Abstract
The regenerative response of skeletal muscle to mechanically induced damage is impaired with age. Previous work in our laboratory suggests this may result from higher proinflammatory signaling in aging muscle at rest and/or a greater inflammatory response to damage. We, therefore, assessed skeletal muscle proinflammatory signaling at rest and 24 h after unaccustomed, loaded knee extension contractions that induced modest muscle damage (72% increase in serum creatine kinase) in a cohort of 87 adults across three age groups (AGE40, AGE61, and AGE76). Vastus lateralis muscle gene expression and protein cell signaling of the IL-6 and TNF-α pathways were determined by quantitative PCR and immunoblot analysis. For in vitro studies, cell signaling and fusion capacities were compared among primary myoblasts from young (AGE28) and old (AGE64) donors treated with TNF-α. Muscle expression was higher (1.5- to 2.1-fold) in AGE76 and AGE61 relative to AGE40 for several genes involved in IL-6, TNF-α, and TNF-like weak inducer of apoptosis signaling. Indexes of activation for the proinflammatory transcription factors signal transducer and activator of transcription-3 and NF-κB were highest in AGE76. Resistance loading reduced gene expression of IL-6 receptor, muscle RING finger 1, and atrogin-1, and increased TNF-like weak inducer of apoptosis receptor expression. Donor myoblasts from AGE64 showed impaired differentiation and fusion in standard media and greater NF-κB activation in response to TNF-α treatment (compared with AGE28). We show for the first time that human aging is associated with muscle inflammation susceptibility (i.e., higher basal state of proinflammatory signaling) that is present in both tissue and isolated myogenic cells and likely contributes to the impaired regenerative capacity of skeletal muscle in the older population.
Keywords: skeletal muscle, inflammation, aging
it is clear that skeletal muscle regenerative capacity declines with advancing age, as shown consistently following overt muscle damage (12, 27). While the root cause(s) is not well understood, it seems likely that well-documented changes in the molecular profile of aging skeletal muscle (39, 43), alterations in the population and phenotype of resident stem cells (6, 8), and aging changes in systemic factors (e.g., cytokines, growth factors) (12) are all contributors. Regardless of the mechanism(s), impaired muscle regenerative capacity is of particular importance among older individuals suffering trauma or undergoing a surgical procedure (e.g., hip replacement, open heart surgery) that induces marked damage to the surrounding skeletal muscles. Compromised regeneration in these older patients may explain the inability to restore mobility function (35) and muscle mass (31) several months to years later. Skeletal muscle regeneration is dependent on resident muscle stem (i.e., satellite) cell proliferation, differentiation, and fusion to form replacement myofibers and/or to donate myonuclei to damaged but surviving fibers, and impairment in any of these processes leads to diminished regeneration. Heightened proinflammatory signaling with advancing age, through cytokines and stress signaling proteins, may contribute to a reduction in myogenesis (12), while upregulated Wnt signaling tips the balance toward fibrogenesis (9).
Using human genomic microarrays, we previously identified candidate pathways altered in the muscles of older adults that might contribute to the blunted regenerative response (39). Two such pathways are those of the putative inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin (IL)-6. TNF-α and IL-6 exert their effects primarily through the transcription factors nuclear factor-κB (NF-κB) and signal transducer and activator of transcription-3 (STAT3), respectively. NF-κB and STAT3 activate numerous proinflammatory and proteolytic genes. In fact, NF-κB is one of the most potent proinflammatory molecules in skeletal muscle. Aberrant NF-κB activation in skeletal muscle can prevent myogenic differentiation necessary for regeneration and can contribute to muscle atrophy by increasing the activity of molecules involved in muscle protein degradation (24). While the role of IL-6 in skeletal muscle regeneration is not yet fully understood, chronic overexpression of IL-6 leads to skeletal muscle atrophy (19). Increased expression of muscle RING finger 1 (MuRF1) by TNF-α promotes muscle protein degradation (1), while IL-6-induced increases in atrogin-1 can also promote atrophy and blunt muscle growth (4, 7). Another potential mediator of skeletal muscle regeneration identified by our microarray analysis was that of the TNF-like weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14). TWEAK was recently identified as a powerful inducer of skeletal muscle atrophy (14). Despite the knowledge that these cytokine signaling pathways are influential in regulating skeletal muscle mass, their potential role in the regeneration impairment of older muscle has not been resolved.
Based on research findings to date from our laboratory and others, our working hypothesis is that a major cause of regenerative impairment in aging muscle is an exaggerated response to inflammatory mediators, or what we call “muscle inflammation susceptibility, or MuIS”. This means that, for a given concentration of cytokines in the circulation or local interstitial compartment, the muscles of old (vs. young) suffer heightened and prolonged proinflammatory signaling that disrupts the local environment, leading to failed myogenesis. To begin to test this working model in humans, here we examined age differences in the skeletal muscle molecular and cell signaling responses to muscle damage. In an effort to most effectively test the concept of MuIS, we utilized a damage model that enabled us to standardize the insult across age groups, and to titrate it in such a way that damage was only modest (to avoid overt proinflammatory activity that might mask proposed age differences). The chosen model was mechanical load-mediated damage in the form of high-intensity, concentric and eccentric muscle contractions against standardized resistance. To gain insight into a potential aging time course for the progression of MuIS, we tested three age groups with mean ages of 40, 61, and 76 yr. To further test our MuIS hypothesis, we isolated primary human myoblasts from the vastus lateralis of young and old subjects and studied the cellular differentiation and signaling responses of young and old donor myoblasts to increasing doses of TNF-α. The in vitro comparative studies of young vs. old donor cells enabled us to determine whether age differences existed in the myogenic cells' properties (exposed to standardized media).
METHODS
Subjects.
Eighty-seven adults were recruited into three age groups: middle-aged (40.4 ± 1.1 yr, n = 38, 19 women, 19 men), older (61.2 ± 0.6 yr, n = 27, 18 women, 9 men), and old (75.5 ± 0.7 yr, n = 22, 15 women, 7 men). Throughout the paper we refer to these groups as AGE40, AGE61, and AGE76, respectively. Because AGE61 and AGE76 were ∼67% female (while AGE40 was 50% female), we removed potential effects of sex from all between-groups comparisons (see Statistical analysis). All participants completed health history and physical activity readiness questionnaires. Volunteers ≥45 yr of age were also screened by comprehensive physical exam and a graded exercise stress test with 12-lead ECG. Subjects were excluded for a history of resistance training, musculoskeletal or other disorder that might influence testing or risk of injury, obesity (body mass index ≥ 30), or any current medications that might influence test results. The study was approved by the Institutional Review Boards of the University of Alabama at Birmingham and the Birmingham Veterans Affairs Medical Center, and all subjects provided written, informed consent in accordance with the Declaration of Helsinki before participation.
Body composition and muscle mass.
Dual-energy X-ray absorptiometry (Lunar Prodigy model no. 8743; GE Lunar, Madison, WI) was performed to determine total body lean mass, limb (bilateral arm + leg) muscle mass, thigh muscle mass (TMM), and body fat percentage using enCORE 2002 software (version 6.10.029), according to the manufacturer's instructions. Measures of muscle mass were normalized to height for standardization across subjects. Skeletal muscle index was calculated as limb muscle mass (kg)/height (m)2. Bilateral TMM (kg) was also adjusted by height (m)2, and we refer to this adjusted value as TMM. Unilateral knee extension strength of the right leg was normalized to muscle mass of the right thigh, and we refer to the latter as unilateral TMM (kg).
Muscle strength and power measurements.
Bilateral and unilateral (right leg) knee extension maximum isometric voluntary contraction (MVC) forces were measured on a dynamometer (Body Solid, Forest Park, IL) using a calibrated load cell (Omegadyne, Sunbury, OH), as described previously (30). Knee extension peak concentric power was determined with an external load equal to 40% of MVC force and using electrogoniometry (model SG150, Biometrics, Gwent, UK) to measure velocity, as described previously (29, 30). Strength and power results were adjusted for TMM to yield relative values.
Mechanically induced muscle damage and tissue collection.
The resistance loading (RL) protocol we used to induce modest muscle damage was described in detail previously (39). Briefly, subjects performed 9 sets × 10 repetitions of unaccustomed, dynamic, bilateral knee extensions against a resistance load equal to 40% MVC, which we have found to be ∼65% of one-repetition maximum strength (39). Contraction intensity is maximized by instructing the subjects to perform the concentric phase of each repetition as rapidly as possible (“explosive”), followed by a controlled eccentric lowering phase. Muscle biopsies and 10-ml blood draws were performed in the morning, fasted state at rest and 24 h after RL. Muscle samples were obtained under local anesthetic (1% lidocaine) from the vastus lateralis by percutaneous needle biopsy. The contralateral limb was used for the post-RL biopsy. Muscle samples were snap frozen in liquid nitrogen and stored at −80°C. Serum was isolated from whole blood by centrifugation and stored at −80°C. To verify muscle damage, we assessed serum creatine kinase (CK) and myoglobin activity before and 24 h after RL. CK activity was determined by a standard enzymatic rate method in the University of Alabama at Birmingham Hospital Chemistry Laboratory with the SYNCHRON DxC 800 system (Beckman-Coulter, Brea, CA), and myoglobin levels were measured using a commercially available ELISA kit (Calbiotech, San Diego, CA).
Immunohistochemistry.
Myofiber type distribution (I, IIa, IIx) and type-specific myofiber size were assessed, as previously described (5), via myosin heavy chain (MHC) isoform immunofluorescence microscopy. In brief, 6-μm muscle serial cross sections were fixed in 3% neutral-buffered formalin at room temperature for 45 min, washed in PBS, and blocked with 5% goat serum for 20 min. Anti-MHC type I (NCL-MHCs, NovoCastra Laboratories, 1:100), anti-MHC type IIa (University of Iowa Hybridoma Bank, 1:80), and anti-laminin (VP-L551, NovoCastra Laboratories, 1:80) primary mouse monoclonal antibodies were used to detect type I myofibers, type IIa myofibers, and basal lamina, respectively (type IIx fibers are the remaining, unstained fibers). Images were captured at ×10 using an Olympus BX51 fluorescent microscope with an Olympus MagnaFire SP camera (S99810). Image analysis (myofiber type and size) was performed by a technician blinded to age and sex using Image-Pro Plus 6.0 software.
Muscle protein and RNA isolation.
Muscle samples (∼30 mg) were homogenized after a 15-min preincubation in 6 μl/mg muscle of ice cold lysis buffer with protease and phosphatase inhibitors and then centrifuged at 15,000 g for 40 min at 4°C. Supernatant was stored at −80°C until assayed for protein content using the bicinchoninic acid technique with BSA as a standard. Total RNA was isolated and further purified from frozen muscle samples (∼30 mg) using Tri-Reagent (Molecular Research Center, Cincinnati, OH) and RNeasy Mini Kits (QIAGEN, Valencia, CA), respectively, following the manufacturer's instructions. RNA quantity and quality were determined using a spectrophotometer (NanoDrop ND-1000, Thermo Scientific, Rockford, IL).
Quantitative PCR.
Skeletal muscle transcript levels for 12 target genes known to be involved in muscle inflammation and/or protein breakdown were measured before and 24 h after RL using quantitative RT-PCR (StepOne System, Applied Biosystems, Foster City, CA) on a subset of subjects (limited by tissue availability) (AGE40, n = 13; AGE61, n = 10; AGE76, n = 10). cDNA was synthesized via reverse transcription using the SuperScript VILO cDNA Synthesis kit (Invitrogen, Carlsbad, CA). Specific mRNAs of interest quantified via quantitative PCR [via Taqman Gene Expression Assays (Applied Biosystems)] included the following: atrogin-1 (Hs01041408_m1); cFOS (Hs00170630_m1); IL-6 (Hs00985639_m1); IL-6 receptor (Hs00794121_m1); receptor IL-6 signal transducer (ST)/GP130 (IL-6ST/gp130) (Hs00174360_m1); MuRF1 (Hs00822397_m1); suppressor of cytokine signaling-3 (SOCS3), which is a negative regulator of IL-6 signal transduction (Hs00269575_s1); TNF-α (Hs00174128_m1); TNF receptor 1A (Hs00533560_m1); TNF receptor 1B (Hs00153550_m1); TWEAK (Hs00356411_m1); and TWEAK receptor (Hs0017993_m1). GAPDH (Hs02758991_g1) expression served as internal control; its expression was not significantly different between age groups, nor did it change from pre- to post-RL. All samples were run in triplicate. Relative amounts of target mRNA (i.e., ΔCT values) were determined using the comparative threshold cycle method via StepOne software version 2.2.2 (Applied Biosystems), and all results are displayed as the relative fold difference (i.e., 2−ΔΔCT) compared with AGE40 at baseline.
Myoblast isolation and culture.
To assess MuIS in vitro, we tested the effects of TNF-α on young and old donor myoblasts during growth and differentiation. Myoblasts were isolated from three young (28 ± 2 yr; AGE28) and three old (64 ± 2 yr; AGE64) subjects, according to previously established procedures (20). Briefly, ≈50 mg muscle tissue from the vastus lateralis were weighed, minced, and subjected to pronase digestion at 37°C for 1 h. Following enzymatic digestion, the disassociated muscle solution was passed through a 100-μm filter to remove large debris. The remaining cell mixture was centrifuged, and the pellet was resuspended in DMEM and preplated onto tissue culture plates for 20 min to remove adherent fibroblasts. The supernatant containing myoblasts was collected, centrifuged, and resuspended in Ham's F-12 containing 20% FBS, 5 ng/ml FGF, 100 μl/ml streptomycin, and 100 U/ml penicillin [growth media (GM)]. Myoblasts were grown on collagen I-coated tissue culture plates and were maintained in a 37°C humid atmosphere with 5% CO2. Cells were subcultured and grown to ≈70% confluence on six-well plates before TNF-α treatment. For growth experiments, AGE28 and AGE64 donor myoblasts were treated with 0, 2, 5, or 10 ng/ml human recombinant TNF-α (Cell Signaling Technology, Danvers, MA) in GM for 24 h. In a subset of these subjects (39), and in separate experiments, we have found human circulating TNF-α concentration to be in the range of 2.5–4.5 pg/ml. While the concentrations used in vitro were much higher, we assume that TNF-α concentration within the skeletal muscle interstitium (i.e., in the satellite cell microenvironment) reaches levels following local muscle damage that far exceed circulating levels due to TNF-α release from myofibers and local inflammatory cells (e.g., M1 macrophages).
In differentiation experiments, after 24 h of growth, differentiation was induced by replacing GM with Ham's F-12 containing 2% horse serum, 100 μl/ml streptomycin, and 100 U/ml penicillin [differentiation media (DM)]. Recombinant TNF-α (0, 2, 5, or 10 ng/ml) was added to DM, and cells were allowed to differentiate for 96 h, with fresh DM provided after the first 48 h. At the conclusion of each experiment, cells were washed twice in PBS, and 100 μl of ice-cold RIPA buffer containing protease and phosphatase inhibitors were added. Cells were scraped from each plate, vortexed, and centrifuged at 5,000 g for 5 min at 4°C. The supernatant was collected and stored at −80°C for future protein lysate analysis.
Immunocytochemistry.
To examine the effects of TNF-α treatment on myogenic fusion, cells that had been differentiating for 96 h were stained to visualize myotubes. Cells were fixed in ice-cold methanol for 20 min, washed in PBS, and blocked in 5% goat serum with 0.1% Triton X-100 for 20 min. Cells were incubated in α-tubulin primary antibody (NB120–11304, Novus Biologicals, Littleton, CO; 1 μg/ml) in 5% goat serum overnight at 4°C. In separate experiments, we confirmed the myotubes yielded after 96 h in DM are myosin positive. Cells were then washed in PBS and incubated in secondary antibody (Alexa Fluor 594 goat anti-mouse, Life Technologies, Grand Island, NY; 1:1000) in 1% goat serum for 1 h at room temperature. After washing with PBS, coverslips were mounted with ProLong Gold (Life Technologies) with 4′,6-diamidino-2-phenylindole to label nuclei. Images were captured at ×10 using an Olympus BX51 fluorescent microscope with an Olympus MagnaFire SP camera (S99810). Image analysis was performed using Image-Pro Plus 6.0 software. A single analyst blinded to age and TNF-α treatment of each sample performed all analyses. For measurement of fusion, ≈500 nuclei were counted within random fields of view for each treatment condition (0, 2, 5, and 10 ng/ml TNF-α) for each donor cell population. The total number of nuclei per cell was determined, and the percentage of multinucleated myotubes containing three or more nuclei was calculated to determine fusion index.
Immunoblotting.
Twenty-five micrograms of skeletal muscle mixed protein lysate, or 15 μg of cell protein lysate, were resolved on 4–12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Immunoblotting of muscle tissue protein lysate was performed on a subset of subjects (limited by lysate availability) to examine protein cell signaling of the IL-6 (AGE40, n = 24; AGE61, n = 16; AGE76, n = 19) and TNF-α (AGE40, n = 34; AGE61, n = 20; AGE76, n = 22) pathways. To assess signaling proteins of interest, antibodies against phosphorylated (Ser32) and total IκBα, phosphorylated inhibitor of κB kinase (IKK)-α/β (S180/181), NF-κB p50/p105, phosphorylated (Ser536) and total NF-κB p65, TNF-α receptor 1, phosphorylated (Ser727) and total STAT3, heat shock protein (HSP) 27, and HSP70 were purchased from Cell Signaling Technologies (Danvers, MA) and used at 1:1,000 dilution in 5% goat serum (monoclonal antibodies) or 2% milk + 2% BSA (polyclonal antibodies). Horseradish peroxidase-conjugated secondary antibody (Pierce Thermoscientific, Rockford, IL) was used at 1:50,000 (wt/vol), followed by chemiluminescent detection in a BioRad (Hercules, CA) ChemiDoc imaging system with band densitometry performed using BioRad Quantity One (software package 4.5.1).
Statistical analysis.
Analysis of covariance was used to test main age group effects for each dependent variable at baseline and after RL, adjusting for the potentially confounding effects of sex (as covariate), since AGE61 and AGE76 were ∼67% women, while AGE40 was 50% women. Planned contrasts between specific age groups were performed using Fisher's least significant difference tests. For within-group comparisons, the difference between baseline and 24-h RL response values were compared using paired t-tests. Results for most all human tissue and phenotyping variables (except mRNAs) are expressed as least squares adjusted means + SE (adjusted for sex). Quantitative PCR results are displayed as the sex-adjusted fold difference (i.e., 2−ΔΔCT) relative to AGE40 at baseline, with error bars representing the maximum relative quantity (i.e., maximum 2−ΔΔCT) of a given transcript within age group and time point. For in vitro experiments, a 2 × 4 (age × TNF-α concentration) factorial ANOVA was used to examine differences in protein levels and myogenic fusion between donor ages, followed by Fisher's least significant difference post hoc analysis to localize effects. In vitro results are shown as means + SE. For all tests, P ≤ 0.05 (two-sided) was considered statistically significant.
RESULTS
Aging muscle atrophy phenotype.
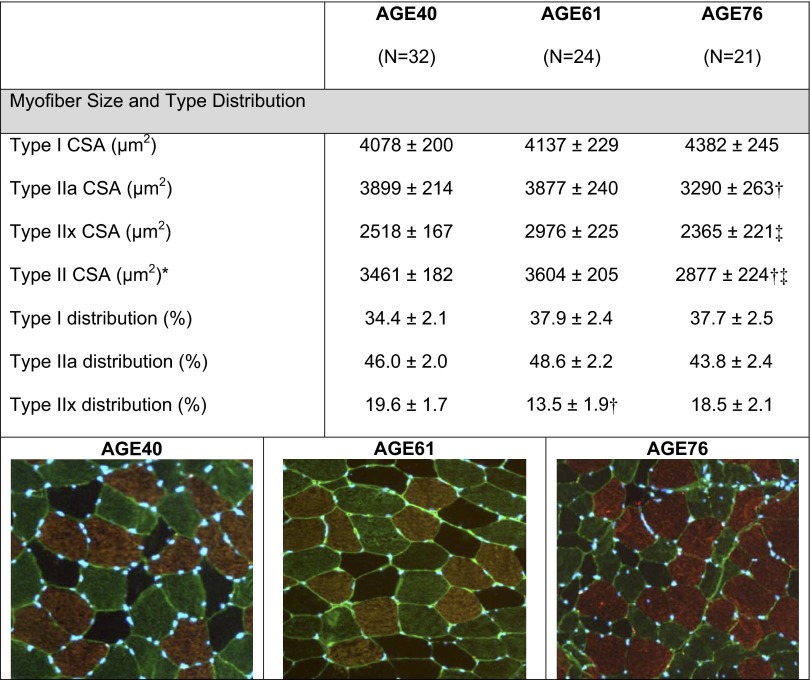
Descriptive characteristics of the age groups can be found in Table 1. We confirmed aging muscle atrophy based on dual-energy X-ray absorptiometry assessments of limb muscle mass normalized to height. Compared with AGE40, skeletal muscle index and TMM were both 9% lower in AGE61 (P < 0.05), while in AGE76 these muscle mass measures were 11 and 13% lower, respectively (P < 0.05). Additionally, the expected type II myofiber atrophy of aging was noted in AGE76 compared with AGE40 (−17%) (P < 0.05; Fig. 1). There were no age group differences in fiber-type distribution between AGE76 and AGE40, although AGE61 had a lower type IIx myofiber distribution compared with AGE40 (P < 0.05; Fig. 1). As expected, age differences in knee extensor strength and power indicate that muscle performance declined with age at a much more precipitous rate than muscle mass. Compared with AGE40, isometric MVC strength was 22% less in AGE61 and 34% lower in AGE76, while knee extension power was 26% and 42% lower in the two older age groups vs. AGE40 (P < 0.05). Absolute strength and power were also significantly lower in AGE76 compared with AGE61 (P < 0.05). Even after adjusting for the involved muscle mass, relative muscle strength and power were lower in the older age group(s) (e.g., relative power was lower in AGE76 and AGE61 vs. AGE40; relative strength was lower in AGE76 vs. AGE40; all P < 0.05).
Table 1.
Descriptive characteristics of each age group
| AGE40 | AGE61 | AGE76 | |
|---|---|---|---|
| n | 38 | 27 | 22 |
| Age, yr | 40.4 ± 1.1 | 61.1 ± 0.6 | 75.5 ± 0.7 |
| Body composition and muscle mass | |||
| BMI, kg/m2 | 25.1 ± 0.4 | 24.7 ± 0.5 | 25.6 ± 0.6 |
| Body fat*, % | 32.4 ± 1.1 | 34.2 ± 1.2† | 36.5 ± 1.4† |
| SMI*, kg/m2 | 7.55 ± 0.11 | 6.87 ± 0.13† | 6.73 ± 0.14† |
| TMM*, kg/m2 | 3.86 ± 0.06 | 3.51 ± 0.70† | 3.35 ± 0.8† |
| Knee extensor strength and power | |||
| Strength (UMVC)*, kg | 55.1 ± 1.6 | 42.8 ± 1.8† | 36.2 ± 2.0†‡ |
| Relative strength (UMVC/UTMM)* | 9.34 ± 0.34 | 8.44 ± 0.40 | 7.37 ± 0.45† |
| Power*, W | 349 ± 14 | 257 ± 17† | 204 ± 19†‡ |
| Relative power*, W/kg TMM | 29.7 ± 1.2 | 24.6 ± 1.4† | 20.8 ± 1.5†‡ |
Values are least squares (adjusted) means ± SE; n, no. of subjects. AGE40, AGE61, AGE76: 40, 61, and 76 yr of age, respectively. BMI, body mass index = weight (kg)/height (m)2; SMI, skeletal muscle index = (bilateral arm + leg lean mass)/height (m)2; TMM, bilateral thigh muscle mass (kg) adjusted by height (m)2; power, maximum bilateral, concentric knee extension power (W); UMVC, unilateral (right) knee extension maximum voluntary contraction strength (kg); UTMM, unilateral thigh muscle mass (kg); TMM, thigh muscle mass.
Main age effect,
different from AGE40, and
different from AGE61: P < 0.05.
Fig. 1.
Myofiber size and type distribution of each age group [40 (AGE40), 61 (AGE61), and 76 yr of age (AGE76)]. CSA, cross-sectional area (μm2). Representative immunohistochemical images: type I = copper; type IIa = green; type IIx = black (negative). Values are least squares (adjusted) means ± SE. *Main age effect, P < 0.05. †Smaller than AGE40, P < 0.005. ‡Smaller than AGE61, P < 0.05.
Muscle damage.
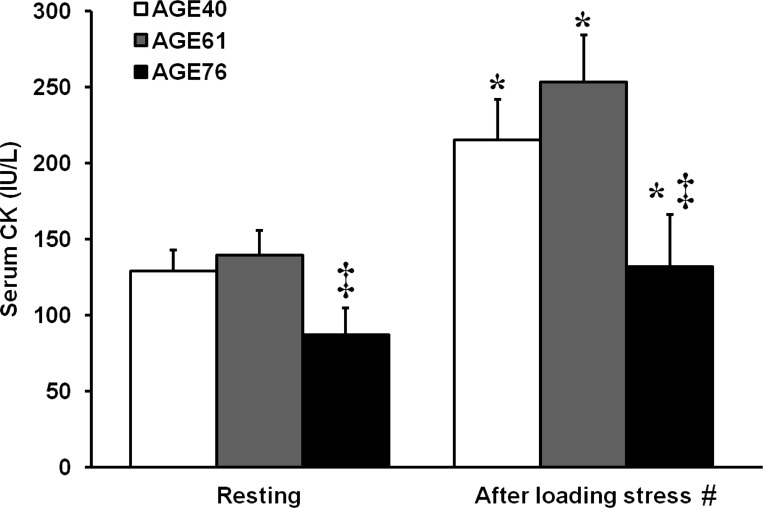
Serum CK is a well-established and reliable surrogate marker of skeletal myofiber membrane wounding (i.e., muscle damage) and is elevated 24–48 h after mechanically induced damage. Serum CK values are shown in Fig. 2. As intended, the standardized RL protocol induced modest muscle damage. Within age groups, serum CK was elevated 24 h after RL in AGE40 (67%, P < 0.001), AGE61 (81%, P < 0.005), and AGE76 (52%, P < 0.001). In a subgroup of AGE40 (n = 9) and AGE76 (n = 10) participants, there were no changes in serum myoglobin concentrations from pre- to 24 h post-RL (AGE40, 24 ± 3 to 31 ± 4 ng/ml; AGE76, 33 ± 5 to 33 ± 3 ng/ml, respectively). Both at rest and after the RL stress, serum CK was lower in AGE76 than in the other two groups, P < 0.05.
Fig. 2.
Serum creatine kinase (CK) activity before and 24 h after unaccustomed knee extensor resistance loading indicating modest muscle damage. Values are least squares (adjusted) means + SE. *Within-age group change in serum CK after resistance loading, P < 0.05. ‡Different from AGE40 and AGE61 at respective time point, P < 0.05. #Main effect of age group, P < 0.05.
Effects of aging and modest muscle damage on IL-6/STAT3 signaling.
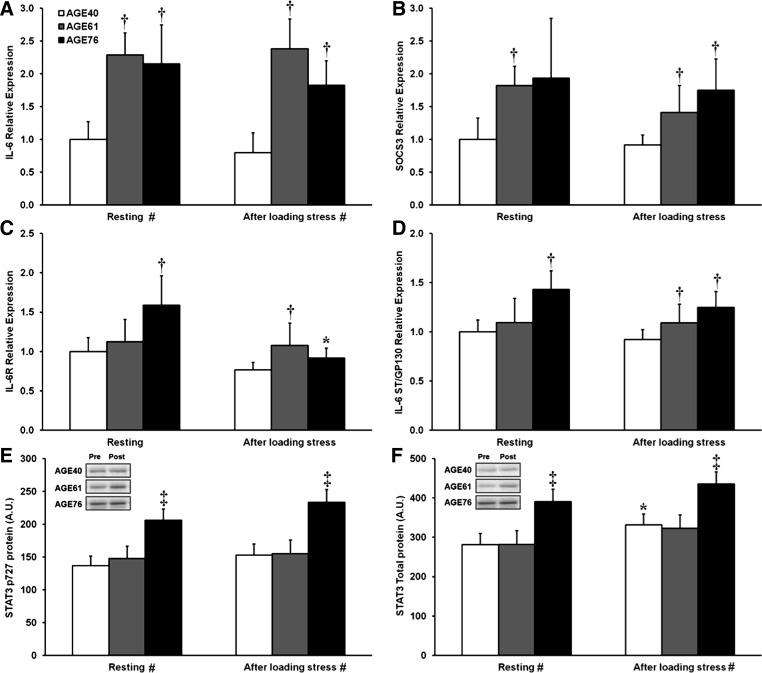
Results for IL-6-related gene expression and protein targets in the IL-6 signaling cascade are shown in Fig. 3. Relative to AGE40, both AGE61 and AGE76 had higher levels (2-fold) of IL-6 mRNA at baseline (resting) and after RL (P < 0.05) (Fig. 3A). At baseline, SOCS3 expression was higher in AGE61 (P < 0.05) and tended to be higher in AGE76 (P = 0.08) compared with AGE40, whereas, after RL, SOCS3 mRNA in both older groups was higher than in AGE40 (P < 0.05) (Fig. 3B). Expression of the IL-6 receptor was higher at rest in AGE76 compared with AGE40 (P < 0.01), whereas, after RL, IL-6 receptor mRNA was higher in AGE61 than AGE40 (P < 0.05) (Fig. 3C). IL-6 receptor expression decreased from pre- to post-RL in AGE76 only (P < 0.001) (Fig. 3C). Compared with IL-6ST/GP130 expression in AGE40, AGE76 had higher baseline expression, and both AGE61 and AGE76 had higher expression after RL (P < 0.05) (Fig. 3D). After binding to the IL-6 receptor, the IL-6 signal is transduced via IL-6ST/GP130 and through phosphorylation and activation of the transcription factor STAT3. While Tyr705 is considered the IL-6-mediated phosphorylation site on STAT3, sequential phosphorylation at Ser727 directs nuclear localization and thus STAT3-induced transcriptional activity. We, therefore, assessed both sites. In both the resting muscle (i.e., baseline) and after RL, AGE76 had a higher STAT3 activation state than the younger groups based on Ser727 (baseline: 51 and 40% higher than AGE40 and AGE61, respectively; after RL: 54 and 51% higher than AGE40 and AGE61, respectively) (Fig. 3E). The same age group differences were found for levels of total STAT3 protein (P < 0.05) (Fig. 3F). RL induced a modest increase in total STAT3 protein in AGE40 only (P < 0.05). No effects were found for STAT3 phosphorylation at Tyr705.
Fig. 3.
Effects of age and unaccustomed resistance loading-induced muscle damage on interleukin (IL)-6/signal transducer and activator of transcription-3 (STAT3) gene expression (A–D) and protein cell signaling (E and F). *Within-age group change 24 h after unaccustomed resistance loading, P < 0.05. †Different from AGE40, P < 0.05. ‡Different from both AGE40 and AGE61, P < 0.05. #Main effect of age group, P < 0.05. In A–D, shown are relative fold differences (i.e., 2−ΔΔCT) compared with AGE40 at baseline, with error bars representing maximum 2−ΔΔCT of a given transcript within age group and time point. In E and F, values are least squares (adjusted) means + SE. AU, arbitrary units; IL-6R, IL-6 receptor; SOCS3, suppressor of cytokine signaling-3; ST, signal transducer.
Effects of aging and modest muscle damage on TNF/NF-κB signaling.
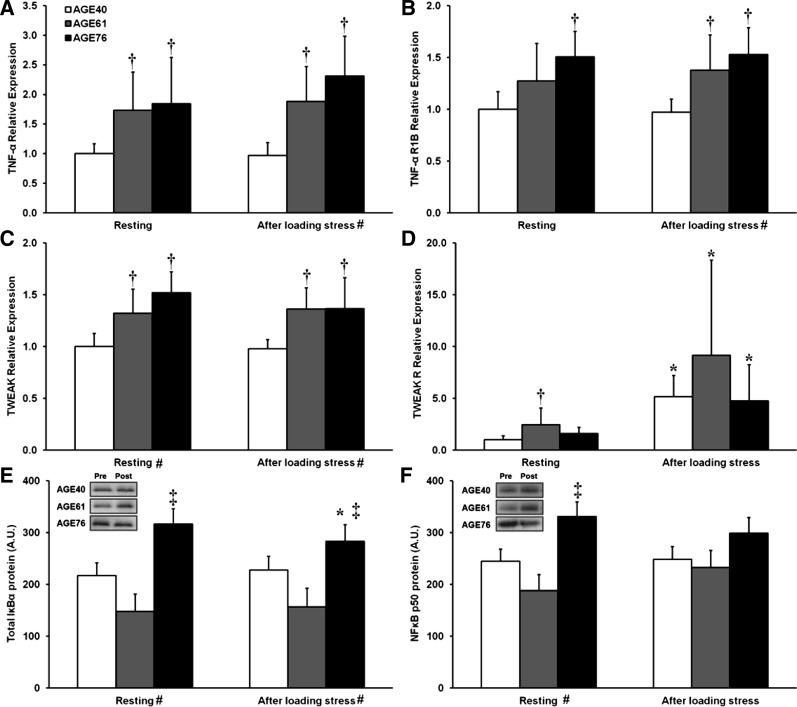
Results for TNF-α-related gene expression and protein analysis of the NF-κB signaling pathway are summarized in Fig. 4. Relative to AGE40, muscle TNF-α mRNA expression was higher in both AGE61 and AGE76 at rest and after RL (≈2-fold) (P < 0.05) (Fig. 4A). TNF receptor 1B was also higher in AGE76 compared with AGE40 at baseline, and higher than AGE40 in both AGE61 and AGE76 after RL (P < 0.05) (Fig. 4B). TWEAK mRNA was ∼40% higher in AGE61 and AGE76 compared with AGE40 at rest and after RL (P < 0.05) (Fig. 4C). Expression of TWEAK receptor, Fn14, was higher in AGE61 compared with AGE40 at baseline (P < 0.01), and expression increased dramatically after RL, up over fivefold in AGE40, over ninefold in AGE61, and nearly fivefold in AGE76 (all P < 0.01) (Fig. 4D). No age or RL effects were found for TNF receptor 1A expression (data not shown). TNF-α signaling is transduced through the classical NF-κB pathway. One axis of NF-κB signaling involves phosphorylation of IKK-α/β, which leads to phosphorylation of the inhibitory IκBα and subsequent release of NF-κB p50, resulting in dimerization (to form either a homodimer, or heterodimer with p65) and nuclear translocation to activate target gene expression. Total IκBα protein in AGE76 was higher than in AGE40 and AGE61 at rest and after RL and decreased after RL (P < 0.05) (Fig. 4E). NF-κB p50 protein content was 35% higher in AGE76 compared with AGE40 and 76% higher compared with AGE61 at baseline (P < 0.05), while no age effects were noted after RL (Fig. 4F). No age effects were found for the phosphorylation states of IKK-α/β and IκBα (data not shown).
Fig. 4.
Effects of age and unaccustomed resistance loading-induced muscle damage on TNF-α/nuclear factor (NF)-κB gene expression (A–D) and protein cell signaling (E and F). *Within-age group change 24 h after unaccustomed resistance loading, P < 0.05. †Different from AGE40, P < 0.05. ‡Different from both AGE40 and AGE61, P < 0.05. #Main effect of age group, P < 0.05. In A–D, shown are relative fold differences (i.e., 2−ΔΔCT) compared with AGE40 at baseline, with error bars representing maximum 2−ΔΔCT of a given transcript within age group and time point. In E and F, values are least squares (adjusted) means + SE. R1B, receptor 1B; TWEAK, TNF-like weak inducer of apoptosis.
Effects of aging and modest muscle damage on atrogenes and stress response proteins.
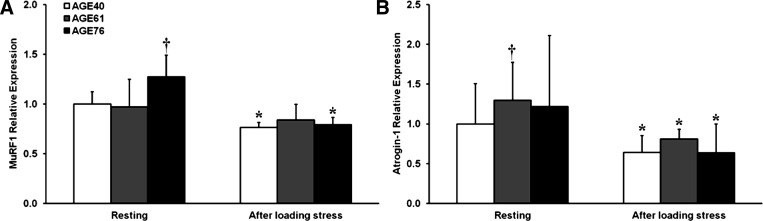
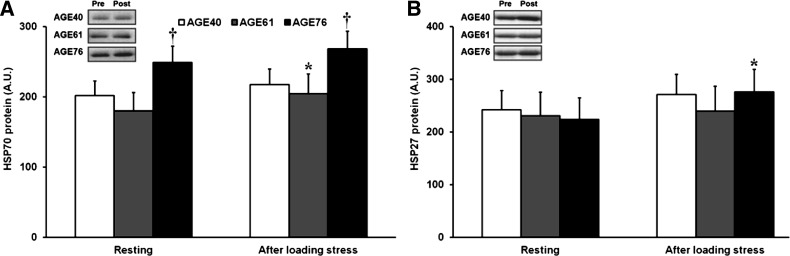
Transcript levels of the skeletal muscle E3 ligases, MuRF1 and atrogin-1, which are typically elevated in atrophy and stress conditions that promote proteolysis, were reduced after RL (Fig. 5). MuRF1 expression was higher in AGE76 compared with AGE40 at baseline (P < 0.05); however, both age groups decreased MuRF1 to similar levels after RL (P < 0.05) (Fig. 5A). Atrogin-1 expression was higher in AGE61 compared with AGE40 at baseline (P < 0.05), and all age groups decreased atrogin-1 expression to similar levels after RL (P < 0.01) (Fig. 5B). Because our RL model was designed to induce modest muscle damage, we also evaluated protein biomarkers of myofiber stress, HSP27 and HSP70. Baseline and post-RL levels of HSP70 were ≈23% higher in AGE76 compared with AGE40 (P < 0.05), and a RL-induced increase in HSP70 in AGE61 was noted (P < 0.05) (Fig. 6A). No age differences existed in HSP27 at baseline, but HSP27 did increase 23% after RL in AGE76 only (P < 0.05) (Fig. 6B).
Fig. 5.
Effects of age and unaccustomed resistance loading-induced muscle damage on atrogene expression. *Within-age group change 24 h after unaccustomed resistance loading, P < 0.05. †Different from AGE40, P < 0.05. Shown are relative fold differences (i.e., 2−ΔΔCT) compared with AGE40 at baseline, with error bars representing maximum 2−ΔΔCT of a given transcript within age group and time point. A: muscle RING finger 1 (MuRF1) relative expression. B: atrogin-1 relative expression.
Fig. 6.
Effects of age and unaccustomed resistance loading-induced muscle damage on heat shock protein (HSP) abundance. A: HSP70. B: HSP27. *Within-age group change 24 h after unaccustomed resistance loading, P < 0.05. †Different from AGE40, P < 0.05. Values are least squares (adjusted) means + SE.
Effects of donor age and TNF-α treatment on human primary myoblast behavior.
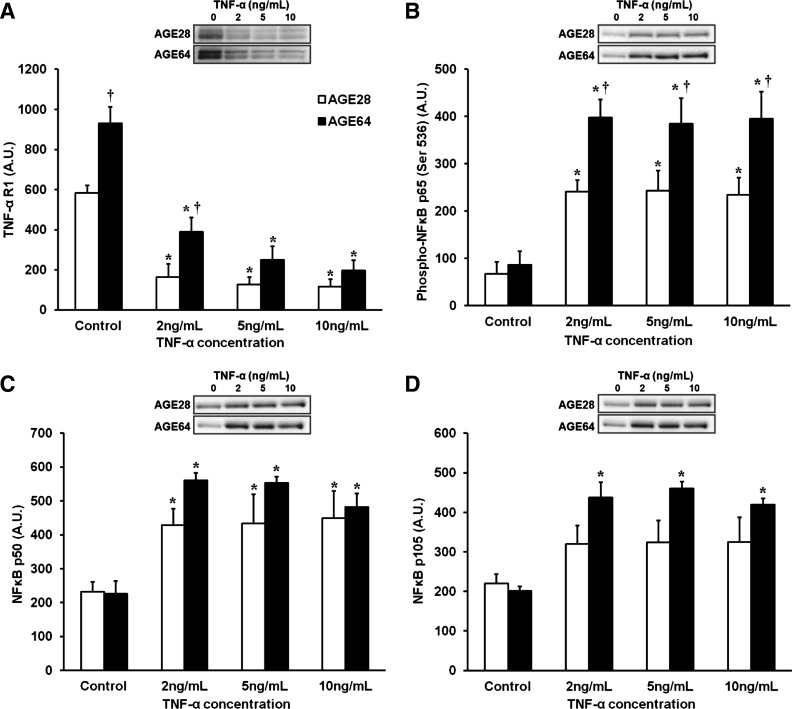
We conducted a series of assays to compare the behavior and inflammation susceptibility of human AGE28 vs. AGE64 donor myoblasts. In standard GM, myoblasts from AGE64 donors expressed a greater abundance of TNF-α receptor 1 protein (+59%, P < 0.001) (Fig. 7A). This age difference remained after 24 h of low-dose (2 ng/mL) TNF-α treatment (+52%, P = 0.01) in GM. Perhaps via negative feedback, in each age group, downregulation of receptor protein expression was noted at each TNF-α treatment dose (P < 0.05). Higher levels of TNF-α receptor protein may largely account for greater TNF-α sensitivity in myoblasts from AGE64 donors, based on heightened activation of the NF-κB pathway at any given TNF-α treatment dose. For example, while NF-κB p65 phosphorylation increased robustly in both AGE28 and AGE64 donor myoblasts in response to TNF-α treatment, the effect was significantly higher in AGE64 (+397%) vs. AGE28 (+270%) myoblasts (P < 0.001) (Fig. 7B). No age or treatment effects were noted for total p65 protein (not shown). A main effect of treatment indicated robustly elevated levels of NF-κB p50 in response to TNF-α (Fig. 7C), and there was a trend toward a greater increase among AGE64 (+147%) vs. AGE28 (+93%) donor myoblasts (P = 0.07). Additionally, increased NF-κB p105 following TNF-α treatment was found only among AGE64 donor myoblasts (+120%, P ≤ 0.001 for all TNF-α concentrations) (Fig. 7D).
Fig. 7.
Effects of 24-h TNF-α treatment on NF-κB signaling in AGE28 and AGE64 donor human myoblasts in growth media. A: TNF-α receptor 1 (R1): main effects of donor age (P < 0.001) and treatment (P < 0.001). A dose-response effect was noted in AGE64 only, as TNF-α R1 content was lower at 10 ng/ml dose compared with 2 ng/ml, P < 0.05. B: phospho-NF-κB p65: main effects of donor age (P < 0.001) and treatment (P < 0.001) and trend toward donor age × treatment interaction, P = 0.054. C: NF-κB p50: main treatment effect, P < 0.001. D: NF-κB p105: main effects of donor age (P < 0.01) and treatment (P < 0.001). Post hoc results in all panels: *Different from control within donor age, P < 0.05. †Different from AGE28 at respective TNF-α treatment dose, P < 0.05. Values are means + SE.
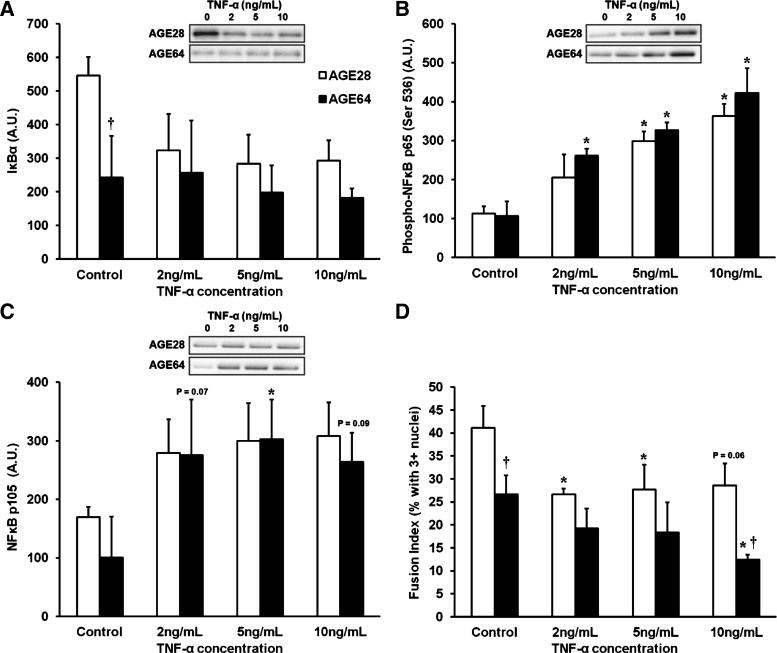
In separate differentiation experiments using the same donor cell populations, myoblasts were differentiated for 96 h in standard DM with and without the various treatment doses of TNF-α. In our hands, after 96 h of differentiation, there is an abundance of myotubes of varying sizes (see fusion index results below); yet many mononucleated myoblasts remain. Immunoblotting results from cells in DM therefore result from cell lysates isolated from a combination of myoblasts/myotubes. After 96 h of differentiation, AGE64 had substantially lower IκBα protein levels (−125%, P < 0.05) than AGE28 without TNF-α treatment, and there was a trend toward AGE64 having lower IκBα protein levels at all TNF-α concentrations compared with AGE28 (age effect, P = 0.053) (Fig. 8A). There was a main effect of TNF-α treatment (P < 0.001) on NF-κB p65 phosphorylation following 96 h of differentiation, and post hoc analysis revealed that only AGE64 significantly increased NF-κB p65 phosphorylation at the lowest dose of TNF-α (2 ng/ml; P = 0.01), and only AGE64 displayed a trend toward further increases in NF-κB p65 phosphorylation from 5 to 10 ng/ml (P = 0.09) (Fig. 8B). Levels of p105 protein tended to rise in the presence of exogenous TNF-α (main treatment effect, P = 0.06) and appeared to increase nearly threefold in AGE64 (Fig. 8C). There were no age or treatment effects for total NF-κB p65, p50, or IKKα/β following differentiation (data not shown).
Fig. 8.
Effects of 96-h TNF-α treatment on NF-κB signaling and myoblast-to-myotube fusion using AGE28 vs. AGE64 donor myoblasts in differentiation media. A: IκBα: trend toward main effect of donor age, P = 0.053. B: phospho-NF-κB p65: main treatment effect, P < 0.001. C: NF-κB p105: trend toward main treatment effect, P = 0.06. D: fusion index: main effects of donor age (P < 0.005) and treatment (P < 0.05). Post hoc results in all panels: *Different from control within donor age, P < 0.05. †Different from AGE28 at respective TNF-α treatment dose, P < 0.05. Trend toward main effect of donor age, P = 0.053. Values are means + SE.
The effects of donor age and TNF-α treatment on the ability of myoblasts to fuse and form multinucleated myotubes were determined after 96 h of differentiation. Overall, AGE64 displayed impaired fusion (% of myotubes with 3+ nuclei) compared with AGE28 (P < 0.01) (Fig. 8D). Fusion was impaired among AGE64 cells, even in the absence of exogenous TNF-α (P < 0.05). TNF-α treatment reduced the fusion capacity of both AGE28 and AGE64 (treatment effect, P < 0.05), and AGE64 donor cells were more sensitive than AGE28 to the fusion-inhibiting effects of the highest TNF-α dose (10 ng/ml) (P < 0.05).
DISCUSSION
Knowledge of the molecular underpinnings responsible for the impaired skeletal muscle regenerative response in aging humans is necessary to improve the course of clinical care following an injury, or surgery common in older adults, that induces marked muscle damage (e.g., trauma, total hip or knee arthroplasty). Age differences in the ability to manage and respond to inflammation within skeletal muscle may be a major cause of this regeneration impairment. In this study, we provide the first combined evaluation of aging on proinflammatory signaling in both human skeletal muscle tissue and human muscle cells in vitro. Results overall indicate that the inflammatory status of muscle rises with advancing age, even in the absence of damage, stress, or heightened extracellular inflammatory mediators, a property we refer to as “muscle inflammation susceptibility,” or MuIS. Furthermore, the detection of greater inflammatory signaling in AGE64 donor myoblasts in the absence of a proinflammatory stimulus (e.g., TNF-α) in the media demonstrated quite clearly that MuIS is driven, at least in part, by changes intrinsic to the aging muscle cell. Exposure to increasing concentrations of TNF-α in vitro indeed differentially affected the cellular behavior and signaling responses of AGE64 vs. AGE28 donor myoblasts and differentiated myotubes. Cells from AGE64 donors were generally more negatively affected by TNF-α treatment with a greater induction of NF-κB signaling and reduced fusion capacity.
In previous work on a subset of the subjects in this report, we found no age differences in the serum levels of IL-6, IL-8, and TNF-α when comparing some of the oldest and youngest subjects both at rest and 24 h after RL-induced muscle damage (39). In light of these prior results, the high levels of inflammatory signaling within the muscles of AGE76 certainly indicate age-related MuIS, and this was supported by the in vitro findings. Since satellite cells are necessary for muscle regeneration, we suspect MuIS with aging could be a significant contributor to the decline in muscle regenerative capacity.
It is well accepted that proinflammatory signaling through both IL-6 and TNF-α pathways has powerful effects on skeletal muscle. In vitro, excess activity of TNF-α, as well as increases in individual signal transducers of the NF-κB pathway, can lead to increased proteolysis, myotube atrophy, and a host of muscle cell-related dysfunctions (36–38, 41). Increased IL-6 and TNF-α have similar effects on individual muscles, as well as on total muscle mass in animal models (11, 19, 21). Strong associations are also evident in humans, and it has been observed that a high level of inflammation is correlated with low muscle mass and decreased muscle function (32–34). Our novel concept of heightened MuIS in the muscles of aging humans is supported by in vitro studies in satellite cells from young vs. aging rats (23).
Elevated muscle TWEAK mRNA in AGE61 and AGE76 relative to AGE40 is an important finding from this study; it is the first report of higher TWEAK expression with advancing age in humans. We are also the first to show a remarkable increase in the expression of TWEAK receptor (i.e., Fn14) detected 24 h after RL, suggesting a delayed or protracted upregulation. These observations could have important implications in the age-associated decline in muscle regenerative responses. The TWEAK/Fn14 system is known to trigger numerous catabolic pathways. Work from the Kumar laboratory has definitively shown the potent effects of elevated TWEAK and Fn14 on myogenic inhibition in vitro (15) and skeletal muscle wasting in mice in vivo (14). Aberrant activation of the system would seemingly have negative consequences on human muscle regeneration.
High muscle transcript levels of IL-6, IL-6 receptor, and IL-6ST/gp130, as well as STAT3 protein activation, certainly suggest MuIS in the muscles of old at rest and most all age group differences in resting muscle remained after RL. Trenerry et al. (40) did not see higher baseline levels in their older group (age 67 yr), and in our hands most IL-6-related markers of MuIS manifested at AGE76, suggesting upregulated IL-6 activity is heightened at advanced ages (i.e., 8th decade). Trenerry et al. (40) found higher STAT3 activation and expression of STAT3-regulated genes in old (vs. young) 2 h after a single bout of resistance exercise (3 sets × 8 repetitions of knee extension). We were somewhat surprised to find no RL-induced increases in IL-6-related signaling and gene expression 24 h after nine sets of RL that induced modest damage. Marked differences in the timing of post-RL biopsy (24 vs. 2 h) is a likely explanation for the differences between the two studies with regard to changes in IL-6-related signaling or gene expression induced by RL.
Age-related MuIS is also supported by TNF-α/NF-κB results in resting muscle tissue. At rest, old expressed much higher transcript levels of TNF-α, TNF receptor (1B), and TWEAK, and protein markers of heightened NF-κB signaling were evident in old (AGE76). Like IL-6/STAT3, most all of the aging differences detected in resting muscle remained after RL. Ligand-mediated activation of TNF-α receptors ultimately leads to phosphorylation and activation of IKK-α/β, which phosphorylates IκBα and releases it from its inhibitory association with NF-κB p50, allowing NF-κB to dimerize and translocate to the nucleus, to then regulate the transcription of numerous genes (24). Interestingly, the phosphorylation states of IKK-α/β and IκBα in resting muscles of AGE61 were significantly lower than both AGE40 and AGE76 (data not shown). It is attractive to speculate that this may serve as a compensatory mechanism to decrease inflammatory signaling in AGE61, despite elevated levels of TNF-α-related gene expression at AGE61 (vs. AGE40), and that this compensatory mechanism is no longer effective in old (AGE76) muscle.
While age-related muscle atrophy is generally not considered a hypercatabolic state (rather, the result of anabolic resistance), we noted subtle elevations in expression of the muscle E3 ligases, or “atrogenes” MuRF1 and muscle-specific ubiquitin ligases muscle atrophy F-box/atrogin-1, in the resting muscle with advancing age. Such small elevations are difficult to interpret, particularly considering that results in aging rats are equivocal (2, 10, 16). The unaccustomed RL bout was used here as a form of mechanical stress to induce modest muscle damage. This generally led to reduced expression of both MuRF1 and atrogin-1. While others have also noted similar downregulation (of atrogin-1) after eccentric damage in humans for as many as 7 days (28), the functional significance is not known. It would be interesting to determine, in future research, the role(s) of these E3 ligases on the elimination of misfolded proteins, etc., during muscle repair.
Overall these in vivo data present compelling evidence for age-related MuIS, and the experiments on cultured myoblasts indicate that age-related MuIS persists independent of the circulating or interstitial milieu. Satellite cells are absolutely necessary for skeletal muscle regeneration following injury, and circulating as well as autocrine/paracrine cytokine signaling can dramatically affect their ability to fuse into damaged fibers or form replacement fibers. It is likely that local cytokine concentrations are severalfold higher than circulating concentrations following muscle damage and may account for a large majority of the proinflammatory signaling within the muscle. We, therefore, treated myoblasts with relatively high doses of TNF-α to reduce myogenic fusion and induce robust increases in NF-κB signaling. Previously, inhibition of NF-κB p65 has been shown to enhance myogenic differentiation in vitro and muscle regeneration in vivo (3, 26), demonstrating the negative role of NF-κB signaling in myogenesis/muscle regeneration. Our present data showing aberrant activation of NF-κB p65 (as well as increases in p50 and p105) in AGE64 myoblasts, along with the reduced fusion capacity of AGE64 vs. AGE28 myoblasts at the highest TNF-α dose, suggest heightened NF-κB signaling plays an important role in age-related MuIS and reduced regeneration.
In addition to heightened activation of the NF-κB pathway in AGE64 vs. AGE28 donor myoblasts in response to TNF-α treatment, we think age differences in levels of key NF-κB signaling proteins in the basal state are equally important indicators of MuIS. For example, in agreement with our muscle tissue gene expression results, AGE64 proliferating myoblasts expressed ∼60% more TNF-α receptor 1 protein than AGE28 myoblasts, which may be a major factor driving heightened NF-κB signaling in old myoblasts at any given concentration of TNF-α. Additionally, differentiated AGE64 donor cells expressed less than one-half the amount of IκBα protein found in AGE28. IκBα is a powerful inhibitor of NF-κB activation; thus AGE64 myoblasts appear to have a lesser ability to squelch NF-κB activity. The effects of IκBα on myogenic differentiation are evident, as expression of an IκBα superrepressor mutant has been shown to inhibit NF-κB activation and enhance myogenesis (17). Both the greater basal TNF-α receptor content and reduced basal IκBα protein content in AGE64 cells likely drive increased NF-κB activation and reduced myogenesis in AGE64 cells at higher doses of TNF-α, as the overall increased NF-κB signaling can reduce myoblast differentiation via reduced expression (18) and destabilization (22) of MyoD, activation of cyclin D1 (17), and increased expression of YY1 (42). The lower levels of IκBα protein in AGE64 vs. AGE28 donor cells are in stark contrast to the high IκBα protein content in whole muscle lysate from AGE76. We speculate that this may be indicative of a compensatory feedback mechanism only noted in vivo.
Some RL-induced changes in muscle were likely missed due to the timing of the single post-RL biopsy (24 h). RL-induced changes in cell signaling and gene expression are of course transient (13, 25), and the time course of RL-mediated effects may differ between young and old. However, among the most compelling findings in this study were the marked differences in gene expression and cell signaling in the resting state, i.e., before RL-induced muscle damage in vivo, and before TNF-α treatment in vitro. In our view, resting state differences shown here for the first time, and across three age groups, clearly establish age-related MuIS. Based on the many known consequences of inflammation on skeletal muscle, this heightened inflammation susceptibility likely contributes to age-associated impairments in the skeletal muscle regenerative response, and as such should be considered when designing and implementing therapeutic strategies for older adults in their recovery from injury or surgery.
GRANTS
This work was supported by a Veterans Affairs Merit Review Grant (M. M. Bamman), National Institute on Aging Grant R01-AG-017896 (M. M. Bamman), and the University of Alabama at Birmingham Center for Clinical and Translational Science (UL1 TR000165).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.M., M.J.S., A.T.-M., and M.M.B. conception and design of research; E.K.M., M.J.S., A.T.-M., D.P.S., D.J.K., and J.-s.K. performed experiments; E.K.M., M.J.S., A.T.-M., S.T.W., J.M.C., D.P.S., S.C.T., D.J.K., J.-s.K., and M.M.B. analyzed data; E.K.M., M.J.S., A.T.-M., S.T.W., J.M.C., D.P.S., S.C.T., D.J.K., J.-s.K., and M.M.B. interpreted results of experiments; E.K.M., M.J.S., A.T.-M., and M.M.B. prepared figures; E.K.M., M.J.S., A.T.-M., S.T.W., J.M.C., and M.M.B. drafted manuscript; E.K.M., M.J.S., A.T.-M., S.T.W., J.M.C., and M.M.B. edited and revised manuscript; E.K.M., M.J.S., A.T.-M., S.T.W., J.M.C., D.P.S., S.C.T., D.J.K., J.-s.K., and M.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to the participants for effort and dedication.
Present address: Edward Merritt, Department of Health, Leisure, and Exercise Science, Appalachian State University, Boone, North Carolina.
REFERENCES
- 1.Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S. Induction of MuRF1 is essential for TNF-alpha-induced loss of muscle function in mice. J Mol Biol 384: 48–59, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, Goldberg AL, Ulfhake B. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem 285: 39597–39608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 180: 787–802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc(−/+) mouse. Pflügers Arch 457: 989–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Barani AE, Durieux AC, Sabido O, Freyssenet D. Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. J Appl Physiol 95: 2089–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol 106: 443–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonavaud S, Thibert P, Gherardi RK, Barlovatz-Meimon G. Primary human muscle satellite cell culture: variations of cell yield, proliferation and differentiation rates according to age and sex of donors, site of muscle biopsy, and delay before processing. Biol Cell 89: 233–240, 1997 [PubMed] [Google Scholar]
- 9.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev 127: 794–801, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43: 120–128, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dalbo VJ, Roberts MD, Hassell SE, Brown RD, Kerksick CM. Effects of age on serum hormone concentrations and intramuscular proteolytic signaling before and after a single bout of resistance training. J Strength Cond Res 25: 1–9, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogra C, Hall SL, Wedhas N, Linkhart TA, Kumar A. Fibroblast growth factor inducible 14 (Fn14) is required for the expression of myogenic regulatory factors and differentiation of myoblasts into myotubes. Evidence for TWEAK-independent functions of Fn14 during myogenesis. J Biol Chem 282: 15000–15010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edstrom E, Altun M, Hagglund M, Ulfhake B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 663–674, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19: 5785–5799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, Cortright RN, Bamman MM, Neufer PD. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med 52: 198–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 56: 49–57, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Langen RC, Van Der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18: 227–237, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Lees SJ, Zwetsloot KA, Booth FW. Muscle precursor cells isolated from aged rats exhibit an increased TNF-alpha response. Aging Cell 8: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med 86: 1113–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lu A, Proto JD, Guo L, Tang Y, Lavasani M, Tilstra JS, Niedernhofer LJ, Wang B, Guttridge DC, Robbins PD, Huard J. NF-kappaB negatively impacts the myogenic potential of muscle-derived stem cells. Mol Ther 20: 661–668, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol 83: 1270–1275, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard A, Vissing K, Overgaard K, Kjaer M, Schjerling P. Expression patterns of atrogenic and ubiquitin proteasome component genes with exercise: effect of different loading patterns and repeated exercise bouts. J Appl Physiol 103: 1513–1522, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Petrella JK, Kim JS, Tuggle SC, Bamman MM. Contributions of force and velocity to improved power with progressive resistance training in young and older adults. Eur J Appl Physiol 99: 343–351, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol 98: 211–220, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Reardon K, Galea M, Dennett X, Choong P, Byrne E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: a pilot study. Intern Med J 31: 7–14, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care 6: 295–299, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 64: 1183–1189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119: 526.e9–526.e17, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Singh JA, Lewallen DG. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J Am Geriatr Soc 58: 2387–2393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145: 4592–4602, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology 147: 4363–4373, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Szalay K, Razga Z, Duda E. TNF inhibits myogenesis and downregulates the expression of myogenic regulatory factors myoD and myogenin. Eur J Cell Biol 74: 391–398, 1997 [PubMed] [Google Scholar]
- 39.Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trenerry MK, Carey KA, Ward AC, Farnfield MM, Cameron-Smith D. Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res 11: 717–724, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J 23: 362–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC. NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol 27: 4374–4387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149–159, 2003 [DOI] [PubMed] [Google Scholar]