Abstract
Neurophysiological tests of anorectal function can provide useful information regarding the integrity of neuronal innervation, as well as neuromuscular function. This information can give insights regarding the pathophysiological mechanisms that lead to several disorders of anorectal function, particularly fecal incontinence, pelvic floor disorders and dyssynergic defecation. Currently, several tests are available for the neurophysiological evaluation of anorectal function. These tests are mostly performed on patients referred to tertiary care centers, either following negative evaluations or when there is lack of response to conventional therapy. Judicious use of these tests can reveal significant and new understanding of the underlying mechanism(s) that could pave the way for better management of these disorders. In addition, these techniques are complementary to other modalities of investigation, such as pelvic floor imaging. The most commonly performed neurophysiological tests, along with their indications and clinical utility are discussed. Several novel techniques are evolving that may reveal new information on brain–gut interactions.
Keywords: anorectal function, anorectal manometry, barostat, cortical evoked potentials, electromyography, neurophysiologic tests, pudendal nerve
Neuroanatomy & physiology of the anorectum
Structure of the anorectum
The rectum is a muscular tube that is 12- to 15-cm long and composed of a continuous layer of longitudinal muscle that interlaces with the underlying circular muscle [1]. The anus is 2- to 4-cm long. At rest, it forms an angle of approximately 90° with the axis of the rectum [1, 2]. During voluntary squeeze, the angle becomes more acute, whereas during defecation, the angle becomes more obtuse. The anal sphincter consists of two muscular components: the internal anal sphincter (IAS), a 0.3- to 0.5-cm thick expansion of the circular smooth muscle layer of the rectum; and the external anal sphincter (EAS), a 0.6- to 1.0-cm thick expansion of the levator ani muscles. Morphologically, both sphincters are separate and heterogeneous. The IAS is a predominantly slow-twitch, fatigue-resistant smooth muscle [1, 2]. The IAS generates mechanical activity, with a frequency of 15–35 cycles/min, and ultra-slow waves at 1.5–3 cycles/min [3]. The IAS contributes approximately 70–85% of the resting sphincter pressure, but only 40% after sudden distention of the rectum and 65% during constant rectal distention [1–3]. Thus, the IAS is primarily responsible for maintaining anal continence at rest. The anus is normally closed by the tonic activity of the IAS. This barrier is reinforced during voluntary squeeze by the EAS. The anal mucosal folds, together with the expansive anal vascular cushions, provide a tight seal. These barriers are further augmented by the puborectalis muscle, which forms a flap-like valve that creates a forward pull and reinforces the anorectal angle.
Rectal distention is associated with a decrease in anal resting pressure known as the rectoanal inhibitory reflex (RAIR) [1–4]. The amplitude and duration of this relaxation increases with the volume of rectal distention. This reflex is mediated by the myenteric plexus and is present in patients with transection of the hypogastric nerves and in patients with spinal cord lesions [5]. It is absent in patients with Hirschsprung’s disease, after circular rectal myotomy and sometimes after lower anterior resection [6, 7]. In addition, it has been shown that the amount of distension required to produce maximum inhibition of the anal sphincter during the RAIR is significantly diminished after radiotherapy [8]. The RAIR is likely to play an important role in the sampling and discrimination of rectal contents; preservation of this reflex correlates with a decrease in the incidence of nocturnal soiling after double-stapled ileoanal reservoir construction [9].
The arrival of flatus mimics sudden rectal distention and this is associated with a decrease in anal pressure. Although the RAIR may facilitate discharge of flatus, rectal distention also induces an anal contractile response – recto anal contractile reflex (RACR), a subconscious reflex effort to prevent release of rectal contents [1, 10]. The RACR involves contraction of the EAS, and it is mediated by the pelvic splanchnic and pudendal nerves. Abrupt increases in intra-abdominal pressure, such as those that occur after coughing or laughing, are associated with simultaneous increases in anal sphincter pressure brought about by reflex contraction of the puborectalis and anal sphincters. These local reflexes are independent of rectal sensation [10]. However, recently it has been described that perception of rectal distension is associated with a unique, consistent and reproducible anal contractile response – the sensory–motor response (SMR) [10].
Innervation & sensory function
The anorectum is richly innervated by sensory, motor and autonomic nerves and by the enteric nervous system. The principal nerve is the pudendal nerve, which arises from the second, third and fourth sacral nerves (S2, S3 and S4) and innervates the EAS. The pudendal nerve is a mixed nerve that subserves both sensory and motor function [1, 2]. Pudendal nerve block creates a loss of sensation in the perianal and genital skin and weakness of the anal sphincter muscle, but does not affect rectal sensation. Pudendal nerve block also abolishes rectoanal contractile reflexes, which suggests that pudendal neuropathy may affect the rectoanal contractile reflex response. The sensation of rectal distention is most likely transmitted along the S2, S3 and S4 parasympathetic nerves. These nerve fibers traverse along the pelvic splanchnic nerves and are independent of the pudendal nerve.
It is not completely understood how humans perceive stool contents in the anorectum. Earlier studies failed to demonstrate rectal sensory nerves. Subsequent studies have confirmed that balloon distention is perceived in the rectum and that such perception plays a role in maintaining continence [11]. Despite the absence of specialized receptors in the rectal mucosa, such as Pacinian corpuscles or Golgi–Mazzoni bodies, there is some evidence to suggest that rectal sensation arises from the stimulation of nerve endings and mechanoreceptors, both in the rectal wall and the adjacent pelvic structures [12]. In addition, recent studies from rat models have confirmed the existence of intraganglionic laminar nerve endings in the myenteric plexus of the rectal wall that are sensitive to mechanical distension [13]. Furthermore, sensory conditioning can improve both hyposensitivity and hypersensitivity of the rectum [14]. Mechanical stimulation of the rectum can produce cerebral evoked responses, which confirms that the rectum is a sensory organ. Although there are no organized nerve endings in the rectal mucosa or in the myenteric plexus, both myelinated and unmyelinated nerve fibers are present [1–3]. These nerves most likely mediate the distention-or stretch-induced sensory responses, as well as the vis-cero–visceral, the recto–anal inhibitory and the recto–anal contractile reflexes. Rectal sensation and the ability to defecate can be abolished completely by resection of the nervi erigentes. If parasympathetic innervation is absent, rectal filling is only perceived as a vague sensation of discomfort. Even paraplegics or persons with sacral neuronal lesions may retain some degree of sensory function, but virtually no sensation is felt if lesions reach the higher spine [1]. Thus, the sacral nerves are intimately involved with the maintenance of continence.
It has been suggested that bowel contents are periodically sensed by anorectal sampling [11, 12], the process by which transient relaxation of the IAS allows the stool contents from the rectum to come into contact with specialized sensory organs, such as the Krause end-bulbs, Golgi–Mazzoni bodies and genital corpuscles, and the sparse Meissner’s corpuscles and Pacinian corpuscles in the upper anal canal [1, 2]. Specialized afferent nerves may exist that subserve sensations of touch, temperature, tension and friction, but these are incompletely understood [15]. Incontinent patients appear to sample rectal contents less frequently than continent subjects. The likely role of anal sensation is to facilitate discrimination between flatus and feces and the fine-tuning of the continence barrier, but its precise role has not been characterized [12].
Neurotransmitters
The IAS is densely innervated by adrenergic nerves in humans [1]. Stimulation of adrenergic α and β receptors can produce contraction and relaxation of human IAS [16]. All three β adrenoreceptor (β1, β2 and β3) agonists relax the opossum internal anal sphincter; the β3 receptor effect is mediated by the cyclic guanylate monophosphate pathway similar to nitric oxide (NO). The IAS is more sensitive to adrenergic compared with cholinergic agonists; cholinergic agonists either contracted or relaxed IAS in humans [2]. Nicotinic agonists also relaxed IAS, probably via nonadrenergic–noncholinergic mechanisms. NO mediates IAS tone and the RAIR [17]. Recent studies suggest that the isoforms of NO synthase – neuronal (nNOS) and endothelial (eNOS) – modulate distinct components of IAS function. Vasoactive intestinal peptide, an inhibitory neurotransmitter also plays a role in RAIR [18].
The act of defecation & the maintenance of continence
Many neuromuscular factors are involved in the act of defecation and maintenance of continence. Defecation begins with the movement of feces from the colon into the rectum [19]. A complex and coordinated sequence of movements is involved in this process, which includes segmental, propagated and retrograde pressure waves, and high-amplitude propagated contractions [19]. The next stage consists of several stereotyped events that are under the control of reflex mechanisms. The basic regulatory mechanisms are present in the newborn but the art of controlled defecation develops through training and is controlled by higher cortical centers. Arrival of stool in the rectum causes rectal distension and induces a desire to defecate along with a decrease in the anal resting pressure – the RAIR [4, 20]. These events allow the rectal contents to come into contact with the sensitive anoderm and, based on the nature of fecal material ‘sampled’ – solid, liquid or gas [21] – an urge to defecate is induced that can only be resisted by vigorous contractions of the EAS and puborectalis muscle. If social and environmental conditions are favorable and voluntary defecation is desired, the subject sits or squats, contracts the diaphragm, abdominal muscles and rectal muscles, and simultaneously relaxes the EAS and puborectalis muscle. These maneuvers open the anus and facilitate stool expulsion. Thus, sensory perception and coordinated movement of stool are important physiologic variables that affect anorectal function [22]. Impairment of these mechanisms may result in functional anorectal disorders.
Neurophysiological tests commonly performed in clinical practice
Anorectal manometry
Anorectal manometry (ARM) provides information regarding anal sphincter function at rest, together with changes that occur during voluntary squeeze or bearing down and reflex activation of the pelvic floor. Adequate measurements of anal sphincter responses can be obtained with either open-tipped or side-opening water-perfused catheters or direct online solidstate microtransducers, or air- or water-filled balloons [23, 24]. Normal anal sphincter pressures vary according to gender, age and testing methodology used. In general, pressures are higher in men and younger persons, but there is a considerable overlap in values [24].
It is the preferred method for defining the functional weakness of the EAS or IAS and for detecting abnormal recto–anal reflexes. These tests may also facilitate biofeedback training. Recently, a novel solid-state manometric assembly with 36 circumferential sensors spaced at 1-cm intervals (4.2 mm outer diameter) has been used to perform anorectal manometry (high-resolution manometry [HRM], Sierra Scientific Instruments, CA, USA) [25]. This device uses novel pressure transduction technology (TactArray) that allows each of the 36 pressure-sensing elements to detect pressure over a length of 2.5 mm and in each of 12 radially dispersed sectors. This HRM provides greater physiologic resolution and minimizes movement artifact. The data can be displayed in isobaric contour plots that can provide a continuous, dynamic representation of pressure changes. In a pilot study, Jones et al. [25] reported good correlation, although anal sphincter pressures were higher with HRM than those recorded with water-perfused manometry.
Clinical utility of ARM testing in fecal incontinence
Patients with incontinence demonstrate several neurophysiological abnormalities, such as disruption or weakness of the EAS and IAS. These are common causes of fecal incontinence.
In addition, the ability of the EAS to contract reflexively during abrupt increases of intra-abdominal pressure, such as when coughing or sneezing is impaired. This can be assessed by having subjects blow up a balloon [24–26]. This reflex response causes the anal sphincter pressure to rise above that of the rectal pressure to preserve continence. The response is triggered by receptors on the pelvic floor and mediated through a spinal reflex arc. In patients with spinal cord lesions above the conus medullaris, this reflex response is present but the voluntary squeeze response may be absent, whereas in patients with lesions affecting the cauda equina or sacral nerve plexus, both the reflex response and the voluntary squeeze response are absent [1, 27].
In a prospective study, anorectal manometry with sensory testing not only confirmed a clinical impression but also provided new information that was not detected clinically [28]. Furthermore, the diagnostic information obtained from these studies influenced both the management and the outcome of patients with incontinence [28]. The gold standard for detecting structural abnormalities are imaging studies, such as anal endosonography or pelvic MRI; these tests are beyond the scope of this review but are described elsewhere [29].
Clinical utility of ARM in chronic constipation
Anorectal manometry detects abnormalities that facilitates the diagnosis of Hirschsprung’s disease and dyssynergic defecation [30]. The absence of a recto–anal inhibitory reflex is considered pathognomonic for Hirschsprung’s disease [30, 31]. In a prospective study of 111 children, anorectal manometry had a sensitivity of 83% and specificity of 93% when compared with rectal suction biopsy (sensitivity 93% and specificity 100%) for detecting Hirschsprung’s disease [31].
When a subject attempts to defecate, there is usually a rise in intrarectal pressure, which is synchronized with a fall in anal sphincter pressure due to relaxation of the puborectalis and EAS [32, 33]. This maneuver is under voluntary control and is primarily a learned response. The inability to perform this coordinated maneuver represents the chief pathophysiological abnormality in patients with dyssynergic defecation [32–34]. This inability may be due to impaired expulsion forces, paradoxical anal contraction or impaired anal relaxation, or a combination of these mechanism(s) [34]. However, during attempted defecation, some subjects may not produce a normal relaxation largely because of the laboratory conditions. For example, in a recent study in 25 healthy volunteers, ARM in the lying position revealed that a third of the subjects had dyssynergia and half could not expel artificial stool [35]. Whereas when sitting with a distended rectum, most showed normal defecation pattern and ability to expel stool. Thus, body position, sensation of stooling and stool characteristics may each influence defecation [35]. Hence, the occurrence of this pattern alone is not diagnostic of dyssynergic defecation. In addition, rectal sensory testing has revealed that the thresholds for first sensation and desire to defecate are impaired in 60% of patients with dyssynergic defecation [34]. Also, a case-controlled study demonstrated that anorectal manometry revealed a dyssynergic defecation pattern in up to 82% of patients with solitary rectal ulcer syndrome [36].
Recommendation
Anorectal manometry is recommended for the routine evaluation of patients with fecal incontinence and chronic constipation, and can reveal neuromuscular dysfunction of the anal sphincter, recto–anal reflexes and recto–anal coordination.
Rectal sensory testing
This test is usually performed by distending the rectum with either an air- or water-filled balloon. Typically, this can be used for the assessment of rectal sensory responses comprised of measuring the thresholds for first perception, a desire or urgent desire to defecate and the maximum tolerable volume (FIGURE 1) [24, 26]. Most investigators test sensory thresholds by rapidly injecting air into a balloon, although some use continuous infusion [37, 38]. The type of inflation (phasic vs continuous) and the speed of inflation can affect the sensory thresholds [38]. The size and shape of the balloon can also affect the threshold volume [38]. Consequently, the normal ranges differ between laboratories. Some of this variability can be reduced by using a highly compliant, computer-controlled balloon-distension device, the barostat.
Figure 1. Rectal sensory testing after balloon distention.
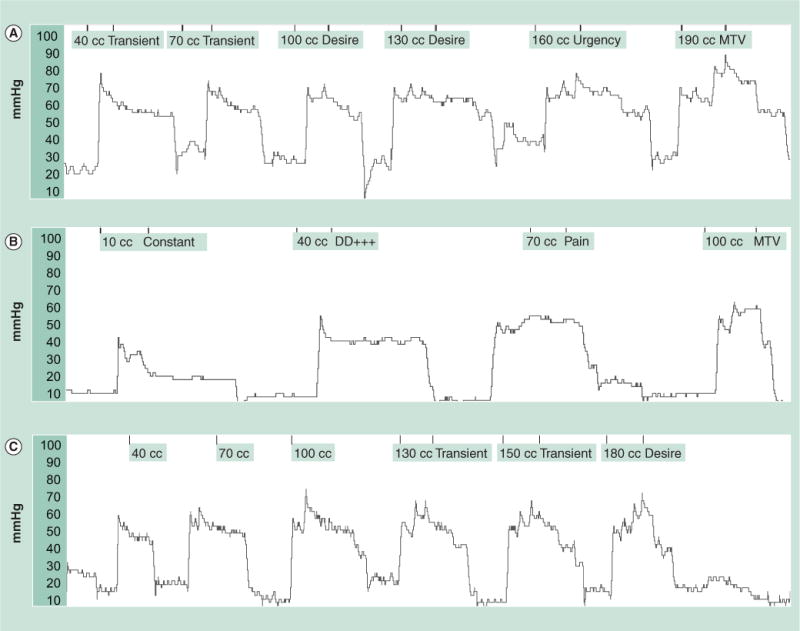
These tracings show intrarectal balloon pressures during balloon distension with increments in balloon volume together with the sensory responses. (A) Rectal balloon distention test in a normal subject, who reported first sensation at less than 40 cc balloon distension, urge to defecate at 160 cc and the MTV at 190 cc. (B) A patient with rectal hypersensitivity with lower sensory thresholds, with an urge to defecate (40 cc), pain (70 cc) and MTV (100 cc). (C) A subject with rectal hyposensitivity with first sensation (130 cc) and a slight desire to defecate at 180 cc and much higher threshold for urge to defecate. DD: Desire to defacate; MTV: Maximal tolerated volume.
In the barostat technique, a highly compliant PVC balloon is placed inside the rectum and stepwise or ramp balloon distensions are performed [39]. The barostat consists of a pump, often a piston located inside a hollow cylinder, a motor and pressure transducers [39]. At preset pressures, the piston drives air out of the cylinder into a highly compliant balloon, which then distends the lumen. Thereafter, intraballoon pressures and intraballoon volumes are continuously recorded and displayed on a monitor. The primary function of the barostat is to maintain a constant intraballoon pressure or volume. When the rectum contracts or its tone increases, the balloon is compressed. This increases the intra-balloon pressure. In order to maintain the preset pressure, the pump withdraws air from the balloon into the cylinder. This changes the intraballoon volume, which is displayed on the recorder as a decrease in the balloon volume. By contrast, when the muscle wall relaxes, the intraballoon pressure falls. In order to maintain the preset pressure the pump pushes air to expand the balloon. Consequently, the intraballoon volume increases. Thus, by plotting the changes in balloon volume, the barostat method can indirectly assess the tonic changes of the luminal wall [39].
To overcome response bias, three distension paradigms have been used: sham distensions randomly interspersed with true distensions, a forced-choice technique and tracking or double-random staircase technique. With the forced-choice technique, patients are told that the distensions will always occur in one of two intervals, and they are to indicate their responses [39].
Although rectal sensation is typically assessed by balloon distension, it can also be evoked by using an electrical stimulus applied to the rectal mucosa. This technique is not only feasible, but is also reproducible and provides comparable information to balloon distensions [40, 41]. In addition, it allows the neurophysiological characterization of visceral afferent pathways between the gut and the brain through the recording of cortical sensory-evoked potentials.
Clinical utility of rectal sensory testing
This test is commonly used in patients with constipation, incontinence, irritable bowel syndrome (IBS) and anorectal pain [14, 26]. The maximum tolerable volume or pain threshold may be reduced in patients who have a noncompliant rectum (e.g., abdominoperineal pull-through, proctitis, rectal ischemia) [26]. The pain threshold may also be reduced in patients with IBS (FIGURE 1) [42–44]. By contrast, a higher threshold for sensory perception suggests impaired rectal sensation or rectal hyposensitivity [14, 26].
Rectal distension also induces the RACR and RAIR [10]. The RAIR is absent in patients with Hirschsprung’s disease, after circular rectal myotomy and after low anterior resection [6–9]. It has been shown that the volume required to induce reflex anal relaxation is lower in incontinent patients than controls [22]. All of the aforementioned local reflexes are independent of rectal sensation. Recently a reproducible anal contractile response – the SMR has been described in association with rectal sensory perception, typically a desire to defecate [10]. Interestingly, the SMR was present in patients with rectal hyposensitivity, but its amplitude, duration and area under the curve were higher than in healthy controls [45]. The SMR could play an integral role in regulating anorectal sensation and function, but its exact role merits further study.
Clinical utility of the barostat
Currently, the barostat is used widely for clinical and research studies. This technique allows the examination of sensory function and compliance of the rectal wall [46]. In addition, the barostat is useful for examining the tonic changes of the rectum after pharmacomodulation.
Technical factors that affect the measurement of compliance include the size and shape of the balloon, and the materials from which the balloon is constructed [39]. With this method, normal values vary according to the range of distention volumes; some investigators have measured pressures over a predetermined range of volumes (50–250 ml), whereas others have measured pressures up to the maximal tolerable volume, which varies greatly in control subjects [39].
Using the barostat, it has been demonstrated that IBS patients have diminished rectal sensory thresholds [47, 48]. Furthermore, subjects with fecal incontinence can demonstrate a hypersensitive and poor compliant rectum [49] or rectal hyposensitivity [14]. By contrast, subjects with dyssynergic defecation demonstrate an increase in rectal tone, abnormal sensory perception and/or altered rectal wall contractility [50].
Alterations in rectal compliance may result in decreased or increased rectal capacity, impaired ability to perceive rectal distention and altered threshold for reflex IAS inhibition. Conditions that decrease rectal compliance include ulcerative colitis [51], radiotherapy [8, 52], surgical replacement of rectum with sigmoid colon or Koch pouch, and drugs [1].
Anal sensory testing
Since sampling of rectal contents by the anal mucosa may play an important role in maintaining continence, quantitative assessment of anal perception using either electrical or thermal [26] stimulation has been tested [53]. In one study, anal mucosal sensation was assessed by recording perception threshold for electrical stimulation of the mid-anal canal using a ring electrode [12, 53].
Clinical utility of anal sensory testing
A combined sensory and motor defect was seen in patients with incontinence [54]. In another study, although anal canal perception was impaired immediately after a vaginal delivery, there was no difference at 6 months [55]. The role of thermosensitivity appears controversial. In one study, the ability of healthy anal mucosa to differentiate between small changes in temperature has been questioned [56]. Hence, under normal conditions it may not be possible to appreciate the temperature of fecal matter passing from the rectum to the anal canal. Whether patients have a pure sensory defect of anus without coexisting sphincter dysfunction or rectal sensory impairment has not been shown.
Recommendation
Rectal sensory testing, preferably using the barostat, is indicated for the detection of rectal hyposensitivity or rectal hypersensitivity. Altered rectal perception is seen in a variety of conditions that include fecal incontinence, chronic constipation, IBS and after rectal surgery.
Electromyography
Electrical recording of the muscle activity from the anal sphincter (electromyography [EMG]) is a useful technique of identifying sphincter injury as well as denervation–reinnervation potentials that can indicate neuropathy [26]. Although anal sphincter EMG activity is variable and subject to significant artifacts, recent studies show that concentric needle EMG provides more robust data without significant artifacts [57, 58]. However, it is still considered to be a research tool and is not routinely used in clinical practice for diagnostic purposes. EMG can be performed using a fine wire needle electrode or a surface electrode such as an anal plug [59–61].
Needle EMG is often used to map the presence or absence of striated muscle within the EAS [26, 59]. This is useful in the assessment of EAS damage (e.g., obstetric injury, surgical or spinal cord injury), and in patients with congenital abnormalities of the anorectum such as imperforate anus. Needle EMG mapping correlates well with anal ultrasonography for identifying EAS injury [62, 63]. Two types of needle electrodes, a concentric needle that samples a large number of motor units simultaneously or a single-fiber electrode whose small recording surface samples the electrical activity of one motor unit at a time, have been used [59]. Concentric needle EMG is usually performed with a disposable electrode that records the spontaneous activity of motor unit potential, and also during voluntary or reflex contraction [59, 64]. The number of motor units recruited during anal squeeze maneuver highly correlates with anal canal squeeze pressure [61].
Single-fiber EMG provides information on fiber density, which is defined as the mean number of muscle fibers that belong to an individual motor unit per detection site [26, 58–61]. The normal fiber density of the EAS is below 2.0. An increase in fiber density is a sensitive indicator of reinnervation following nerve or muscle injury, for example, in patients with fecal incontinence [26, 59, 65–67].
Although, needle EMG provides more accurate information, surface EMG has its own advantages [68]. Surface EMG recorded from the anal canal is painless when compared with needle EMG, and can provide qualitative information. The quality and reliability of anal sphincter EMG using surface electrodes depends on the orientation, size and number of electrodes. Recently, an anal plug holding an array of 16 equally spaced silver bar electrodes has been tested [69], that may provide important pathophysiological information.
Clinical utility of EMG
Abnormal EMG activity, such as fibrillation potentials and high-frequency spontaneous discharges, provide evidence of chronic denervation [26, 59]. For example, the finding of polyphasic motor unit potentials is indicative of denervation and reinnervation. Anal sphincter denervation is commonly seen in patients with fecal incontinence secondary to pudendal nerve injury or cauda equina syndrome [70]. The EAS may be affected in multiple system atrophy [71, 72]. However, the interpretation of EMG findings requires specialized training and experience.
Single fiber EMG is useful for the measurement of jitter, which is the variability of consecutive muscle fiber discharges. This correlates with the stability of terminal motor axons and neuromuscular transmission [59]. Jitter is most helpful in detecting disorders of neuromuscular junction, for example, myasthenia gravis, and only rarely in anorectal dysfunction.
Surface EMG can be combined with anorectal manometry to reveal abnormal patterns of muscle activation in patients with dyssynergic defecation because it can determine the presence of inappropriate sphincter relaxation during defecation [73]. In one study, inability to relax the anal sphincter detected by EMG correlated with inability to expel a balloon in 82% of subjects (dyssynergic defecation) [74]. Finally, insufficient anal sphincter activation during attempts to retain a manometric balloon in the anorectum can be seen in fecal incontinence [59].
Recommendation
Surface, needle and concentric needle EMG techniques have been used to define an underlying neuropathy or muscle dysfunction in selected cases and in research studies. They are recommended for specialist use but not for routine clinical practice.
Pudendal nerve terminal motor latency
The pudendal nerve terminal motor latency (PNTML) measures neuromuscular integrity between the terminal portion of the pudendal nerve and the anal sphincter (FIGURE 2). PNTML may be useful in the assessment of patients with fecal incontinence prior to anal sphincter repair and is particularly helpful in predicting the outcome of surgery [26, 59, 75]. However, other studies have shown that this technique may be useful in the evaluation of perineal descent [76, 77] and constipation [78]. An injury to the pudendal nerve leads to denervation of the anal sphincter muscle and muscle weakness. Thus, measurement of PNTML can identify if a weak sphincter muscle is due to muscle injury or nerve injury. A disposable electrode (St Mark’s electrode; Dantec-Medtronics, MN, USA) is commonly used [79].
Figure 2. Normal and abnormal right-sided pudendal nerve terminal motor latency recordings.
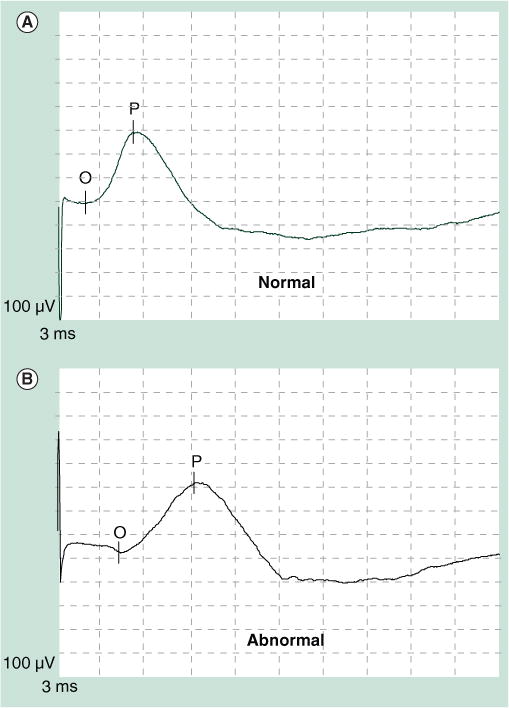
(A) A healthy subject and (B) in a patient with fecal incontinence.
Clinical utility of PNTML
A prolonged nerve latency time suggests pudendal neuropathy. Women who delivered vaginally and had a prolonged second stage of labor or had forceps-assisted delivery were more likely to have prolonged PNTML when compared with women who delivered by cesarian section [80]. Furthermore, after an obstetric injury, women who develop fecal incontinence have been shown to have both pudendal neuropathy and anal sphincter defects. Fecal incontinence is often the end result of both nerve and muscle injury. In one study, women with obstetrical injury developed fecal incontinence only when there was associated pudendal neuropathy [81]. Thus, PNTML by itself cannot identify the underlying mechanism for fecal incontinence. However, in conjunction with manometry and/or anal endosonography, it can provide the missing link. In a retrospective study of 55 patients with fecal incontinence, secondary to obstetric trauma and who underwent surgery, five patients with intact anal sphincter and six with a nonintact anal sphincter had a poor surgical outcome [81]. Thus, neither anal endosonography nor PNTML could predict surgical outcome. Others have shown that no single test of anorectal function has a high enough discriminatory value or predictive value for identifying the underlying pathophysiology [82]. One study showed that surgical repair produced a good-to-excellent result in 80% of women with fecal incontinence but without pudendal neuropathy compared with 11% of women with neuropathy [83]. Thus, it appears that women with sphincter defects alone fare better following sphincter repair than women with both sphincter defects and neuropathy.
The American Gastroenterological Association technical review did not recommend PNTML for the evaluation of patients with fecal incontinence because it correlates poorly with clinical symptoms and histology findings; it does not discriminate muscle weakness caused by nerve or muscle injury; it has poor sensitivity and specificity; it was operator dependent (e.g., a short latency could be due to stimulation of the nerve more distally); and that it does not predict surgical outcome [26]. However, reviews of eight uncontrolled studies reported that patients with pudendal neuropathy generally have a poor surgical outcome when compared with those without neuropathy [84, 85]. A normal PNTML does not exclude pudendal neuropathy, because the presence of a few intact nerve fibers can give a normal result, whereas an abnormal latency time is more significant. Thus, when interpreting the PNTML result, it is important to consider whether a patient has muscle injury or neurogenic injury or mixed injury. In a patient with only muscle injury, there may be little or no nerve damage whereas in a patient with only neurogenic injury there may be little or no muscle disruption. In the vast majority of patients, however, there is mixed injury. If so, the prognostic value of PNTML will depend to some extent on the degree of each type of injury, the age of the patient and other coexisting problems. A well-designed multicenter prospective controlled trial is needed to better define the utility of this test, both for diagnostic purposes and for predicting the clinical outcome of therapeutic intervention(s).
Recommendation
PNTML may be useful in the assessment of patients with fecal incontinence, particularly when considering surgical intervention.
Novel & emerging neuropysiological tests
Somatosensory cortical evoked potentials
Efferent and afferent neuronal pathways between the brain and gut are intimately involved in mediating sensations and reflexes that govern anal and rectal function [86–89]. Thus, dysfunction of the efferent or afferent pathways from the rectum or the pelvic floor could lead to disorders of defecation, such as constipation and fecal incontinence [86]. Today, we can investigate the bidirectional neural pathways between the anal canal, rectum, spinal cord and brain using magnetic or electrical or mechanical stimulation [89–93].
The afferent neural circuitry can be studied using either cortical evoked potentials (CEP) or by examining PET or functional MRI (fMRI), or magnetic encephalography (MEG) techniques [86, 92, 93]. CEP recording provides an objective, quantifiable tool for the evaluation of sensory disorders that affect the afferent tracts, the spinal cord and the cerebral cortex. Neurophysiologists have used CEP to study somatosensory, visual, auditory and pain pathways for over 50 years. This technique involves a brief sensory stimulation, which is time and phase locked to the electroencephalogram recording via surface electrodes placed on the scalp. The event-related signal is small in amplitude but occurs at the same moment in time following each stimulus while the large amplitude background electroencephalogram occurs randomly. In order to extract the desired signal, repeated stimuli are given and the subsequent brain activity is averaged. The resultant waveform represents the brain’s response to a stimulus as it changes with each millisecond. CEP responses are reproducible and have been recorded in response to stimulation of many regions of the GI tract [89–92]. A recent study described a ‘human visceral homunculus’ to electrical evoked pain in the esophagus, stomach, duodenum and colon and anorectum (FIGURE 3) [90].
Figure 3. Cortical evoked potentials after anal electrical stimulation.
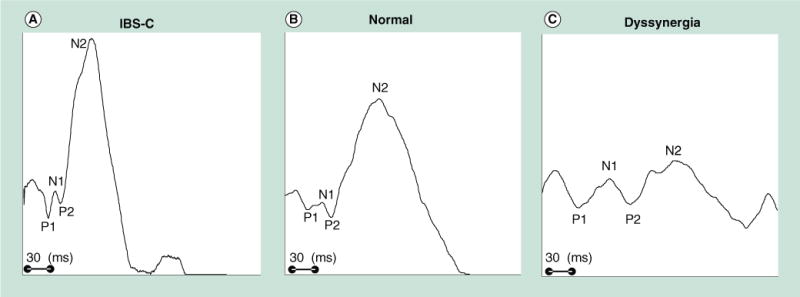
Typical examples from (A) one patient with predominant IBS-C, (B) a healthy control and (C) a dyssynergic constipated patient. The anal cortical evoked potentials latencies are shorter and the amplitudes are higher in IBS-C than control or dyssynergia patient. By contrast, the anal cortical evoked potential latency for P1 is prolonged, and the N2 amplitude is lower in the subject with dyssynergia. These preliminary data suggest that the gut–brain axis is altered in subjects with functional anorectal disorders. IBS-C: Predominant constipation irritable bowel syndrome.
The advantages of the CEP over other brain imaging techniques include inexpensive equipment, wide availability and the ability to incorporate this in physiology laboratories. However, CEP represents a summation of cortical activity, which makes it difficult to precisely localize the neural correlates of the evoked response.
Clinical utility of CEP
Two studies have concluded that CEP responses to rectal stimulation may provide useful pathophysiological information in patients with IBS [91, 92]. Chan et al. recorded CEP in response to rhythmic rectal distensions in 22 pairs of age-matched healthy females and IBS patients [91]. IBS patients demonstrated higher prevalence of cerebral evoked potential early peaks (latency: 100 ms) postprandially, and uniformly shorter cerebral evoked potential latencies, both before and after a meal. In another study, patients with IBS had shorter latency and increased amplitude compared with controls [92]. These findings provide supporting evidence for visceral afferent hypersensitivity.
Loening-Baucke et al. studied anorectal CEP responses in children with constipation and encopresis and found that the latencies of the early-onset evoked potentials were prolonged [93]. Recently, we have found that adult patients with dyssynergic defecation exhibit prolonged latencies as well as significantly attenuated amplitudes of both the anal and rectal CEP responses when compared with healthy controls. This shows that the afferent neuronal transmission between the gut and the brain is impaired in patients with dyssynergic defecation [94].
Motor evoked potentials
The integrity of the efferent motor pathways that control anorectal function can be assessed by recording the motor evoked potentials (MEPs) of the rectum and anal sphincter response to magnetic stimulation of the motor cortex (transcranial magnetic stimulation [TMS]) [95, 96]. TMS is a new noninvasive technique of magnetic stimulation of the cortical neurons and with minimal discomfort [95]. It relies on Faraday’s principle, which states that in the presence of a changing electrical field, a magnetic field is generated. Consequently, when a current is rapidly discharged through a conducting coil, a magnetic flux is produced around the coil. The magnetic flux causes stimulation of neural tissue. Recently, TMS has been used to map the cortical location of human anorectal musculature (FIGURE 4) [96]. Cortical mapping with transcranial magnetic stimulation suggests that rectal and anal responses are bilaterally represented on the superior motor cortex – that is, Brodmann area 4 [46].
Figure 4. Anal motor evoked potentials after transcranial magnetic stimulation.
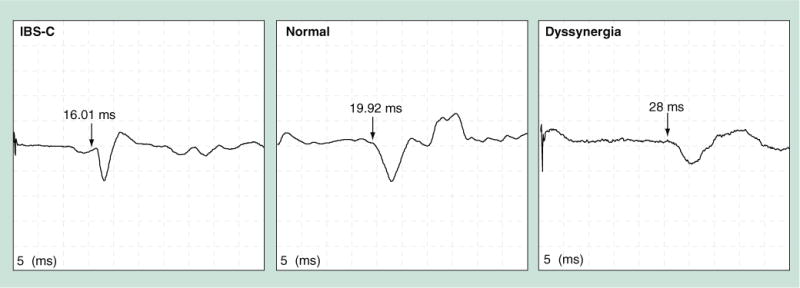
A patient with IBS-C has shorter latency whereas a subject with dyssynergea has prolonged latency of motor evoked potentials response compared with a normal subject. IBS-C: Predominant constipation irritable bowel syndrome.
In addition, the peripheral component of the efferent pathways can be assessed by stimulating the lumbo–sacral nerve roots (translumbar magnetic stimulation [TLMS]), and transsacral magnetic stimulation (TSMS) [97–100]. Magnetic stimulation of the lumbosacral roots (TLMS and TSMS) may allow more precise localization of the motor pathways between the brain and the anal sphincter, as well as subcomponent analysis of the efferent nervous system between the brain and end organ [97–100].
Clinical utility of MEP
Electric or magnetic stimulation of the lumbosacral nerve roots facilitates measurement of the conduction time within the cauda equina and diagnose sacral motor radiculopathy, as a possible cause of fecal incontinence [101–103]. Recently, a combined technique of CEP and MEP evaluation has been described in healthy humans [100]. This combined technique provides a novel, integrated, comprehensive and objective method of assessment of gut–brain–gut interactions but further prospective studies are awaited.
Positron emission tomography
Positron emission tomography relies on the principle of detecting positron-emitting radionuclides that are intravenously injected into a subject [104, 105]. PET has a high spatial resolution and sources of brain activity can be identified with sufficient accuracy. Furthermore, unlike MEG and CEP, subcortical brain structures can also be identified. PET can also be used to determine the size of an area where there is metabolic change [104, 105]. The main limitation of PET is that it requires radioactive compounds and is therefore invasive, repeat studies are problematic, it is expensive and sophisticated, and the equipment is only available in specialized centers.
Clinical utility of PET
Positron emission tomogrphy is essentially a research tool for assessing the brain–gut axis. In healthy subjects, perception during either actual or simulated delivery of painful rectal stimulation is associated with increased activity of the anterior cingulate cortex [104]. In patients with IBS, anterior cingulate cortex activation failed to occur and, by contrast, the left prefrontal cortex was activated, suggesting an aberrant CNS processing [104, 105]. Further studies in patients with IBS also suggest that rectal hypersensitivity induced by repetitive distention of the sigmoid colon correlates closely with an increase in blood flow in the thalamus and that an abnormal thalamic response to pain may be responsible for the abnormal sensitization [105]. Although it reveals novel pathophysiological mechanisms, the clinical relevance of these findings is uncertain and its clinical use has not been tested.
Functional MRI
Whereas MRI is a well-established technique for imaging brain structure, fMRI is a technique for correlating brain structure and function. fMRI produces images of active brain regions by detecting indirect effects of neural activity on local blood flow, volume and oxygen saturation, giving detailed information regarding the functional neuroanatomy of the brain [104, 105]. The most commonly used fMRI technique relies on the detection of deoxygenated hemoglobin, due to its paramagnetic properties within the blood, and to identify changes in its regional distribution during conditions wthat change neuronal activation. Similar to PET, fMRI is largely used in research laboratories and clinical trials to examine the brain–gut interactions in IBS patients [103, 104]. It has not been tested for clinical use.
Expert commentary
Disorders of the anorectum affect 15–20% of the population and most of these are a consequence of altered neuromuscular function [106]. Hence, neurophysiological testing can provide information regarding the physiology and pathophysiology of common anorectal disorders, such as fecal incontinence, dyssynergic defecation, rectal hyposensitivity, rectal hypersensitivity and pelvic neuropathy.
In this review, we examined the functional neuroanatomy and physiology of the anorectum, and provided detailed description(s), including indications and clinical utility of several commonly performed neurophysiological tests, as well as those that are regarded as novel and emerging techniques. These tests are complementary to other methods of investigation, such as pelvic floor imaging. Currently, several new techniques are being tested that, hopefully, may provide novel insights regarding the brain–gut interactions.
Five-year view
Although many of the neurophysiological tests discussed previously are at present mainly used in tertiary care centers and for research purposes, it is possible that over the next 5 years some of these tests could become more widely available and prove useful in the diagnosis of anorectal disorders. However, prospective, well-controlled clinical trails are required to assess their feasibility and clinical utility. Furthermore, the techniques should be standardized and made more user friendly for community use. It is likely that some of the emerging techniques may provide a more accurate and reliable diagnosis of anorectal disorders that could conceivably lead to more effective treatment approaches.
Key issues.
Neurophysiological tests, such as anorectal manometry, rectal sensory and compliance testing with a barostat, electromyography of the anal sphincter and pudendal nerve terminal motor latency, are useful for defining the pathophysiology of anorectal disorders and, in some instances, for facilitating better treatment of these disorders. However, many of these tests lack well-controlled, prospective evaluations particularly with regards to their clinical utility and impact on treatment outcome. Such systematic studies are eagerly awaited.
No single neurophysiological test will provide comprehensive information regarding the pathophysiology of anorectal disorders since these disorders are heterogeneous and often multifactorial.
Today, several innovative techniques, such as PET, functional MRI, cortical evoked potentials and transcranial magnetic stimulation, are available and are being tested in research centers. These innovative tests may provide novel information regarding the neuronal connections between the anorectum and the brain as well as the brain–gut axis. A better understanding of the underlying neurophysiological mechanisms could pave the way for more rational therapy for many of these disorders.
Acknowledgments
We would like to acknowledge the excellent technical support provided by Ms Jessica Paulson.
Footnotes
Financial & competing interests disclosure
Dr Remes-Troche was supported by the American Gastroenterological Association, Jon I Isenberg International Scholar Award. Dr Rao was supported by the R01DK057100 grant from the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
of interest
of considerable interest
Contributor Information
Jose M Remes-Troche, Email: joremes@uv.mx, Digestive Physiology and Motility Department, Medical-Biological Research Institute, University of Veracruz, Veracruz, Mexico, Tel.: +52 229 202 1231, Fax: +52 229 202 1231.
Satish SC Rao, Email: satish-rao@uiowa.edu, Section of Neuro gastroenterology, Division of Gastroenterology–Hepatology, Department of Internal Medicine, University of Iowa College of Medicine, Iowa City, IA 52242, USA, Tel.: +1 319 353 6602, Fax: +1 319 353 6399.
References
- 1.Rao SSC. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–S22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2••.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. Excellent and updated review that includes traditional and novel concepts regarding pelvic floor anatomy and function. [DOI] [PubMed] [Google Scholar]
- 3.Wankling WJ, Brown BH, Collins CD, et al. Basal electrical activity in the anal canal in man. Gut. 1968;9:457–460. doi: 10.1136/gut.9.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gowers WR. The automatic action of the sphincter ani. Proc R Soc Lond. 1877;126:77–84. [Google Scholar]
- 5.Sun WM, Read NW, Miner PB, et al. The role of transient internal sphincter relaxation in fecal incontinence. Int J Colorectal Dis. 1990;5:31–36. doi: 10.1007/BF00496147. [DOI] [PubMed] [Google Scholar]
- 6.Sangwan YP, Solla JA. Internal anal sphincter. Advances and insights. Dis Colon Rectum. 1998;41:1297–1311. doi: 10.1007/BF02258232. [DOI] [PubMed] [Google Scholar]
- 7.Cortesini C, Pucciani F, Carassale G, et al. Anorectal physiology after anterior resection and pull-through operation. Eur Surg Res. 1983;15:176–183. doi: 10.1159/000128350. [DOI] [PubMed] [Google Scholar]
- 8.Lewis WG, Williamson ME, Kuzu A, et al. Potential disadvantages of post-operative adjuvant radiotherapy after anterior resection for rectal cancer: a pilot study of sphincter function, rectal capacity and clinical outcome. Int J Colorectal Dis. 1995;10:133–137. doi: 10.1007/BF00298533. [DOI] [PubMed] [Google Scholar]
- 9.Saigusa N, Belin BM, Choi HJ, et al. Recovery of the rectoanal inhibitory reflex after restorative proctocolectomy: does it correlate with nocturnal continence? Dis Colon Rectum. 2003;46:168–172. doi: 10.1007/s10350-004-6519-z. [DOI] [PubMed] [Google Scholar]
- 10.De Ocampo S, Remes-Troche JM, Miller M, et al. Rectoanal sensori-motor response in humans during rectal distension. Dis Colon Rectum. 2007;50(10):1639–1646. doi: 10.1007/s10350-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 11.Read MG, Read NW. Role of anorectal sensation in preserving continence. Gut. 1982;23:345–347. doi: 10.1136/gut.23.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers J. Anal and rectal sensation. Baillieres Clin Gastroenterol. 1992;6:179–191. doi: 10.1016/0950-3528(92)90026-b. [DOI] [PubMed] [Google Scholar]
- 13.Zagorodnyuk VP, Lynn P, Costa M, et al. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–G406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- 14•.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol. 2006;101(5):1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. Comprehensive review regarding rectal hyposensitivity, considered to be a key factor in the pathogenesis of most anorectal disorders. [DOI] [PubMed] [Google Scholar]
- 15.Penninckx F, Lestar B, Kerremans R. The internal anal sphincter: mechanisms of control and its role in maintaining anal continence. Baillieres Clin Gastroenterol. 1992;6:193–213. doi: 10.1016/0950-3528(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 16.Parks AG, Fishlock DJ, Cameron JD, et al. Preliminary investigation of the pharmacology of the human internal anal sphincter. Gut. 1969;10:674–677. doi: 10.1136/gut.10.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rattan S, Sarkar A, Chakder S. Nitric oxide pathway in rectoanal inhibitory reflex of opossum internal anal sphincter. Gastroenterolgy. 1992;103:43–50. doi: 10.1016/0016-5085(92)91093-j. [DOI] [PubMed] [Google Scholar]
- 18.Nurko S, Rattan S. Role of vasoactive intestinal peptide in the internal anal sphincter of the opossum. J Clin Invest. 1988;81(4):1146–1153. doi: 10.1172/JCI113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SS. Constipation. In: Rao SS, editor. Gastrointestinal Motility: Tests and Problem-Oriented Approach. Kluwer Academic/Plenum Publishers; NY, USA: 1999. pp. 197–211. [Google Scholar]
- 20.Denny Brown D, Robertson EG. An investigation of the nervous control of defecation. Brain. 1935;58:256–210. doi: 10.1111/j.1463-1318.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller R, Bartolo DCC, Cervero F, et al. Anorectal sampling: a comparison of normal and incontinent patients. Br J Surg. 1988;75:44–47. doi: 10.1002/bjs.1800750116. [DOI] [PubMed] [Google Scholar]
- 22.Read NW, Harford WV, Schmulen AC, et al. A clinical study of patients with fecal incontinence and diarrhea. Gastroenterology. 1979;76:747–756. [PubMed] [Google Scholar]
- 23•.Rao SSC, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:1605–1615. doi: 10.1111/j.1572-0241.2005.41845.x. Systematic review assessing the clinical utility of diagnostic tests in adult patients with chronic constipation. According to this review, evidence to support the use of blood tests, radiography or endoscopy in the routine work up of patients with constipation without alarm features is lacking. Colonic transit, anorectal manometry and balloon expulsion tests reveal physiologic abnormalities in many selected patients with refractory constipation. [DOI] [PubMed] [Google Scholar]
- 24•.Rao SS, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Mot. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. Proposed the minimal uniform standards for performing and interpreting anorectal manometry according to the American and European motility societies. [DOI] [PubMed] [Google Scholar]
- 25.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water perfused manometry. Am J Gastroenterol. 2007;102:850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 26•.Diamant ND, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. Reviewed the indications, utility and advantages of the anorectal testing techniques recommended by the American Gastroenterological Association. [DOI] [PubMed] [Google Scholar]
- 27.Sun W, MacDonagh R, Forster D, et al. Anorectal function in patients with complete spinal transection before and after sacral posterior rhizotomy. Gastroenterology. 1998;108:990–998. doi: 10.1016/0016-5085(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 28.Rao SSC, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469–475. [PubMed] [Google Scholar]
- 29.Bartram C. Radiological evaluation of anorectal disorders. In: Rao SSC, editor. Disorders of Anorectum. 1. Vol. 30. WB Saunders; 2001. pp. 55–76. (Gastroenterol Clin North Am). [DOI] [PubMed] [Google Scholar]
- 30.Reid JR, Buonomo C, Moreira C, et al. The barium enema in constipation: comparison with rectal manometry and biopsy to exclude Hirschsprung’s disease after the neonatal period. Pediatr Radiol. 2001;30:681–684. doi: 10.1007/s002470000298. [DOI] [PubMed] [Google Scholar]
- 31.De Lorijn, Reitsma JB, Voskuijk WP, et al. Diagnosis of Hirschprung’s disease: a prospective, comparative accuracy study of common tests. J Pediatr. 2005;146:787–792. doi: 10.1016/j.jpeds.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:1–8. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 33.Rao SSC. Dyssynergic defecation: disorders of the anorectum. Gastroenterol Clin North Am. 2001;31:97–114. doi: 10.1016/s0889-8553(05)70169-2. [DOI] [PubMed] [Google Scholar]
- 34.Rao SSC, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 35.Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006;101(2):2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 36.Rao SS, Ozturk R, De Ocampo S, et al. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol. 2006;101:613–618. doi: 10.1111/j.1572-0241.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 37.Whitehead WE, Schuster MM. Anorectal physiology and pathophysiology. Am J Gastroenterol. 1987;82:487–497. [PubMed] [Google Scholar]
- 38.Sun WM, Read NW, Prior A, et al. Sensory and motor responses to rectal distension vary according to rate and pattern of balloon inflation. Gastroenterology. 1999;99:1008–1015. doi: 10.1016/0016-5085(90)90620-g. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research, UK. Dig Dis Sci. 1997;42:223–241. doi: 10.1023/a:1018885028501. [DOI] [PubMed] [Google Scholar]
- 40.Harris ML, Hobson AR, Hamdy S, et al. Neurophysiological evaluation of healthy human anorectal sensation. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G950–G958. doi: 10.1152/ajpgi.00010.2006. [DOI] [PubMed] [Google Scholar]
- 41.Remes-Troche JM, Paulson J, Miller MJ, et al. Cortical evoked potentials (CEP) and transcranial motor evoked potentials (MEP) – a novel test of brain–gut axis in humans. Am J Gastroenterol. 2006;101:S480–S480. [Google Scholar]
- 42.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 43.Corsetti M, Cesana B, Bhoori S, et al. Rectal hyperreactivity to distention in patients with irritable bowel syndrome: role of distention rate. Clin Gastroenterol Hepatol. 2004;2(1):49–56. doi: 10.1016/s1542-3565(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 44.Nozu T, Kudaira M, Kitamori S, et al. Repetitive rectal painful distention induces rectal hypersensitivity in patients with irritable bowel syndrome. J Gastroenterol. 2006;41(3):217–222. doi: 10.1007/s00535-005-1748-z. [DOI] [PubMed] [Google Scholar]
- 45.Remes-Troche JM, De Ocampo S, Miller M, et al. Investigation of sensori–motor, recto anal inhibitory and contractile reflexes in rectal hyposensitivity. Gastroenterology. 2006;130(Suppl. A115):940. [Google Scholar]
- 46.Felt-Bersma RJ, Sloots CE, Poen AC, et al. Rectal compliance as a routine measurement: extreme volumes have direct clinical impact and normal volumes exclude rectum as a problem. Dis Colon Rectum. 2000;12:1732–1738. doi: 10.1007/BF02236859. [DOI] [PubMed] [Google Scholar]
- 47.Lee KJ, Kim JH, Cho SW. Relationship of underlying abnormalities in rectal sensitivity and compliance to distension with symptoms in irritable bowel syndrome. Digestion. 2006;73:133–141. doi: 10.1159/000094099. [DOI] [PubMed] [Google Scholar]
- 48.Steens J, Van Der Schaar PJ, Penning C, et al. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:41–47. doi: 10.1046/j.1365-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 49.Sproudhis L, El Abkari M, El Aloui M, et al. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Aliment Pharmacol Ther. 2006;23:989–996. doi: 10.1111/j.1365-2036.2005.02675.x. [DOI] [PubMed] [Google Scholar]
- 50.Schouten WR, Gosselink MJ, Boerma MO, et al. Rectal wall contractility in response ton an evoked urge to defecate in patients with obstructed defecation. Dis Colon Rectum. 1998;41:473–479. doi: 10.1007/BF02235762. [DOI] [PubMed] [Google Scholar]
- 51.Rao SS, Read NW, Davison PA, et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1983;93:1270–1275. doi: 10.1016/0016-5085(87)90255-1. [DOI] [PubMed] [Google Scholar]
- 52.Yeoh EE, Holloway RH, Fraser RJ, et al. Anorectal dysfunction increases with time following radiation therapy for carcinoma of the prostate. Am J Gastroenterol. 2004;99:361–369. doi: 10.1111/j.1572-0241.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 53.Felt-Bersma RJ, Poen AC, Cuesta MA, et al. Anal sensitivity test: what does it measure and do we need it? Cause or derivative of anorectal complaints. Dis Colon Rectum. 1997;40:811–816. doi: 10.1007/BF02055438. [DOI] [PubMed] [Google Scholar]
- 54.Rogers J, Henry MM, Misiewicz JJ. Combined motor and sensory deficit in primary neuropathic fecal incontinence. Gut. 1988;29:5–9. doi: 10.1136/gut.29.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornes H, Bartolo DC, Stirrat GM. Changes in anal canal sensation after childbirth. Br J Surg. 1991;78(1):74–77. doi: 10.1002/bjs.1800780123. [DOI] [PubMed] [Google Scholar]
- 56.Rogers J, Hayward MP, Henry MM, et al. Temperature gradient between the rectum and anal canal: evidence against the role of temperature sensation as a sensory modality in the anal canal of normal subjects. Br J Surg. 1988;75:1082–1085. doi: 10.1002/bjs.1800751111. [DOI] [PubMed] [Google Scholar]
- 57.Podnar S. Which patients need referral for anal sphincter electromyography? Muscle Nerve. 2006;33:278–282. doi: 10.1002/mus.20472. [DOI] [PubMed] [Google Scholar]
- 58.Podnar S, Rodi Z, Lukanovic A, et al. Standardization of anal sphincter EMG: technique of needle examination. Muscle Nerve. 1999;22:400–403. doi: 10.1002/(sici)1097-4598(199903)22:3<400::aid-mus14>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 59.Lefaucher JP. Neurophysiological testing in anorectal disorders. Muscle Nerve. 2006;33:324–333. doi: 10.1002/mus.20387. [DOI] [PubMed] [Google Scholar]
- 60.Sorensen M, Nielsen MB, Pedersen JF, et al. Electromyography of the internal anal sphincter performed under endosonographic guidance. Description of a new method. Dis Colon Rectum. 1994;37:138–143. doi: 10.1007/BF02047535. [DOI] [PubMed] [Google Scholar]
- 61.Lopez A, Nilsson BY, Mellgren A, et al. Electromyography of the external anal sphincter: comparison between needle and surface electrodes. Dis Colon Rectum. 1999;42:482–485. doi: 10.1007/BF02234172. [DOI] [PubMed] [Google Scholar]
- 62.Law PJ, Kamm MA, Bartram CI. A comparison between electromyography and anal endosonography in mapping external anal sphincter defects. Dis Colon Rectum. 1990;33:370–373. doi: 10.1007/BF02156260. [DOI] [PubMed] [Google Scholar]
- 63.Cheong DMO, Vaccaro CA, Salanga VD, et al. Electrodiagnostic evaluation of fecal incontinence. Muscle Nerve. 1995;18:612–619. doi: 10.1002/mus.880180608. [DOI] [PubMed] [Google Scholar]
- 64.Jesel M, Isch-Treussard C, Isch F. Electromyography of striated muscle of anal and urethral sphincters. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Vol. 2. Karger Publishers; Basel, Switzerland: 1973. pp. 406–420. [Google Scholar]
- 65.Podnar S. Electrodiagnosis of the anorectum: a review of techniques and clinical applications. Tech Coloproctol. 2003;7(2):71–76. doi: 10.1007/s10151-003-0012-x. [DOI] [PubMed] [Google Scholar]
- 66.Thomas C, Lefaucheur JP, Galula G, et al. Respective value of pudendal nerve terminal motor latency and anal sphincter electromyography in neurogenic fecal incontinence. Neurophysiol Clin. 2002;31(1):85–90. doi: 10.1016/s0987-7053(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 67.Sangwan YP, Coller JA, Barrett RC, et al. Prospective comparative study of abnormal distal rectoanal excitatory reflex, pudendal nerve terminal motor latency, and single fiber density as markers of pudendal neuropathy. Dis Colon Rectum. 1996;39:794–798. doi: 10.1007/BF02054446. [DOI] [PubMed] [Google Scholar]
- 68.Gee AS, Jones RS, Durdey P. On-line quantitative analysis of surface electromyography of the pelvic floor in patients with faecal incontinence. Br J Surg. 2000;87:814–818. doi: 10.1046/j.1365-2168.2000.01416.x. [DOI] [PubMed] [Google Scholar]
- 69.Merletti R, Bottin A, Cescon C, et al. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69:112–122. doi: 10.1159/000077877. [DOI] [PubMed] [Google Scholar]
- 70.Infantino A, Melega E, Negrin P, et al. Striated anal sphincter electromyography in idiopathic fecal incontinence. Dis Colon Rectum. 1995;38:27–31. doi: 10.1007/BF02053853. [DOI] [PubMed] [Google Scholar]
- 71.Gilad R, Giladi N, Korczyn AD, et al. Quantitative anal sphincter EMG in multisystem atrophy and 100 controls. J Neurol Neurosurg Psychiatry. 2001;71:596–599. doi: 10.1136/jnnp.71.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palace J, Chandiramani VA, Fowler CJ. Value of sphincter electromyography in the diagnosis of multiple system atrophy. Muscle Nerve. 1997;20:1396–1403. doi: 10.1002/(sici)1097-4598(199711)20:11<1396::aid-mus7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 73.Pfeifer J, Teoh TA, Salanga VD, et al. Comparative study between intra-anal sponge and needle electrode for electromyographic evaluation of constipated patients. Dis Colon Rectum. 1998;41:1153–1157. doi: 10.1007/BF02239438. [DOI] [PubMed] [Google Scholar]
- 74.Jones PN, Lubowski DZ, Swash M, et al. Is paradoxical contraction of puborectalis muscle of functional importance? Dis Colon Rectum. 1987;30:667–670. doi: 10.1007/BF02561685. [DOI] [PubMed] [Google Scholar]
- 75.Rao SS. American College of Gastroenterology Practice Parameters Committee. Diagnosis and management of fecal incontinence. Am J Gastroenterol. 2004;99(8):1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 76.Jones PN, Lubowski DZ, Swash M, et al. Relation between perineal descent and pudendal nerve damage in idiopathic faecal incontinence. Int J Colorectal Dis. 1987;2:93–95. doi: 10.1007/BF01647699. [DOI] [PubMed] [Google Scholar]
- 77.Snooks SJ, Henry MM, Swash M. Anorectal incontinence and rectal prolapse: differential assessment of the innervation to puborectalis and external anal sphincter muscles. Gut. 1985;26:470–476. doi: 10.1136/gut.26.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaccaro CA, Cheong DM, Wexner SD, et al. Pudendal neuropathy in evacuatory disorders. Dis Colon Rectum. 1995;38:166–171. doi: 10.1007/BF02052445. [DOI] [PubMed] [Google Scholar]
- 79.Kiff ES, Swash M. Slowed conduction in the pudendal nerves in idiopathic (neurogenic) faecal incontinence. Br J Surg. 1984;71:614–616. doi: 10.1002/bjs.1800710817. [DOI] [PubMed] [Google Scholar]
- 80.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329(26):1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 81.Tetzschner T, Sorensen M, Lose G, et al. Anal and urinary incontinence in women with obstetric anal sphincter rupture. Br J Obstet Gynecol. 1996;103:1034–1040. doi: 10.1111/j.1471-0528.1996.tb09557.x. [DOI] [PubMed] [Google Scholar]
- 82.Engel AF, Kamm MA, Sultan AH, et al. Anterior anal sphincter repair in patients with obstetric trauma. Br J Surg. 1994;81:1231–1234. doi: 10.1002/bjs.1800810853. [DOI] [PubMed] [Google Scholar]
- 83.Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SGM. Investigation of anorectal function. Br J Surg. 1988;75:53–55. doi: 10.1002/bjs.1800750119. [DOI] [PubMed] [Google Scholar]
- 84.Laurberg S, Swash M, Henry MM. Delayed external sphincter repair for obstetric tear. Br J Surg. 1988;75:786–788. doi: 10.1002/bjs.1800750821. [DOI] [PubMed] [Google Scholar]
- 85.Rothholtz NA, Wexner SD. Surgical treatment of constipation and fecal incontinence. Gastroenterol Clin North Am. 2001;30:131–166. doi: 10.1016/s0889-8553(05)70171-0. [DOI] [PubMed] [Google Scholar]
- 86.Aziz Q, Thompson DG. Brain–gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- 87•.Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding? Gut. 2004;53:1198–1206. doi: 10.1136/gut.2003.035642. Useful paper describing many of the current and evolving techniques for studying the brain–gut axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loening-Baucke V, Read NW, Yamada T. Cerebral evoked potentials after rectal stimulation. Electroencephalogr Clin Neurophysiol. 1991;80:490–495. doi: 10.1016/0168-5597(91)90130-p. [DOI] [PubMed] [Google Scholar]
- 89.Hobday DI, Hobson AR, Sarkar S, et al. Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol. 2002;283:335–339. doi: 10.1152/ajpgi.00230.2001. [DOI] [PubMed] [Google Scholar]
- 90.Drewes AM, Dimcevski G, Sami SA, et al. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res. 2006;174:443–452. doi: 10.1007/s00221-006-0480-0. [DOI] [PubMed] [Google Scholar]
- 91.Chan YK, Herkes GK, Badcock C, et al. Alterations in cerebral potentials evoked by rectal distension in irritable bowel syndrome. Am J Gastroenterol. 2001;96:2413–2417. doi: 10.1111/j.1572-0241.2001.04088.x. [DOI] [PubMed] [Google Scholar]
- 92.Sinhamahaptra P, Saha SP, Chowdhury A, et al. Visceral afferent hypersensitivity in irritable bowel syndrome – evaluation by cerebral evoked potential after rectal stimulation. Am J Gastroenterol. 2001;96:2150–2157. doi: 10.1111/j.1572-0241.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- 93.Loening-Baucke V, Yamada T. Is the afferent pathway from the rectum impaired in children with chronic constipation and encopresis. Gastroenterology. 1991;109:397–403. doi: 10.1016/0016-5085(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 94.Remes-Troche JM, Paulson J, Yamada T, et al. Anorectal–cortical function is impaired in patients with dyssynergic defecation. Gastroenterology. 2007;132(Suppl. 1) [Google Scholar]
- 95.Barker AT, Jalinous R, Freeston IL. Noninvasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 96•.Turnbull G, Hamdy S, Aziz Q, et al. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117:32–39. doi: 10.1016/s0016-5085(99)70547-0. Describes the technique and validation of assessing the cortical evoked potentials from the anorectum in humans. [DOI] [PubMed] [Google Scholar]
- 97.Herdmann J, Bielefeldt K, Enck P. Quantifications of motor pathways to the pelvic floor in humans. Am J Physiol. 1991;260:G720–G723. doi: 10.1152/ajpgi.1991.260.5.G720. [DOI] [PubMed] [Google Scholar]
- 98.Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral stimulation: normative data. J Neurol Sci. 1997;149:69–72. doi: 10.1016/s0022-510x(97)05388-4. [DOI] [PubMed] [Google Scholar]
- 99.Hamdy S, Enck P, Aziz Q, et al. Spinal and pudendal nerve modulation of human corticoanal motor pathways. Am J Physiol. 1998;274(2 Pt 1):G419–G423. doi: 10.1152/ajpgi.1998.274.2.G419. [DOI] [PubMed] [Google Scholar]
- 100.Remes-Troche JM, Paulson J, Attaluri A, et al. A comprehensive assessment of the efferent motor pathways to the anorectum in humans. Neuorgastroenterol Motil. 2007;19:A330. [Google Scholar]
- 101.Rao SSC, Tantiphlachiva K, Attaluri A, Troche-Remes J, Paulson J, Yamada T. Translumbar and transsacral magnetic stimulation – a novel test of assessing anorectal neuropathy in fecal incontinence. Gastroenterology. 2008;134:A278. [Google Scholar]
- 102.Maccabee PJ, Lipitz ME, Desudshit T, et al. A new method using neuromagnetic stimulation to measure conduction time within the cauda equina. Electroencephalogr Clin Neurophysiol. 1996;101:153–166. doi: 10.1016/0924-980x(95)00264-l. [DOI] [PubMed] [Google Scholar]
- 103.Morren GL, Walter S, Lindehammar H, et al. Evaluation of the sacroanal motor pathway by magnetic and electric stimulation in patients with fecal incontinence. Dis Colon Rectum. 2001;44:167–172. doi: 10.1007/BF02234288. [DOI] [PubMed] [Google Scholar]
- 104.Silverman DHS, Munakata JA, Ennes H, et al. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 105.Munakata J, Silvermann DHS, Hoh CK, et al. Rectosigmoid sensitization induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 106.Remes-Troche JM, Rao SS. Defecation disorders: neuromuscular aspects and treatment. Curr Gastroenterol Rep. 2006;8:291–299. doi: 10.1007/s11894-006-0049-x. [DOI] [PubMed] [Google Scholar]


