Abstract
Although some central aspects of pulmonary function (ventilation and perfusion) are known to be heterogeneous, the distribution of diffusive gas exchange remains poorly characterized. A solution is offered by hyperpolarized 129Xe magnetic resonance (MR) imaging, because this gas can be separately detected in the lung's air spaces and dissolved in its tissues. Early dissolved-phase 129Xe images exhibited intensity gradients that favored the dependent lung. To quantitatively corroborate this finding, we developed an interleaved, three-dimensional radial sequence to image the gaseous and dissolved 129Xe distributions in the same breath. These images were normalized and divided to calculate “129Xe gas-transfer” maps. We hypothesized that, for healthy volunteers, 129Xe gas-transfer maps would retain the previously observed posture-dependent gradients. This was tested in nine subjects: when the subjects were supine, 129Xe gas transfer exhibited a posterior-anterior gradient of −2.00 ± 0.74%/cm; when the subjects were prone, the gradient reversed to 1.94 ± 1.14%/cm (P < 0.001). The 129Xe gas-transfer maps also exhibited significant heterogeneity, as measured by the coefficient of variation, that correlated with subject total lung capacity (r = 0.77, P = 0.015). Gas-transfer intensity varied nonmonotonically with slice position and increased in slices proximal to the main pulmonary arteries. Despite substantial heterogeneity, the mean gas transfer for all subjects was 1.00 ± 0.01 while supine and 1.01 ± 0.01 while prone (P = 0.25), indicating good “matching” between gas- and dissolved-phase distributions. This study demonstrates that single-breath gas- and dissolved-phase 129Xe MR imaging yields 129Xe gas-transfer maps that are sensitive to altered gas exchange caused by differences in lung inflation and posture.
Keywords: hyperpolarized 129Xe, dissolved phase, radial pulse sequence, supine, prone
the introduction of hyperpolarized (HP) gas magnetic resonance (MR) imaging (MRI) over the last decade has enabled rapid, noninvasive, and relatively high-resolution three-dimensional (3D) imaging of pulmonary function in a single breath hold (36). The most well-developed clinical applications of HP gas MRI use the regional density of the isotope 3He to visualize regions of impaired ventilation, altered ventilation dynamics (13), and regional gas trapping (34) in the lungs. Furthermore, MRI of gaseous HP 3He in the pulmonary air spaces can be used to assess other aspects of lung biology, including pulmonary microstructure, by mapping the apparent diffusion coefficient of 3He (28) and Po2 by mapping the MR relaxation of 3He (8). Unfortunately, because of its increasing cost and scarcity, 3He has not achieved widespread clinical dissemination. However, HP 129Xe MRI has recently been shown to be well tolerated in human subjects (7) and to display sensitivity similar to that of 3He to changes in ventilation (36) and pulmonary microstructure through diffusion-weighted imaging (20).
Additionally, HP 129Xe is advantageous, in that it is moderately soluble in the pulmonary tissues and capillary blood (∼10% solubility). Because 129Xe follows the same physical gas-transfer pathway as O2 to reach the red blood cells (RBCs) in the pulmonary capillary bed, 129Xe enables MRI of pulmonary function beyond the air spaces (3). Moreover, when 129Xe is dissolved in the blood-gas barrier tissues and the RBCs, its MR frequency shifts dramatically (several kHz at the magnetic field strengths commonly used for MRI). This frequency shift enables 129Xe atoms dissolved in the barrier tissues and RBCs to be separately detected from those remaining in the alveolar gas spaces (6). Although these “dissolved-phase” 129Xe atoms represent only ∼2% of the total magnetization pool at any given instant, we and others recently demonstrated that it is possible to obtain a 3D image of the uptake of 129Xe gas in human subjects in a breath hold (3, 21). These initial dissolved-phase 129Xe images were characterized by strong gravitational gradients and notable isogravitational heterogeneity, even in healthy subjects (3). Hence, these findings suggest that 129Xe MRI is well suited to show regional pulmonary gas transfer. However, the physical origins of the dissolved-phase 129Xe signal are substantially different from more standard physiological approaches to measurement of gas transfer.
Inhaled 129Xe enters the pulmonary capillary beds by diffusion from the alveolar spaces, and these 129Xe atoms very quickly saturate the thin (∼10-μm) alveolar septa on a time scale of ∼150–200 ms (24). Imaging of these dissolved atoms employs selective, relatively large radio-frequency (RF) flip angles (10–30°), and this rapidly depletes their signal. However, this dissolved magnetization is rapidly replenished by continuous diffusion of fresh HP 129Xe into the tissues from the alveolar gas spaces (3, 6). Hence, these combined properties of alveolar structure and a properly designed MR acquisition scheme ensure that dissolved 129Xe MR signal arises almost exclusively from the lung's gas exchange tissues and contains virtually no contribution from the larger downstream vasculature. Moreover, as estimated by Cleveland et al. (3), ∼40–50% of the dissolved 129Xe signal originates from the capillary blood, both RBCs and plasma, and the remainder from the adjacent lung parenchyma.
Like O2 transfer, 129Xe gas transfer increases in regions where the well-perfused capillary bed is in contact with well-ventilated lung. By contrast, low dissolved 129Xe intensity can arise from poorly ventilated or well ventilated, but poorly perfused, regions of the lungs. However, unlike O2 or CO, 129Xe does not bind chemically to hemoglobin. Thus 129Xe lacks the complexity of the reactive conductance component, and 129Xe transfer begins to resemble a spatially resolved measure of the membrane diffusing capacity. However, because 129Xe is an inert gas, it is essentially a perfusion-limited, and not a diffusion-limited, marker.
Because dissolved-phase 129Xe image intensity is affected by ventilation and perfusion, it cannot be interpreted in isolation. For instance, in our initial work, two separate images of the dissolved- and gas-phase HP 129Xe distributions were acquired from two separate 1-liter breaths of 129Xe (3). Unfortunately, the batch-to-batch polarization of 129Xe and the breath-to-breath distributions of an inhaled gas can vary substantially, especially when lung volumes are between functional residual capacity (FRC) and total lung capacity (TLC). Thus it was not possible to obtain an accurate estimate of the “source” gas-phase magnetization with use of this two-breath imaging approach, and only qualitative comparisons between images (and subjects) were possible.
A preferred approach for assessing the spatial distribution of dissolved HP 129Xe is to image gaseous and dissolved 129Xe in the same breath hold. Recently, such single-breath images of the gas- and dissolved-phase 129Xe were elegantly achieved by Mugler et al. (21), who took advantage of the chemical-shift “artifact” between dissolved- and gas-phase 129Xe. This study employed a Cartesian gradient-recalled echo sequence (21) with carefully tuned acquisition parameters, allowing the gas and dissolved phases to appear side-by-side in each image slice. Although this work provided important initial quantitative confirmation of the observed gradients in dissolved 129Xe images, it employed a relatively long echo time (TE), which sacrificed significant signal-to-noise ratio (SNR) because of the relatively short (∼2-ms) of dissolved 129Xe (22). An additional complication is that the of the gas phase is ∼10 times longer than that of the dissolved phase, which compromises quantitative comparisons at longer TEs.
Therefore, the first goal of this work was to develop a robust, 3D isotropic approach to image dissolved 129Xe in the resting lung, which would enable quantitative study of the 129Xe gas-transfer distribution. Our technical approach is designed to overcome specific limitations of the previously described gradient-recalled echo acquisition by use of a 3D radial acquisition scheme to alternatively excite the gas- and dissolved-phase 129Xe resonances. This approach permits acquisition with submillisecond TEs to counteract the signal losses due to the short dissolved-phase 129Xe (22). Moreover, this acquisition strategy is robust to motion (e.g., cardiac motion) (10) and to undersampling (29) and acquires 3D isotropic images of gas- and dissolved-phase 129Xe that are inherently coregistered. These distributions are then normalized, and the ratio of dissolved- to gas-phase signal intensity provides a unitless, but still quantitative, measure of regional 129Xe gas transfer. Moreover, these 129Xe gas-transfer maps reflect the extent of “matching” of the signal intensity distributions of gas-phase (a surrogate for ventilation) and dissolved-phase (a surrogate for diffusive gas exchange) 129Xe.
With a robust 129Xe gas-transfer acquisition in hand, our second goal was to develop a fundamental understanding of the physiologically relevant information provided by these images by performing 129Xe MRI of the lungs under a set of conditions that are known to alter the underlying pattern of physiological gas exchange. Thus 129Xe gas transfer was imaged in the supine and prone positions for all subjects. Such postural changes are known to alter the resting perfusion distribution (26) and diffusing capacity for O2 (19). Moreover, our previous efforts to image the dissolved 129Xe distribution showed that the images were characterized by a strong postural gradient, which favored the dependent lung. We thus hypothesized that, upon development of a single-breath method of imaging 129Xe gas transfer, a postural gradient would be detectable in the individual 129Xe gas- and dissolved-phase distributions and that gravitational gradients would still be retained in the normalized 129Xe gas-transfer maps. Furthermore, we hypothesized that gradients in the gas and dissolved phases, as well as in 129Xe gas transfer, would reverse when subjects were repositioned from the supine to the prone position. Such imaging of the underlying variability of 129Xe gas transfer in healthy subjects provides a necessary platform to probe basic lung physiology and eventually understand the pathophysiological changes that arise with the onset of pulmonary diseases.
METHODS
Subject inclusion and exclusion criteria.
Studies were approved by the Duke Institutional Review Board and conducted under US Food and Drug Administration Investigational New Drug Application 109490. Written, informed consent was obtained from all the subjects prior to the scan. All subjects (8 men, 1 woman) were ≥18 yr of age (average 45.6 ± 18.7), had a <5 pack-yr smoking history, had not smoked in the last 5 yr, and had no diagnosed pulmonary disorders. The body mass index for the subjects was 19–29.6 (average 24.3 ± 3.6). Subjects were excluded if they were unable to hold their breath for 16 s, had a history of cardiac arrhythmias, were pregnant or lactating, or had a respiratory illness within 30 days of MRI. At 1 h prior to MR studies, subjects underwent pulmonary function testing, including measures of TLC, which were used to interpret aspects of the MR images. TLC was calculated as the sum of residual volume, which was measured by body plethysmography, and vital capacity, which was measured by spirometry.
Xe polarization and delivery.
Each subject received two 1-liter doses of xenon (isotopically enriched to 86% 129Xe; Linde Gases, Stewartsville, NJ) produced by rubidium vapor spin-exchange optical pumping (5) and cryogenically accumulated using a commercially available polarizer (model 9800, Polarean, Durham, NC). Xe was then thawed into a 1-liter ALTEF bag (Jensen Inert Products, Coral Springs, FL), and polarization (∼8–10%) was determined using a polarization measurement station (model 2881, Polarean). Prior to gas inhalation, subjects were instructed to inhale to TLC and exhale to FRC twice. They then inhaled the gas through 0.95-cm-ID Tygon tubing (Saint Gobain Performance Plastics, Akron, OH) and held their breath for a maximum of 16 s while the images were acquired. Throughout the imaging session, blood oxygenation and heart rate were monitored using a MR-compatible monitoring system (GE Healthcare, Helsinki, Finland).
MR acquisition and work flow.
Studies were conducted on a 1.5-T scanner (EXCITE 15M4, GE Healthcare Waukesha, WI) equipped with a 2-kW broadband (8–70 MHz) amplifier for the multinuclear RF chain (CPC, Hauppauge, NY). Subjects were fitted in a quadrature 129Xe vest coil (Clinical MR Solutions, Brookfield, WI) tuned to 17.66 MHz and proton-blocked to permit 1H MRI of the thoracic cavity with the scanner's body coil, with no change in body position.
Single-breath gas- and dissolved-phase HP 129Xe images were acquired by collection of each radial view of k-space twice (Fig. 1). First the transmit and receive frequencies were set on the gas-phase resonance (0 Hz) and then on the dissolved-phase resonance (+3,832 Hz). Imaging parameters included sinc pulse duration = 1.2 ms, TE/TR = 0.932/7.5 ms, matrix = 32 × 32 × 32, 1,001 rays for each frequency, gas flip angle = 0.5°, dissolved flip angle = 22°, bandwidth = 15.625 kHz, and field of view = 40 cm. k-Space data for each 129Xe resonance was reconstructed separately using a nonuniform fast Fourier transform algorithm (30). Prior to this study, dissolved 129Xe MRI acquisition parameters and, thus, image quality were rigorously optimized with regard to the frequency selectivity of the RF excitation (see appendix). Radial 1H images of the thoracic cavity (breath hold = 16 s) were acquired using a 274-μs hard pulse, TE/TR = 0.336/2.4 ms, matrix = 64 × 64 × 64, 5,647 rays, 512 dummy pulses, flip angle = 5°, bandwidth = 15.625 kHz, and field of view = 40 cm.
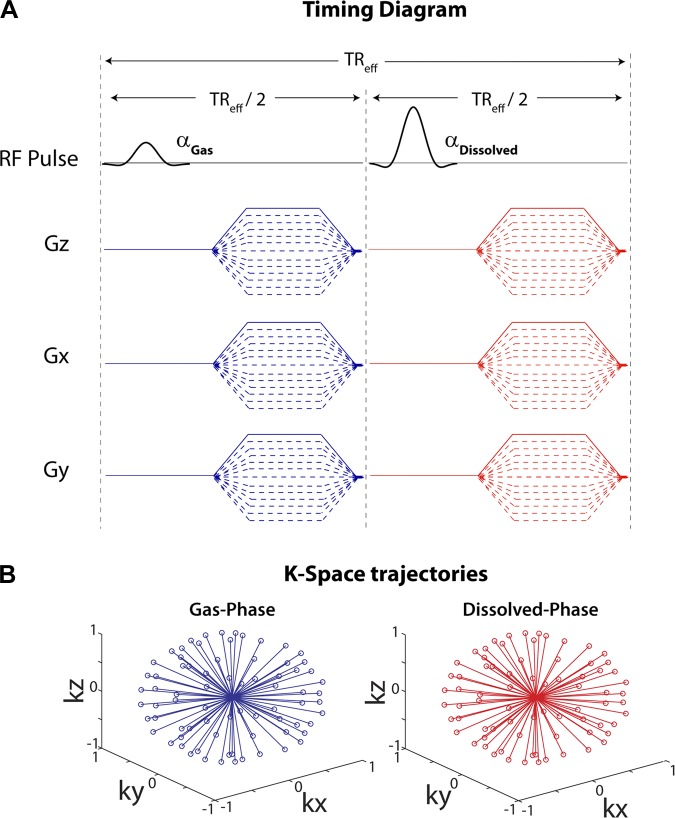
Fig. 1.
Pulse sequence diagram for single-breath, 3-dimensional (3D) radial imaging of gas- and dissolved-phase 129Xe. A: pulse sequence diagram. A given ray of k-space was acquired after excitation of the gas phase (α = 0.5°), and then a second ray with an identical k-space trajectory was acquired after excitation of the dissolved phase (α = 22°). TReff, repetition time to apply 2 successive radio-frequecy pulses, one on the gas phase and one on the dissolved phase of 129Xe. B: 3D k-space trajectories for both resonances, which were reconstructed separately to yield the images.
The study work flow proceeded as follows: after a free-breathing localizer scan in the supine position, 1H radial thoracic cavity images were acquired after the subject had inhaled 1 liter of room air from a polyethylene bag starting from FRC. This was followed by administration of 1 liter of 129Xe for single-breath acquisition of both the gas- and dissolved-phase distributions. The subject was then transferred to the prone position with no change in the coil orientation, and a second, 1-liter single-breath scan of the gas- and dissolved-phase distributions was acquired. Finally, while the subject was still in the prone position, a second radial breath-hold 1H scan of the thoracic cavity was acquired.
Image and statistical analysis.
The 1H images were used to confine the analysis of the HP 129Xe images to the thoracic cavity (36). To match the resolution of the thoracic cavity images, the dual-acquisition gas- and dissolved-phase 129Xe images were up-sampled from their native 32 × 32 × 32 matrix to a 64 × 64 × 64 matrix by bilinear interpolation using ImageJ 1.44p (National Institutes of Health, Bethesda, MD). The 1H and gaseous 129Xe images were then registered using a multiresolution, affine transformation, with joint entropy as the cost function and a cubic-spline interpolation using the Image Registration Toolkit (31). Because these dual-acquisition gas- and dissolved-phase 129Xe images are inherently coregistered, this transformation also registered the dissolved-phase and 1H images. The thoracic cavity image was then segmented using the region-growing algorithm in 3DSlicer (36) to create a binary thoracic cavity “mask” (Fig. 2). This mask was manually segmented to remove the trachea and main stem bronchi and morphologically closed using a spherical structuring element (7-voxel diameter); then an additional filling operation in MATLAB R2011a (MathWorks, Natick, MA) was performed.
Fig. 2.
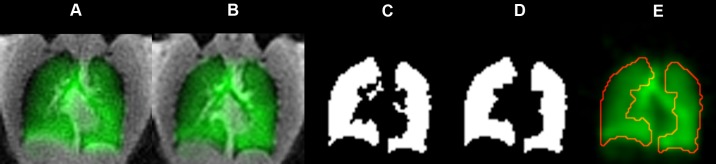
Image processing used to limit the analysis to the thoracic cavity. A: gas-phase image (green) overlaid on the corresponding thoracic cavity image (grayscale) prior to registration. Postregistration thoracic cavity image (B) was used to create a thoracic cavity mask that defines the data analysis region (C). Airways in the mask were manually segmented, and the mask was then subjected to morphological closing with a spherical structuring element (7-pixel diameter) and a filling operation to yield the final mask (D). E: outline of the processed thoracic cavity mask on the gas-phase image.
Both gas- and dissolved-phase images were normalized by the sum of their respective voxel intensities within the thoracic cavity mask (25), and the resulting normalized dissolved-phase images were divided on a voxel-by-voxel basis by the corresponding normalized gas-phase images to create the 129Xe gas-transfer maps. Images were analyzed for posterior-anterior signal intensity gradients by a linear regression (weighted by voxel count) of the mean signal intensity of each slice as a function of slice position. 129Xe gas-transfer maps and source images were further analyzed for heterogeneity by calculating the coefficient of variation (CV) for the whole lung.
Changes in the posterior-anterior gradients of the gas and dissolved phases were tested for overall statistical significance using a repeated-measures ANOVA. Post hoc tests for significance of the individual gradients (gas phase, dissolved phase, and 129Xe gas transfer) were done using a matched-pair t-test.
RESULTS
Signal intensity gradients.
Isotropic, single-breath gas- and dissolved-phase HP 129Xe images were successfully acquired in both postures for all nine subjects. Figure 3 shows a typical example of these images acquired from a 29-yr-old healthy male subject in the supine position. Similar to the trends observed in earlier studies (3, 21), axial slices from these images reveal that gas- and dissolved-phase signal intensities are greatest in the gravitationally dependent posterior lung and diminish in the anterior lung. Although gas- and dissolved-phase images individually exhibit gravitational gradients, these gradients persist in the normalized dissolved phase-to-gas phase ratio (129Xe gas transfer) maps. Hence, the 129Xe gas transfer itself exhibits a gravitational gradient beyond what can be attributed simply to the gas-phase 129Xe distribution.
Fig. 3.

Representative single-breath, isotropic gas- and dissolved-phase images and the corresponding gas-transfer map, defined as the normalized dissolved-phase image-to-gas-phase image ratio.
The effects of subject posture on the uptake of HP 129Xe are illustrated in Fig. 4, which depicts coronal 129Xe gas-transfer maps from a second subject. When supine (Fig. 4A), this subject exhibited decreasing 129Xe gas-transfer values from the posterior to the anterior lung. However, when the subject was repositioned in the prone position (Fig. 4B), the gradient in 129Xe transfer reversed, with higher values in the anterior, now gravitationally dependent, lung and lower values in the posterior, now nondependent, lung. The 129Xe gas-transfer distributions for both positions are better appreciated when plotted as a function of slice position (Fig. 4C). In addition to quantitatively depicting gradient reversal, Fig. 4C shows the scale of 129Xe gas-transfer variability for this subject. With the subject supine, mean 129Xe transfer decreased 43% from the posterior to the anterior lung, but with the subject prone, 129Xe transfer increased 27% from the posterior to the anterior lung. However, in both postures, 129Xe transfer was nonmonotonic and increased markedly in slices isogravitational with the main pulmonary arteries. This increase was evident for seven of the nine subjects, with two other subjects displaying a gas-transfer plateau in the central regions of the lungs. The functional gradients for all subjects, as well as their demographics, are summarized in Table 1.
Fig. 4.

A and B: representative 129Xe gas-transfer map (arranged from posterior to anterior lung) from a healthy volunteer (subject 2) in the supine and prone positions. C: mean 129Xe gas transfer in each slice, plotted as a function of lung position, showing higher gas transfer in the dependent lung for both postures.
Table 1.
Subject demographics and postural gradients
| Mean Gradient, %/cm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supine |
Prone |
Mean 129Xe Gas Transfer |
||||||||
| Subj No. | Age, yr | TLC, liters | Gas | Dissolved | 129Xe Gas Transfer | Gas | Dissolved | 129Xe Gas Transfer | Supine | Prone |
| 1 | 29 | 9.16 | −3.51 | −5.30 | −2.28 | −1.16 | 2.34 | 3.24 | 0.997 | 1.017 |
| 2 | 60 | 8.05 | −2.43 | −4.85 | −2.56 | 3.37 | 5.55 | 2.45 | 1.003 | 1.001 |
| 3 | 68 | 7.00 | −2.19 | −4.15 | −2.32 | 1.13 | 2.69 | 1.94 | 1.001 | 0.999 |
| 4 | 23 | 7.84 | −3.28 | −4.84 | −2.08 | −3.88 | −0.93 | 2.95 | 0.991 | 1.011 |
| 5 | 59 | 7.56 | −2.54 | −3.57 | −1.36 | 2.07 | 4.49 | 2.78 | 0.988 | 0.997 |
| 6 | 65 | 7.17 | 2.40 | 0.54 | −1.98 | −0.21 | 0.10 | 0.46 | 1.008 | 1.008 |
| 7 | 51 | 5.74 | −2.17 | −3.42 | −1.34 | 1.90 | 3.96 | 2.32 | 0.996 | 1.003 |
| 8 | 22 | 6.71 | −1.71 | −2.13 | −0.80 | −1.66 | −0.74 | 1.42 | 0.998 | 1.005 |
| 9 | 33 | 7.96 | −1.81 | −5.30 | −3.24 | 0.95 | 2.34 | −0.08 | 1.026 | 1.010 |
TLC, total lung capacity.
The population means of the gradients in 129Xe distribution for all subjects are summarized in Table 2 and Fig. 5. Gas-phase images exhibited a modest population-mean posterior-anterior gradient of −1.92 ± 1.73%/cm for supine subjects that reversed significantly and diminished to 0.28 ± 2.23%/cm when the subjects were prone (P = 0.042). Dissolved-phase 129Xe images exhibited a larger posterior-anterior gradient of −3.46 ± 1.91%/cm for supine subjects that also reversed significantly (P = 0.0034) to 2.18 ± 2.47%/cm when the subjects were prone. These gradients persisted in the 129Xe gas-transfer maps and also reversed significantly (P < 0.001) when subjects changed from the supine (−1.99 ± 0.74%/cm) to the prone (1.94 ± 1.14%/cm) position.
Table 2.
Mean gradients and coefficients of variation
| Posture | Gas | Dissolved | 129Xe Gas Transfer |
|---|---|---|---|
| Gradient, %/cm | |||
| Supine | −1.92 ± 1.73 | −3.46 ± 1.91 | −2.00 ± 0.74 |
| Prone | 0.28 ± 2.23 | 2.18 ± 2.47 | 1.94 ± 1.14 |
| Coefficient of variation | |||
| Supine | 0.34 ± 0.05 | 0.38 ± 0.05 | 0.19 ± 0.04 |
| Prone | 0.32 ± 0.04 | 0.36 ± 0.05 | 0.20 ± 0.04 |
Fig. 5.
Signal intensity and gas-transfer gradients for all subjects. Gas and dissolved phases showed a significant gradient reversal (P < 0.001, by F-test) when subjects were repositioned from the supine to the prone position (P = 0.042 for gas and P = 0.0034 for dissolved). The gradient in gas transfer also reversed significantly when subjects were repositioned from supine to prone (P < 0.0001).
Heterogeneity analysis.
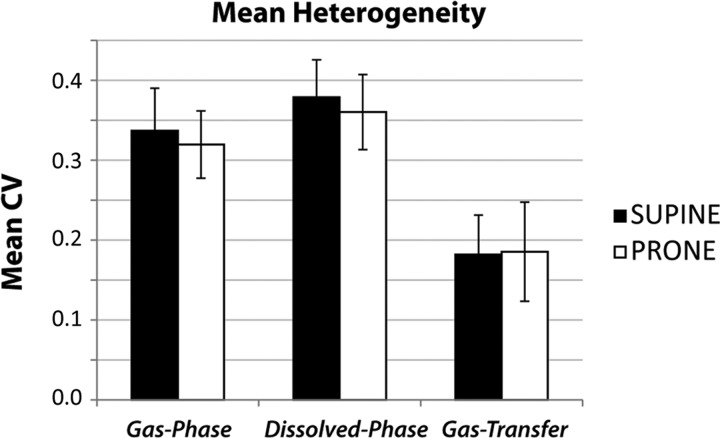
In addition to gravitational gradients, gas-phase, dissolved-phase, and 129Xe transfer maps showed significant in-plane and overall heterogeneity. Whole lung heterogeneity, as measured by the CV, is summarized in Table 2 and Fig. 6. The population-mean CVs of the gas- and dissolved-phase 129Xe images were similar and not significantly impacted by posture (P = 0.21 for gas, P = 0.22 for dissolved, and P = 0.45 for 129Xe gas transfer). However, for both postures, the whole lung CVs of the dissolved-phase image were modestly higher than those of the gas-phase images (P = 0.0001 for supine and P = 0.0028 for prone). Interestingly, the CV of the normalized 129Xe gas-transfer maps relative to the gas- or dissolved-phase images alone was roughly 45% lower in both positions. Despite the pronounced spatial variation in the 129Xe gas-transfer maps, the 129Xe gas-transfer value averaged over the entire lung remained near unity for all subjects (see Table 1) and displayed no significant posture dependence: 1.00 ± 0.01 in the supine position and 1.01 ± 0.01 in the prone position (P = 0.25).
Fig. 6.
Population means for whole lung coefficient of variation (CV). Mean gas-transfer CV was almost half that of the gas- and dissolved-phase images. The CV was significantly higher for dissolved-phase than gas-phase images for both postures (P = 0.0001 for supine and P = 0.003 for prone). No significant posture-dependent change in CV was observed in gas-phase (P = 0.22) and dissolved-phase (P = 0.21) images or 129Xe gas-transfer maps (P = 0.34).
The distribution of gas transfer was also impacted by lung inflation. In the supine position, subjects with larger TLC tended to exhibit greater gas-transfer heterogeneity than those with smaller TLC. Because all subjects in this study received the same 1-liter volume of HP 129Xe (i.e., range 10.9–17.4% of TLC), subjects with larger lung volume underwent imaging at a lower degree of lung inflation. For supine subjects (Fig. 7A), 129Xe gas-transfer CV correlated significantly with TLC (r = 0.77, P = 0.015). However, in the prone position (Fig. 7B), these same subjects exhibited no correlation between CV and TLC (r = 0.18, P = 0.64). Notably, the polarization-corrected SNR (SNR/polarization) was not significantly different between postures (P = 0.55 for gas phase and P = 0.42 for dissolved phase). Thus TLC-dependent heterogeneity in the supine position does not appear to be explained by lower image SNR resulting from greater 129Xe dilution but, instead, reflects fundamental aspects of 129Xe gas-transfer distribution.
Fig. 7.
Whole lung 129Xe gas-transfer CV as a function of total lung capacity (TLC). A: 129Xe gas-transfer CVs in supine subjects. Note significant (P = 0.015) increase in CV with increasing TLC in supine subjects. B: 129Xe gas-transfer CVs in prone subjects. CV in prone subjects showed no significant correlation with TLC (P = 0.64).
DISCUSSION
Gravitational gradients.
The acquisition-and-analysis method described here accounts for the contribution of the gas-phase 129Xe distribution to the dissolved-phase 129Xe image, permitting quantitative regional mapping of 129Xe gas transfer. These maps corroborate our hypothesis that 129Xe gas transfer is heterogeneous in normal subjects and is subject to gravitational gradients that reverse with subject posture. Indeed, 129Xe gas transfer varies by 30–40% across the lung field. These single-breath 129Xe gas-transfer images show trends that are consistent with observations from other imaging modalities and experimental techniques that image tissue density (32) and perfusion (23, 25, 26) and provide a unique window on the regional variability of lung function in healthy subjects.
It is important to note that the 129Xe gas-transfer imaging signal arises from physical origins that are fundamentally different from established physiological measures of respiratory gas transfer. First, since 129Xe gas-transfer images are acquired in a single breath, they will report primarily on well-ventilated regions of the lung. Second, 129Xe gas-transfer signal arises almost exclusively from the alveolar septa, where diffusion from the alveolar spaces continuously replenishes the dissolved 129Xe magnetization. Given that the harmonic mean thickness of the barrier is 1 μm (L) and the diffusion coefficient of 129Xe in the barrier is 0.33 × 10−5 cm2/s (D), 129Xe takes only ∼1.5 ms [t = (L2/2D)] to diffuse across the interstitial barrier and into the RBCs and only ∼150 ms longer to saturate the septum (24). Furthermore, 40–50% of the dissolved 129Xe signal originates from the capillary blood, which transits through the capillary network in 0.75 s. Consequently, although 129Xe gas-transfer intensity is influenced by perfusion, it must closely reflect local capillary blood volume. Since local blood volume tracks with perfusion (18), 129Xe transfer imaging may be thought of as approximating local ventilation-perfusion matching. Hence, in this regard, 129Xe transfer MRI is a closer surrogate for studying the O2 transfer pathway than other imaging modalities that infer this information indirectly through ventilation and perfusion metrics.
The postural gradients in 129Xe gas transfer in this study are consistent with tissue density, ventilation, and perfusion gradients reported by other imaging modalities, which are summarized in Table 3. For supine subjects, lung tissue density has been reported to exhibit a posterior-anterior gradient of −4.33%/cm by CT (32) and −4.9 ± 1.9%/cm by MRI (14). By comparison, the mean 129Xe gas-transfer gradient of −2.00 ± 0.74%/cm for supine subjects was of the same sign but roughly half as large. This is because 129Xe transfer is affected by tissue density gradients, but these gradients also cause a gradient in the intrapleural pressure, which similarly increases ventilation in the dependent lung. Since 129Xe gas transfer is “ventilation-normalized,” 129Xe gas transfer exhibits a smaller gradient than the dissolved signal or tissue density alone. Comparison of 129Xe gas-transfer distribution with perfusion distributions measured by other modalities revealed a gravitational gradient that was of the same sign as, but somewhat smaller than, the perfusion gradient of −3.10 ± 1.50%/cm observed for supine subjects by single-photon-emission CT (SPECT) (25) and roughly half the −4.40 ± 3.20%/cm gradient reported by PET (23). However, the supine 129Xe gas-transfer gradient was very similar to the perfusion gradient reported by arterial spin labeling (−2.10 ± 4.32%/cm), albeit with reduced intersubject variability. For prone subjects, the 129Xe gas-transfer gradient was reversed and exhibited a positive slope of 1.94 ± 1.14%/cm, which is comparable to the density gradient of 2.72%/cm measured by CT (32), as well as perfusion gradients of 2.70 ± 1.50%/cm measured by SPECT (25) and 1.11 ± 1.68%/cm measured by arterial spin labeling MRI (26). However, it was less than half the perfusion gradient of 5.20 ± 4.30%/cm reported by PET (23). While perfusion and tissue density contribute significantly to the gas-transfer distribution, the differences in the gravitational gradients can be attributed to the sensitivity of the dissolved-phase signal to the capillary blood volume (not flow) and the tissue density primarily in the acinar region of the lung.
Table 3.
Comparison of 129Xe gas-transfer gradients with other modalities
| Perfusion |
|||||
|---|---|---|---|---|---|
| 129Xe GasTransfer | ASL | PET | SPECT | Tissue Density CT | |
| Supine | −2.00 ± 0.74 | −2.10 ± 4.32 | −4.40 ± 3.20 | −3.10 ± 1.50 | −4.33 |
| Prone | 1.94 ± 1.14 | 1.11 ± 1.68 | 5.20 ± 4.30 | 2.70 ± 1.50 | 2.72 |
Values are expressed as %/cm. ASL, arterial spin labeling (calculated using data in Ref. 26); PET, positron emission tomography (reported in Ref. 23); SPECT, single-photon-emission computed tomography (reported in Ref. 25); tissue density CT, tissue density computed tomography (reported in Ref. 32).
Although all subjects demonstrated a consistent trend of increasing 129Xe gas transfer from nondependent to dependent lung in both positions, this behavior was distinctly nonmonotonic and unique to each subject (Fig. 8). For example, when scanned prone, subjects 6, 8, and 9, exhibited prominently increased 129Xe gas transfer in the central image slices that decreased toward the anterior lung. In fact, even in supine subjects, a subtle increase in 129Xe gas transfer was seen in the central lung at the plane of the main pulmonary arteries. This was evident for seven of the nine subjects, with two other subjects displaying a 129Xe gas-transfer plateau in the central regions of the lungs. Such nonmonotonic behavior has also been reported in tissue density (32) and perfusion (40) distributions, but their origins have not been probed extensively. As a result of the nonmonotonic increase in the 129Xe gas transfer in the more central slices of the lung, the full degree of 129Xe gas-transfer variability in this population was poorly reflected by a simple linear gradient metric.
Fig. 8.
129Xe gas-transfer pattern for subjects 1–9.
The general gravitational trend observed in our study is consistent with the classic model of West and Dollery (39), which predicts a larger fraction of capillary blood volume in the gravitationally dependent lung (39) and, hence, provides a larger sink for 129Xe gas transfer. Of course, as shown by Hopkins et al. (14), the remaining tissues of the lung also follow a gravitational “slinky”-like density pattern that favors the dependent lung.
Additional sources of heterogeneity.
Beyond gravity-induced changes in distribution, additional factors contribute significantly to 129Xe gas-transfer heterogeneity in resting healthy subjects (9, 11). One consideration may be the shape of an individual's chest cavity, which impacts regional lung expansion and alveolar recruitment (32). To this end, some regions may exhibit slower ventilatory filling, which gives rise to lower gas-phase signal intensity and, consequently, lower dissolved-phase intensity.
Additionally, the effect of the heart must also be considered, because its weight compresses the pulmonary tissue beneath it, increasing tissue density in this region (1). As a greater portion of the heart rests over the left lung, we might expect differences in the 129Xe gas-transfer gradient between the right and left lung. However, no such differences were observed in our study, which may suggest that ventilation is equally increased in these areas of high tissue density. Finally, the increased 129Xe transfer in regions isogravitational with the main pulmonary arteries suggests that those capillary beds may be filled preferentially, even relative to regions experiencing greater gravitational compression.
For supine subjects, 129Xe gas transfer was also affected by the extent of lung expansion. Specifically, 129Xe gas-transfer heterogeneity as measured by whole lung CV was found to be highest for supine subjects with larger TLC, who were therefore imaged at a lower level of lung inflation after inhaling 1 liter of 129Xe. This is consistent with CT studies that have shown almost complete elimination of tissue-density gradients when the lung is fully expanded to TLC (35). By contrast, lower lung inflation may lead to alveolar derecruitment and small airway closure (38), which could contribute to the increased CV in subjects with higher TLC. However, for prone subjects, 129Xe transfer CV did not correlate with lung volume (r = 0.2), which may reflect more uniform lung expansion in that position (12).
Gas-transfer matching.
Although 129Xe gas-transfer images exhibited significant heterogeneity and varying gradient patterns, the mean 129Xe gas-transfer ratio for all the subjects, regardless of posture, was ∼1. This finding is noteworthy considering that 129Xe gas-transfer values in a given gravitational plane ranged from as low as 0.6 to as high as 1.4 and exhibited an even greater range at the voxel level. Much like the well-established ventilation-to-perfusion ratio, the 129Xe gas-transfer ratio appears to exhibit a wide range of values in healthy subjects and, yet, to retain nearly perfect “matching” when averaged over the entire lung. The substantial regional variability in 129Xe gas transfer suggests that the healthy lung at rest possesses significant “reserve capacity,” a notion that has also been put forth to explain why stereology-based estimates of pulmonary diffusing capacity consistently predict diffusing capacities that exceed the experimentally measured values (4, 37). In fact, pulmonary diffusing capacity of CO (DlCO) is known to increase with cardiac output (15). Thus it could be intriguing to conduct 129Xe gas-transfer MRI in subjects undergoing exercise-induced or physiological stress, because these conditions should make the perfusion-dependent capillary bed filling more uniform and would be expected to result in correspondingly uniform 129Xe gas-transfer maps and to exhibit a narrower range.
Technical aspects of radial 129Xe gas-transfer imaging.
Although not a primary focus of this work, the MR acquisition approach developed here represents a significant technical extension of prior human dissolved-phase imaging studies (3, 21). By employing a radial, center-out k-space trajectory, we enable submillisecond TE imaging that largely overcomes the short dissolved-phase 129Xe of ∼2 ms (22) and retains greater SNR, despite the relatively modest 129Xe polarization (∼8–10%). Furthermore, this 3D acquisition is intrinsically isotropic and robust against the undersampling of k-space (29), and the dense oversampling of the center of k-space ensures that the images are largely unaffected by the motion of the heart (10). This approach does require overcoming two challenges compared with the approach of Mugler et al. (21), who deliberately excited both phases together. The interleaved acquisition described here requires twice as many RF excitations and readouts to individually acquire each ray of k-space for gas- and dissolved-phase 129Xe, but the potentially longer acquisition time is offset by undersampling k-space and by using shorter TE afforded by the radial sequence.
A second challenge is the need to apply highly selective RF excitation pulses to avoid exciting the 50-fold larger gas-phase magnetization pool to minimize off-resonance artifacts. This requirement is confounded by the nonlinear response of the RF amplifiers used in MRI systems that tend to distort the prescribed shape of the selective pulse and cause undesired off-resonance excitation. As outlined in the appendix, an adequate solution was developed by characterizing the off-resonance excitation profile of the scanner to empirically determine a pulse length and a power that minimizes off-resonant excitation while providing the large flip angle needed for dissolved-phase 129Xe MRI.
The interleaved radial acquisition approach outlined here is also well suited to aid in the interpretation of the underlying gas-exchange physiology. Creation of 3D images of both distributions in a single breath, with short TE, enables casting of dissolved- and gas-phase distributions into a unified map of 129Xe gas transfer. This approach for calculating regional 129Xe gas transfer has the further technical advantage of eliminating most coil-induced B1 inhomogeneity, because to first order, it affects gas- and dissolved-phase 129Xe residing in a given image voxel equally. Moreover, the short TE enabled by radial imaging reduced SNR differences caused by the 10-fold longer of gas-phase relative to dissolved-phase 129Xe, which simplifies the quantification of 129Xe gas-transfer images.
Conclusions.
This work has demonstrated the feasibility of acquiring 3D, isotropic images of gas- and dissolved-phase HP 129Xe in a single breath. This acquisition, coupled with appropriate normalization of the gas- and dissolved-phase 129Xe images, enables mapping of regional 129Xe gas transfer from air space to pulmonary blood and tissues and, thus, provides a surrogate for studying the regional distribution O2 transfer in the lungs. This study has confirmed our primary hypothesis that 129Xe transfer in the healthy lung at rest is heterogeneous and favors the gravitationally dependent lung. Although the distribution of 129Xe gas transfer can be characterized by a linear gravitational gradient that reverses when subjects are imaged prone, rather than supine, the posterior-anterior variation distribution is distinctly nonmonotonic, with particularly increased values in the plane of the main pulmonary arteries. In aggregate, this study points to the notion that healthy individuals possess a significant reserve capacity for gas transfer, which results in its heterogeneous distribution under basal conditions.
Given that 129Xe gas transfer from air space into blood follows the same pathway into the blood as O2 and standard test gases such as CO, it is tempting to consider whether 129Xe gas-transfer MRI could develop into the regional equivalent of a DlCO examination (15). There are enough similarities to warrant this comparison, but there are also sufficient differences to warrant caution. For example, pathologies such as emphysema, which may lower the alveolar surface area, will reduce the CO uptake and similarly reduce 129Xe gas transfer in those areas. Similarly, DlCO increases with increasing capillary blood volume (16), and 129Xe gas-transfer signal should increase similarly.
However, a key difference with physiological gases is that 129Xe is a freely diffusible gas that binds only transiently to hemoglobin (33), meaning that, unlike O2 or CO, 129Xe gas transfer does not involve a reactive conductance, nor is 129Xe gas-transfer MRI a measurement of physical 129Xe uptake. Rather, it is a measure of dissolved 129Xe magnetization in a pseudo steady state with magnetization in the air spaces and, to first order, should be purely perfusion-limited. This stands in contrast to a measurement such as DlCO, which is diffusion-limited and is known to diminish in the presence of fibrosis or alveolitis (17). Under conditions of inflammation or increased barrier thickness, 129Xe gas transfer could actually increase, because more tissue is present to take up 129Xe. Hence, although the similarities between 129Xe gas transfer and DlCO are tantalizing, significant additional work must be done to fully characterize their relationships.
These limitations point to the future value of further separating the dissolved 129Xe signal into its parenchymal tissue and RBC components (6), the groundwork for which has been laid by the acquisition methodology introduced here. Recently, extraordinary progress in such separation was made by Qing et al. (27), paving a path toward direct imaging of conditions where increased thickness of barrier tissue impairs diffusive transport of 129Xe to the RBCs. As a final note, because 129Xe gas-transfer MRI uses no ionizing radiation, this single-breath imaging approach is well suited to repeated studies and could lead to a better understanding of pulmonary gas-exchange distribution in healthy subjects and individuals with pulmonary disease.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant 1R01 HL-105643 and partly by the Center for In Vivo Microscopy National Institute of Biomedical Imaging and Bioengineering Grant P41 EB-015897, Duke Clinical and Translational Science Award UL1 RR-024128 from the National Center for Research Resources, and National Institute of Allergy and Infectious Diseases Grant AI-081672.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.K., Z.I.C., and B.D. are responsible for conception and design of the research; S.S.K., M.S.F., J.D., J.S., R.S.V., S.H.R., M.H., K.T.K., and B.D. performed the experiments; S.S.K. analyzed the data; S.S.K., M.S.F., Z.I.C., S.H.R., W.M.F., and B.D. interpreted the results of the experiments; S.S.K. and Z.I.C. prepared the figures; S.S.K. drafted the manuscript; S.S.K., M.S.F., Z.I.C., R.S.V., S.H.R., M.H., K.T.K., W.M.F., H.P.M., and B.D. edited and revised the manuscript; S.S.K., M.S.F., Z.I.C., J.D., J.S., R.S.V., S.H.R., M.H., K.T.K., W.M.F., H.P.M., and B.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Gary Cofer for advice on pulse-sequence programming, Jerry Dahlke for MR hardware expertise, and Sally Zimney for proofreading the manuscript.
Appendix
Minimizing off-resonance excitation.
As discussed by Cleveland et al. (3), production of dissolved-phase 129Xe images without off-resonance artifacts requires selective excitation of the dissolved 129Xe with a relatively large flip angle (∼10–20°), without excitation of the much larger gas-phase 129Xe magnetization pool. This selectivity must be achieved using an RF excitation pulse that is sufficiently brief to limit the signal losses caused by the short (∼2 ms) of dissolved-phase 129Xe (22). Although the dissolved-phase 129Xe resonance frequency is separated from the gas phase by ∼3.8 kHz at 1.5 T, avoidance of gas-phase excitation remains challenging because of the nonlinearity of the broadband RF amplifiers on most MRI scanners, which can cause significant gain-dependent warping of the desired RF pulse shape (2). Therefore, we empirically characterized the excitation profile to identify the RF pulse duration and transmit power that minimized unwanted gas-phase 129Xe excitation.
Characterization was performed by placement of a 1-liter ALTEF bag containing HP 129Xe in the coil and application of RF excitation pulses 3.832 kHz above gas-phase 129Xe resonance, where the 129Xe RBC resonance would occur in vivo. Because the bag phantom exhibits no dissolved-phase signal, any 129Xe signal results from unwanted gas-phase 129Xe excitation and measures the degree of RF pulse imperfection. The approach, which is illustrated in Fig. 9A, used a three-lobe windowed sinc pulse, ranging in duration from 1 to 1.5 ms and swept over an 11- to 388-W range of RF power. As shown in Fig. 9B, for pulses <1.2 ms, the gas-phase 129Xe excitation increased with RF power before slightly decreasing at the highest powers. However, for the 1.2-ms pulse, a narrow band of RF power exists where gas-phase excitation is minimized. As the pulse duration was increased to 1.4 ms, off-resonance excitation was somewhat diminished and the local minimum shifted to a lower power.
Fig. 9.
Characterization of off-resonant excitation. A: schematic showing the method used to map the off-resonance excitation pattern. To mimic selective dissolved-phase excitation using a 1-liter bag of HP 129Xe gas, 3-lobed sinc pulses of varying amplitudes (flip angles) were used to pulse 3.832 kHz above the gas-phase peak (on the RBC resonance). B: gas-phase signal intensity resulting from off-resonance excitation using various radio-frequency flip angles and pulse lengths. The 1,200-μs sinc pulse showed a reduction in the gas-phase excitation with a flip angle of ∼22°, and this pulse length and flip angle were chosen as optimum for all experiments.
RF characterization was then repeated in three healthy volunteers to confirm that the same optimum occurs in vivo. On the basis of these tests, all dissolved-phase imaging results were acquired with a 1.2-ms sinc pulse at 244 W, which resulted in a flip angle of ∼22°. Under these conditions, the unwanted gas-phase excitation was ∼20% of the dissolved-phase excitation and provided the optimal balance between high-frequency selectivity needed to excite the dissolved-phase without gaseous contamination and a short pulse length needed to combat the short of dissolved 129Xe.
REFERENCES
- 1.Baryishay E, Hyatt RE, Rodarte JR. Effect of heart-weight on distribution of lung surface pressures in vertical dogs. J Appl Physiol 61: 712–718, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Chan F, Pauly J, Macovski A. Effects of RF amplifier distortion on selective excitation and their correction by prewarping. Magn Reson Med 23: 224–238, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kaushik SS, Kraft M, Wolber J, Kelly KT, McAdams HP, Driehuys B. Hyperpolarized Xe-129 MR imaging of alveolar gas uptake in humans. PLos One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crapo JD, Crapo RO. Comparison of total lung diffusion capacity and the membrane component of diffusion capacity as determined by physiologic and morphometric techniques. Respir Physiol 51: 183–194, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Driehuys B, Cates GD, Miron E, Sauer K, Walter DK, Happer W. High-volume production of laser-polarized Xe-129. Appl Phys Lett 69: 1668–1670, 1996 [Google Scholar]
- 6.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized Xe-129 MRI. Proc Natl Acad Sci USA 103: 18278–18283, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driehuys B, Martinez-Jimenez S, Cleveland ZI, Metz GM, Beaver DM, Nouls JC, Kaushik SS, Firszt R, Willis C, Kelly KT, Wolber J, Kraft M, McAdams HP. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized Xe-129 MR imaging in healthy volunteers and patients. Radiology 262: 279–289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer MC, Spector ZZ, Ishii M, Yu J, Emami K, Itkin M, Rizi R. Single-acquisition sequence for the measurement of oxygen partial pressure by hyperpolarized gas. MRI Magn Reson Med 52: 766–773, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Glenny RW. Determinants of regional ventilation and blood flow in the lung. Intensive Care Med 35: 1833–1842, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 28: 275–289, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Grassino AE, Forkert L, Anthonisen NR. Configuration of chest wall during increased gravitational stress in erect humans. Respir Physiol 33: 271–278, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Hoffman EA. Effect of body orientation on regional lung expansion: a computed tomography approach. J Appl Physiol 59: 468–480, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Holmes JH, Korosec FR, Du J, Sorkness RL, Grist TM, Kuhlman JE, Fain SB. Imaging of lung ventilation and respiratory dynamics in a single ventilation cycle using hyperpolarized He-3 MRI. J Magn Reson Imaging 26: 630–636, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai T, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the slinky effect. J Appl Physiol 103: 240–248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest 122: 1774–1783, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hughes JM, Bates DV. The carbon monoxide diffusing capacity (DlCO) and its membrane (D-M) and red cell (θV̇c) components. Respir Physiol Neurobiol 138: 115–142, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hughes JM, Lockwood DN, Jones HA, Clark RJ. Dlco/Q̇ and diffusion limitation at rest and on exercise in patients with interstitial fibrosis. Respir Physiol 83: 155–166, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RL, Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol 15: 893–902, 1960 [DOI] [PubMed] [Google Scholar]
- 19.Jolliet P, Bulpa P, Chevrolet JC. Effects of the prone position on gas exchange and hemodynamics in severe acute respiratory distress syndrome. Crit Care Med 26: 1977–1985, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Kaushik SS, Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kraft M, Auffermann W, Wolber J, McAdams HP, Driehuys B. Diffusion-weighted hyperpolarized Xe-129 MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65: 1155–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugler JP, Altes TA, Ruset IC, Dregely IM, Mata JF, Miller GW, Ketel S, Ketel J, Hersman FW, Ruppert K. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci USA 107: 21707–21712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugler JP, Altes TA, Ruset IC, Miller GW, Mata JF, Qing K, Tsentalovich I, Hersman FW, Ruppert K. Image-based measurement of for dissolved-phase Xe129 in the human lung. Proc Int Soc Magn Reson Med. In press [Google Scholar]
- 23.Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol 93: 1841–1851, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Patz S, Muradyan I, Hrovat MI, Dabaghyan M, Washko GR, Hatabu H, Butler JP. Diffusion of hyperpolarized Xe-129 in the lung: a simplified model of Xe-129 septal uptake and experimental results. New J Phys 13: 2011 [Google Scholar]
- 25.Petersson J, Rohdin M, Sanchez-Crespo A, Nyren S, Jacobsson H, Larsson SA, Lindahl SG, Linnarsson D, Neradilek B, Polissar NL, Glenny RW, Mure M. Posture primarily affects lung tissue distribution with minor effect on blood flow and ventilation. Respir Physiol Neurobiol 156: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Prisk GK, Yamada K, Henderson AC, Arai TJ, Levin DL, Buxton RB, Hopkins SR. Pulmonary perfusion in the prone and supine postures in the normal human lung. J Appl Physiol 103: 883–894, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing K, Ruppert K, Jiang Y, Mata JF, Miller GW, Shim YM, Wang C, Ruset IC, Hersman FW, Altes TA, Mugler JP. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP. Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes: initial experience. Radiology 222: 252–260, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Scheffler K, Hennig J. Reduced circular field-of-view imaging. Magn Reson Med 40: 474–480, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Song JY, Liu YH, Gewalt SL, Cofer G, Johnson GA, Liu QH. Least-square NUFFT methods applied to 2-D and 3-D radially encoded MR image reconstruction. IEEE Trans Biomed Eng 56: 1134–1142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studholme C, Hill DL, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit 32: 71–86, 1999 [Google Scholar]
- 32.Tawhai MH, Nash MP, Lin CL, Hoffman EA. Supine and prone differences in regional lung density and pleural pressure gradients in the human lung with constant shape. J Appl Physiol 107: 912–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilton RF, Kuntz ID. Nuclear magnetic-resonance studies of Xe-129 with myoglobin and hemoglobin. Biochemistry 21: 6850–6857, 1982 [DOI] [PubMed] [Google Scholar]
- 34.van Beek EJ, Wild JM, Kauczor HU, Schreiber W, Mugler JP, de Lange EE. Functional MRI of the lung using hyperpolarized 3-helium gas. J Magn Reson Imaging 20: 540–554, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Verschakelen JA, Vanfraeyenhoven L, Laureys G, Demedts M, Baert AL. Differences in CT density between dependent and nondependent portions of the lung: influence of lung volume. Am J Roentgenol 161: 713–717, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Virgincar RS, Cleveland ZI, Kaushik SS, Freeman MS, Nouls J, Cofer G, Martinez-Jimenez S, He M, Kraft M, Wolber J, McAdams HP, Driehuys B. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR Biomed. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weibel ER. What makes a good lung? The morphometric basis of lung function. Swiss Med Wkly 139: 375–386, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Melo MF. Effect of regional lung inflation on ventilation heterogeneity at different length scales during mechanical ventilation of normal sheep lungs. J Appl Physiol 113: 947–957, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West JB, Dollery CT. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive CO2. J Appl Physiol 15: 405–410, 1960 [DOI] [PubMed] [Google Scholar]
- 40.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung: relation to vascular and alveolar pressures. J Appl Physiol 19: 893–898, 1964 [DOI] [PubMed] [Google Scholar]