Abstract
Controlled mechanical ventilation (CMV) is known to result in rapid and severe diaphragmatic dysfunction, but the recovery response of the diaphragm to normal function after CMV is unknown. Therefore, we examined the time course of diaphragm function recovery in an animal model of CMV. Healthy rats were submitted to CMV for 24–27 h (n = 16), or to 24-h CMV followed by either 1 h (CMV + 1 h SB, n = 9), 2 h (CMV + 2 h SB, n = 9), 3 h (CMV + 3 h SB, n = 9), or 4–7 h (CMV + 4–7 h SB, n = 9) of spontaneous breathing (SB). At the end of the experiment, the diaphragm muscle was excised for functional and biochemical analysis. The in vitro diaphragm force was significantly improved in the CMV + 3 h SB and CMV + 4–7 h SB groups compared with CMV (maximal tetanic force: +27%, P < 0.05, and +59%, P < 0.001, respectively). This was associated with an increase in the type IIx/b fiber dimensions (P < 0.05). Neutrophil influx was increased in the CMV + 4–7 h SB group (P < 0.05), while macrophage numbers remained unchanged. Markers of protein synthesis (phosphorylated Akt and eukaryotic initiation factor 4E binding protein 1) were significantly increased (±40%, P < 0.001, and ±52%, P < 0.01, respectively) in the CMV + 3 h SB and CMV + 4–7 h SB groups and were positively correlated with diaphragm force (P < 0.05). Finally, also the maximal specific force generation of skinned single diaphragm fibers was increased in the CMV + 4–7 h SB group compared with CMV (+45%, P < 0.05). In rats, reloading the diaphragm for 3 h after CMV is sufficient to improve diaphragm function, while complete recovery occurs after longer periods of reloading. Enhanced muscle fiber dimensions, increased protein synthesis, and improved intrinsic contractile properties of diaphragm muscle fibers may have contributed to diaphragm function recovery.
Keywords: controlled mechanical ventilation, diaphragm contractile properties, reloading, inflammation, protein synthesis
in ventilated patients, failure in discontinuing mechanical ventilation (MV) may be due to a variety of factors, but ventilator-induced diaphragmatic dysfunction (VIDD) appears to be a major determinant in this process (16, 20, 28). In this regard, animal studies have consistently demonstrated that prolonged controlled MV (CMV) results in a rapid and time-dependent reduction of the force generated by the diaphragm (6, 13, 32, 33, 48), associated with a reduction in diaphragm fiber dimensions (13, 37), decreased protein synthesis, and increased activity of all the proteolytic systems and oxidative stress in the diaphragm (36, 37). In ventilated patients, twitch transdiaphragmatic pressure has been shown to be reduced (22, 46), and the duration of MV was associated with a progressive (21) and a logarithmic (17) decline in diaphragm force. Prolonged CMV in humans also resulted in diaphragmatic atrophy, with an increase in expression of proteolysis- and atrophy-related genes (24, 25) and activation of the autophagy-lysosome pathway (18).
The recovery response of the diaphragm after MV to the resumption of spontaneous muscle activity may play an important role in weaning from the ventilator. Diaphragm disuse associated with CMV may increase susceptibility to subsequent contraction-induced injury, once respiratory efforts are resumed. According to reloading data of locomotor skeletal muscles (11, 12, 40), a further decrease in muscle force and muscle fiber membrane damage is expected with reloading. Whether this also occurs in the diaphragm has been investigated by Van Gammeren et al. (42). This study showed that 2 h of diaphragm reloading after CMV did not exacerbate diaphragm contractile dysfunction, nor did it induce muscle fiber injury, despite enhanced neutrophil infiltration (42). It is not yet known whether VIDD is reversible, and, if so, the time course for the diaphragm to recover to its normal function has not yet been determined. These data are relevant as they may provide indications on whether diaphragm function recovery is feasible while resuming spontaneous breathing (SB) after MV and whether this is associated with diaphragm injury. But the diaphragm differs in several ways from the other skeletal muscles. In contrast to locomotor muscles, the diaphragm is continuously active, and this would explain why it is particularly sensitive to disuse (e.g., MV) and activity. On the other hand, because the diaphragm is so important for life, it might be protected from reloading-induced injury, as suggested by the data of Van Gammeren et al. (42). Furthermore, active contraction of the diaphragm after a period of MV seems to be potent in slowing down the deleterious effects of disuse on the diaphragm, as shown with intermittent SB trials applied during MV in animals (14). Even a relatively short period of SB was associated with beneficial effects (14), suggesting that the diaphragm response to active contraction is fast. Taken together, these data suggest that reloading the diaphragm with SB after MV might result in progressive improvement of diaphragm force with no further damage, and the time scale for the diaphragm to full recovery might be rapid.
The aim of this study was to investigate the time course of diaphragm function recovery after CMV in an animal model and to examine the potential mechanisms responsible for diaphragm function recovery. We hypothesized that VIDD is reversible, and recovery would be quick and occur far more rapidly than in locomotor skeletal muscles, knowing the particularities of the diaphragm muscle. To address this issue, healthy rats were submitted to 24 h of CMV followed by 1, 2, 3, or 4 up to 7 h of SB. Function, size, and type of muscle fibers and inflammatory cell infiltration were measured in the diaphragm. Whether oxidative stress, proteolysis, protein synthesis, or intrinsic contractile properties of the diaphragm were involved in diaphragm function recovery was also assessed.
MATERIALS AND METHODS
Experimental Design
Anesthetized healthy adult male Wistar rats were randomly assigned to one of five groups: 24- to 27-h CMV (CMV; n = 16), and 24-h CMV followed by 1, 2, 3, or 4–7 h of SB [CMV + 1 h SB (n = 9), CMV + 2 h SB (n = 9), CMV + 3 h SB (n = 9), and CMV + 4–7 h SB (n = 9), respectively]. For the latter group, duration of SB ranged from 4 to 7 h with the mean value of SB being 5.5 h. The study was approved by the Animal Experiments Committee of the Medical Faculty of the Katholieke Universiteit, Leuven, Belgium.
Experimental Procedure
Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (60 mg/kg body wt) and tracheotomized. Body temperature was continuously controlled and maintained at 37°C using a heating blanket. The right jugular vein and carotid artery were cannulated for infusion of pentobarbital sodium (1.5 mg·100 g−1·ml−1·h−1) and heparin (2.8 U·ml−1·h−1) during the MV protocol, respectively. The anesthetized animals were mechanically ventilated with a volume-driven small-animal ventilator (Harvard Apparatus model 665A, Holliston, MA) (control mode, tidal volume ± 0.6 ml/100 g, frequency of breathing 55–60 breaths/min), and breathed humidified air, maintained at 37°C and enriched with O2. Ventilatory parameters were chosen to maintain blood gases within the normal range. Throughout the study duration, animals received enteral nutrition, via a gastric tube, as previously described (14). Arterial blood pressure was monitored during the study period, and blood gases were measured after 12 h of CMV and at the end of the study. SB was achieved by disconnecting the animals from the ventilator while breathing the same gas mixture as during CMV. All animals remained completely anesthetized throughout the study duration.
On completion of the protocol, one muscle bundle was dissected from each hemidiaphragm, parallel to the longitudinal axis of the muscle fibers for the measurement of in vitro contractile properties, as described previously (14). From some animals [CMV (n = 6) and CMV + 4–7 h SB (n = 6)], a third muscle bundle was dissected from the right hemidiaphragm and stored at 4°C in a relaxing solution, containing 50% glycerol (vol/vol). After 24 h, the muscle strip was stored at −20°C for later analysis of single-fiber contractile properties. The remaining segments of the costal diaphragm were snap frozen in liquid nitrogen-cooled isopentane and stored at −80°C for histochemical and biochemical analysis. In addition, the diaphragm and the gastrocnemius muscle were weighed at the completion of the experiments.
Histological and Immunohistochemical Analysis of the Diaphragm
Histological analysis.
Serial sections (10 μm) of the costal diaphragm were stained with hematoxylin and eosin and analyzed for structural abnormalities. Other sections were stained for myofibrillar adenosine triphosphatase to determine the cross-sectional area (CSA) and proportions of the different muscle fiber types (14).
Immunohistochemical assessment of diaphragm inflammation.
To identify neutrophils, ED1+ macrophages, and ED2+ macrophages, immunohistochemistry was performed on serial sections (10 μm) of the costal diaphragm. Therefore, the sections were incubated with one of the following primary antibodies: 1) anti-ED1+; 2) anti-ED2+; or 3) anti-W3/13 (AbD Serotec, Dusseldorf, Germany). Primary antibody was replaced with PBS solution for a negative control. Afterwards, the sections were incubated with a biotinylated horse anti-mouse IgG (rat absorbed) (Vector Laboratories, Burlingame, CA), followed by incubation with R.T.U. VectaStain Elite ABC Kit and NovaRed solution (Vector Laboratories, Burlingame, CA). Cell densities were expressed as the number of labeled cells per cubic millimeter.
Western Blot
Diaphragm tissue samples were homogenized 1:10 (wt/vol) in 5 mM Tris·HCl and 5 mM EDTA buffer (pH 7.5) with a protease inhibitor cocktail (Roche, Complete) and centrifuged at 1,500 g for 10 min at 4°C. After collection of the resulting supernatant, diaphragmatic protein content was assessed by the method of Bradford (Bio-Rad). Total protein concentration was expressed as micrograms of protein per milligram diaphragm muscle.
For all different Western blot protocols described below, proteins were separated on a polyacrylamide gel and transferred onto a polyvinyl difluoride membrane. Blots were incubated overnight at 4°C with a primary antibody and subsequently with the appropriate secondary antibody. Ponceau S staining was performed for each blot to ensure equal loading and proper transfer of the proteins. Proteins were visualized with Chemiluminescent Peroxidase Substrate (Sigma-Aldrich, Bornem, Belgium) and analyzed with the software package (Bio 1D) of the imaging system (Photo print, Vilber, France).
Proteolysis in the Diaphragm
In MV-induced diaphragmatic atrophy, the cysteine proteases calpain and caspase-3 are essential in dissociating intact actomyosin complexes. Therefore, degradation of αII-spectrin, an intracellular substrate of calpain (150-kDa degradation product) and caspase-3 (120-kDa degradation product), was investigated in the diaphragm by Western blot. Ratio of degradation of αII-spectrin to intact protein was used as an indirect measurement of in vivo calpain and caspase-3 activity. A mouse monoclonal primary antibody against αII-spectrin (Enzo Life Sciences, Antwerp, Belgium) and a horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-mouse secondary antibody (Dako, Heverlee, Belgium) were used for detection.
Protein Synthesis in the Diaphragm
The Akt/mammalian target of rapamycin (mTOR) pathway directly regulates skeletal muscle protein synthesis. Therefore, we measured the phosphorylation of two key proteins [Akt and eukaryotic initiation factor 4E binding protein (4E-BP1)] in this pathway as indirect markers of muscle protein synthesis by Western blot. For detection, a rabbit polyclonal primary antibody against total Akt, and rabbit monoclonal primary antibodies against total 4E-BP1, phosphorylated Ser473-Akt, and phosphorylated Thr37/46-4E-BP1 (Cell Signaling Technology, Danvers, MA), and a HRP-conjugated polyclonal swine anti-rabbit secondary antibody (Dako, Heverlee, Belgium) were used for detection. First, the phosphorylated proteins were detected. After detection, the antibodies were stripped from the membrane (Restore Western Blot Stripping Buffer, Thermo Scientific, Rockford, IL), after which the total Akt and total 4E-BP1 proteins were detected. The intensity of the bands of phosphorylated Akt (p-Akt) and 4E-BP1 (p-4E-BP1) was expressed as a percentage of the intensity of the bands of total Akt and 4E-BP1, to assess the amount of p-Akt and p-4E-BP1, respectively.
Oxidative Stress Measurements
Diaphragm protein oxidation.
Oxidative modification of proteins by free radical species or other reactive species results in the formation of carbonyl groups into amino acid side chains. Diaphragmatic protein oxidation was measured by assaying the levels of these protein carbonyls using the Oxyblot Protein Oxidation Detection Kit (Chemicon, Temecula, CA) with Western blot (following the manufacturer's instructions). Data are normalized with Ponceau S values to correct for equal loading.
Diaphragm lipid oxidation.
During the lipid peroxidation cascade, the primary adduct that is formed is 4-hydroxynonenal (4-HNE). Therefore, 4-HNE was measured by Western blot as an indication of oxidative stress. A polyclonal rabbit anti-4-HNE antibody (R&D Systems, Minneapolis, MN) was used, followed by a HRP-conjugated polyclonal swine anti-rabbit secondary antibody (Dako, Heverlee, Belgium). α-Tubulin was used as a loading control.
Single-Fiber Contractile Properties
Relaxing and activating solutions consisted of 1 mM MgCl2, 4 mM Na2ATP, 5 mM EGTA, 10 mM imidazole, 15 mM creatine phosphate, and sufficient KCl to adjust the total ionic strength to 150 mM at pH 7.0. The negative logarithm of the free Ca2+ concentration (pCa) of the relaxing solution was ∼9.0, while in activating solutions the pCa was ∼4.5.
Single-fiber contractile measurements were performed according to previously described methods (43). In short, muscle fiber membranes were permeabilized by 1% Triton X-100. Single fibers were isolated from the muscle bundle, and the fiber ends were attached to two stainless steel hooks, connected to a force transducer (model AE-801; SensoNor, Horten, Norway) and a servomotor (model 308B, Aurora Scientific) in flow-through acrylic chamber. In relaxing solution, sarcomere length was set at 2.4 μm as the optimal length for force generation (5, 49). MIDAC software (Radboud University, Nijmegen, The Netherlands) and a data acquisition board were used to record force signals. Muscle fiber length, width, and depth were measured under an inverted microscope (model IX-70, Olympus, Amsterdam, The Netherlands). Maximum isometric force was determined by measuring force after perfusing the experimental chamber with, successively, pCa 9.0 and pCa 4.5 solutions. Maximum specific force was derived from dividing maximum isometric force by fiber CSA.
In both the CMV and CMV + 4–7 h SB groups, diaphragm single fibers were isolated from six animals, and from each animal, two to four fibers were analyzed. In total, 17 fibers were analyzed in the CMV group, and 16 fibers in the CMV + 4–7 h SB group. Fibers were classified as fast-typed muscle fibers by means of SDS-gel electrophoresis, as described previously (45).
Statistics
Statistical analysis was performed with the SAS statistical package (version 9.2; SAS Institute, Cary, NC). Normal distribution was tested with D'Agostino-Pearson omnibus normality test. Comparisons between the groups were made by a one-way analysis of variance or a Student's t-test, when appropriate. Differences between means were assessed with a Tukey post hoc test. Relationships between parameters were made by using Pearson correlation coefficients, and, subsequently, linear regressions were performed. Significance was established at P < 0.05. Values are expressed as means ± SD.
RESULTS
Systemic and Biological Response to CMV
See Table 1. Values for arterial blood pressure and blood gas/pH homeostasis were maintained within a physiological range during the study period. The arterial blood pressure of the CMV + 4–7 h SB group was significantly higher than that of the CMV + 1 h SB and CMV + 2 h SB groups, and the pH of the arterial blood was higher in the CMV group than in the CMV + 1 h SB group at the time of dissection (Table 1). These values were, however, within a physiological range and will not affect the outcomes of the study. No significant differences were observed in body weight or in diaphragm and gastrocnemius weights. Also the levels of pentobarbital sodium used during the experiments were similar between the groups (1.39 ± 0.17 mg·h−1·100 g−1, pooled values).
Table 1.
Systemic data and body and muscle weights
| CMV | CMV + 1 h SB | CMV + 2 h SB | CMV + 3 h SB | CMV + 4–7 h SB | |
|---|---|---|---|---|---|
| Arterial blood pressure and blood gases after 12-h CMV | |||||
| Arterial blood pressure, mmHg | 139 ± 23 | 138 ± 10 | 134 ± 12 | 136 ± 25 | 140 ± 21 |
| PaO2, Torr | 140 ± 37 | 147 ± 29 | 130 ± 24 | 165 ± 16 | 128 ± 16 |
| PaCO2, Torr | 31 ± 7 | 31 ± 14 | 27 ± 9 | 29 ± 7 | 28 ± 5 |
| pH | 7.39 ± 0.07 | 7.34 ± 0.08 | 7.29 ± 0.08 | 7.30 ± 0.04 | 7.42 ± 0.04 |
| Arterial blood pressure and blood gases at dissection time | |||||
| Arterial blood pressure, mmHg | 125 ± 31 | 114 ± 20 | 114 ± 15 | 115 ± 16 | 144 ± 14* |
| PaO2, Torr | 150 ± 34 | 135 ± 27 | 135 ± 34 | 136 ± 23 | 144 ± 43 |
| PaCO2, Torr | 31 ± 9 | 31 ± 9 | 36 ± 9 | 29 ± 12 | 37 ± 8 |
| pH | 7.45 ± 0.18† | 7.27 ± 0.17 | 7.29 ± 0.10 | 7.31 ± 0.08 | 7.43 ± 0.07 |
| Body weight and muscle weights at dissection time | |||||
| Body weight, g | 454 ± 36 | 453 ± 28 | 454 ± 26 | 451 ± 30 | 469 ± 33 |
| Diaphragm weight, g | 0.60 ± 0.06 | 0.57 ± 0.06 | 0.55 ± 0.06 | 0.57 ± 0.07 | 0.62 ± 0.05 |
| Gastrocnemius weight, g | 2.10 ± 0.20 | 2.12 ± 0.22 | 2.12 ± 0.19 | 2.00 ± 0.15 | 2.17 ± 0.23 |
Values are means ± SD. Shown are arterial blood pressure and blood-gas data after 12 h of controlled mechanical ventilation (CMV) and at dissection time, and starting body weight and muscle masses at dissection time in the CMV group (n = 16) and CMV groups followed by 1, 2, 3, or 4–7 h of spontaneous breathing (SB) (CMV + 1 h SB, n = 9; CMV + 2 h SB, n = 9; CMV + 3 h SB, n = 9; and CMV + 4–7 h SB, n = 9; respectively). PaO2, arterial Po2; PaCO2, arterial Pco2
P < 0.05, CMV + 4–7 h SB vs. CMV + 1 h SB and CMV + 2 h SB. †P < 0.05, CMV vs. CMV + 1 h SB.
Diaphragm In Vitro Contractile Properties
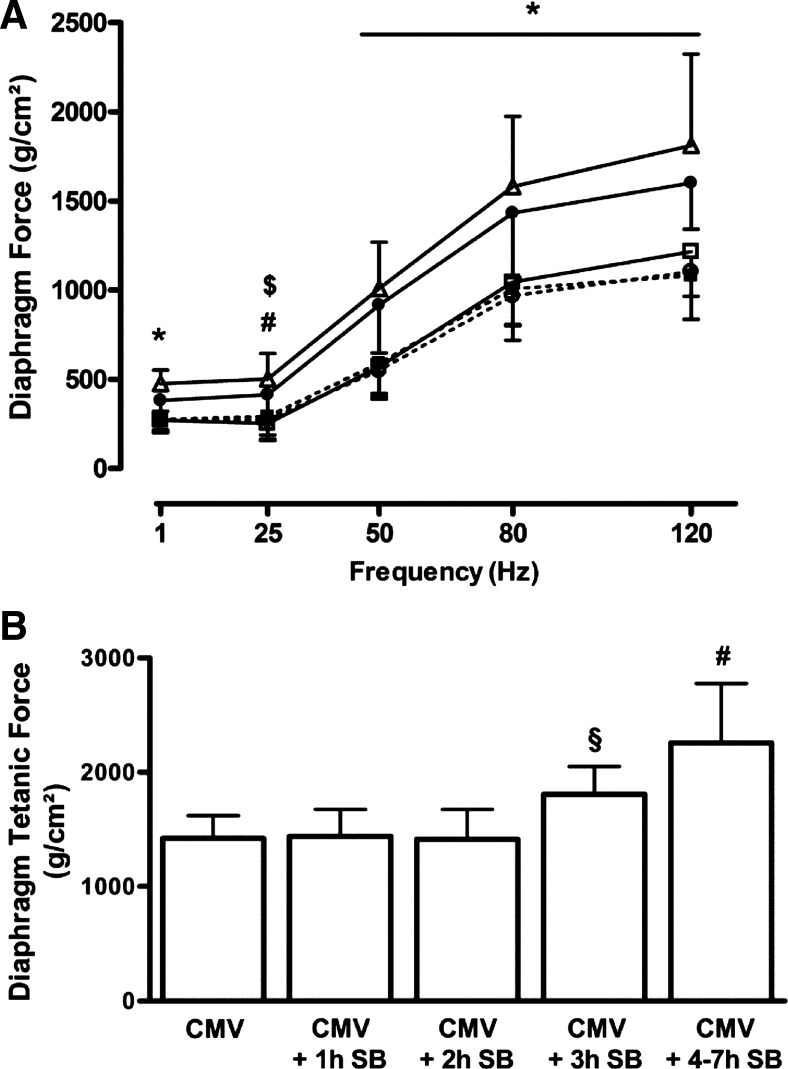
The force generated by the diaphragm was similar at all stimulation frequencies in the CMV, CMV + 1 h SB, and CMV + 2 h SB groups (Fig. 1A). By contrast, the diaphragmatic force generated in the CMV + 3 h SB and CMV + 4–7 h SB groups was significantly higher at all stimulation frequencies compared with the other groups (P < 0.05). The same pattern was observed for the maximal tetanic force (Po), that was significantly higher in the CMV + 3 h SB group (+27% vs. CMV, P < 0.05) and in the CMV + 4–7 h SB group (+59% vs. CMV, P < 0.001) (Fig. 1B). A positive correlation was found between the diaphragm Po and the duration of SB after CMV (r = 0.65, P < 0.0001) (Table 2).
Fig. 1.
A: force-frequency relationship in the diaphragm of controlled mechanical ventilation (CMV) rats (CMV, □, n = 16), and CMV followed by 1, 2, 3, or 4–7 h of spontaneous breathing (SB): CMV + 1 h SB (■ and dashed line, n = 9), CMV + 2 h SB (○ and dashed line, n = 9), CMV + 3 h SB (●, n = 9), CMV + 4–7 h SB (△, n = 9). B: diaphragm maximal tetanic force of the same experimental groups. Maximal tetanic force was measured at 160 Hz at the beginning of the protocol. Values are means ± SD. *P < 0.05, CMV + 3 h SB and CMV + 4–7 h SB vs. others. #P < 0.01, CMV + 4–7 h SB vs. CMV, CMV + 1 h SB and CMV + 2 h SB. $P < 0.01, CMV + 3 h SB vs. CMV. §P < 0.05, CMV + 3 h SB vs. CMV and CMV + 4–7 h SB.
Table 2.
Pearson correlation coefficients
| Diaphragm Po | p-Akt/total Akt | p-4E-BP1/total 4E-BP1 | CSA of Diaphragm Type IIx/b Fibers | |
|---|---|---|---|---|
| Duration of spontaneous breathing, h | r = 0.65*, P < 0.0001 | r = 0.81, P < 0.0001 | r = 0.68, P < 0.001 | |
| Diaphragm Po | r = 0.45, P < 0.05 | r = 0.54, P < 0.05 | r = 0.45*, P < 0.01 |
Correlations between duration of spontaneous breathing or diaphragm tetanic force (Po) and diaphragm Po, the ratio of phosphorylated Akt (p-Akt) to total Akt, the ratio of phosphorylated eukaryotic initiation factor 4E binding protein (p-4E-BP1) to total 4E-BP1, and the cross-sectional area (CSA) of the diaphragm type IIx/b fibers
Correlations are made with all groups considered in the analysis.
Histological Analysis of Diaphragm Muscle Fibers
No structural abnormalities were detected on the hematoxylin and eosin stained sections of the diaphragm in any of the groups.
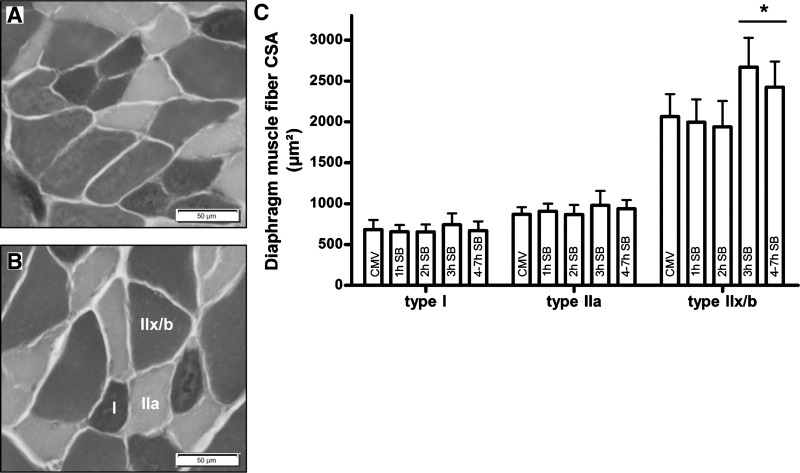
The proportions of the different fiber types were similar between all groups. The CSA of the type I and type IIa fibers in the diaphragm was similar in all groups, whereas the CSA of the type IIx/b fibers was significantly higher in the CMV + 3 h SB (+31% vs. CMV, P < 0.01) and CMV + 4–7 h SB (+ 21% vs. CMV, P < 0.05) groups compared with all other groups (Fig. 2). CSA of the type IIx/b fibers was positively correlated with the Po of the diaphragm (Table 2).
Fig. 2.
Representative photographs of a histochemical ATPase staining of the diaphragm muscle fibers in the CMV group (A) and the CMV + 3 h SB group (B) are shown on the left. Photographs are taken at magnification ×20, and type I, type IIa, and type IIx/b fibers are indicated in B. C: diaphragm cross-sectional area (CSA) of type I, IIa, and IIx/b muscle fibers in the CMV animals (CMV, n = 8) and CMV + 1 h SB (1 h SB, n = 8), CMV + 2 h SB (2 h SB, n = 9), CMV + 3 h SB (3 h SB, n = 7), and CMV + 4–7 h SB (4–7 h SB, n = 9). Values are means ± SD. *P < 0.05, CMV + 3 h SB and CMV + 4–7 h SB vs. others.
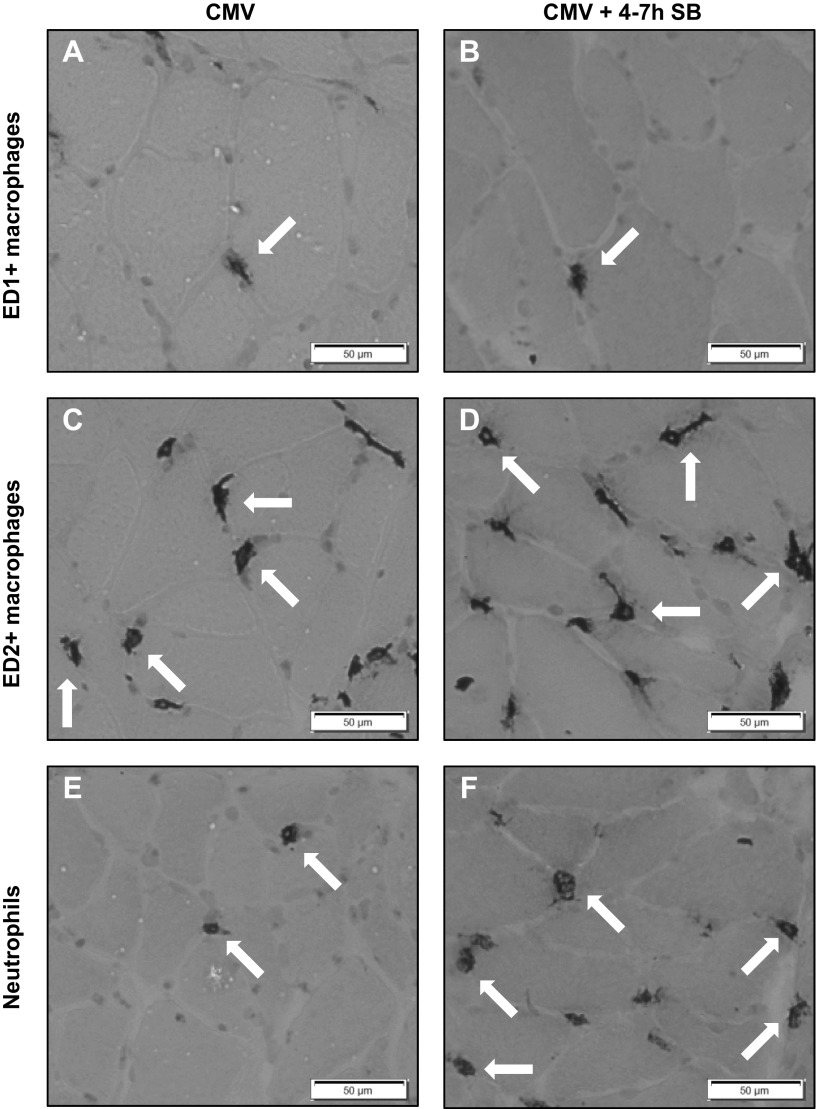
Immunohistochemical analyses indicated no differences in the number of ED1+ or ED2+ macrophages in the diaphragm of the different groups (Table 3 and Fig. 3, A–D). However, a significant increase in the number of neutrophils was present in the CMV + 4–7 h SB group compared with all other groups (P < 0.05) (Table 3 and Fig. 3, E and F).
Table 3.
Diaphragmatic inflammation
| CMV | CMV + 1 h SB | CMV + 2 h SB | CMV + 3 h SB | CMV + 4–7 h SB | |
|---|---|---|---|---|---|
| ED1+ macrophages, cells/mm3 | 5,822 ± 1,515 | 5,666 ± 1,544 | 5,927 ± 1,556 | 5,016 ± 976 | 6,339 ± 1,201 |
| ED2+ macrophages, cells/mm3 | 17,101 ± 1,952 | 18,070 ± 2,054 | 18,618 ± 3,410 | 16,485 ± 1,901 | 17,283 ± 2,540 |
| Neutrophils, cells/mm3 | 1,154 ± 226 | 1,159 ± 247 | 1,720 ± 551 | 1,829 ± 540 | 2,872 ± 1,278* |
Values are means ± SD. Shown are amount of ED1+ macrophages, ED2+ macrophages, and neutrophils in the diaphragm in the CMV group (n = 8), and CMV + 1 h SB (n = 9), CMV + 2 h SB (n = 9), CMV + 3 h SB (n = 9), and CMV + 4–7 h SB (n = 9) groups, expressed as no. of cells/mm3
P < 0.05, CMV + 4–7 h SB vs. others.
Fig. 3.
Top: representative photographs of an immunohistochemical staining for ED1+ macrophages in the diaphragm of the CMV group (A) and the CMV + 4–7 h SB group (B). Middle: representative photographs are shown of an immunohistochemical staining for ED2+ macrophages in the diaphragm of the CMV group (C) and the CMV + 4–7 h SB group (D). Bottom: representative photographs of an immunohistochemical staining for neutrophils (W3/13) in the diaphragm of the CMV group (E) and the CMV + 4–7 h SB group (F). All photographs are taken at magnification ×20; several cells are indicated with arrows.
Because the data on diaphragm force, fiber dimensions, and inflammatory cells in the CMV + 1 h SB and CMV + 2 h SB groups did not differ from that of the CMV group, the results of these groups are not shown in the next analyses to simplify the figures, especially because they were not significantly different from that of the CMV group.
Proteolytic Activity Of Calpain and Caspase-3 in the Diaphragm
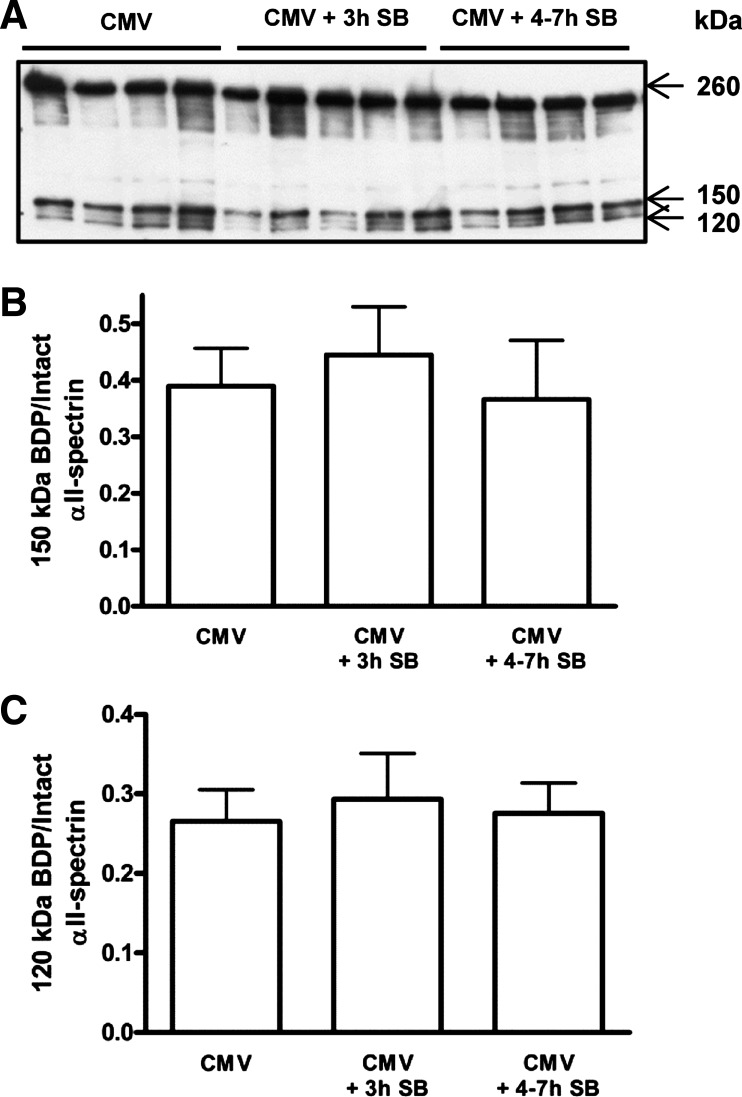
Calpain activity in the diaphragm, measured by the ratio of 150-kDa calpain-specific cleaved αII-spectrin to intact αII-spectrin, was similar in all groups (Fig. 4, A and B). Also, diaphragmatic caspase-3 activity, measured by the ratio of 120-kDa caspase-3-specific cleaved αII-spectrin to intact αII-spectrin, did not change between the groups (Fig. 4, A and C).
Fig. 4.
A: representative immunoblot for the analysis of intact and cleaved αII-spectrin in the diaphragm of CMV (n = 8) and CMV + 3 h SB (n = 9) and CMV + 4–7 h SB (n = 8). B: mean densitometric values for αII-spectrin degradation by calpain. Values are shown as the ratio of the 150-kDa breakdown product (BDP) to intact αII-spectrin (260 kDa). C: mean densitometric values for αII-spectrin degradation by caspase-3. Values are shown as the ratio of the 120-kDa BDP to intact αII-spectrin. Values are means ± SD.
Oxidative Stress in the Diaphragm
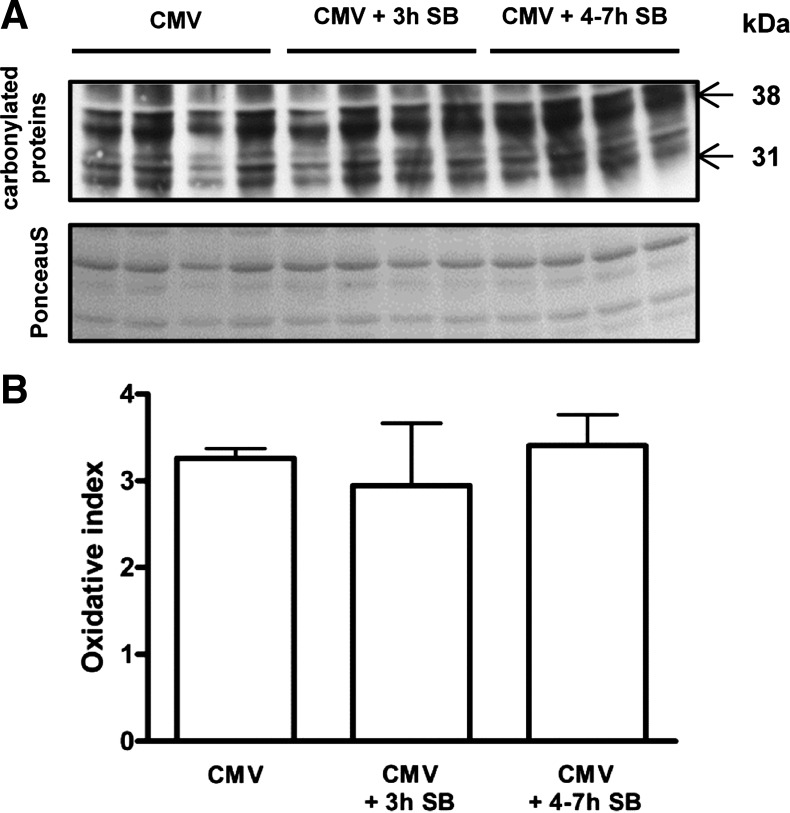
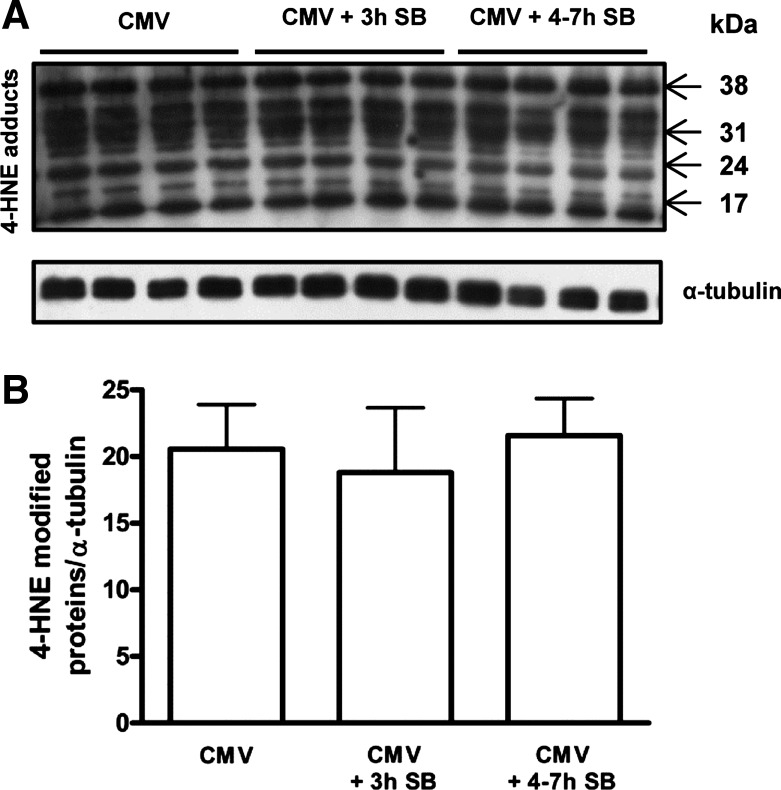
Diaphragm protein oxidation was similar in all groups (Fig. 5). Also, lipid peroxidation, measured by 4-HNE protein adducts, was the same between the groups (Fig. 6).
Fig. 5.
A: representative immunoblot for the analysis of the amount of carbonylated proteins, a marker for protein oxidation. The Ponceau S staining, as a loading control, is also shown. B: mean densitometric values for protein oxidation in the diaphragm of CMV (n = 8), CMV + 3 h SB (n = 7), and CMV + 4–7 h SB (n = 9). Values are shown as the ratio of the amount of carbonylated proteins on Western blot to Ponceau S staining. Values are means ± SD.
Fig. 6.
A: representative immunoblot for the analysis of the amount of 4-hydroxynonenal (4-HNE) adducts, a marker for lipid peroxidation. α-Tubulin was used as a loading control. B: mean densitometric values for lipid peroxidation in the diaphragm of CMV (n = 7), CMV + 3 h SB (n = 7), and CMV + 4–7 h SB (n = 5). Values are shown as the ratio of the amount of 4-HNE adducts to α-tubulin. Values are means ± SD.
Protein Synthesis in the Diaphragm
No differences in total diaphragm protein content were found between the CMV and CMV + 4–7 h SB groups (29.5 ± 3.49 and 30.5 ± 5.61 μg protein/mg diaphragm muscle, respectively).
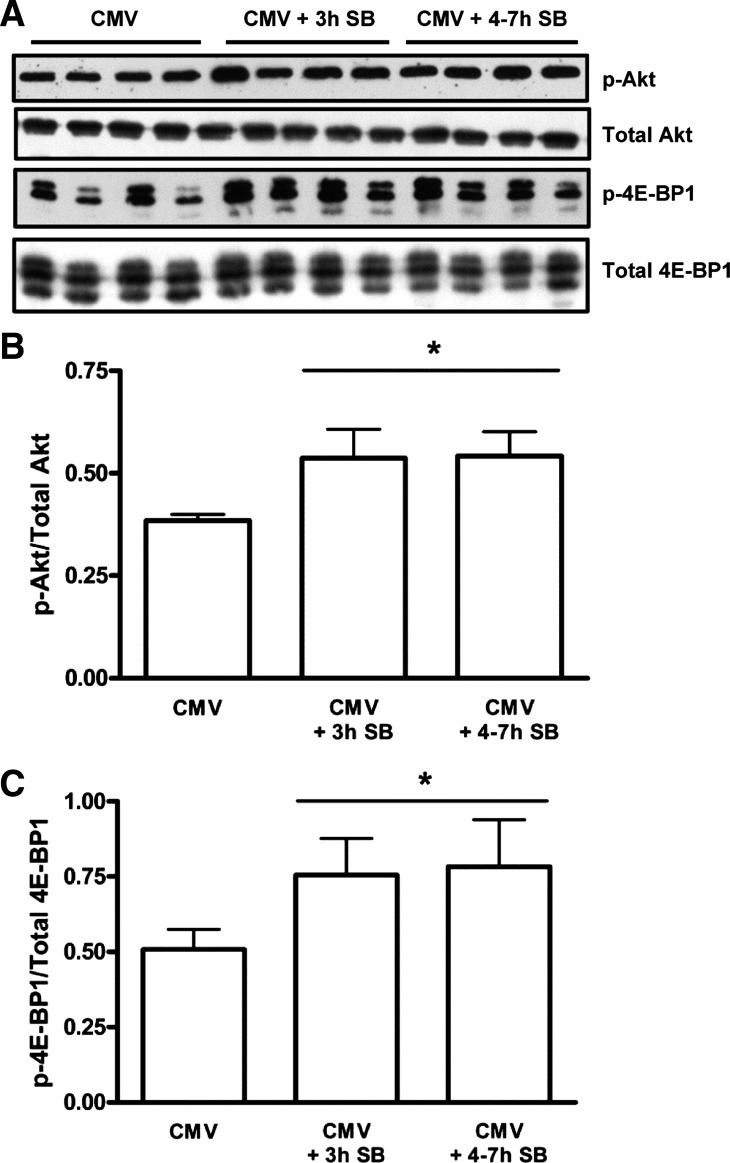
The ratio of p-Akt to total Akt increased by 40% after 3 h of SB (P < 0.001) and by 41% in the CMV + 4–7 h SB group (P < 0.001) compared with the CMV group (Fig. 7, A and B). Similarly, the ratio of p-4E-BP1 to total 4E-BP1 increased by 49% in the CMV + 3 h SB group (P < 0.01) and by 54% in the CMV + 4–7 h SB group (P < 0.001) compared with the CMV group (Fig. 7, A and C). p-Akt and p-4E-BP1 were positively correlated to the duration of SB after CMV and also to the diaphragm Po (Table 2).
Fig. 7.
A: representative immunoblots for phosphorylated Akt (p-Akt), total Akt, phosphorylated eukaryotic initiation factor 4E binding protein (p-4E-BP1), and total 4E-BP1. B: mean densitometric values for the amount of activated Akt. Values are shown as the ratio of p-Akt to total Akt, from CMV (n = 7), CMV + 3 h SB (n = 6), and CMV + 4–7 h SB (n = 7) diaphragms, measured on the same blotting membrane, and are expressed as a percentage of CMV. B: mean densitometric values for the amount of p-4E-BP1. Values are shown as the ratio of p-4E-BP1 to total 4E-BP1, from CMV (n = 7), CMV + 3 h SB (n = 6), and CMV + 4–7 h SB (n = 7) diaphragms, measured on the same blotting membrane. Values are means ± SD. *P < 0.01, CMV + 3 h SB and CMV + 4–7 h SB vs. CMV.
Diaphragm Single-Fiber Contractile Properties
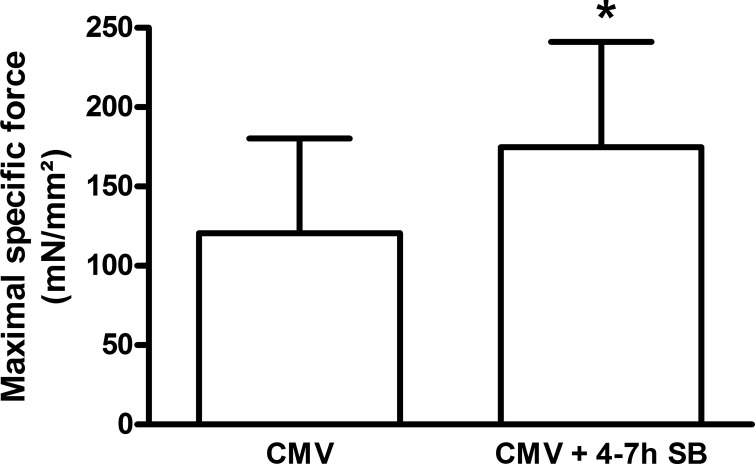
Diaphragm single-fiber force-generating capacity was enhanced by 45% in the CMV + 4–7 h SB group compared with CMV alone (P < 0.05) (Fig. 8).
Fig. 8.
Maximal specific force generation of skinned fast-type diaphragm fibers from mechanically ventilated rats (CMV, n = 6) and rats that breathed spontaneously for 4–7 h following CMV (n = 6), corrected for the CSA of each muscle fiber. Values are means ± SD. *P < 0.05, CMV + 4–7 h SB vs. CMV.
DISCUSSION
Overview of Principal Findings
The major findings of the present study are as follows. 1) In healthy rats, 3 h of diaphragm reloading by SB after 24 h of CMV are sufficient to improve CMV-induced diaphragm contractile dysfunction, while full recovery occurs after only 4–7 h of reloading. 2) The rapid increase in diaphragm force was correlated with an increase in the CSA of the type IIx/b diaphragm muscle fibers, while reloading during this time frame had no effect on calpain and caspase-3 induced αII-spectrin degradation, nor on the amount of oxidative stress markers in the diaphragm. 3) Increased protein synthesis and improved intrinsic contractile properties of skinned diaphragm muscle fibers may have contributed to diaphragm function recovery.
Reloading the Diaphragm with SB Improves CMV-Induced Diaphragm Contractile Dysfunction and Atrophy
It is well known that inactivating the diaphragm by prolonged CMV leads to a rapid and time-dependent diaphragm contractile dysfunction (6, 13, 32, 33, 48). Furthermore, also in studies with locomotor skeletal muscle unloading and disuse, contractile dysfunction was reported (11, 15), although the time course of these effects is longer for locomotor skeletal muscles (11) than for the diaphragm (23). In this regard, reloading of unloaded locomotor skeletal muscles has also been associated with reloading-induced muscle injury with an additional decrease in muscle force, before the start of muscle function recovery (11, 29). In the present study, reloading the diaphragm for 1 or 2 h after 24 h of CMV did not exacerbate or improve CMV-induced diaphragm dysfunction, which is in agreement with the study of Van Gammeren et al. (42), where the same results were obtained. Furthermore, we found a significant increase in diaphragm force from 3 h of SB on, reaching normal function (2, 42) after 4–7 h of SB. The increased diaphragm force was positively correlated with the time of SB. We hereby indicate that VIDD is reversible, which has never been shown before, and the time course of this recovery shows that reversibility takes only a few hours, at least in healthy animals. This is in contrast with what is observed in locomotor skeletal muscles, where functional recovery following unloading takes up to several weeks (11). There are several possible explanations for these discrepancies between the diaphragm and the locomotor skeletal muscles. First, it is possible that the load applied for reloading the diaphragm while resuming SB is less than the load necessary to reload the soleus muscle by resuming ambulation (30). Another possible explanation is that, at higher workloads (increase in ventilation demand), additional ventilatory muscles are recruited, and, because of this, the mechanical load is divided between several different muscles (30). Also the fact that the diaphragm is a chronically active muscle (i.e., contracting 80–100 times/min in the rat) can play an important role in the discrepancy with locomotor skeletal muscles, which are only active during active movements. Furthermore, in the present study, the CMV-induced atrophy of the type IIx/b fibers in the diaphragm significantly improved after only 3 h of diaphragm reloading, and the size of the type IIx/b muscle fibers was positively correlated with diaphragm force. This recovery from atrophy is extremely rapid, especially compared with the time course of recovery in unloaded locomotor skeletal muscles, in which the CSA is still 30% lower than in controls after 5 wk of reloading (19). In the study of Van Gammeren et al. (42), a significant increase in muscle water content or edema was measured in the diaphragm after 2 h of reloading, but this was, however, not measured on histological sections. In the present study, only the type IIx/b diaphragm fibers show an increase in CSA, which makes the effect of edema on the increase of muscle fiber size unlikely because this is expected to affect all fiber types.
Reloading the Diaphragm Has No Effect on Proteolysis
During prolonged MV, MV-induced diaphragmatic atrophy develops as a result of an increase in oxidative stress in the muscle fibers (1, 31, 47), which leads to a decrease in protein synthesis (36) and an increase in protein degradation during which all proteolytic pathways are activated (26, 37, 47). This is also the case in locomotor skeletal muscles after unloading (3, 9), and, furthermore, it is well known that early recovery of these muscles is associated with enhanced oxidative stress (35), proteolysis, and protein synthesis (37) compared with the unloaded muscle. To date, it is not known whether any of these systems also play a role in the recovery of the diaphragm following CMV. In this regard, we investigated markers of oxidative stress and the specific breakdown of αII-spectrin by calpain and caspase-3 during recovery of the diaphragm from MV. The latter because, in ventilator-induced diaphragm dysfunction, the Ca2+-dependent calpain system and the caspase-3 system have been shown to be one of the most important proteolytic systems, as they are necessary for the initial degradation of actomyosin complexes. In addition, their inhibition has been shown to result in a prevention of the deleterious effects of CMV on the diaphragm (1, 26, 27). However, no changes could be detected in these markers of protein degradation. Moreover, also no differences could be detected in the markers of oxidative stress in the diaphragm. One possible explanation for this is that there indeed could be a decrease in oxidative stress and proteolysis in the diaphragm, but that the modified proteins are still present in the diaphragm, and that changes, therefore, could not be detected with the methods we have used in this study. Another possibility is that reloading does not cause an additional increase in oxidative stress, which might explain the fact that the markers of proteolytic activity remained similar to CMV after reloading since oxidative stress is an essential upstream signal for calpain and caspase-3 activation in the diaphragm during prolonged CMV (47).
Reloading the Diaphragm Is Associated With Increased Protein Synthesis
Prolonged MV leads to a decrease in protein synthesis in the diaphragm (36). Concerning protein synthesis in skeletal muscles, it is known that this is directly regulated by the Akt/mTOR pathway (4). This pathway is activated by several growth hormones and cytokines, which leads to activation of Akt by phosphorylation. Akt then indirectly activates mTOR, which activates eukaryotic translation initiation factor 4E by the phosphorylation of the inhibitory binding protein 4E-BP1, and thereby allowing translation initiation (for review, see Ref. 34). The results of our study indicate an increase in the activity of the Akt/mTOR pathway with reloading, and we thus assume an increase in protein synthesis during the recovery of the diaphragm from CMV. This is also the case in reloaded locomotor skeletal muscles, where protein synthesis was increased with 65% after 18 h of reloading following 9 days of unloading, and this increase was maintained for several days (38). However, our measurements of total diaphragm protein content did not show any changes between the CMV and CMV + 4–7 h SB groups. But the method used to measure total muscle protein content is a very crude method, and as such it cannot detect subtle or specific alterations in protein content.
Additionally, our measurements of the contractile properties at the single-muscle fiber level showed a significant increase in specific muscle fiber force in the 4- to 7-h reloading group compared with the CMV group. These single-muscle fiber preparations allow direct measurement of contractile function in muscle cells with an intact myofilament lattice, but without the confounding effects of nerves, excitation-contraction coupling, fiber architecture, and intercellular connective tissue. This means that, using this technique, we measure the effect of reloading at the level of the contractile proteins. It has been previously shown that MV leads to impaired diaphragm single-fiber-specific force-generating capacity, which is the absolute force divided by the CSA of the muscle fiber (44). Van Hees et al. (44) also showed that contractile protein loss is a strong determinant of diaphragm weakness on MV, since a decrease in myosin concentration contributed to the reduced force generation of the diaphragm fibers. In the present study, the improvement in specific muscle fiber force probably indicates an increase in the myosin content in the diaphragm. This was, however, not measured. The increase in protein synthesis that we have measured in the reloaded diaphragm may, therefore, be associated with a possible increase in myosin synthesis and content and, therefore, with an increased muscle force.
Increased Influx of Neutrophils During Diaphragm Reloading Is Not Associated With Additional Diaphragm Dysfunction
A final point of interest is that an increase in the influx of neutrophils was found in the diaphragm after 4–7 h of reloading. No changes in numbers of macrophages were observed. These data are in agreement with those of Van Gammeren et al. (42), obtained after 2 h of reloading. By contrast, hindlimb reloading following unloading was shown to be characterized by an infiltration of neutrophils followed by macrophages (7, 11). In this context, macrophages are believed to promote muscle membrane repair and regeneration of muscle fibers (8, 41), while the exact role of neutrophils remains to be determined. Very little muscle fiber damage has been reported in hindlimb muscle reloading models, despite the presence of high numbers of neutrophils following reloading (10, 11, 40). No correlation was found between the number of neutrophils and the loss in muscle force associated with hindlimb unloading and reloading (11). In our study too, the increased neutrophil numbers in the diaphragm after reloading were not associated with a further deterioration of diaphragm function, and this was also not reported in the study by Van Gammeren et al. (42). Since diaphragm function was improved in our study after reloading, it might be possible that, during diaphragm recovery, neutrophils may play a role in muscle repair or remodeling in the diaphragm [e.g., phagocytosis of tissue debris to accelerate the regenerative process (39) or activation of satellite cells].
Limitations of the Experimental Model
The animal model used in the present study is a simplified model of VIDD, since the animals are healthy, not taking any (harmful) medication, and being ventilated in a fully controlled mode, although it is not the most commonly used mode of ventilation in the intensive care unit. The reason that this simplified animal model was chosen is to dissociate the reloading effect from effects related to disease or medication. Therefore, the data of the present study cannot be extended directly to patients, but at least they show that recovery of the diaphragm function occurred quickly and is not associated with additional negative effects.
This study, however, only examined the short-term reloading effects on diaphragm function. Future studies are required to examine the effects of reloading on longer term, e.g., 12–24 h of reloading. Furthermore, also the recovery response in animals that are submitted to longer periods of MV, which results in more extensive diaphragmatic dysfunction and atrophy, needs to be investigated.
Conclusions
In this study, we investigated the time course of the recovery response of the diaphragm following 24 h of CMV in healthy rats. We have shown that reloading the diaphragm for only a few hours after CMV was sufficient to reverse the CMV-induced diaphragmatic dysfunction, leading to a time-dependent increase in diaphragm force, which is considered as a very quick recovery compared with unloaded and reloaded locomotor skeletal muscles. The observed improvement in diaphragm function might be due to an increase in protein synthesis and improved intrinsic contractile properties of the diaphragm muscle fibers, probably indicating an increase in myosin content. The data of the present study are relevant as they indicate that VIDD is reversible, and this does not result in additional reloading-induced diaphragm injury, at least in healthy animals.
GRANTS
This study was supported by AstraZeneca Pharmaceuticals and FWO-Vlaanderen G.0893.11.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.T., K.M., A.A., and H.W.v.H. performed experiments; D.T., K.M., and H.W.v.H. analyzed data; D.T., K.M., A.A., L.M.H., R.D., M.D., H.W.v.H., and G.G.-R. interpreted results of experiments; D.T. and K.M. prepared figures; D.T. and K.M. drafted manuscript; D.T., K.M., A.A., L.M.H., R.D., M.D., H.W.v.H., and G.G.-R. edited and revised manuscript; D.T., K.M., A.A., L.M.H., R.D., M.D., H.W.v.H., and G.G.-R. approved final version of manuscript; M.D. and G.G.-R. conception and design of research.
ACKNOWLEDGMENTS
We sincerely thank Petra Weckx for cutting and staining the histological sections.
REFERENCES
- 1.Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. N-acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med 39: 777–782, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Agten A, Maes K, Thomas D, Cielen N, van Hees HW, Dekhuijzen RP, Decramer M, Gayan-Ramirez G. Bortezomib partially protects the rat diaphragm from ventilator-induced diaphragm dysfunction. Crit Care Med 40: 2449–2455, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Andrianjafiniony T, Dupre-Aucouturier S, Letexier D, Couchoux H, Desplanches D. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. Am J Physiol Cell Physiol 299: C307–C315, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204: 1529–1536, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Capdevila X, Lopez S, Bernard N, Rabischong E, Ramonatxo M, Martinazzo G, Prefaut C. Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med 29: 103–110, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Dumont N, Bouchard P, Frenette J. Neutrophil-induced skeletal muscle damage: a calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol 295: R1831–R1838, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am J Pathol 176: 2228–2235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enns DL, Raastad T, Ugelstad I, Belcastro AN. Calpain/calpastatin activities and substrate depletion patterns during hindlimb unweighting and reweighting in skeletal muscle. Eur J Appl Physiol 100: 445–455, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol 156: 2103–2110, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenette J, St-Pierre M, Cote CH, Mylona E, Pizza FX. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am J Physiol Regul Integr Comp Physiol 282: R351–R357, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve 32: 605–612, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med 29: 825–833, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gayan-Ramirez G, Testelmans D, Maes K, Racz GZ, Cadot P, Zador E, Wuytack F, Decramer M. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med 33: 2804–2809, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Guillot C, Steinberg JG, Delliaux S, Kipson N, Jammes Y, Badier M. Physiological, histological and biochemical properties of rat skeletal muscles in response to hindlimb suspension. J Electromyogr Kinesiol 18: 276–283, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Haitsma JJ. Diaphragmatic dysfunction in mechanical ventilation. Curr Opin Anaesthesiol 24: 214–218, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care 14: R127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 182: 1377–1386, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Itai Y, Kariya Y, Hoshino Y. Morphological changes in rat hindlimb muscle fibres during recovery from disuse atrophy. Acta Physiol Scand 181: 217–224, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction–human studies confirm animal model findings! Crit Care 15: 206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183: 364–371, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 149: 1539–1544, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Levine S, Biswas C, Dierov J, Barsotti R, Shrager JB, Nguyen T, Sonnad S, Kucharchzuk JC, Kaiser LR, Singhal S, Budak MT. Increased proteolysis, myosin depletion and atrophic AKT-FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med 183: 483–490, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Maes K, Testelmans D, Cadot P, Deruisseau K, Powers SK, Decramer M, Gayan-Ramirez G. Effects of acute administration of corticosteroids during mechanical ventilation on rat diaphragm. Am J Respir Crit Care Med 178: 1219–1226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 175: 1134–1138, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 16: 19–25, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Pottle D, Gosselin LE. Impact of mechanical load on functional recovery after muscle reloading. Med Sci Sports Exerc 32: 2012–2017, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Powers SK, Criswell D. Adaptive strategies of respiratory muscles in response to endurance exercise. Med Sci Sports Exerc 28: 1115–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 92: 1851–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 92: 2585–2595, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1: 4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol 102: 1702–1707, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Shanely RA, Van Gammeren D, DeRuisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 170: 994–999, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Taillandier D, Aurousseau E, Combaret L, Guezennec CY, Attaix D. Regulation of proteolysis during reloading of the unweighted soleus muscle. Int J Biochem Cell Biol 35: 665–675, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol 65: 492–498, 1999 [PubMed] [Google Scholar]
- 41.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578: 327–336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Gammeren D, Falk DJ, DeRuisseau KC, Sellman JE, Decramer M, Powers SK. Reloading the diaphragm following mechanical ventilation does not promote injury. Chest 127: 2204–2210, 2005 [DOI] [PubMed] [Google Scholar]
- 43.van Hees HW, Ottenheijm CA, Granzier HL, Dekhuijzen PN, Heunks LM. Heart failure decreases passive tension generation of rat diaphragm fibers. Int J Cardiol 141: 275–283, 2010 [DOI] [PubMed] [Google Scholar]
- 44.van Hees HW, Schellekens WJ, ndrade Acuna GL, Linkels M, Hafmans T, Ottenheijm CA, Granzier HL, Scheffer GJ, van der Hoeven JG, Dekhuijzen PN, Heunks LM. Titin and diaphragm dysfunction in mechanically ventilated rats. Intensive Care Med 38: 702–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Watson AC, Hughes PD, Louise HM, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol 108: 1376–1382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Luo J, Bourdon J, Lin MC, Gottfried SB, Petrof BJ. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am J Respir Crit Care Med 166: 1135–1140, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Zuurbier CJ, Heslinga JW, Lee-de Groot MB, Van der Laarse WJ. Mean sarcomere length-force relationship of rat muscle fibre bundles. J Biomech 28: 83–87, 1995 [DOI] [PubMed] [Google Scholar]