Abstract
It has been ∼40 yr since the discovery that PGs are produced by exercising skeletal muscle and since the discovery that inhibition of PG synthesis is the mechanism of action of what are now known as cyclooxygenase (COX)-inhibiting drugs. Since that time, it has been established that PGs are made during and after aerobic and resistance exercise and have a potent paracrine and autocrine effect on muscle metabolism. Consequently, it has also been determined that orally consumed doses of COX inhibitors can profoundly influence muscle PG synthesis, muscle protein metabolism, and numerous other cellular processes that regulate muscle adaptations to exercise loading. Although data from acute human exercise studies, as well as animal and cell-culture data, would predict that regular consumption of a COX inhibitor during exercise training would dampen the typical muscle adaptations, the chronic data do not support this conjecture. From the studies in young and older individuals, lasting from 1.5 to 4 mo, no interfering effects of COX inhibitors on muscle adaptations to resistance-exercise training have been noted. In fact, in older individuals, a substantial enhancement of muscle mass and strength has been observed. The collective findings of the PG/COX-pathway regulation of skeletal muscle responses and adaptations to exercise are compelling. Considering the discoveries in other areas of COX regulation of health and disease, there is certainly an interesting future of investigation in this re-emerging area, especially as it pertains to older individuals and the condition of sarcopenia, as well as exercise training and performance of individuals of all ages.
Keywords: PGE2, PGF2α, acetaminophen, ibuprofen, sarcopenia
pgs produced by the cyclooxygenase (COX) enzyme are ubiquitous in human physiology and regulate numerous processes (105, 108), including muscle protein metabolism (78, 93, 106, 126, 128). As a result, PGs regulate adaptations to muscular exercise, and COX-inhibiting drugs can alter the acute and chronic responses to exercise. Because of the continuum of acute responses and chronic adaptations that exists from lower-intensity, longer-duration exercise to higher-intensity, shorter-duration exercise, it is difficult to adopt a “one-mechanism-fits-all” approach to PG and COX involvement in skeletal muscle exercise adaptations. This is also true when distinguishing between muscle injury and muscular exercise for health and performance.
COX-inhibiting drugs are one of the most commonly consumed classes of drugs in the world. In the United States, the COX inhibitors, acetaminophen, ibuprofen, and aspirin, are the top three consumed drugs of young, middle-aged, and older individuals, with ∼50 million individuals using each of these drugs during any given week (52, 129). Interestingly, two of these drugs (acetaminophen and aspirin) have been used for over 100 yr, originating in the 1800s (4). Understanding the role of PGs in skeletal muscle adaptation to activity is important because of the widespread use and potential influence of COX-inhibiting drugs, for better or worse, and so we can understand more completely the mechanisms that control muscle adaptation.
The focus of this review will be on those studies that have used COX inhibition in human studies of skeletal muscle metabolism and adaptations to exercise. Distinction between studies using exercise paradigms that would be used for health benefits and those more focused on injury will be made when necessary and when appropriate animal and cell-culture studies will be discussed. A historical overview of the PG and COX pathway, with specific reference to skeletal muscle metabolism, will also be presented.
PGs, THE COX PATHWAY, AND SKELETAL MUSCLE
Historical context.
PGs were discovered in the 1930s from extracts of the prostate gland and named by von Euler (136). Subsequent studies, over the next 40 yr, elucidated many of the tenets of PG structural biology and physiology, resulting in the Nobel Prize being awarded to Bergström, Samuelsson, and Vane in 1982 (10, 97, 135). There are numerous PGs (e.g., PGA-J) that are produced from arachidonic acid (53), which is liberated from the membrane of cells by PLA2 (19, 32, 38) (Fig. 1). The enzyme PG G/H synthase, more commonly known as COX, is a dual-function enzyme that converts arachidonic acid to PGG2 and then PGH2 (105, 107), which is then rapidly converted to a specific PG (e.g., PGD2, PGE2, PGF2α, PGI2) by PG synthases (70, 107, 137). Additionally, there are two well-known isoforms of COX—COX-1 and COX-2 (44, 105, 107, 141)—and a possible third isoform (an intron-retaining variant of COX-1, referred to as COX-1b or COX-3) may exist in some tissues (22, 86, 101, 138).
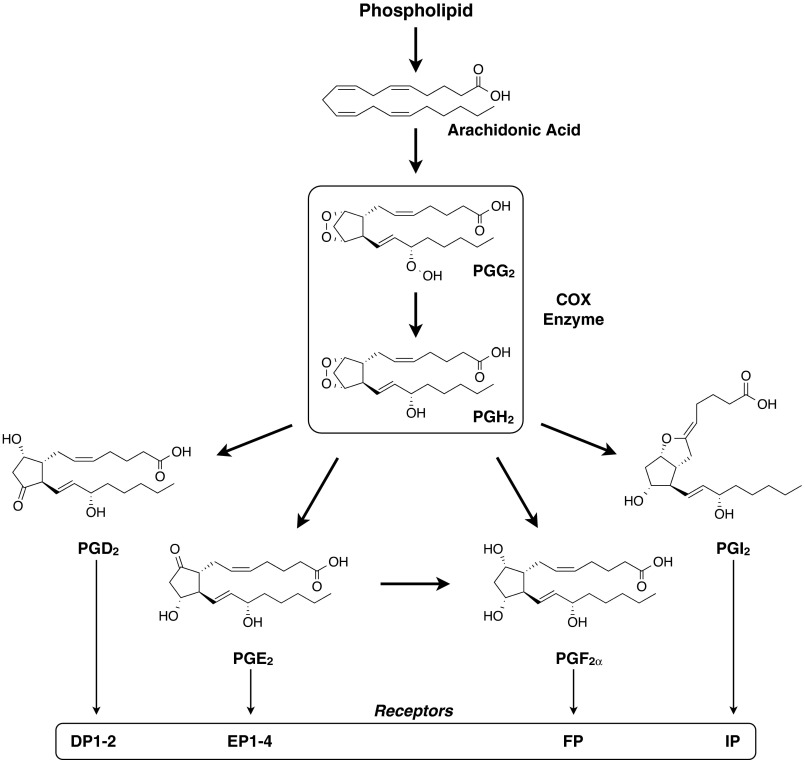
Fig. 1.
General schematic of the biosynthesis of the primary PGs in the PG/cyclooxygenase (COX) pathway and their associated receptors (53, 107, 108). The thicker arrows reflect conversions that are catalyzed by specific enzymes. Several other PGs are also made from the conversion (enzymatic or nonenzymatic) of those listed here: PGJ2, Δ12-PGJ2, 15-deoxy-Δ12,14-PGJ2, and 11-epi-PGF2α are derived from PGD2; PGA2, PGB2, and PGC2 are derived from PGE2; 15-keto-PGF2α is derived from PGF2α; 6-keto-PGF1α and 6-keto-PGF1 are derived from PGI2. PGs are lipid molecules with a general chemical formula of C20H28–34O3–6 and molecular weight range of 317–371 Da. See text for specific references related to the numerous aliases that exist for the variants and isoforms of the enzymes and receptors and for further understanding of the nomenclature. Although not a PG, thromboxane A2 is also derived from the enzymatic conversion of PGH2. This review focuses on the 2 primary PGs that influence skeletal muscle protein turnover (PGF2α and PGE2), which are discussed in the text and presented in further detail (see Fig. 2). DP1–2, EP1–4, FP, and IP, PG receptors.
PGs work in an autocrine and paracrine fashion through receptors specific to each PG, some of which have multiple isoforms (1, 17, 32, 62, 72, 112). PGs are relatively transient molecules with a half-life, typically, of only seconds to minutes (32). For example, ∼90% of PGE2 and PGF2α is removed from the blood in one pass through the pulmonary circulation (85). PGs are also potent in relatively small amounts (9, 32). As a result, circulating PG levels are typically low, and in response to stimulation, tissue production can be increased tremendously [e.g., 10 times the total resting tissue content can be generated every minute (135)].
The first studies of PG production and release by skeletal muscle in response to muscular work were published by Herbaczynska-Cedro and colleagues (41–43, 47) in 1974 and 1976 (Table 1). These studies were focused on identifying muscle-produced vasodilators and blood flow regulation during simulated exercise in dogs and showed that more than one PG was produced by exercising skeletal muscle (suggested as at least PGE2 and PGF2α), which could be eliminated by the COX inhibitor indomethacin. These authors also speculated that the mechanism of the PG release was the distortion of the muscle cell membrane but showed that the PG release occurred during and after the exercise bouts. Soon thereafter, a study in humans using a static and dynamic exercise forearm model showed similar results (54). Subsequent arteriovenous studies by Nowak and Wennmalm (75) showed no net release or uptake of PGs across the leg at rest, but cycling exercise at ∼75% maximal oxygen consumption substantially increased net release of PGs from the leg. Whereas PGs were reported to be in resting human skeletal muscle as early as 1967 (51), Berlin et al. (11) showed in 1979, with radiolabeled arachidonic acid added to homogenates of skeletal muscle biopsy samples, that skeletal muscle could produce PGD2, PGE2, PGF2α, and PGI2. PGE2 was the predominant PG produced (11), but this could be shifted to PGF2α if the assay environment was altered (74).
Table 1.
Noteworthy studies of PGs, skeletal muscle, and exercise adaptations
| Author | Year | Species | Key Findings |
|---|---|---|---|
| Early studies | |||
| Karim et al. (51) | 1967 | Humans | PGs are located in skeletal muscle. |
| Herbaczynska-Cedro and colleagues (41–43, 47) | 1974, 1976 | Dogs | Skeletal muscle PG release increased during and after simulated exercise; eliminated with COX inhibitor. |
| Kilbom and Wennmalm (54) | 1976 | Humans | Forearm skeletal muscle PG release increased during and after static and dynamic exercise; reduced with COX inhibitor (indomethacin). |
| Nowak and Wennmalm (75) | 1978 | Humans | Leg skeletal muscle PG release increased during cycling exercise at 75% maximal oxygen consumption. |
| Berlin et al. (11) | 1979 | Humans | Skeletal muscle had the enzymatic capacity to produce PGD2, PGE2, PGF2a, and PGI2 from arachidonic acid. |
| Young et al. (142) | 1981 | Monkeys | Aging increased skeletal muscle PG production. |
| Rodemann and Goldberg (93) | 1982 | Rats | Arachidonic acid increased skeletal muscle PGF2a and PGE2, which increased muscle protein synthesis and degradation, respectively; reduced or eliminated with COX inhibitors. |
| Palmer et al. (78) | 1983 | Rabbits | Simulated exercise (intermittent stretching) increased skeletal muscle PGF2a and protein synthesis; reduced or eliminated with COX inhibitors. PGF2a and protein synthesis levels correlated. |
| Smith et al. (106) | |||
| Gibson et al. (33) | 1991 | Humans | Reduced skeletal muscle PGF2a levels associated with reduced muscle protein synthesis and type I and II muscle fiber size. |
| Contemporary studies | |||
| Trappe et al. (126, 128) | 2001, 2002 | Humans | Increased skeletal muscle PGF2a, PGE2, and protein synthesis after resistance exercise; eliminated with over-the-counter, orally consumed COX inhibitors (acetaminophen and ibuprofen). |
| Karamouzis et al. (49, 50) | 2001 | Humans | Aerobic exercise increased intramuscular PGE2; increased production with increased workload. |
| Boushel et al. (15) | 2002 | Humans | Confirmed findings of Karamouzis et al. (49, 50) and showed that an oral dose of COX inhibitor (indomethacin) reduced intramuscular PGE2 during aerobic exercise by 90%. |
| Höffner et al. (45) | 2003 | Humans | An oral dose of COX inhibitor (aspirin) reduced intramuscular PGE2 production at rest by nearly 90% within 1 h. |
| Trappe et al. (123) | 2006 | Humans | Both young and old individuals increased intramuscular PGF2a in the hours after resistance exercise; no apparent effect of age on resting or postexercise levels. |
| Mikkelsen et al. (67) | 2008 | Humans | Local intramuscular low-dose COX inhibitor (indomethacin) delivery blocked PGE2 production by ∼85% during and after resistance exercise. |
| Burd et al. (18) | 2010 | Humans | COX-2-specific inhibitor (celecoxib) did not eliminate or reduce the increase in skeletal muscle protein synthesis after resistance exercise. |
| Paulsen et al. (79) | 2010 | Humans | COX-2-specific inhibitor (celecoxib) did not influence intramuscular PGE2 levels or satellite cell activity after resistance exercise. |
| Petersen et al. (82) | 2011 | Humans | COX inhibitor (ibuprofen) did not influence skeletal muscle protein synthesis 24 h after aerobic exercise in older osteoarthritic patients. |
| Kreiner and Galbo (55) | 2011 | Humans | Resting intramuscular PGE2 levels 20 times higher than plasma. |
| Standley et al. (110) | 2013 | Humans | PGE2 stimulated transcription of the skeletal muscle mass regulators IL-6 and muscle RING finger-1. |
| COX inhibitor effects on chronic exercise adaptations | |||
| Krentz et al. (56) | 2008 | Humans | Duration: 6 wk; Training: resistance exercise 2–3 days/wk; Drug dose: ibuprofen 400 mg, 2–3 days/wk (training days); Participants: 24 yr, men and women, resistance exercise trained (∼6 yr); no effect on muscle mass or strength adaptations compared with placebo-consuming resistance exercise group |
| Petersen et al. (81) | 2011 | Humans | Duration: 12 wk; Training: resistance exercise 3 days/wk; Drug dose: ibuprofen 1,200 mg/day; Participants: 50–70 yr, men and women, knee osteoarthritis patients; no effect on muscle mass, increased muscle strength compared with placebo-consuming resistance exercise group |
| Trappe et al. (125, 127) | 2011, 2013 | Humans | Duration: 12 wk; Training: resistance exercise 3 days/wk; |
| Drug dose: acetaminophen 4 g/day or ibuprofen 1,200 mg/day; Participants: 60–78 yr, men and women, healthy, untrained; enhanced muscle mass and strength gains 25–50% above placebo-consuming resistance exercise group | |||
| Jankowski et al. (48) | 2012 | Humans | Duration: 16 wk; Training: resistance exercise ∼3 days/wk; Drug dose: acetaminophen 1 g, 3 days/wk (training days); Participants: 64 yr, men, healthy, untrained; no effect on fat-free mass or muscle-strength adaptations compared with placebo-consuming resistance exercise group |
COX, cyclooxygenase.
The initial reports of PG regulation of skeletal muscle protein turnover were in the early 1980s when Rodemann and Goldberg (93) showed that arachidonic acid supplementation to rat muscle in vitro increased muscle protein synthesis and degradation, which could be replicated with PGF2α and PGE2 supplementation, respectively, and reduced or eliminated with COX inhibition. Arachidonic acid supplementation increased muscle production of both PGs, but about twice as much PGE2 was synthesized compared with PGF2α. At the same time, Palmer and colleagues (78, 106) showed that with simulated exercise in rabbit muscle, PGF2α was produced by intermittent muscle stretch (+105%), which in turn, stimulated muscle protein synthesis (+70%), both of which were reduced or eliminated by COX inhibition. In addition, the increase in muscle PGF2α production and protein synthesis was sustained following the cessation of simulated exercise.
Over the next 10–15 yr following these seminal studies, many investigations in animal and cell-culture models built on these findings (5, 7, 34, 35, 39, 40, 64, 65, 77, 90, 94, 113, 132–134). Although the animal- and cell-culture data were compelling with regard to PG and COX-inhibitor regulation of skeletal muscle protein turnover, very few studies in humans were completed during this time, and none focused on exercise. Gibson et al. (33) showed that women (mean age of 58 yr) with rheumatoid arthritis receiving steroid treatment (which lowers PG production via COX-independent PLA2 inhibition; mean treatment time: 8 yr) had reduced muscle PGF2α levels, muscle protein-synthesis rates, and type I and II muscle fiber size compared with a group of controls (mean age of 70 yr). In addition, the rheumatoid arthritis patients had elevated intramuscular PGE2, resulting in ∼12 times more PGE2 than PGF2α compared with approximately three times in the controls. McNurlan et al. (65) showed that a single dose of the COX inhibitor indomethacin was unable to influence whole-body protein synthesis in the fed and fasted states, but given that muscle protein turnover constitutes <30% of whole-body turnover (71), it is unclear if there was an influence on muscle protein turnover (as they had shown in rats).
Contemporary information.
What is known about PG and COX-inhibiting drug regulation of muscle protein turnover with exercise in humans has been obtained since ∼2000 (Table 1). Several studies using animal models of simulated exercise and muscle growth have also been completed during this time period and have provided additional insight. Also during this time frame, the amount of basic information about the PG/COX pathway has increased substantially, including characterization of the structure and enzymology of the PG synthases (107), leading the way for new drug development (8, 96). Much more has also been learned from continued investigation of the COX-specific inhibitors engineered in the 1990s and of many of the “classic” COX inhibitors in other areas of health and disease.
Studies completed during the 1990s using isotopically labeled amino acids showed that muscle protein synthesis increased after resistance exercise for up to 48 h in humans (23, 58, 84), and the accumulation of these acute increases in muscle protein synthesis was generally understood to be, at least in part, the underlying basis of muscle hypertrophy. Perturbations (e.g., pharmaceutical, nutrition, a specific exercise regimen) that altered this response would alter the benefits of strength training. With the consideration of the aforementioned studies showing that PGF2α was a regulator of muscle protein synthesis, it was realized that orally consumed COX inhibitors might interfere with the normal muscle protein-synthesis response to resistance exercise. This interfering effect might be particularly important after damaging exercise that caused muscle soreness, which is, at least in part, a result of increases in intramuscular PGE2 (3, 55, 66) and would further increase the likelihood of COX-inhibitor consumption. Indeed, this was shown to be the case when individuals consumed an over-the-counter dose of the COX inhibitor acetaminophen (4 g/day) or ibuprofen (1.2 g/day) in the 24 h following a single bout of excessive resistance exercise [10–14 sets of 10 eccentric (lowering) contractions; thus the work equivalent of ∼70 typical repetitions] that caused muscle soreness (126, 128). Muscle protein synthesis (+76%) and intramuscular PGF2α (+77%) were increased significantly, 24 h after the exercise bout, and these increases were eliminated with the consumption of the COX-inhibiting drugs. Intramuscular PGE2 was also increased (+64%), 24 h after the exercise, and this increase was also eliminated with COX inhibition. This study presented some interesting initial findings regarding PG and COX regulation of skeletal muscle responses to exercise and raised several interesting questions. First, the continued, increased production of intramuscular PGF2α and PGE2, 24 h after a single bout of resistance exercise, considering the short half-life of PGs, highlighted that it would be important to understand the typical PG levels in human skeletal muscle and how they are affected by exercise (i.e., time course, magnitude, type of exercise). Second, considering the pharmacokinetic and metabolic complexities of orally consumed COX inhibitors, it was interesting that doses of the most commonly consumed COX inhibitors could apparently produce intramuscular drug levels that inhibited intramuscular COX to the extent that PGF2α and PGE2 production and muscle protein metabolism after exercise were inhibited. Given the large number of COX-inhibiting drugs available for human consumption, it followed that knowledge regarding the efficacy of these drugs and doses in relation to the COX isoforms found in skeletal muscle at rest and after exercise would be necessary. Third, the obvious question from this investigation was: what are the impacts of chronic COX-inhibitor consumption on exercise-training adaptations? Whereas there is much yet to be discovered in this area, several studies have added to our understanding and started to address some of the key questions.
Intramuscular PGs and exercise.
Muscle biopsy-derived measures of PGs that regulate protein turnover show PGE2 to be the dominant PG in young and old human muscle (11, 33, 51, 74, 126). Intramuscular PGE2 levels are three to four times higher than PGF2α at rest and after resistance exercise in young individuals, suggesting equivalent, relative increases in both PGs after exercise (126). This same ratio of PGE2 to PGF2α appears to hold in resting skeletal muscle of older individuals as well (33). However, studies in skeletal muscle of rhesus monkeys show that PG production is approximately twofold higher in older muscle compared with young (142). Several studies in humans using the microdialysis technique to sample the muscle interstitial fluid provide additional information on intramuscular levels of the PGs (primarily PGE2). Few studies report both intramuscular and plasma levels of PGs, but it has been shown that resting vastus lateralis and trapezius intramuscular PGE2 levels are ∼20 times higher than plasma levels (55), albeit in older individuals. However, the intramuscular levels reported in that study are similar to those reported in middle-aged and younger individuals (29, 49, 50). In addition, low-level muscular work does not increase PGE2 levels (30, 50), whereas resistance and aerobic exercise stimulate the muscle to produce PGE2 and/or PGF2α during and/or after exercise (15, 49, 50, 67, 123, 126). Furthermore, increasing the workload during aerobic exercise increases the muscle production of PGE2 (15, 49). Specifically, going from rest to cycling exercise at 100 W and 150 W increased intramuscular PGE2 levels by ∼400% and ∼600%, respectively (49). No information is available on the possible workload effects on PG production following resistance exercise; however, there does not appear to be an age effect on PGF2α production during the 24 h following resistance exercise (123).
COX inhibitors and skeletal muscle COX.
There are somewhat limited data on PG synthesis and muscle protein metabolism at rest or after exercise in response to oral consumption of over-the-counter COX inhibitors (82, 126, 128). Mikkelsen et al. (67) have shown that an intramuscular infusion of a relatively low dose of the prescription COX inhibitor indomethacin reduced PGE2 levels in the muscle during and after resistance exercise by ∼85%. This same COX-inhibitor delivery approach during and for a few hours after resistance exercise did not influence muscle protein synthesis measured near the infusion site 24 h later (69). Furthermore, numerous studies of PG regulation of skeletal muscle blood flow at rest and in response to exercise have used over-the-counter- and prescription-strength COX inhibitors (15, 45, 100), and these studies provide some additional insight. For example, Höffner et al. (45) showed that a single oral dose of 1,000 mg acetylsalicylic acid (aspirin), an irreversible inhibitor of COX, inhibits resting skeletal muscle PGE2 production by nearly 90% within 1 h. In addition, a single oral dose (100 mg) of indomethacin given to individuals, 16 h before exercise, eliminated 90% of the intramuscular PGE2 production during aerobic exercise (15). Clearly, doses typical for human consumption can impact intramuscular PG production, but more data are needed in this area and in the context of muscle protein turnover.
The efficacy of these over-the-counter and prescription COX inhibitors should also be considered in the context of the COX enzymes found in skeletal muscle (Table 2). That is, COX-inhibiting drugs are commonly classified based on their specificity toward the two main isoforms of COX—COX-1 and COX-2 (24, 105). The aforementioned drugs that have been shown to reduce intramuscular PG production in humans (acetaminophen, ibuprofen, indomethacin, aspirin) are all generally considered to be nonspecific COX inhibitors (i.e., they block both COX-1 and COX-2 to some degree). Confusion can arise, however, if this classification is considered to hold across all tissues and physiological conditions. That is, different tissues under different stresses express different levels of the COX isoforms and have different cellular environments that appear to affect drug efficacy. In healthy individuals at rest and following exercise, human skeletal muscle expresses COX-1 almost exclusively at the transcript and protein level (18, 125, 138). Interestingly, there is a relatively ignored variant of the COX-1 isoform that is the most abundant transcript in human skeletal muscle, and it is responsive to exercise (18, 125, 138). COX-2 transcript levels in healthy human skeletal muscle at rest or after exercise are very low, and similarly, the amount of detectable, enzymatically active COX-2 protein is questionable (18, 116, 125, 138). However, intramuscular COX-2 transcript levels do increase in response to COX-2-specific and nonspecific COX inhibitors (18, 69). The COX-3 (i.e., COX-1b) isoform in human skeletal muscle has been ruled out as a contributor to PG production (18, 125, 138). Animal models of muscle adaptation (13, 14, 27, 73, 102–104, 109) suggest that COX-2 plays a substantial role in PG production in skeletal muscle, but these models appear to be more reflective of muscle injury and not necessarily human exercise (18, 125). Overall, the COX-2 isoform in skeletal muscle appears to be more responsive to injury-related stimuli, which is consistent with the large induction of skeletal muscle COX-2 protein levels in humans with septic myopathy (87). Further support for this notion comes from two separate studies showing that a COX-2-specific inhibitor designed for human consumption did not influence the skeletal muscle responses to a single bout of eccentric resistance exercise (18, 79). Burd et al. (18) showed that the COX-2 inhibitor was unable to block the normal increase in muscle protein synthesis following exercise, as was shown previously with two different, nonspecific COX inhibitors (128). Similarly, Paulsen et al. (79) were unable to show an effect of the COX-2 inhibitor on intramuscular PGE2 levels, satellite cell activity, or autologous-radiolabeled leukocyte accumulation.
Table 2.
COX enzymes in healthy human skeletal muscle in relation to exercise and COX-inhibiting drugs
| Isoform | Variant(s) | Comments |
|---|---|---|
| COX-1 | Variant 1 (−1v1) | Relatively abundant at the transcript and protein level at rest and after acute and chronic exercise. The protein product commonly believed to interact with nonspecific COX-inhibiting drugs. Acute exercise increases transcript levels; chronic exercise training increases transcript and protein levels. |
| COX-1 | Variant 2 (−1v2) | A truncated transcript of COX-1v1 (missing 111 bases from exon 9) that may not generate a functional protein product. Most abundant COX transcript but specific role in skeletal muscle unknown. Acute exercise and chronic exercise training increase transcript levels. |
| COX-1 | Variant b (−1b) | Also known as COX-3. An intron-1-retaining version of COX-1 with 3 splice variants: −1b1, −1b2, −1b3. Apparently sensitive to common COX-inhibiting drugs in other tissues. Nondetectable or very low transcript levels at rest and nonresponsive to acute and chronic exercise. Unlikely involved in exercise adaptations or related COX-inhibitor effects. |
| COX-2 | Very low or nondetectable transcript and enzymatically active protein levels at rest and after acute and chronic exercise. Although low, transcript levels increase with ingestion or infusion of COX-2-specific or nonspecific COX inhibitors after acute exercise, as well as with chronic exercise training. |
See text and related studies (18, 69, 105, 116, 125, 138) for further discussion of skeletal muscle COX.
Chronic effects of COX inhibitors on exercise adaptations.
Four recent studies (48, 56, 81, 125) provide the initial evidence as to whether chronic consumption of commonly consumed COX inhibitors negatively impacts chronic exercise adaptations (Table 1).
Krentz et al. (56) showed in young men and women that 400 mg ibuprofen taken after six sets of biceps curls [three sets of eight to 10 concentric repetitions at 70% one repetition maximum (1RM) and three sets of four to six eccentric repetitions at 100% 1RM], 2–3 days/wk for 6 wk, did not influence muscle growth or strength adaptations. The discrepancy between the acute suppression of muscle protein synthesis in younger individuals by ibuprofen (128) and these chronic findings is not completely clear. The lower dose of ibuprofen (1,200 mg/day vs. 800–1,200 mg/wk); the somewhat short duration of training, limiting the time for the COX inhibitor to have an effect; and the possible influence on muscle protein breakdown are plausible explanations.
Petersen et al. (81) recently showed that older patients with osteoarthritis (mean age ∼62 yr), taking ibuprofen (1,200 mg/day) and completing 12 wk of progressive resistance training, 3 days/wk (four to five sets of eight to 15 repetitions at 70–80% of 1RM), had no effect on muscle mass gains, but muscle strength was enhanced in those individuals consuming the COX inhibitor. The authors suggest that this effect on muscle function was related to the pain relief obtained from the drug consumption. These findings are corroborated by this same group’s data showing that ibuprofen (1,200 mg/day) does not influence the muscle protein-synthesis response to exercise in older osteoarthritis patients (mean age ∼62 yr) (82). Interestingly, they also reported that 12 wk of training and taking ibuprofen did inhibit the increase in muscle satellite cell number induced with training in the placebo group (81). These results are in accordance with reports of COX-inhibiting drugs interfering with satellite cell activity after exercise, which is apparently mediated through COX-1 (61, 68, 79). These findings raise the question of whether satellite cells are necessary for muscle hypertrophy, at least the amount that is generally elicited with exercise-training paradigms used for health and wellness of older individuals and the treatment of sarcopenia. This general question has been debated recently (63, 76), and the answer is apparently not yet at hand (46, 60, 83).
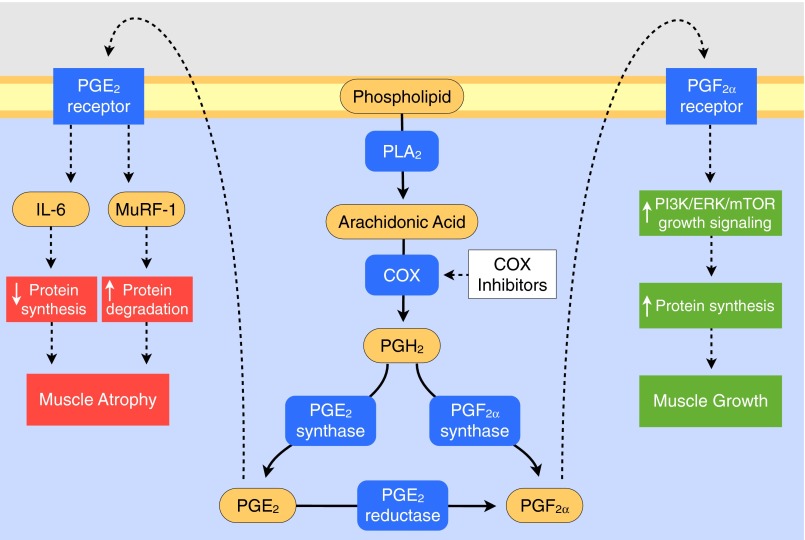
With the use of doses of acetaminophen (4 g/day) or ibuprofen (1.2 g/day) in healthy, older men and women (60–78 yr) completing resistance-exercise training 3 days/wk (three sets of 10 repetitions at ∼75% of 1RM/day) for 12 wk, Trappe et al. (125) unexpectedly showed an enhancement of muscle mass and strength gains of 25–50% over a placebo-consuming group. Follow-up studies on muscle biopsies obtained from these individuals (125, 127) and subsequent ex vivo studies (110) provide some mechanistic clarity about these unexpected COX-inhibitor effects (Fig. 2). It appears the COX inhibitors reduced the PGE2 production (126) and resultant stimulation of intramuscular IL-6 and muscle RING finger protein-1 (MuRF-1) production (57, 88, 111), which increased net protein balance in response to each exercise bout throughout the exercise program. This hypothesis is based on the data that show that low-level increases in IL-6 acutely inhibit muscle protein turnover (131), chronically promote muscle atrophy (12, 37), and are associated with a reduction in muscle mass and functional independence in older individuals (6, 25, 28, 99), as well as the proteolytic nature of the ubiquitin ligase MuRF-1 (20, 98). The COX-inhibitor consumption also promoted an upregulation of the PGF2α receptor in the muscle of the drug groups (127). This increase coupled with a general training increase in COX-1 and the PGF2α-producing enzymes (PGF2α synthase and PGE2-to-PGF2α reductase) (125, 127) would make the muscle less susceptible to the same, daily COX-inhibiting drug doses and more sensitive to any PGF2α that was produced following exercise. Whether these responses and muscle adaptations are specific to older individuals and any potential basal inflammatory state or exaggerated response following exercise (21, 26, 36, 80, 89, 117, 118, 124) is unclear and needs further investigation. Interestingly, COX-inhibitor consumption did not promote muscle growth in the nonexercising hamstring muscles, suggesting an exercise-loading and/or stretch-related mechanism. This nonexercise finding is somewhat in conflict with the data from Rieu et al. (92), showing that chronic consumption of ibuprofen limits sarcopenia through restoration of the muscle protein-synthesis response to feeding in older rats. The discrepancy between these studies is possibly due to the longer-term dosing of the animals (20% vs. 0.4% of the lifespan) and the higher dose of the drug (30 vs. 14 mg·kg body wt−1·day−1). However, these collective findings have implications, not only for use of COX inhibitors during resistance-exercise training for the treatment of sarcopenia but also for the potential chronic use of low-level, long-term, exercise-independent consumption of COX inhibitors for the treatment of sarcopenia, as is promoted or contemplated for several other conditions, such as cardiovascular disease, dementia, and certain types of cancer (91, 95, 105, 130).
Fig. 2.
Schematic of the portion of the PG/COX pathway involved in the regulation of skeletal muscle protein metabolism and adaptation. See text for specific references related to the enzymes, intermediates, and receptors of the COX pathway, as well as the studies that have delineated the factors and cellular processes regulated by the PGE2 and PGF2α receptors that influence skeletal muscle mass. See Trappe et al. (127) for nomenclature related to the PG synthases, reductase, and receptors. MuRF-1, muscle RING finger protein-1; PI3K/ERK/mTOR, phosphoinositide 3-kinase/ERK/mammalian target of rapamycin (62).
It is interesting to note that acetaminophen is not commonly considered an nonsteroidal, anti-inflammatory drug because of its relative lack of COX-inhibitory or anti-inflammatory effect in many peripheral tissues, yet it is a potent analgesic, fever reducer, and COX inhibitor within the central nervous system (22, 31). Nonetheless, acetaminophen clearly inhibits PG synthesis in human skeletal muscle (126). Chronic acetaminophen consumption in animals has also been shown to influence skeletal muscle fiber size and glucose metabolism (139, 140). The basis for this tissue specificity is not clear but may be related to the cellular environment (16, 138).
Finally, Jankowski et al. (48) showed recently that 16 wk of progressive resistance-exercise training, 3–5 days/wk (three sets of five to 12 repetitions at 60–80% 1RM coupled with stair-climbing and jumping exercises), combined with the COX-inhibitor acetaminophen (1,000 mg only on days of exercise, average of 3 days/wk) had no effect on fat-free mass or muscle-strength gains in older men (mean age 64 yr). The large difference in acetaminophen dosing (28 g/wk vs. 3 g/wk) likely explains the different responses in this study and those reported by Trappe et al. (125).
Collectively, these chronic studies, albeit of a limited number, highlight three main points: 1) chronic consumption of commonly consumed COX inhibitors at over-the-counter doses during exercise training does not appear to interfere with the muscle mass and strength gains expected from typical resistance-exercise-training regimens; 2) there appears to be a threshold of the amount of drug that is needed to influence skeletal muscle metabolism and adaptation; and 3) there may be differences between the acute and chronic COX-inhibitor effects on muscle metabolism between younger and older individuals.
It should also be noted that the animal studies in this area clearly show an interfering effect of COX inhibitors on muscle growth and adaptation (14, 59, 73, 109). The discrepancy between these studies and the aforementioned human exercise-training and COX-inhibitor studies is most likely due to the animal studies not necessarily reflecting typical human exercise stimuli, as eluded to previously (18). The consideration of the stress placed on the muscle in the different models is also important in understanding the potential role of COX isoforms, as well as PG and inflammatory regulation of muscle adaptation. For example, typical and highly effective resistance-exercise programs in humans only need to load the muscle for a few minutes every 2–3 days (2, 115, 119–122, 125), resulting in <10 min of muscle loading/wk. Whole muscle-growth rates typical for these types of training paradigms are ∼0.5%/wk, and the highest reported muscle-growth rate reported in the literature is ∼1%/wk (114). These rates are in contrast to the animal models of hypertrophy—some of which have almost constant loading and elicit edema—that result in muscle growth of 25–40%/wk. Considering the potential use of the animal studies and particularly, the PG/COX-pathway genetically modified animals, development of appropriate animal exercise models could facilitate significant advancements in this area.
CONCLUDING REMARKS
The research field of PG and COX-inhibitor regulation of health and disease has grown enormously over the last 80 yr of existence. That skeletal muscle responses and adaptations are regulated by PGs synthesized by the muscle that produced them is now firmly established. It is also clear that one of the most commonly consumed classes of drugs in the world—COX inhibitors—can alter several cellular processes that regulate skeletal muscle responses to acute exercise loading and chronic exercise training. There is much research yet to be done in this complex area to better understand the role of PGs and COX inhibitors, specifically as they pertain to older individuals and the condition of sarcopenia, as well as exercise training and performance of individuals of all ages. The PG/COX-pathway research being conducted in other areas of health and disease will no doubt continue to add significantly to our understanding of this research area in skeletal muscle.
GRANTS
Support for this research was provided by the National Institute on Aging (AG-020532 and AG-00831).
DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: T.A.T. conception and design of research; T.A.T. and S.Z.L. analyzed data; T.A.T. and S.Z.L. interpreted results of experiments; T.A.T. and S.Z.L. prepared figures; T.A.T. and S.Z.L. drafted manuscript; T.A.T. and S.Z.L. edited and revised manuscript; T.A.T. and S.Z.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all who contributed to the PG and COX-related research associated with the authors.
REFERENCES
- 1.Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, Slipetz DM, Grygorczyk R. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem 269: 2632–2636, 1994 [PubMed] [Google Scholar]
- 2.Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci 32: 819–825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronoff DM. Aspirin and Reye’s syndrome: discovery of aspirin and paracetamol. Drug Saf 25: 751, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 308: 553–558, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab 284: E481–E487, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Barnett JG, Ellis S. Prostaglandin E2 and the regulation of protein degradation in skeletal muscle. Muscle Nerve 10: 556–559, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Bauman DR, Rudnick SI, Szewczuk LM, Jin Y, Gopishetty S, Penning TM. Development of nonsteroidal anti-inflammatory drug analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: potential antineoplastic agents that work independently of cyclooxygenase isozymes. Mol Pharmacol 67: 60–68, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom S, Duner H, von EU, Pernow B, Sjovall J. Observations on the effects of infusion of prostaglandin E in man. Acta Physiol Scand 45: 145–151, 1959 [DOI] [PubMed] [Google Scholar]
- 10.Bergström SK. Nobel Lecture: The Prostaglandins: from the Laboratory to the Clinic (Online). Nobelprize.org http://www.nobelprize.org/nobel_prizes/medicine/laureates/1982/bergstrom-lecture.html [8 December 1982].
- 11.Berlin T, Cronestrand R, Nowak J, Sonnenfeld T, Wennmalm A. Conversion of arachidonic acid to prostaglandins in homogenates of human skeletal muscle and kidney. Acta Physiol Scand 106: 441–445, 1979 [DOI] [PubMed] [Google Scholar]
- 12.Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol 106: 443–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 287: C475–C483, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 290: C1651–C1659, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc Natl Acad Sci USA 99: 7130–7135, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE, Jr, Trappe TA. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab 298: E354–E361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23: 49–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Caldow MK, Cameron-Smith D, Levinger P, McKenna MJ, Levinger I. Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol 113: 509–517, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA 99: 13926–13931, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73: 1383–1388, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 104: 413–421, 1998 [DOI] [PubMed] [Google Scholar]
- 25.de Gonzalo-Calvo D, de Luxan-Delgado B, Rodriguez-Gonzalez S, Garcia-Macia M, Suarez FM, Solano JJ, Rodriguez-Colunga MJ, Coto-Montes A. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine 58: 193–198, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Dennis RA, Zhu H, Kortebein PM, Bush HM, Harvey JF, Sullivan DH, Peterson CA. Muscle expression of genes associated with inflammation, growth, and remodeling is strongly correlated in older adults with resistance training outcomes. Physiol Genomics 38: 169–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupouy VM, Ferre PJ, Uro-Coste E, Lefebvre HP. Time course of COX-1 and COX-2 expression during ischemia-reperfusion in rat skeletal muscle. J Appl Physiol 100: 233–239, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Flodgren GM, Crenshaw AG, Alfredson H, Fahlstrom M, Hellstrom FB, Bronemo L, Djupsjobacka M. Glutamate and prostaglandin E2 in the trapezius muscle of female subjects with chronic muscle pain and controls determined by microdialysis. Eur J Pain 9: 511–515, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Flodgren GM, Hellstrom FB, Fahlstrom M, Crenshaw AG. Effects of 30 versus 60 min of low-load work on intramuscular lactate, pyruvate, glutamate, prostaglandin E(2) and oxygenation in the trapezius muscle of healthy females. Eur J Appl Physiol 97: 557–565, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature 240: 410–411, 1972 [DOI] [PubMed] [Google Scholar]
- 32.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Gibson JN, Poyser NL, Morrison WL, Scrimgeour CM, Rennie MJ. Muscle protein synthesis in patients with rheumatoid arthritis: effect of chronic corticosteroid therapy on prostaglandin F2α availability. Eur J Clin Invest 21: 406–412, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Goldberg AL, Baracos V, Rodemann P, Waxman L, Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc 43: 1301–1306, 1984 [PubMed] [Google Scholar]
- 35.Goldberg AL, Kettelhut IC, Furuno K, Fagan JM, Baracos V. Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin 1 or other known monokines. J Clin Invest 81: 1378–1383, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 15: 475–482, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Haq S, Kilter H, Michael A, Tao J, O’Leary E, Sun XM, Walters B, Bhattacharya K, Chen X, Cui L, Andreucci M, Rosenzweig A, Guerrero JL, Patten R, Liao R, Molkentin J, Picard M, Bonventre JV, Force T. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat Med 9: 944–951, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Hasselgren PO, Warner BW, Hummel RP, 3rd, James JH, Ogle CK, Fischer JE. Further evidence that accelerated muscle protein breakdown during sepsis is not mediated by prostaglandin E2. Ann Surg 207: 399–403, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasselgren PO, Zamir O, James JH, Fischer JE. Prostaglandin E2 does not regulate total or myofibrillar protein breakdown in incubated skeletal muscle from normal or septic rats. Biochem J 270: 45–50, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbaczynska-Cedro K, Staszewska-Barczak J. Proceedings: muscular work and prostaglandin release. Br J Pharmacol 52: 454P–455P, 1974 [PMC free article] [PubMed] [Google Scholar]
- 42.Herbaczynska-Cedro K, Staszewska-Barczak J, Janczewska H. Muscular work and the release of prostaglandin-like substances. Cardiovasc Res 10: 413–420, 1976 [DOI] [PubMed] [Google Scholar]
- 43.Herbaczynska-Cedro K, Staszewska-Barczak J, Janczewska H. The release of prostaglandin-like substances during reactive and functional hyperemia in the hind leg of the dog. Pol J Pharmacol Pharm 26: 167–170, 1974 [PubMed] [Google Scholar]
- 44.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA 89: 7384–7388, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Höffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in human skeletal muscle interstitium. J Physiol 550: 217–225, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janczewska H, Herbaczynska-Cedro K. Effect of indomethacin on vascular responses to vasoactive agents in working skeletal muscles in the dog. Pol J Pharmacol Pharm 26: 159–166, 1974 [PubMed] [Google Scholar]
- 48.Jankowski CM, Gozansky WS, Maclean PS, Shulman B, Wolfe P, Schwartz RS, Kohrt WM. N-Acetyl-4-aminophenol and musculoskeletal adaptations to resistance exercise training. Eur J Appl Physiol 113: 1127–1136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karamouzis M, Karamouzis I, Vamvakoudis E, Ampatzidis G, Christoulas K, Angelopoulou N, Mandroukas K. The response of muscle interstitial prostaglandin E2 (PGE2), prostacyclin I2 (PGI2) and thromboxane A2 (TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids 64: 259–263, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Karamouzis M, Langberg H, Skovgaard D, Bulow J, Kjaer M, Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiol Scand 171: 71–76, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Karim SM, Sandler M, Williams ED. Distribution of prostaglandins in human tissues. Br J Pharmacol Chemother 31: 340–344, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 287: 337–344, 2002 [DOI] [PubMed] [Google Scholar]
- 53.KEGG (Kyoto Encyclopedia of Genes and Genomes) Arachidonic Acid Metabolism–Reference Pathway (Online). Kyoto University, Kyoto, Japan: http://www.genome.jp/kegg/pathway/map/map00590.html [8 February 2013]. [Google Scholar]
- 54.Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol 257: 109–121, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreiner F, Galbo H. Elevated muscle interstitial levels of pain-inducing substances in symptomatic muscles in patients with polymyalgia rheumatica. Pain 152: 1127–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Krentz JR, Quest B, Farthing JP, Quest DW, Chilibeck PD. The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training. Appl Physiol Nutr Metab 33: 470–475, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 58.MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 20: 480–486, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Machida M, Takemasa T. Ibuprofen administration during endurance training cancels running-distance-dependent adaptations of skeletal muscle in mice. J Physiol Pharmacol 61: 559–563, 2010 [PubMed] [Google Scholar]
- 60.Mackey AL, Holm L, Reitelseder S, Pedersen TG, Doessing S, Kadi F, Kjaer M. Myogenic response of human skeletal muscle to 12 weeks of resistance training at light loading intensity. Scand J Med Sci Sports 21: 773–782, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Mackey AL, Kjaer M, Dandanell S, Mikkelsen KH, Holm L, Dossing S, Kadi F, Koskinen SO, Jensen CH, Schroder HD, Langberg H. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol 103: 425–431, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Markworth JF, Cameron-Smith D. Prostaglandin F2α; stimulates PI3K/ERK/mTOR signaling and skeletal myotube hypertrophy. Am J Physiol Cell Physiol 300: C671–C682, 2011 [DOI] [PubMed] [Google Scholar]
- 63.McCarthy JJ, Esser KA. Counterpoint: satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103: 1100–1102, 2007 [DOI] [PubMed] [Google Scholar]
- 64.McMillan DN, Reeds PJ, Lobley GE, Palmer RM. Changes in protein turnover in hypertrophying plantaris muscles of rats: effect of fenbufen—an inhibitor of prostaglandin synthesis. Prostaglandins 34: 841–852, 1987 [DOI] [PubMed] [Google Scholar]
- 65.McNurlan MA, McHardy KC, Broom J, Milne E, Fearns LM, Reeds PJ, Garlick PJ. The effect of indomethacin on the response of protein synthesis to feeding in rats and man. Clin Sci (Lond) 73: 69–75, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res 196: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Mikkelsen UR, Helmark IC, Kjaer M, Langberg H. Prostaglandin synthesis can be inhibited locally by infusion of NSAIDS through microdialysis catheters in human skeletal muscle. J Appl Physiol 104: 534–537, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107: 1600–1611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mikkelsen UR, Schjerling P, Helmark IC, Reitelseder S, Holm L, Skovgaard D, Langberg H, Kjaer M, Heinemeier KM. Local NSAID infusion does not affect protein synthesis and gene expression in human muscle after eccentric exercise. Scand J Med Sci Sports 21: 630–644, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat 68–69: 383–399, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci 50 Spec No: 107–112, 1995 [DOI] [PubMed] [Google Scholar]
- 72.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest 108: 25–30, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novak ML, Billich W, Smith SM, Sukhija KB, McLoughlin TJ, Hornberger TA, Koh TJ. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol 296: R1132–R1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nowak J, Bohman SO, Alster P, Berlin T, Cronestrand R, Sonnenfeld T. Biosynthesis of prostaglandins in microsomes of human skeletal muscle and kidney. Prostaglandins Leukot Med 11: 269–279, 1983 [DOI] [PubMed] [Google Scholar]
- 75.Nowak J, Wennmalm A. Effect of exercise on human arterial and regional venous plasma concentrations of prostaglandin E. Prostaglandins Med 1: 489–497, 1978 [DOI] [PubMed] [Google Scholar]
- 76.O’Connor RS, Pavlath GK. Point:counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103: 1099–1100, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids 39: 95–104, 1990 [DOI] [PubMed] [Google Scholar]
- 78.Palmer RM, Reeds PJ, Atkinson T, Smith RH. The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J 214: 1011–1014, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulsen G, Egner IM, Drange M, Langberg H, Benestad HB, Fjeld JG, Hallen J, Raastad T. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand J Med Sci Sports 20: e195–e207, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Petersen SG, Beyer N, Hansen M, Holm L, Aagaard P, Mackey AL, Kjaer M. Nonsteroidal anti-inflammatory drug or glucosamine reduced pain and improved muscle strength with resistance training in a randomized controlled trial of knee osteoarthritis patients. Arch Phys Med Rehabil 92: 1185–1193, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Petersen SG, Miller BF, Hansen M, Kjaer M, Holm L. Exercise and NSAIDs: effect on muscle protein synthesis in patients with knee osteoarthritis. Med Sci Sports Exerc 43: 425–431, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 85.Piper PJ, Vane JR, Wyllie JH. Inactivation of prostaglandins by the lungs. Nature 225: 600–604, 1970 [DOI] [PubMed] [Google Scholar]
- 86.Qin N, Zhang SP, Reitz TL, Mei JM, Flores CM. Cloning, expression and functional characterization of human COX-1 splicing variants: evidence for intron 1 retention. J Pharmacol Exp Ther 315: 1298–1305, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Rabuel C, Renaud E, Brealey D, Ratajczak P, Damy T, Alves A, Habib A, Singer M, Payen D, Mebazaa A. Human septic myopathy: induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology 101: 583–590, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol 62: 1407–1412, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reeds PJ, Palmer RM. The possible involvement of prostaglandin F2α in the stimulation of muscle protein synthesis by insulin. Biochem Biophys Res Commun 116: 1084–1090, 1983 [DOI] [PubMed] [Google Scholar]
- 91.Reid CM, Storey E, Wong TY, Woods R, Tonkin A, Wang JJ, Kam A, Janke A, Essex R, Abhayaratna WP, Budge MM. Aspirin for the prevention of cognitive decline in the elderly: rationale and design of a neuro-vascular imaging study (ENVIS-ion). BMC Neurol 12: 3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587: 5483–5492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem 257: 1632–1638, 1982 [PubMed] [Google Scholar]
- 94.Rodemann HP, Waxman L, Goldberg AL. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem 257: 8716–8723, 1982 [PubMed] [Google Scholar]
- 95.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31–41, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 59: 207–224, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Samuelsson BI. Nobel Lecture: from Studies of Biochemical Mechanisms to Novel Biological Mediators: Prostaglandin Endoperoxides, Thromboxanes and Leukotrienes (Online). Nobelprize.org. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1982/samuelsson-lecture.html [8 December 1982]. [DOI] [PubMed] [Google Scholar]
- 98.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology 23: 160–170, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol 64: 1183–1189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol 109: 768–777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwab JM, Beiter T, Linder JU, Laufer S, Schulz JE, Meyermann R, Schluesener HJ. COX-3—a virtual pain target in humans? FASEB J 17: 2174–2175, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Shen W, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol 167: 1105–1117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol 214: 405–412, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Shen W, Prisk V, Li Y, Foster W, Huard J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2α. J Appl Physiol 101: 1215–1221, 2006 [DOI] [PubMed] [Google Scholar]
- 105.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387–437, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Smith RH, Palmer RM, Reeds PJ. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J 214: 153–161, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111: 5821–5865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res 50, Suppl: S423–S428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soltow QA, Betters JL, Sellman JE, Lira VA, Long JH, Criswell DS. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc 38: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 110.Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot Essent Fatty Acids 88: 361–364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283: E1272–E1278, 2002 [DOI] [PubMed] [Google Scholar]
- 112.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Templeton GH, Padalino M, Moss R. Influences of inactivity and indomethacin on soleus phosphatidylethanolamine and size. Prostaglandins 31: 545–559, 1986 [DOI] [PubMed] [Google Scholar]
- 114.Tesch PA, Ekberg A, Lindquist DM, Trieschmann JT. Muscle hypertrophy following 5-week resistance training using a non-gravity-dependent exercise system. Acta Physiol Scand 180: 89–98, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol 96: 1451–1458, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Testa M, Rocca B, Spath L, Ranelletti FO, Petrucci G, Ciabattoni G, Naro F, Schiaffino S, Volpe M, Reggiani C. Expression and activity of cyclooxygenase isoforms in skeletal muscles and myocardium of humans and rodents. J Appl Physiol 103: 1412–1418, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288: E883–E891, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol 57: B138–B143, 2002 [DOI] [PubMed] [Google Scholar]
- 122.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000 [DOI] [PubMed] [Google Scholar]
- 123.Trappe T, Raue U, Williams R, Carrithers J, Hickner R. Effects of age and resistance exercise on skeletal muscle interstitial prostaglandin F2α. Prostaglandins Leukot Essent Fatty Acids 74: 175–181, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol 554: 803–813, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrinol Metab 86: 5067–5070, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 304: R198–R205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002 [DOI] [PubMed] [Google Scholar]
- 129.United States Census Bureau State & County QuickFacts (Online). U.S. Department of Commerce, Washington, DC: http://quickfacts.census.gov/qfd/states/00000.html [14 March 2013]. [Google Scholar]
- 130.United States Preventative Services Task Force Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 150: 396–404, 2009 [DOI] [PubMed] [Google Scholar]
- 131.van Hall G, Steensberg A, Fischer C, Keller C, Moller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008 [DOI] [PubMed] [Google Scholar]
- 132.Vandenburgh HH, Hatfaludy S, Sohar I, Shansky J. Stretch-induced prostaglandins and protein turnover in cultured skeletal muscle. Am J Physiol Cell Physiol 259: C232–C240, 1990 [DOI] [PubMed] [Google Scholar]
- 133.Vandenburgh HH, Shansky J, Karlisch P, Solerssi RL. Mechanical stimulation of skeletal muscle generates lipid-related second messengers by phospholipase activation. J Cell Physiol 155: 63–71, 1993 [DOI] [PubMed] [Google Scholar]
- 134.Vandenburgh HH, Shansky J, Solerssi R, Chromiak J. Mechanical stimulation of skeletal muscle increases prostaglandin F2α production, cyclooxygenase activity, and cell growth by a pertussis toxin sensitive mechanism. J Cell Physiol 163: 285–294, 1995 [DOI] [PubMed] [Google Scholar]
- 135.Vane JR. Nobel Lecture: Adventures and Excursions in Bioassay: the Stepping Stones to Prostacyclin (Online). Nobelprize.org. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1982/vane-lecture.html [8 December 1982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.von Euler US. On the specific vaso-dilating and plain muscle stimulating substances from accessory genital glands in man and certain animals (prostaglandin and vesiglandin). J Physiol 88: 213–234, 1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watanabe K. Prostaglandin F synthase. Prostaglandins Other Lipid Mediat 68–69: 401–407, 2002 [DOI] [PubMed] [Google Scholar]
- 138.Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, Lemoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol 292: R2241–R2248, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Wu M, Desai DH, Kakarla SK, Katta A, Paturi S, Gutta AK, Rice KM, Walker EM, Jr, Blough ER. Acetaminophen prevents aging-associated hyperglycemia in aged rats: effect of aging-associated hyperactivation of p38-MAPK and ERK1/2. Diabetes Metab Res Rev 25: 279–286, 2009 [DOI] [PubMed] [Google Scholar]
- 140.Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS One 4: e6430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun 165: 888–894, 1989 [DOI] [PubMed] [Google Scholar]
- 142.Young MK, Bocek RM, Herrington PT, Beatty CH. Aging: effects on the prostaglandin production by skeletal muscle of male rhesus monkeys (Macaca mulatta). Mech Ageing Dev 16: 345–353, 1981 [DOI] [PubMed] [Google Scholar]