Abstract
Numerous studies have pointed to the role of Programmed Death-1 Ligand 1 (PD-L1) in regulating tolerance, chronic infection, and tumor immunity. Recently, we have identified murine B7-1 as a new binding partner for murine PD-L1. Human and mouse B7-1 share only 46% identity, leading us to question whether human B7-1 and PD-L1 can participate in a similar interaction. Here we show that human B7-1 can interact with human PD-L1 with affinity greater than that of B7-1 with CD28, but less than that of B7-1 with CTLA-4 or of PD-L1 with PD-1. We characterize a series of anti-human PD-L1 monoclonal antibodies and identify antibodies that can block interactions of PD-L1 with B7-1, PD-1, or both. Since PD-L1 and CD28 on T cells may compete for B7-1 as a binding partner and CD8 T cells may express high or low levels of CD28, we examined when PD-L1 and CD28 are co-expressed on CD8 T cells. We compared the time-course and extent of PD-L1 induction on CD8 CD28high versus CD28low T cells following stimulation with anti-CD3. We show that PD-L1 is induced to a higher level on CD28high T cells than on CD28low T cells upon activation. These results suggest that PD-L1 may play an important and undervalued role on human T cells.
Keywords: CD80, CD274, surface plasmon resonance, adhesion, antibody
Introduction
Members of the B7-CD28 family play important roles in regulating T cell activation and tolerance. B7-1 (CD80) and PD-L1 (CD274, B7-H1) are well-established as playing vital roles in controlling T cell responses in a variety of contexts, from development to autoimmunity to microbial pathogenesis to tumor immunity. B7-1 is inducibly expressed on many types of antigen presenting cells and also upregulated on activated T cells. B7-1 and B7-2 ligate the stimulatory receptor CD28 and the inhibitory receptor CTLA-4. Like B7-1, PD-L1 is expressed on antigen presenting cells and on T cells. PD-L1 is constitutively expressed on murine APCs and T cells, and upregulated to higher levels upon activation. Constitutive expression of PD-L1 is lower in humans than in mice. PD-L1 also is expressed on wide range of non-hematopoetic cells, including vascular endothelial cells, and pancreatic islet cells. PD-L1, and its sister molecule PD-L2, engage the inhibitory PD-1 receptor. Integration of positive signals through CD28 and negative signals through CTLA-4 and PD-1 shapes the outcome of T cell encounter with antigen, leading to T cell activation versus tolerance.
PD-L1 is minimally expressed on resting naïve human CD4 and CD8 T cells(Brown et al., 2003) as compared to constitutive expression on naïve mouse T cells, an important difference between mice and humans. PD-L1-mediated signaling adjusts the threshold of activation for thymocytes(Keir et al., 2005; Nishimura et al., 1996) and T cells(Freeman et al., 2000; Keir et al., 2007). PD-L1 also regulates both the induction and maintenance of tolerance(Fife et al., 2006; Freeman et al., 2000; Keir et al., 2008; Tsushima et al., 2007). PD-L1 has been shown to have a key role in chronic viral infections, regulating the delicate balance between anti-microbial immune defenses and immune-mediated tissue damage(Barber et al., 2006; Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006). PD-1 is highly expressed on “exhausted” virus-specific T cells, which have suppressed cytokine and proliferative responses to their antigen. In a model of chronic LCMV infection, blockade of PD-1 or PD-L1 interactions restores virus-specific T cell responses and reduces viral load. These findings have been extended to HIV, Hepatitis B and Hepatitis C, stimulating interest in treating chronic viral infections by blockade of PD-1 or PD-L1 mediated signals.
We recently showed that ligation of PD-L1 on T cells by B7-1 inhibits T cell responses using a reductionist approach with mouse T cells(Butte et al., 2007). We questioned whether this interaction may hold for the human molecules, because while human PD-L1 and mouse PD-L1 are highly homologous with 70% identical amino acids, human B7-1 shares only 46% identity with mouse B7-1. In particular, human B7-1 and mouse B7-1 are more similar in their IgC-like domains (59% identity) than in their IgV-like domains (48% identity). This finding is noteworthy because we showed, using cross-linking and mass spectrometry, that the IgV-like domain comprises most of the interaction interface between murine B7-1 and PD-L1(Butte et al., 2007). As shown by crystallography, these IgV-like domains are also central to the interfaces of the B7-1:CTLA-4(Stamper et al., 2001) and the PD-L1:PD-1(Lin et al., 2008) interactions. These differences between mouse and human B7-1 led us to investigate if human B7-1 could bind human PD-L1.
In this paper, we characterize the interaction between human B7-1 and PD-L1. We demonstrate that their interactions are comparable to the mouse proteins with a dissociation constant of ~1.4 µM. We characterize anti-human PD-L1 mAbs and identify distinct classes that can block PD-L1 interactions with PD-1, B7-1, or both. We also examine the co-expression of CD28 and PD-L1 on human CD8 T cells. We find that PD-L1 is induced to a higher level on CD8 T cells expressing high levels of CD28 than on those with low CD28. These findings together raise the importance of B7-1 and PD-L1 on human T cells.
Experimental Materials and Methods
Biacore
Protein-protein interactions were measured with a BIAcore 3000 surface plasmon resonance instrument (BIAcore AB, Uppsala, Sweden). Proteins were covalently coupled to the sensor chip as follows: 0.1 M N-hydroxysuccinimide (NHS) and 0.4 M N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide (EDC) were mixed 1:1 and injected at 10 µL/min for 10 min over the flow cells. The protein to be coupled was diluted in 0.1 M sodium acetate buffer pH 5.5 to a concentration of 50 µg/mL and subsequently injected at 10 µL/min for 10 min. One flow cell per chip did not receive any protein at this step and served as a reference channel. To quench any remaining NHS molecules, 1 M ethanolamine was injected at 10 µL/min for 10 min into all flow cells. The channels were subsequently washed with PBS until a stable baseline was observed. Subtracting this baseline from the Resonance Units (RU) obtained prior to adding protein gave a measure of protein immobilized in each flow cell. The flow cells revealed capture of ~7,000 RU of PD-L1-Ig (R&D Systems, hPD-L1-hIgG1Fc), PD-L2-Ig (R&D Systems, hPD-L2-hIgG1Fc), and control hIgG1Fc (BioXCell)
All Biacore experiments were performed at 25 °C. Analyte samples of B7-1-Ig, B7-2-Ig, PD-1-Ig (all from R&D Systems, fusion proteins of hIgG1Fc), or hIgG1Fc control fusion protein (BioXCell) were injected in randomized order in triplicate. To prevent extreme sample consumption, a flow rate of 5 or 10 µL/min was used throughout the binding and dissociation experiments. To provide a double reference, we injected buffer blanks intermittently throughout the course of an experiment – at least 40 such blanks were averaged to provide a reference. Samples or blanks were injected using the KINJECT command for 6 min and then dissociation was followed for 6 min. Equilibrium binding data were analyzed using Scrubber software (Univ. of Utah Protein Interactions Group) (Roden and Myszka, 1996) and graphs were made using Statistica (Statsoft) and Adobe Illustrator.
Anti-PD-L1 antibodies
The following anti-human PD-L1 mAbs were used, listed by clone, isotype, and source: Clone 130002 (mIgG2a, R&D Systems), 29E.11D12 (mIgG2a), 29E.2A3 (mIgG2b), 29E.5A9 (mIgG1)(Brown et al., 2003), MIH1 (mIgG1, Ebioscience), and MIH3 (mIgG1, MBL International). Prior to use, monoclonal antibodies were spun at 15,000 rpm to remove aggregates and their concentrations were determined using Quant-IT Protein reagent (Invitrogen).
Adhesion Assay
The mouse pre-B cell line 300.19 was transfected with human PD-L1 cDNA in the pEF6 expression vector encoding blasticidin resistance. Cells were selected in media containing 5 µg/mL blasticidin, sorted, and subcloned. Cell-surface expression of PD-L1 was verified by flow cytometry (not shown). The cells were loaded with BCECF (2', 7'-(bis-2-carboxyethy1)-5-(and-6)-carboxyfluorescein) fluorescent dye by incubating cells in a 10 µM solution as per the manufacturer's directions (Invitrogen). A 96 well Maxisorp plate (Nunc) was coated with PD-1-hIgG1Fc (R&D Systems), B7-1-hIgG1Fc (R&D Systems), or control hIgG1Fc (BioXCell) overnight at 4 °C, then washed with PBS, and blocked with 2% BSA and 0.002% sodium azide in PBS for 2 hr at 37 °C. For studies that characterized anti-human PD-L1 mAbs, 300.19 cells were suspended in 0.2% BSA in PBS along with antibodies that bound PD-L1 (40 µg/mL) and cultured for 20 min at 37 °C. Cells plus mAbs were then loaded into the wells (3×104/well), centrifuged briefly at 300 rpm (20×g), and incubated for 20 min at 37 °C to allow spreading and adhesion. Each well was read in a fluorescence plate reader exciting at 488 nm and reading at 520 nm, indicating the number of cells present. The plate was then washed in a microplate washer (BioTek ELx405 Select CW) using settings to wash loosely adherent cells, after which the plate was read again. Plates were gently washed in this manner until the negative control wells showed < 10% signal (typically ~5 washes), then read one final time at which point adhesion data were calculated. Experiments were conducted in sextuplet wells, and some wells were excluded if a large air bubble became visible (0–2/wells per plate). Percentage bound was calculated by dividing the fluorescence before and after the washes. Results are reported +/− SEM by Statistica (StatSoft), and statistical significance was determined by two-tailed t-test between samples and their respective isotype control mAb.
Flow cytometery
Peripheral blood mononuclear cells (PBMCs) were obtained from human blood leukopacks depleted of platelets, with informed consent and approval by the Dana Farber Cancer Institute IRB. Cells were purified by Ficoll gradient and stained with fluorescently labeled mAbs for PD-L1 (clones MIH1, eBioscience), CD45RO (clone UCHL1 , eBioscience), CD28 (clone CD28.2, eBioscience), and CD8 (OKT8, eBioscience). Flow cytometric data were collected on a LSR II flow cytometer (BD Biosciences).
Results and Discussion
Interaction properties of human B7-1 and PD-L1
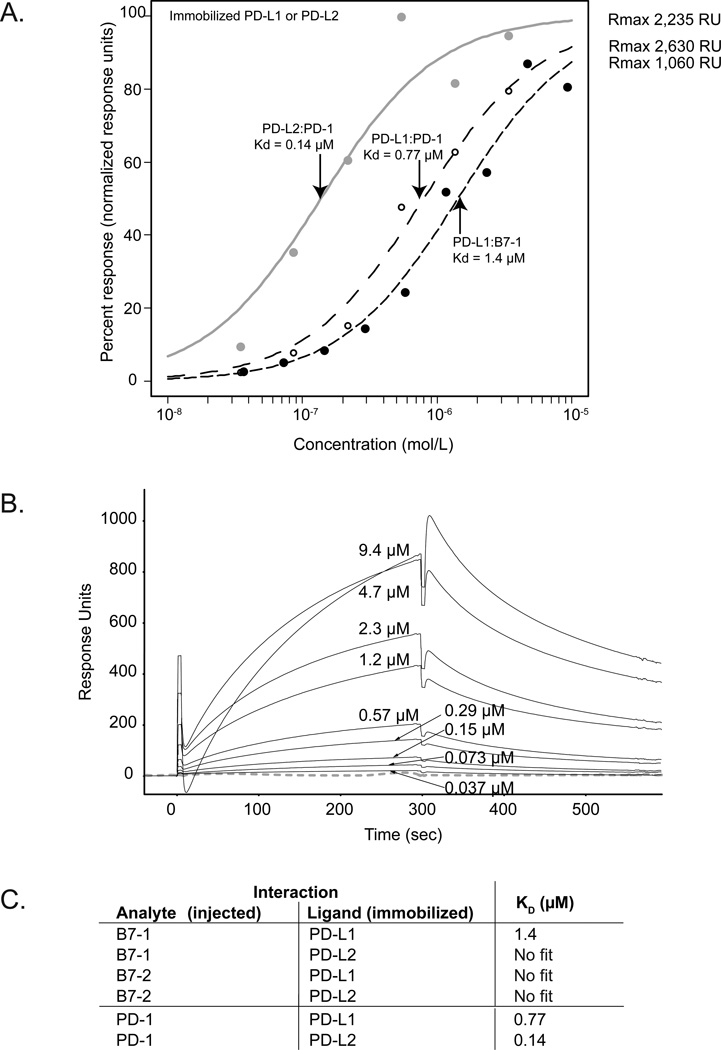
To investigate whether human B7-1 can associate with human PD-L1, we used surface plasmon resonance to monitor the interactions of PD-L1-Ig, B7-1-Ig, or control proteins. We immobilized PD-L1-Ig and PD-L2-Ig and injected B7-1-Ig, B7-2-Ig, or PD-1-Ig at various concentrations in triplicate. We calculated dissociation constants (KD) from equilibrium binding to eliminate considerations of mass-transport and to avoid the complexities of kinetic analyses of divalent ligands. We found a specific interaction between B7-1 and PD-L1 with a KD of 1.4 µM. Equilibrium binding responses (Figure 1A) and typical sensorigrams for a B7-1 injection series (Figure 1B) were similar across three replicate injection series. By comparison, the binding affinities of PD-1 to PD-L1 or PD-L2 were 3–10 times stronger (KD of 0.77 µM and 0.14 µM, respectively). Our dissociation constants were similar to those published for human PD-L1 and PD-L2 to PD-1 (0.52 µM and 0.26 µM, respectively) (Youngnak et al., 2003). There was no specific interaction between human B7-1 and PD-L2, between human B7-2 and PD-L1 or PD-L2, nor with control proteins. These results show a specific and unique interaction between PD-L1 and B7-1, with an affinity (~1.4 µM) intermediate between the affinities of B7-1 for CD28 (4 µM, (van der Merwe et al., 1997)) and CTLA-4 (0.4 µM, (van der Merwe et al., 1997)), and PD-L1 for PD-1 (0.5 µM) (our result). Thus, the interaction between B7-1 and PD-L1 is conserved, suggesting that this interaction may play parallel roles across the murine and human immune systems.
Figure 1.
A) Equilibrium binding responses from surface plasmon resonance measurements of immobilized PD-L1 or PD-L2 interacting with fusion proteins of B7-1, B7-2, and PD-1. No binding response was seen for B7-2 to PD-L1 or PD-L2 (not shown). The dissociation constants (Kd) for the three important interactions are shown (arrows). B) Surface plasmon resonance data collected for immobilized hPD-L1-Ig with hB7-1-Ig as the analyte injected at concentrations between 0.0366 and 9.375 µM (concentrations rounded to two significant figures). Pre-injection baseline has been subtracted, and the data were corrected for the reference channel. Average of ~40 buffer blanks is shown (grey dashed line); response units for these blanks do not exceed 9 response units. C) Summary of dissociation constants obtained from Biacore equilibrium binding experiments.
Even though the B7-1 IgV-like domains are not well conserved overall between mice and humans, it is possible that conservation between particular residues of the IgV-like domains (e.g., in the interface) may override lack-of-conservation between other residues. For example, we find that there is 75.7% identity between mouse and human residues in the GFCC’C” strands of PD-L1, which we previously identified as being an important region for interactions with B7-1. We find 51.2% identity between mouse and human residues in the GFCC’C” strands of B7-1, a region of likewise importance. These strands have (slightly) higher identity than their entire IgV-like domains (70% and 48% for B7-1 and PD-L1, respectively). Future studies will be needed to show whether specific residues of the B7-1:PD-L1 interface are conserved, by site-directed mutagenesis or by examination of a B7-1:PD-L1 co-crystal structure. Another possibility is that our previous findings, using chemical cross-linking and mass spectrometry to identify the B7-1:PD-L1 binding interface, may have overestimated the role of the IgV-like domain.
PD-L1 monoclonal antibodies block PD-1 and/or B7-1
To further characterize the interaction between human B7-1 and PD-L1 when these proteins are expressed on living cells, we used an avidity-based adhesion assay, which facilitates interrogation of mAbs for their ability to specifically block interactions(Butte et al., 2007). We questioned whether PD-L1 mAbs could block interactions with PD-1, B7-1, or both. and characterized anti-human PD-L1 mAbs that are either commercially available or generated in our laboratory.
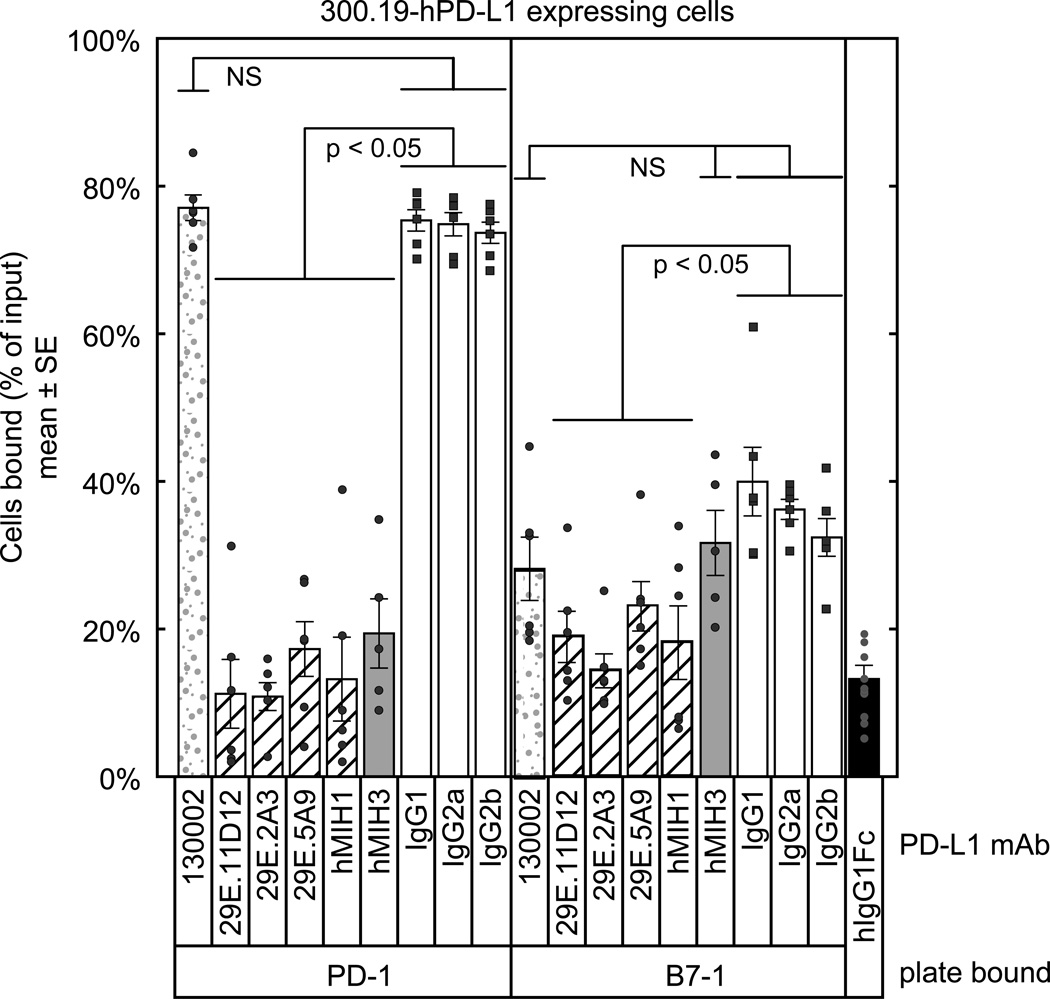
We found that hPD-L1 transfectants of 300.19 cells adhered to PD-1-Ig-coated wells (~74% of cells bound) and hB7-1-Ig (~37% of cells bound) but not to B7-2-Ig or hIgG1-Fc coated wells (<10%) (Figure 3A). Untransfected 300.19 cells did not adhere to any of the wells (< 10%, not shown). To test PD-L1 mAbs for their ability to block interactions, we pre-incubated the cells with PD-L1 mAbs (clones 29E.11D12, 29E.2A3, 29E.5A9, MIH1, MIH3, 130002) or the appropriate isotype control mAbs. We found that the 29E.11D12, 29E.2A3, 29E.5A9, and MIH1 mAbs blocked the interaction between PD-L1 on the 300.19-hPD-L1 cells and B7-1 on the plate (p < 0.05), as well as the interaction between PD-L1 on the 300.19-hPD-L1 cells and PD-1 on the plate (p < 0.05). In contrast, the MIH3 mAb blocked the interaction between PD-L1 and PD-1 (p < 0.05), but did not block the interaction between PD-L1 and B7-1 to a significant extent (p > 0.05). The clone 130002 mAb did not block the interactions between PD-L1 and either PD-1 or B7-1. These results indicate that there are distinct classes of anti-human PD-L1 mAb, which differ in their capacity to block PD-L1 interactions with PD-1 or B7-1. Each of these classes of antibodies may engage different epitopes on PD-L1, some of which block interaction with B7-1 and some of which block interaction with PD-1. The epitope for clone 130002 does not overlap with either of these binding sites. These results together confirm that human B7-1 and PD-L1 expressed on cell surfaces can interact, and that this interaction can be specifically blocked.
Figure 3.
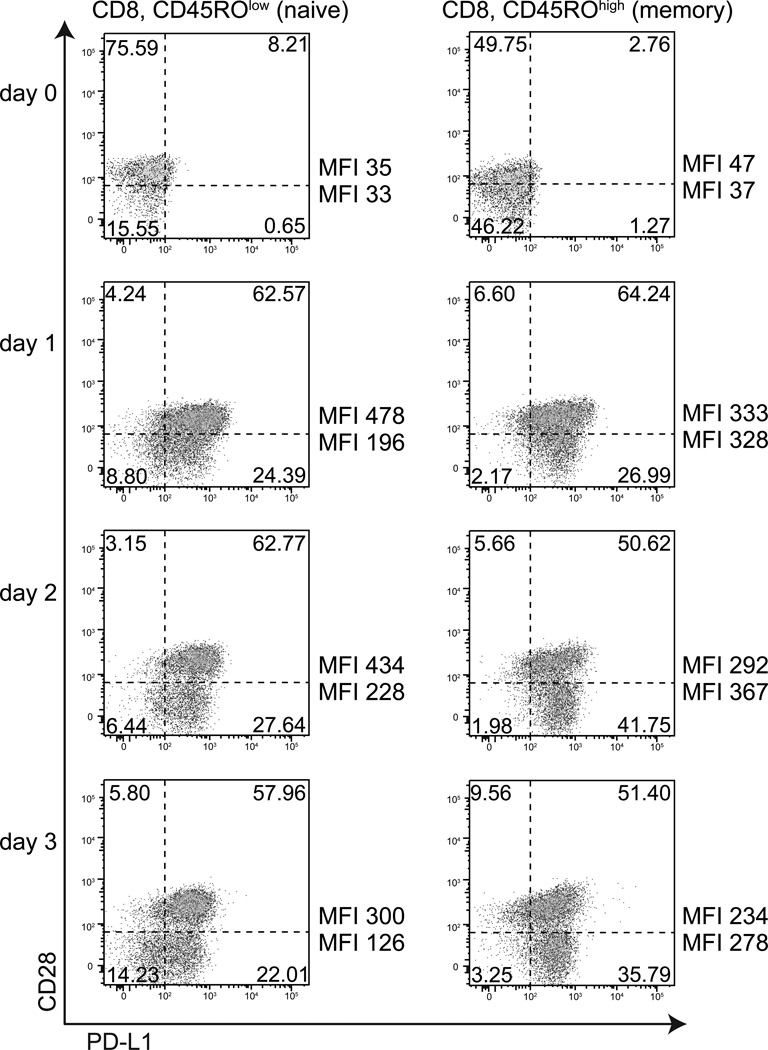
Flow cytometric analysis of PD-L1 expression on CD28high and CD28low CD8 T cells from human peripheral blood, ex vivo and after 1–3 days of stimulation with anti-CD3. PD-L1 is induced on activated T cells. Events shown have been gated on live cells by forward and side scatter, then gated on CD8+ cells. CD45RO expression was gated into high and low subgroups. The quadrant gates shown were defined from unstained or control samples. These results were representative of three donors.
Co-expression of CD28 and PD-L1 on human T cells
While both CD28 and PD-L1 are expressed on mouse T cells constitutively, PD-L1 is inducibly expressed on human T cells, and CD28 is expressed on ~95% of human CD4 T cells but only on ~60% of human CD8 T cells. In particular, a subset of human CD8 memory T cells expresses little CD28, and these T cells are hypo-responsive to stimulation with anti-CD3(Azuma et al., 1993). The identification of the B7-1:PD-L1 interaction indicates that B7-1 on the APC has the potential to engage PD-L1 as well as CD28 and CTLA-4 on T cells. Could the hyporesponsiveness of CD28low CD8 T cells reflect a shift in B7-1-mediated signals from stimulation (due to little CD28) to inhibition (due to PD-L1 and/or CTLA-4 engagement)? An important first step towards addressing this question is to examine the kinetics of PD-L1 expression on human CD8 CD28low and CD8 CD28high T cell populations.
To investigate the relationship between CD28 expression and PD-L1 expression on CD8 T cells, we performed flow cytometry on human peripheral blood CD8 T cells ex vivo and after one, two, and three days of activation with anti-CD3. We found that approximately 36% of CD8 T cells isolated from peripheral blood lacked detectable CD28 expression (called here CD28low, range 14–52%), with a higher percentage of CD28low cells among CD45ROhigh cells than CD45ROlow cells (Figure 3). These ranges are in concordance with previously published estimates of CD28low CD8 T cells(Azuma et al., 1993).
PD-L1 expression on CD8 T cells directly ex vivo was equally low among CD28high cells (MFI 35–47, ~8% positive for PD-L1) and CD28low cells (MFI 33–37, ~1% positive for PD-L1) as well as CD45ROlow cells and CD45ROhigh cells. Following anti-CD3 stimulation, there was a marked increase in the percentage of CD8 T cells expressing PD-L1 as well as in the level of PD-L1. After 24 hours of activation with soluble anti-CD3 mAb, PD-L1 expression was induced by about 13 fold on CD28high CD45ROlow CD8 T cells (MFI 478, ~93% positive for PD-L1) and by about 6 fold on CD28low CD45ROlow CD8 T cells (MFI 196, ~73% positive for PD-L1). In contrast, among CD45ROhigh cells both the CD28low and CD28high CD8 T cells (~91% positive for PD-L1) showed equal induction of PD-L1 of about 7 fold.
On days 2 and 3 of stimulation, a new pattern emerged. Among CD45ROlow CD8 T cells, expression of PD-L1 decreased slightly for both the CD28high and CD28low cells, though the CD28high CD45ROlow cells still retained an almost 2-fold higher level of PD-L1 (and maintained higher percent-positive PD-L1) compared to the CD28low CD45ROlow cells. These findings suggest that the level of PD-L1 expression on CD45ROlow CD8 T cells peaks by 24 hours post stimulation. Among CD45ROhigh cells on day 2 and 3, however, the CD28low CD45ROhigh cells showed slightly greater induction of PD-L1 than did the CD28high CD45ROhigh cells (although the percentages of positive PD-L1 T cells remained high and were roughly equal between these groups at 85–94%). These patterns were observed across three healthy adult donors. In summary, we find: 1) CD45ROlow CD28low CD8 T cells show lower PD-L1-induction than did CD45ROlow CD28high cells; 2) CD45ROhigh CD28low CD8 T cells showed slightly more PD-L1 induction, but at later timepoints, than CD45ROhigh CD28high cells; and 3) The percentage of PD-L1-positive CD8 T cells was markedly induced upon activation, from 5–9% of naïve cells to about 85–91% of activated cells.
CD28 is a crucial costimulatory molecule which is required for optimal initiation of T cell responses(Lenschow et al., 1996). In view of the PD-L1:B7-1 interaction and the bimodal expression of CD28 on human CD8 T cells, we examined co-expression of CD28 and PD-L1 on CD8 T cells. Relatively few CD8 T cells expressed PD-L1 directly ex vivo, but the numbers of PD-L1 expressing CD8 cells and the levels of PD-L1 expression on CD8 T cells were markedly increased following stimulation with anti-CD3. Intriguingly CD28high and CD28low CD8 T cells distinctly modulate PD-L1 expression. PD-L1 is induced to a higher level on CD8 T cells expressing high levels of CD28 than those with low CD28. The higher expression of PD-L1 on CD28high versus CD28low cells may provide a means to balance positive signals through CD28 and inhibitory signals mediated by PD-L1 on T cells. In addition, CD28low cells, especially within the CD45ROhigh group, were slower to upregulate PD-L1 expression (peaked on day 2 rather than day 1). These results are consistent with the previous observations that these CD28low cells have a higher threshold for activation and diminished cytokine responses(Azuma et al., 1993). Whether signals downstream of PD-L1 activation directly oppose those from CD28, or whether there are separate pathways that mediate CD28 stimulatory signals and PD-L1 inhibitory signals, remain to be seen. Further studies are also required to understand the relationship between CTLA-4 and PD-L1 on activated T cells and their interactions with B7-1 on APC.
Conclusions
In summary, we demonstrate that human B7-1 can interact with PD-L1. This new interaction necessitates a reassessment of the roles of B7-1 and PD-L1 in regulating the activation and inhibition of immune responses, as well as their therapeutic manipulation. The identification of the B7-1:PD-L1 interaction gives impetus to investigate how this interaction may modify specific T cell responses, and regulate the balance between T cell tolerance versus activation. The PD-L1:B7-1 interaction also may have important therapeutic implications. Microbes and tumors appear to have exploited PD-1 and PD-L1 to evade eradication by the immune system. Differences in anti-PD-L1 blocking specificities may help explain distinct functional outcomes of blockade of PD-1 and PD-L1 in disease models in vivo. Notably, anti-PD-L1 mAb had greater effects than anti-PD-1 mAb in reinvigorating “exhausted” T cells during chronic LCMV infection (Barber et al., 2006), and the anti-PD-L1 mAb used in this study (10F.9G2) blocked both PD-L1:PD-1 and PD-L1:B7-1 interactions. Further studies are needed to compare the functional effects of anti-PD-L1 mAb that block solely PD-L1:PD-1 or PD-L1:B7-1 interactions with mAbs that block both interactions. Blockade of PD-L1 interactions with PD-1 or B7-1 alone may be more beneficial, in terms of augmenting immunity while minimizing the risk of immunopathology. Our identification of PD-L1 monoclonal antibodies that can be categorized as single-specific blockers (i.e., specifically blocking PD-L1: B7-1 or PD-L1:PD-1) or dual-specific blockers (i.e., blocking both interactions) will support future studies to dissect the contributions of PD-L1:B7-1 or PD-L1:PD-1 interactions individually and together in regulating T cell activation and tolerance.
Figure 2.
Adhesion assay with 300.19-hPD-L1 cells shows specific binding to hPD-1 and hB7-1. Wells were coated with B7-1-Ig, PD-1-Ig, or hIgG1-Fc (negative control) and blocked. 300.19-hPD-L1 cells were labeled with BCECF, incubated with 40 µg/mL of the indicated mAbs, introduced into the wells, and fluorescence was measured before and after washing. Untransfected 300.19 cells showed less than 10% binding (not shown). Patterns of binding are represented by the shading pattern: mAb that block neither the PD-L1:B7-1 or PD-L1:PD-1 interaction (gray stipple), mAbs that block both PD-L1:B7-1 and PD-L1:PD-1 interactions (diagonal lines), mAbs that block primarily the PD-L1:PD-1 interaction but not the PD-L1:B7-1 interaction (solid gray), isotype control mAbs (white), and the negative control (black). Specific p-values (n=6) for each clone are: 130002 (PD-1: 0.622, B7-1: 0.11), 29E.11D12 (PD-1: < 2x10−16, B7-1: 0.001), 29E.2A3 (PD-1: < 2x10−16, B7-1: 0.0007), 29E.5A9 (PD-1: < 2x10−16, B7-1: 4.7x10−6), MIH1 (PD-1: < 2x10−16, B7-1: 6.3x10−5), MIH3 (PD-1: 5.1x10−16, B7-1: 0.12).
Acknowledgements
We are grateful to the Dana Farber Cancer Institute Blood Bank for blood samples. GJF draws royalties from patents regarding PD-L1. Funding for this project came from the NIH/NIAID (R01 38310, R01 46414, P01 AI56299 and BAA 05-11) and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors disclose no conflicts of interest.
References
- Azuma M, Phillips JH, Lanier LL. CD28-T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and Its Ligands in Tolerance and Immunity. Annu Rev Immunol. 2008 doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Freeman GJ, Sharpe AH. PD-1 Regulates Self-Reactive CD8+ T Cell Responses to Antigen in Lymph Nodes and Tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, Garboczi DN. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden LD, Myszka DG. Global analysis of a macromolecular interaction measured on BIAcore. Biochem Biophys Res Commun. 1996;225:1073–1077. doi: 10.1006/bbrc.1996.1297. [DOI] [PubMed] [Google Scholar]
- Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, Omura K, Yagita H, Pardoll DM, Chen L, Azuma M. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]