMed] [Google Scholar]
- 64.Navalkar S, Parthasarathy S, Santanam N, et al. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosis. J Am Coll Cardiol. 2001;37(2):440–444. doi: 10.1016/s0735-1097(00)01138-4. [DOI] [PubMed] [Google Scholar]
- 65.Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101(12):1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 66.Siragy HM, Awad A, Abadir P, et al. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-alpha in diabetic rats. Endocrinology. 2003;144(6):2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsutamoto T, Wada A, Maeda K, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol. 2000;35(3):714–721. doi: 10.1016/s0735-1097(99)00594-x. [DOI] [PubMed] [Google Scholar]
- 68.Beasley D. Phorbol ester and interleukin-1 induce interleukin-6 gene expression in vascular smooth muscle cells via independent pathways. J Cardiovasc Pharmacol. 1997;29(3):323–330. doi: 10.1097/00005344-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84(6):695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 70.Hahn AW, Jonas U, Buhler FR, et al. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347(2–3):178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- 71.Weidanz JA, Jacobson LM, Muehrer RJ, et al. ATR blockade reduces IFN-gamma production in lymphocytes in vivo and in vitro. Kidney Int. 2005;67(6):2134–2142. doi: 10.1111/j.1523-1755.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 72.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42(4):600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 73.Nickenig G, Sachinidis A, Michaelsen F, et al. Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation. 1997;95(2):473–478. doi: 10.1161/01.cir.95.2.473. [DOI] [PubMed] [Google Scholar]
- 74.Omura T, Yoshiyama M, Takeuchi K, et al. Angiotensin blockade inhibits SIF DNA binding activities via STAT3 after myocardial infarction. J Mol Cell Cardiol. 2000;32(1):23–33. doi: 10.1006/jmcc.1999.1051. [DOI] [PubMed] [Google Scholar]
- 75.Costantino L, Barlocco D. STAT 3 as a target for cancer drug discovery. Curr Med Chem. 2008;15(9):834–843. doi: 10.2174/092986708783955464. [DOI] [PubMed] [Google Scholar]
- 76.Chappell VL, Le LX, LaGrone L, et al. Stat proteins play a role in tumor necrosis factor alpha gene expression. Shock. 2000;14(3):400–402. doi: 10.1097/00024382-200014030-00027. [discussion: 402–3]. [DOI] [PubMed] [Google Scholar]
- 77.Schrader LI, Kinzenbaw DA, Johnson AW, et al. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27(12):2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 78.Wassmann S, Stumpf M, Strehlow K, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 79.Burrell LM, Johnston CI. Angiotensin II receptor antagonists. Potential in elderly patients with cardiovascular disease. Drugs Aging. 1997;10(6):421–434. doi: 10.2165/00002512-199710060-00003. [DOI] [PubMed] [Google Scholar]
- 80.Basso N, Paglia N, Stella I, et al. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128(3):247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 81.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369(9568):1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 82.Xu J, Carretero OA, Liu YH, et al. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension. 2002;40(3):244–250. doi: 10.1161/01.hyp.0000029095.23198.ad. [DOI] [PubMed] [Google Scholar]
- 83.Jugdutt BI, Menon V. AT2 receptor and apoptosis during AT1 receptor blockade in reperfused myocardial infarction in the rat. Mol Cell Biochem. 2004;262(1–2):203–214. doi: 10.1023/b:mcbi.0000038236.59905.8b. [DOI] [PubMed] [Google Scholar]
- 84.Mancia G, Zanchetti A, Agabiti-Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation. 1997;95(6):1464–1470. doi: 10.1161/01.cir.95.6.1464. [DOI] [PubMed] [Google Scholar]
- 85.Schmieder RE. The role of non-haemodynamic factors of the genesis of LVH. Nephrol Dial Transplant. 2005;20(12):2610–2612. doi: 10.1093/ndt/gfi190. [DOI] [PubMed] [Google Scholar]
- 86.Mazzolai L, Nussberger J, Aubert JF, et al. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31(6):1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 87.Mazzolai L, Pedrazzini T, Nicoud F, et al. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000;35(4):985–991. doi: 10.1161/01.hyp.35.4.985. [DOI] [PubMed] [Google Scholar]
- 88.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 89.Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 90.Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) trial. Circulation. 2004;110(11):1456–1462. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 91.Wachtell K, Hornestam B, Lehto M, et al. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):705–711. doi: 10.1016/j.jacc.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 92.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 93.Schmieder RE, Kjeldsen SE, Julius S, et al. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertens. 2008;26(3):403–411. doi: 10.1097/HJH.0b013e3282f35c67. [DOI] [PubMed] [Google Scholar]
- 94.Lithell H, Hansson L, Skoog I, et al. The Study on COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add-on therapy after randomization. J Hypertens. 2004;22(8):1605–1612. doi: 10.1097/01.hjh.0000133730.47372.4c. [DOI] [PubMed] [Google Scholar]
- 95.Lithell H, Hansson L, Skoog I, et al. The Study on COgnition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Skoog I, Lithell H, Hansson L, et al. Effect of baseline cognitive function and anti-hypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE) Am J Hypertens. 2005;18(8):1052–1059. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 97.Chrysant SG. Possible pathophysiologic mechanisms supporting the superior stroke protection of angiotensin receptor blockers compared to angiotensin-converting enzyme inhibitors: clinical and experimental evidence. J Hum Hypertens. 2005;19(12):923–931. doi: 10.1038/sj.jhh.1001916. [DOI] [PubMed] [Google Scholar]
- 98.Farmer JA, Torre-Amione G. The renin angiotensin system as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2001;3(2):117–124. doi: 10.1007/s11883-001-0047-2. [DOI] [PubMed] [Google Scholar]
- 99.Kjeldsen SE, Julius S, Mancia G, et al. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high-risk hypertensive patients: the VALUE trial. J Hypertens. 2006;24(7):1405–1412. doi: 10.1097/01.hjh.0000234122.55895.5b. [DOI] [PubMed] [Google Scholar]
- 100.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab. 2004;30(6):487–496. doi: 10.1016/s1262-3636(07)70146-5. [DOI] [PubMed] [Google Scholar]
- 101.Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28(9):2261–2266. doi: 10.2337/diacare.28.9.2261. [DOI] [PubMed] [Google Scholar]
- 102.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 103.Lewis EJ, Lewis JB. Treatment of diabetic nephropathy with angiotensin II receptor antagonist. Clin Exp Nephrol. 2003;7(1):1–8. doi: 10.1007/s101570300000. [DOI] [PubMed] [Google Scholar]
- 104.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 105.Gard PR. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008;9(Suppl 2):S15. doi: 10.1186/1471-2202-9-S2-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wright JW, Harding JW. The brain RAS and Alzheimer’s disease. Exp Neurol. 2010;223(2):326–333. doi: 10.1016/j.expneurol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 107.Wright JW, Harding JW. The angiotensin AT4 receptor subtype as a target for the treatment of memory dysfunction associated with Alzheimer’s disease. J Renin Angiotensin Aldosterone Syst. 2008;9(4):226–237. doi: 10.1177/1470320308099084. [DOI] [PubMed] [Google Scholar]
- 108.Unger T, Gohlke P, Paul M, et al. Tissue renin-angiotensin systems: fact or fiction? J Cardiovasc Pharmacol. 1991;18(Suppl 2):S20–S25. [PubMed] [Google Scholar]
- 109.Atlas SA. The renin-angiotensin system revisited: classical and nonclassical pathway of angiotensin formation. Mt Sinai J Med. 1998;65(2):87–96. [PubMed] [Google Scholar]
- 110.Danser AH. Local renin-angiotensin systems. Mol Cell Biochem. 1996;157(1–2):211–216. doi: 10.1007/BF00227900. [DOI] [PubMed] [Google Scholar]
- 111.Montgomery H, Clarkson P, Barnard M, et al. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet. 1999;353(9152):541–515. doi: 10.1016/S0140-6736(98)07131-1. [DOI] [PubMed] [Google Scholar]
- 112.Perez-Castrillon JL, Justo I, Silva J, et al. Relationship between bone mineral density and angiotensin converting enzyme polymorphism in hypertensive post-menopausal women. Am J Hypertens. 2003;16(3):233–235. doi: 10.1016/s0895-7061(02)03263-6. [DOI] [PubMed] [Google Scholar]
- 113.Perez-Castrillon JL, Silva J, Justo I, et al. Effect of quinapril, quinapril-hydrochlorothiazide, and enalapril on the bone mass of hypertensive subjects: relationship with angiotensin converting enzyme polymorphisms. Am J Hypertens. 2003;16(6):453–459. doi: 10.1016/s0895-7061(03)00845-8. [DOI] [PubMed] [Google Scholar]
- 114.Rejnmark L, Vestergaard P, Mosekilde L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens. 2006;24(3):581–589. doi: 10.1097/01.hjh.0000203845.26690.cb. [DOI] [PubMed] [Google Scholar]
- 115.Woods D, Onambele G, Woledge R, et al. Angiotensin-I converting enzyme genotype-dependent benefit from hormone replacement therapy in isometric muscle strength and bone mineral density. J Clin Endocrinol Metab. 2001;86(5):2200–2204. doi: 10.1210/jcem.86.5.7514. [DOI] [PubMed] [Google Scholar]