Abstract
The group II metabotropic glutamate receptors (group II mGluRs) have emerged as the new drug targets for the treatment of mental disorders like schizophrenia. To understand the potential mechanisms underlying the antipsychotic effects of group II mGluRs, we examined their impact on NMDA receptors (NMDARs), since NMDAR hypofunction has been implicated in schizophrenia. The activation of group II mGluRs caused a significant enhancement of NMDAR currents in cortical pyramidal neurons, which was associated with increased NMDAR surface expression and synaptic localization. We further examined whether these effects of group II mGluRs are through the regulation of NMDAR exocytosis via SNARE proteins, a family of proteins involved in vesicle fusion. We found that the enhancing effect of APDC, a selective agonist of group II mGluRs, on NMDAR currents was abolished when botulinum toxin was delivered into the recorded neurons to disrupt the SNARE complex. Inhibiting the function of two key SNARE proteins, SNAP-25 and syntaxin 4, also eliminated the effect of APDC on NMDAR currents. Moreover, the application of APDC increased the activity of Rab4, a small Rab GTPase mediating fast recycling from early endosomes to the plasma membrane, and enhanced the interaction between syntaxin 4 and Rab4. Knockdown of Rab4 or expression of dominant-negative Rab4 attenuated the effect of APDC on NMDAR currents. Taken together, these results have identified key molecules involved in the group II mGluR-induced potentiation of NMDAR exocytosis and function.
Key points
Activation of group II metabotropic glutamate receptors (mGluRs) enhances NMDA receptor (NMDAR)-mediated currents in cortical pyramidal neurons.
In this study, we found that group II mGluR-induced enhancement of NMDAR currents was associated with increased NMDAR surface expression and synaptic localization.
Inhibition of SNAP-25 or knockdown of syntaxin 4 blocked the enhancement of NMDAR currents by group II mGluRs.
Group II mGluRs increase the activity of Rab4 small GTPase. Rab4 knockdown or dominant negative Rab4 abolished the enhancing effect of Group II mGluRs on NMDAR currents.
These results suggest that SNARE proteins and Rab4 are key molecules involved in the enhancement of NMDAR exocytosis and function by group II mGluRs. Identification of key molecules involved in NMDAR up-regulation could provide novel drug targets for schizophrenia treatment.
Introduction
The metabotropic glutamate receptors (mGluRs) are G-protein-coupled receptors playing an important role in the regulation of synaptic functions (Conn & Pin, 1997; Anwyl, 1999). Currently, mGluRs have emerged as the promising drug targets for several neurological and psychiatric disorders (Krystal et al. 2010). In particular, group II mGluRs, which comprise mGluR2 and mGluR3, have been implicated in the treatment of schizophrenia, anxiety disorders and depression (Krystal et al. 2010). Rodent studies suggest that the agonists of group II mGluRs reduce the disruption of working memory and abnormal locomotor activity in schizophrenia models (Moghaddam & Adams, 1998; Harich et al. 2007), exhibit anxiolytic efficacy in several stress and anxiety models (Schoepp et al. 2003; Nordquist et al. 2008), and inhibit ischaemia-induced neuronal death in hippocampus (Pizzi et al. 1996). Furthermore, preliminary human studies have shown that the agonists of group II mGluRs effectively alleviate schizophrenia symptoms (Patil et al. 2007).
Group II mGluRs are highly expressed in forebrain regions including frontal cortex (Ohishi et al. 1993a,b; Petralia et al. 1996). The mGluR2 is expressed in neurons not only presynaptically but also postsynaptically, while mGluR3 is localized pre- and postsynaptically in both neurons and glial cells (Neki et al. 1996; Petralia et al. 1996; Tamaru et al. 2001). The physiological roles of group II mGluRs in inhibition the release of glutamate or other neurotransmitters have been well studied in presynaptic terminals (Kamiya et al. 1996; Schoepp, 2001). However, the function of group II mGluRs in postsynaptic neurons is still elusive. There have been reports showing that the activation of the postsynaptic group II mGluRs fine-tune baroreceptor signal transmission in nucleus tractus solitarius (Sekizawa et al. 2009), depolarize neurons and enhance network activity in hippocampus (Ster et al. 2011), and reduce calcium currents in cerebellar granule cells (Chavis et al. 1994). In addition, a previous study in our lab revealed that the activation of group II mGluRs potentiated NMDA receptor currents in frontal cortical pyramidal neurons (Tyszkiewicz et al. 2004).
NMDA receptors (NMDARs) are ionotropic glutamate receptors that play a critical role in regulating synaptic plasticity and cognitive processes. NMDAR dysfunction has been associated with psychiatric disorders, neurodegenerative diseases, and neurodevelopmental illnesses (Gonda, 2012). In particular, NMDAR hypofunction is considered as a fundamental pathophysiology in schizophrenia (Kantrowitz and Javitt, 2012). Treating animals with NMDAR antagonists produces abnormal behaviours that resemble the positive, negative and cognitive symptoms of schizophrenia (Rung et al. 2005; Bubenikova-Valesova et al. 2008). Thus, pharmacological agents, such as group II mGluRs agonists, that enhance NMDAR function could potentially be used as antipsychotics.
The trafficking of NMDARs between intracellular compartments is dynamically regulated by various proteins (Wenthold et al. 2003; Prybylowski & Wenthold, 2004; Lau & Zukin, 2007). Recently, the SNARE proteins (comprising families of membrane-associated proteins, i.e. the synaptobrevin/VAMP, syntaxin and SNAP-25 families), which regulate vesicle transport and docking (Gerst, 1999) and mediate membrane fusion (Jahn & Scheller, 2006), have been implicated in the delivery of NMDAR vesicles at postsynaptic sites (Lau & Zukin, 2007; Suh et al. 2010). In this study, we examined the involvement of SNAREs and interacting proteins in the group II mGluR-induced regulation of NMDARs. Identification of key molecules involved in NMDAR up-regulation could provide novel drug targets for schizophrenia treatment.
Methods
Animals and reagents
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo. Pregnant rats were anaesthetized with isoflurane vapour and immediately killed. The frontal cortex was dissected from rat embryos (E18) and used for the cortical culture preparation.
The mGluR ligand (2R, 4R)-4-aminopyrrolidine-2, 4-dicarboxylate (APDC) was from Tocris (Ballwin, MO, USA). It was made up as concentrated stocks and stored at −20°C. The final DMSO concentration in all applied solutions was less than 0.1%. Stocks were thawed and diluted immediately prior to use. SNAP-25 (synaptosomal-associated protein 25) blocking peptide was designed as QSFFSGLFGGSSKIEEACE, which mimics the NH2-terminal 19aa of SNAP-25 (Lledo et al. 1998). SNAP-23 blocking peptide was designed as MDDLSPEEIQLRAHQVTD, which mimics the NH2-terminal domain of SNAP-23 (Vaidyanathan et al. 2001). The scrambled peptide (GFAESLFQSIEKESGFSCG) serves as a negative control (Lledo et al. 1998).
Transfection and DNA constructs
To suppress the expression of various proteins, small interfering RNA (siRNA) or small hairpin RNA (shRNA) was transfected into cultured neurons using the Lipofectamine 2000 method (Invitrogen, Carlsbad, CA, USA). The shRNA oligonucleotide targeting rat syntaxin 4 (CCTGCGAGAGGAGATCAAA; Kennedy et al. 2010) was cloned into pLKO.3G vector (Addgene, Cambridge, MA, USA), which contains an enhanced green fluorescent protein marker (eGFP). The siRNA (20 nm) targeting Rab4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was co-transfected with EGFP (0.3 ng μl−1). Dominant-negative Rab4 (DN-Rab4, Rab4-S27N; Odley et al. 2004) was constructed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). All constructs were verified by DNA sequencing. At 2–3 days after transfection, electrophysiological recording was conducted in GFP-positive neurons.
Electrophysiological recordings in cultured neurons
Cortical cultures from E18 embryos were prepared as previously described (Yuen et al. 2005, 2011, 2012). Cultures were maintained in Neurobasal with B27 supplement (Invitrogen Grand Island, NY, USA). Recordings of whole-cell NMDA-elicited currents employed similar techniques to those described previously (Gu et al. 2005, 2012; Yuen et al. 2005). The internal solution contained (in mm): 180 N-methyl-d-glucamine, 40 Hepes, 4 MgCl2, 0.1 BAPTA, 12 phosphocreatine, 3 Na2ATP, 0.5 Na2GTP, and 0.1 leupeptin (pH 7.2–7.3, 265–270 mosmol l−1). The external solution contained (in mm): 127 NaCl, 20 CsCl, 10 Hepes, 5 BaCl2, 12 glucose, 1 CaCl2, 20 glycine, and 0.001 TTX (pH 7.3–7.4, 300–305 mosmol l−1). Recordings were obtained with the Axopatch 200B amplifier (Molecular Device, Sunnyvale, CA, USA) controlled and monitored with a PC running pCLAMP with a DigiData 1322A series interface. Electrode resistances were typically 3–5 MΩ in the bath. After sealing rupture, series resistance (4–10 MΩ) was compensated (60–70%) and periodically monitored. The cell membrane potential was held at −60 mV. NMDA (100 μm) was applied for 2 s every 30 s to minimize desensitization-induced decrease of current amplitude. Drugs were applied with a gravity-fed ‘sewer pipe’ system. Solution changes were affected by the SF-77B fast-step solution stimulus delivery device (Warner Instruments, Hamden, CT, USA). Data analyses were performed with Clampfit (Molecular Device) and KaleidaGraph (Albeck Software, Reading, PA, USA).
Biochemical measurement of surface and total proteins
The surface and total NR2A, NR2B and NR1 receptors were detected as previously described (Yuen et al. 2009, 2012). In brief, after treatment, rat cortical slices were incubated with PBS containing 1 mg ml−1 sulfo-N-hydroxysuccinimide-LC-biotin (Pierce Chemical Co., Rockford, IL, USA) for 20 min on ice. Slices were then rinsed three times in Tris-buffered saline to quench the biotin reaction, followed by homogenization in modified radioimmunoprecipitation assay buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mm Na3PO4, 150 mm NaCl, 2 mm EDTA, 50 mm NaF,10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, and 1 mg ml−1 leupeptin). The homogenates were centrifuged at 14,000 g for 15 min at 4°C. Protein (15 μg) was removed to measure total NR2A, NR2B and NR1. For surface protein, 150 μg of protein was incubated with 100 μl of 50% NeutrAvidin Agarose (Pierce Chemical Co.) overnight at 4°C. Bound proteins were resuspended in 100 μl of 2× loading buffer and boiled. Western blots were performed on both total and biotinylated (surface) proteins using anti-NR2A (1:500, EMD Millipore CO., Billerica, MA, USA, 07–632), anti-NR2B (1:500,, 06–600), or anti-NR1 antibody (1:1000, Cell Signaling Technology Inc., Danvers, MA, USA, 5704).
Co-immunoprecipitation
After treatment, rat cortical slices were collected and homogenized in NP-40 lysis buffer (0.5% NP-40, 10% glycerol, 50 mm Tris, pH 7.6, 150 mm NaCl, 30 mm sodium pyrophosphate, 50 mm NaF, 0.1 mm EDTA, 0.1 mm Na3VO4, and 1 mm phenylmethylsulfonyl fluoride, with protease inhibitor tablet). Lysates were ultracentrifuged (100,000 g) at 4°C for 1 h. Supernatant fractions were incubated with anti-Rab4 antibody (1:200, BD Bioscience, San Jose, CA, USA, 610889) or anti-rabaptin-5 antibody (2 μg, Santa Cruz Biotechnology, sc-15351) overnight at 4°C, followed by incubation with 50 μl of protein A/G plus agarose (Santa Cruz Biotechnology) for 1 h at 4°C. Immunoprecipitates were washed three times with lysis buffer, then boiled in 2x SDS loading buffer for 5 min, and separated on 10% SDS-polyacrylamide gels. Western blotting experiments were performed with anti-syntaxin 4 antibody (1:1000, Millipore, AB5330) or anti-Rab4 antibody (1:1000, BD Biosciences, 610889).
Immunocytochemical staining
Neurons grown on coverslips were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature and then washed three times with PBS. For the detection of surface NR1 receptors, cortical cultures were blocked and incubated with the anti-NR1 antibody (1:500, NeuroMab, Davis, CA, USA, 75–272) at 4°C overnight. After washing, cultures were incubated with an Alex568-conjugated secondary antibody (1:500, Invitrogen) for 1 h at room temperature. To co-stain NR1 with PSD-95, neurons were fixed and permeabilized with 0.1% Triton X-100 in PBS for 20 min, followed by 1 h incubation with 5% bovine serum albumin (BSA) to block non-specific staining. Neurons were then incubated with the anti-PSD-95 antibody (1:1000, NeuroMab, 2507) and anti-NR1 antibody (1:300, Cell Signalling, 5704) at 4°C overnight. After three washes, they were incubated with an Alex568 (red) or Alex488 (green) conjugated secondary antibody (1:1000, Invitrogen) at room temperature for 1 h. To detect syntaxin 4, neurons were fixed and permeabilized with 0.1% Triton X-100 and then incubated with anti-syntaxin 4 (1:500, Millipore, AB5330), followed by Alex568 (red) conjugated secondary antibody (1:500, Invitrogen). After washing in PBS, the coverslips were mounted on slides with VECTASHIELD mounting media.
Fluorescent images were obtained using a 100× objective with a cooled CCD camera mounted on a Nikon microscope. The surface NR1 clusters, total NR1, total PSD-95 and their localizations were measured using Image J software as previously described (Yuen et al. 2011; Gu et al. 2012). All specimens were imaged and analysed under identical conditions and parameters. To define dendritic clusters, a single threshold was chosen manually, so that clusters corresponded to puncta of at least twofold greater intensity than the diffuse fluorescence on the dendritic shaft. Three to four independent experiments for each of the treatments were performed. On each coverslip, the cluster density, size, and fluorescence intensity of four to six neurons (2–3 dendritic segments of at least 50 μm length per neuron) were measured. Quantitative analyses were conducted blind (without knowledge of experimental treatment).
Statistics
All data are expressed as the mean ± SEM. Experiments with two groups were analysed statistically using unpaired Student's t tests. Experiments with more than two groups were subjected to one-way ANOVA, followed by post hoc Tukey tests.
Results
Group II mGluRs regulate NMDAR surface expression and synaptic localization
To study the regulation of NMDA receptors by group II mGluRs, we firstly examined the effect of APDC, a highly selective mGluR2/3 agonist (Schoepp et al. 1996; Carlton et al. 2011), on NMDAR currents in cultured cortical neurons. As shown in Fig. 1A–C, application of APDC (50 μm) caused a significant and reversible enhancement of NMDAR currents (19.2 ± 0.6%, n= 20, P < 0.001, ANOVA). To identify the receptors involved, we examined the ability of LY341495, a selective antagonist of group II mGluRs (Kingston et al. 1988), to prevent the action of APDC. Application of LY341495 alone (0.5 μm) had no effect on the amplitude of NMDAR currents (−1.3 ± 1.5%, n= 16, P > 0.05, ANOVA). However, the potentiation of NMDAR currents by APDC (50 μm) was effectively abolished in the presence of LY341405 (1.2 ± 1.1%, n= 16, P < 0.001, ANOVA). These results are consistent with our previous study showing that group II mGluRs mediate the enhancing effect of APDC on NMDAR currents in acutely dissociated neurons (Tyszkiewicz et al. 2004).
Figure 1. Activation of Group II mGluRs increases NMDAR currents and NMDAR surface expression and synaptic localization.
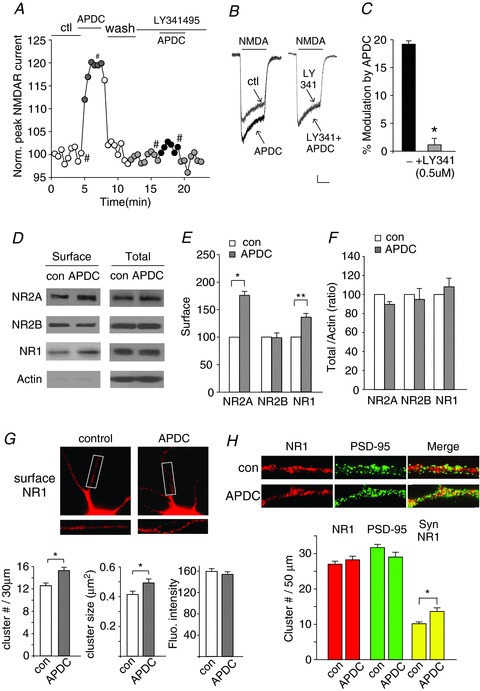
A, plot of peak NMDAR currents as a function of time and drug application in cultured cortical pyramidal neurons. APDC (50 μm): a selective group II mGluR agonist; LY341495 (0.5 μm): a selective group II mGluR antagonist. B, representative current traces taken from the recordings used to construct panel A (at time points denoted by #). Scale bars: 0.1 nA, 1 s. C, cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by APDC in the absence or presence of LY341495. *P < 0.001, ANOVA. D–F, immunoblots (D) and quantitative analysis (E and F) of the surface and total NMDAR subunits in cortical slices treated without (con) or with APDC (50 μm, 10 min). *P < 0.001, **P < 0.01, t test. G, immunocytochemical images and quantitative analysis of surface NR1 in cortical cultures treated without (control) or with APDC (50 μm, 10 min). Enlarged versions of the boxed regions of dendrites are shown beneath each of the images. Surface NR1 cluster density, size and fluorescence intensity were analysed. *P < 0.05, t test. H, immunocytochemical images and quantitative analysis (cluster size) of total NR1 clusters (red puncta), PSD-95 clusters (green puncta) and synaptic NR1 (PSD-95 colocalized, yellow puncta) along dendrites of cortical cultures treated without or with APDC (50 μm, 10 min). *P < 0.05, t test.
The group II mGluR-induced enhancement of NMDAR currents could be a result of increased level of NMDARs on the plasma membrane. To test this possibility, we examined the surface expression of NMDAR subunits in cortical slices in the absence or presence of APDC (50 μm, 10 min), using the surface biotinylation and Western blotting methods (Yuen et al. 2011, 2012). As shown in Fig. 1D and E, surface levels of NR2A and NR1 subunits were significantly increased by APDC treatment (NR2A: 76.2 ± 6.9%, n= 5, P < 0.001, t test; NR1: 36.4 ± 7%, n= 5, P < 0.01, t test), whereas surface NR2B subunits remained unchanged (−2 ± 8%, n= 5, P > 0.05, t test). The total protein levels of NMDAR subunits in cortical slices were not altered by APDC treatment (Fig. 1D and F). These data indicate that group II mGluRs primarily regulate the trafficking of NR1/NR2A-containing NMDARs. Consistently, our previous electrophysiological and pharmacological studies have shown that ifenprodil, a selective NR2B inhibitor, failed to block the enhancing effect of APDC on NMDAR currents (Tyszkiewicz et al. 2004), suggesting that NR1/NR2A channels are the main targets of group II mGluRs (Tyszkiewicz et al. 2004).
Next, immunocytochemical experiments were performed to confirm the effect of APDC on NMDAR trafficking. As shown in Fig. 1G, APDC (50 μm, 10 min) caused a large increase in the surface NR1 cluster density (number of clusters per 30 μm dendrite; control: 12.6 ± 0.5, n= 31; APDC: 15.3 ± 0.5, n= 31, P < 0.05, t test). The cluster size (μm2) of surface NR1 was also up-regulated by APDC treatment (control: 0.42 ± 0.02, n= 31; APDC: 0.50 ± 0.03, n= 31, P < 0.05, t test). Fluorescence intensity of surface NR1 cluster was unchanged (control: 159.5 ± 5.0, n= 31; APDC: 154.0 ± 5.0, n= 31, P > 0.05, t test). These results further suggest that NMDAR clusters on the plasma membrane are increased by the activation of group II mGluRs.
Since NMDARs are expressed both synaptically and extrasynaptically, we investigated the effect of APDC on NMDARs at synapses. Synaptic NMDAR clusters were measured by detecting NR1 colocalized with the synaptic marker PSD-95. As shown in Fig. 1H, APDC treatment (50 μm, 10 min) induced a remarkable enhancement of synaptic NR1 (colocalized with PSD-95) cluster density (number of clusters per 50 μm dendrite) in cultured cortical neurons (control: 10.2 ± 0.5, n= 48; APDC: 13.6 ± 1.0, n= 30, P < 0.05, t test). The total NR1 and PSD-95 cluster intensity remained unchanged by APDC. These results indicate that APDC increases the number of synaptic NMDARs.
SNAP-25 is involved in group II mGluR-induced enhancement of NMDAR currents
The group II mGluR-induced potentiation of NMDAR currents is accompanied by increased surface NMDARs at synapses, suggesting that the activation of group II mGluRs might influence the membrane delivery of NMDARs. It is known that SNAREs are the key protein family driving membrane fusion in all eukaryotic cells (Jahn & Scheller, 2006). During intracellular vesicle fusion, SNAREs, including SNAP-25, syntaxins (t-SNARE, located at target membrane) and VAMP/synaptobrevin (v-SNARE, located at vesicle membrane), assemble into stable complexes that force membranes to link tightly together (O’Connor et al. 1994; Jahn & Scheller, 2006). It has been well characterized that SNAREs regulate synaptic vesicle exocytosis in presynaptic terminals (O’Connor et al. 1994). However, recent studies have also shown that SNAREs mediate membrane fusion events in postsynaptic neurons and are critical in the control of synaptic strength (Lledo et al. 1998; Kennedy et al. 2010; Suh et al. 2010).
To study the involvement of SNAREs in the NMDAR trafficking regulated by group II mGluRs, we dialysed neurons with botulinum toxin (BoTx), which disrupts the SNARE complex by cleaving the v-SNAREs of VAMP/synaptobrevin family proteins (Schiavo et al. 1993). As shown in Fig. 2A–C, dialysis with BoTx (0.5 μm) abolished the enhancing effect of APDC on NMDAR currents (control: 19.0 ± 1.2%, n= 10; +BoTx: 6.0 ± 2.5%, n= 7, P < 0.01, t test), indicating the participation of SNAREs in the group II mGluR-induced potentiation of NMDARs.
Figure 2. Inhibiting the SNARE protein SNAP-25 blocks group II mGluR-induced enhancement of NMDAR currents.
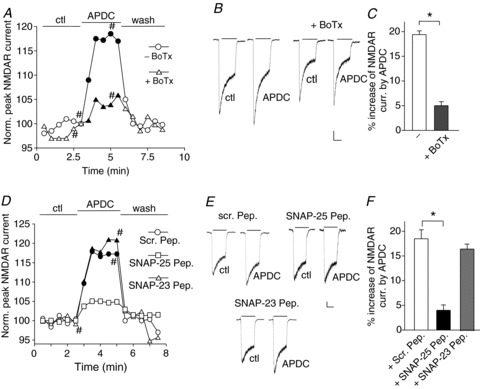
A, plot of normalized peak NMDAR currents showing the effect of APDC (50 μm) in neurons dialysed without or with botulinum toxin (BoTx, 0.5 μm). B, representative current traces taken from the records used to construct panel A (at time points denoted by #). Scale bars: 0.1 nA, 1 s. C, cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by APDC in the absence or presence of BoTx. *P < 0.01, t test. D, plot of normalized peak NMDAR currents showing the effect of APDC (50 μm) in neurons dialysed with a scrambled peptide (1 mm), a SNAP-25 blocking peptide (1 mm) or a SNAP-23 blocking peptide (1 mm). E, representative current traces taken from the records used to construct D (at time points denoted by #). Scale bars: 0.1 nA, 1 s. F, cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by APDC in the presence of various peptides. *P < 0.01, ANOVA.
Next, we investigated the role of SNAP-25, a key member of the SNARE complex (Jahn & Scheller, 2006). SNAP-25 is predominately expressed in neuronal systems (Oyler et al. 1989) and is involved in protein kinase C (PKC)-dependent incorporation of surface NMDARs (Lau et al. 2010). We dialysed neurons with a blocking peptide that mimics the N-terminal domain of SNAP-25, which disrupts the interaction of SNAP-25 with N-ethylmaleimide-sensitive factor (NSF) that is required for the assembly–disassembly cycle of SNAREs (Lledo et al. 1998; Jahn & Fasshauer, 2012). The scrambled peptide serves as a negative control (Lledo et al. 1998). As shown in Fig. 2D–F, dialysis with the SNAP-25 blocking peptide (1 mm, Lledo et al. 1998) significantly attenuated the enhancing effect of APDC on NMDAR currents (scrambled peptide: 19.4 ± 0.8%, n= 12; SNAP-25 peptide: 4.1 ± 1.1%, n= 11, P < 0.01, ANOVA).
SNAP-23, a homologue of SNAP-25 (Vaidyanathan et al. 2001), associates with syntaxin 4 in the SNARE complexes (St-Denis et al. 1999) and regulates postsynaptic glutamate receptors (Suh et al. 2010). We next examined the participation of SNAP-23 by dialysing neurons with a blocking peptide that mimics the N-terminal domain of SNAP-23. This peptide disrupts the interaction of SNAP-23 with syntaxin 4 (Vaidyanathan et al. 2001), which is required for vesicle exocytosis (Predescu et al. 2005; Kawaguchi et al. 2010). As shown in Fig. 2D–F, the SNAP-23 blocking peptide (1 mm) failed to abolish the enhancing effect of APDC on NMDAR currents (16.6 ± 1.0%, n= 12). These data suggest that SNAP-25, but not SNAP-23, plays a role in the group II mGluR-induced increase of functional NMDARs.
Syntaxin 4 is required for the group II mGluR-induced enhancement of NMDAR currents
The syntaxin family of proteins is another key component of SNARE complex. Among syntaxin members, syntaxin 4 (Stx4) is localized at the plasma membrane in the postsynaptic terminals (Sherry et al. 2006; Kennedy et al. 2010). Previous studies have shown that syntaxin 4 binds to SNAP-25 (Hu et al. 2007) and is critical for the activity-dependent exocytosis of AMPA receptors in dendritic spines (Kennedy et al. 2010). To examine whether syntaxin 4 is involved in the group II mGluR-induced regulation of NMDARs, we used a shRNA (Kennedy et al. 2010) to knockdown the endogenous syntaxin 4 in cultured cortical neurons. Previous studies have demonstrated that the syntaxin 4 shRNA led to a significant suppression of syntaxin 4 expression in 3T3 cultures and hippocampal neurons (Kennedy et al. 2010). In this study, the knockdown efficacy of syntaxin 4 shRNA was also confirmed in cultured cortical neurons (Fig. 3A; ∼90% suppression in GFP-positive neurons, n= 12). Cellular knockdown of syntaxin 4 abolished the enhancing effect of APDC on NMDAR currents (Fig. 3B–D; GFP: 16.3 ± 0.8%, n= 12; Stx4 shRNA: 5.3 ± 0.6%, n= 12, P < 0.01, t test). These results indicate that syntaxin 4 is required for the group II mGluR-induced potentiation of NMDARs.
Figure 3. Knockdown of syntaxin 4 blocks group II mGluR-induced enhancement of NMDAR currents.
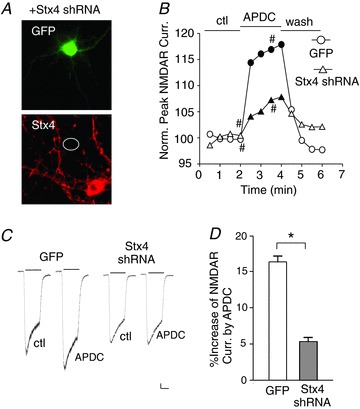
A, immunocytochemical staining of Stx4 in cultured cortical neurons co-transfected with syntaxin 4 shRNA (Stx4 shRNA) and GFP. B, plot of normalized peak NMDAR currents showing the effect of APDC (50 μm) in neurons transfected with GFP or Stx4 shRNA. C, representative current traces taken from the records used to construct panel B (at time points denoted by #). Scale bars: 0.1 nA, 1 s. D, cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by APDC in neurons with different transfections. *P < 0.01, t test.
Rab4-mediated NMDAR recycling underlies the group II mGluR-induced enhancement of NMDAR currents
In addition to SNAREs, several protein families are suggested to be involved in vesicle trafficking and fusion. Among them, Rab family of small GTPases has been shown to specifically regulate the vesicle transport between organelles and acts as the upstream molecule of SNAREs (Gerst, 1999; Zerial M, 2001). In particular, Rab4 controls a rapid direct recycling route from early endosomes to cell surface (van der Sluijs et al. 1992), which could potentially be involved in the group II mGluR-induced regulation of NMDAR trafficking. To test this hypothesis, we performed siRNA knockdown of Rab4 in cortical neurons. The knockdown efficacy of the Rab4 siRNA has been demonstrated in our previous study (Yuen et al. 2011). As shown in Fig. 4A and B, Rab4 knockdown remarkably eliminated the APDC-induced increase of NMDAR currents (scrambled siRNA: 19.2 ± 1.0%, n= 10; Rab4 siRNA: 3.4 ± 0.5%, n= 12, P < 0.01, ANOVA), but had no effect on basal NMDAR current density (pA pF−1; scrambled siRNA: 31.7 ± 6.1, n= 8; Rab4 siRNA: 34.5 ± 4.7, n= 8, P > 0.05, t test). To further confirm the involvement of Rab4, a dominant-negative Rab4 (DN-Rab4, Rab4-S27N) was used (Odley et al. 2004). As shown in Fig. 4C, APDC failed to potentiate NMDAR currents in neurons transfected with DN-Rab4 (5.8 ± 1.7%, n= 8, P < 0.01, ANOVA). These data suggest that Rab4 is critical for the potentiation of NMDAR currents by group II mGluRs.
Figure 4. Rab4 is required for APDC enhancement of NMDAR currents.
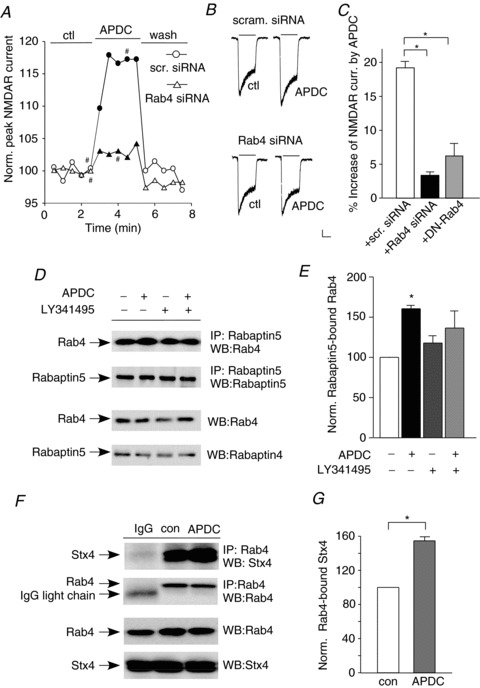
A, plot of normalized peak NMDAR currents showing the effect of APDC (50 μm) in neurons transfected with a scrambled siRNA or Rab4 siRNA. B, representative current traces taken from the records used to construct panel A (at time points denoted by #). Scale bars: 0.1 nA, 1 s. C, cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by APDC in neurons transfected with a scrambled siRNA, Rab4 siRNA or dominant-negative Rab4 (DN-Rab4). *P < 0.01, ANOVA. D, representative co-immunoprecipitation blots showing the effect of APDC (50 μm, 10 min) on active (Rabaptin5-bound) Rab4 in cortical slices pretreated without or with the mGluR2/3 antagonist LY341495 (0.5 μm, 10 min). E, quantitative analysis (mean ± SEM) showing the level of active (Rabaptin5-bound) Rab4 with different treatments. *P < 0.01, ANOVA. F, representative co-immunoprecipitation blots showing the effect of APDC (50 μm, 10 min) on the interaction between syntaxin 4 and Rab4 in cortical slices. G, quantitative analysis (mean ± SEM) showing the level of Rab4-bound syntaxin 4 in control vs. APDC-treated cortical slices. *P < 0.01, t test.
Next we examined the impact of group II mGluRs on Rab4 activity. Since Rabaptin-5 only binds to the GTP-bound active Rab4 at its N terminus (Vitale et al. 1998), co-immunoprecipitation experiments were performed to measure the Rabaptin-5-bound active Rab4. As shown in Fig. 4D and E, APDC treatment (50 μm, 10 min) induced a significant enhancement of active (Rabaptin-5-bound) Rab4 in rat cortical slices (1.62 ± 0.09-fold of control, n= 4, P < 0.01, ANOVA), and this effect was significantly attenuated by LY341495 (0.5 μm, LY: 1.18 ± 0.10-fold of control; APDC+LY: 1.36 ± 0.21-fold of control, n= 4, P>0.05, ANOVA). These results indicate that group II mGluRs enhance Rab4 activity, which could facilitate the Rab4-mediated protein recycling to the plasma membrane.
Previous studies have shown that Rab4 directly binds to syntaxin 4 when it adopts an active open conformation, which allows syntaxin 4 to enter the SNARE complexes leading to the membrane fusion (Li et al. 2001). We then examined whether Rab4–syntaxin 4 interaction is regulated by group II mGluRs. Co-immunoprecipitation experiments indicated that Rab4 was bound to syntaxin 4 in cortical slices, and their association was significantly increased by APDC treatment (Fig. 4F and G; 54.6 ± 4.4%, n= 5, P < 0.001, t test). It further suggests that the activation of group II mGluRs facilitates Rab4–SNARE-mediated exocytosis of target proteins.
Discussion
In this study, we have revealed the key molecules involved in the regulation of NMDA receptors by group II mGluRs (Fig. 5). Previous studies suggested that the agonists of group II mGluRs could be potential antipsychotics due to their inhibition of presynaptic glutamate release (Kamiya et al. 1996; Schoepp, 2001). In this study, we found that activation of group II mGluRs in postsynaptic neurons significantly increased the surface and synaptic NR1/NR2A-containing NMDAR clusters. NR2A subunit is mainly expressed in the synaptic sites of mature neurons and shows different properties from NR2B subunit (Yashiro & Philpot, 2008). It has been found that NR2A plays a dominant role in the phencyclidine-induced apoptosis and the development of schizophrenia-like behaviours (Anastasio et al. 2009). NR2A is selectively altered in the medial prefrontal cortex of rats reared in isolation, a preclinical model of schizophrenia (Turnock-Jones et al. 2009). Moreover, the aberrant gamma activity in the schizophrenia model induced by NMDAR antagonist is primarily mediated by NR2A-containing NMDARs (Kocsis, 2012). Hence, the selective up-regulation of NR2A-containing NMDARs by group II mGluRs agonists may account for their beneficial effects as potential antipsychotics.
Figure 5. Schematic diagram showing the potential mechanism underlying the regulation of NMDARs by group II mGluRs.
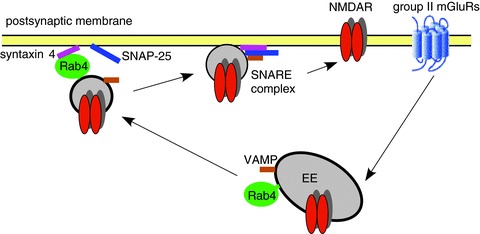
Activation of group II mGluRs increases Rab4 activity and the interaction between Rab4 and syntaxin 4, facilitating the formation of SNARE complexes composed of SNAP-25, syntaxin 4 and VAMP at postsynaptic sites, which leads to the increased exocytosis of NMDARs. Consequently, NMDAR surface expression and synaptic function are up-regulated by the activation of group II mGluRs.
Mounting evidence suggests that diverse protein families are involved in the regulation of NMDAR trafficking (Wenthold et al. 2003; Prybylowski & Wenthold, 2004; Lau & Zukin, 2007). Dendritic SNARE proteins play a critical role in the late stage of synaptic vesicle exocytosis (Ovsepian & Dolly, 2011), including the regulation of postsynaptic glutamate receptor trafficking (Lledo et al. 1998). SNAP-25, a key component of SNARE complex, is expressed in excitatory neurons and localized at both presynaptic and postsynaptic terminals (Schwab et al. 2001). Previous studies have shown that SNAP-25 participates in the postsynaptic fusion events contributing to LTP (Lledo et al. 1998) and PKC-dependent insertion of synaptic NMDARs (Lan et al. 2001; Lau et al. 2010). In agreement with these results, we revealed that SNAP-25 is involved in the group II mGluR-induced potentiation of NMDAR currents.
A homologue of SNAP-25, SNAP-23 (59% identical with SNAP-25), is expressed in the brain (Chen et al. 1999) and binds to multiple syntaxins and synaptobrevins (Ravichandran et al. 1996). Recent studies indicate that SNAP-23 is predominantly localized at dendritic spines (Suh et al. 2010). Loss of SNAP-23 led to a marked decrease of surface NMDARs (Suh et al. 2010). However, we did not find any involvement of SNAP-23 in the group II mGluR-induced regulation of NMDAR currents. The underlying reason for this is unclear. Probably it is because SNAP-23 is more involved in the basal recycling of NMDARs (Suh et al. 2010), or because SNAP-23 binds to other SNARE family members less efficiently than SNAP-25 (Vaidyanathan et al. 2001).
Syntaxins, a key component of the SNARE complex, contain 16 members in mammalian cells (Gerst, 1999). Among them, syntaxins 1–4 are localized to the plasma membrane (Bennett et al. 1993), and only syntaxin 1 and syntaxin 4 are highly expressed in the brain. While syntaxin 1 is present presynaptically and its role in regulating transmitter release has been well studied, the specific syntaxin member involved in postsynaptic membrane fusion is unclear. Syntaxin 4, which had been shown to be present postsynaptically (Sherry et al. 2006; Kennedy el al. 2010), was recently shown to direct the membrane fusion of AMPAR-containing recycling vesicles at hippocampal synapses (Kennedy et al. 2010; Mohanasundaram & Shanmugam, 2010). In this study, we showed that syntaxin 4 was required for the group II mGluR-induced enhancement of NMDAR currents, suggesting that syntaxin 4 plays an important role in regulating the exocytosis of NMDARs.
Rab family GTPases, suggested as the SNARE regulators acting upstream of SNARE complex assembly, mediate directional transport of vesicles between different organelles (Gerst, 1999; Pfeffer, 2001). Rab proteins associate with membranes in their GTP-bound active form and are dissociated in their GDP-bound inactive form. This dynamic cycling is essential for their functioning (Fukuda, 2008). Among Rab proteins, Rab4 is the central player in the fast recycling of μ-opioid receptor (Roman-Vendrell et al. 2012), β2-adrenergic receptor (Yudowski et al. 2009) and glutamate receptors (Yuen et al. 2011). Rab4 binds to syntaxin 4 when it adopts an active open conformation (Li et al. 2001). The binding of Rab proteins to SNAREs leads to the dissociation of negative regulators for SNARE complex formation (Gerst, 1999). Here, we found that knockdown of Rab4 or expression of dominant-negative Rab4 blocked the group II mGluR-induced enhancement of NMDAR currents, indicating that Rab4 is important for the regulation of NMDAR exocytosis. Furthermore, we found that the interaction between Rab4 and syntaxin 4 was increased by group II mGluRs agonist, suggesting that group II mGluRs activation may facilitate the formation of stable SNARE complexes mediating membrane fusion.
In addition to coupling to the Gi–PKA pathway, group II mGluRs are also linked to phospholipase C (Klein et al. 1997; Otani et al. 2002), which will lead to the activation of PKC. Our previous study has shown that APDC enhances NMDAR currents via a PKC-dependent mechanism (Tyszkiewicz et al. 2004). Since Rab4, SNAP25 and syntaxin 4 are all regulated by PKC (Chung et al. 2000; Lan et al. 2001; Imamura et al. 2003; Lau et al. 2010), group II mGluRs may enhance the SNARE-mediated NMDAR exocytosis through PKC signalling.
In summary, our findings have shown that SNARE complexes, composed of SNAP-25 and syntaxin 4, and the Rab4 protein are critically involved in the increased membrane delivery of NMDA receptors by group II mGluRs in cortical pyramidal neurons. Given the role of NMDAR hypofunction in the pathology of schizophrenia (Kantrowitz & Javitt, 2012), the up-regulation of NMDAR function could be one of the mechanisms underlying the antipsychotic effects of group II mGluRs.
Acknowledgments
We thank Xiaoqing Chen and Dr Jing Wei for excellent technical support.
Glossary
- APDC
(2R, 4R)-4-aminopyrrolidine-2,4-dicarboxylate;
- BoTx
botulinum toxin
- GFP
green fluorescent protein
- mGluR
metabotropic glutamate receptor
- NMDAR
N-methyl-d-aspartate receptor
- PKC
protein kinase C
- PSD-95
postsynaptic density protein 95
- siRNA
small interference RNA
- shRNA
short hairpin RNA
- SNAP-25
synaptosomal-associated protein 25
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
All the experiments were performed in the research laboratory of Z.Y. in the Department of Physiology and Biophysics, State University of New York at Buffalo. Conception and design of the experiments: Z.Y., and J.C.; collection, analysis and interpretation of data: J.C., W.L. and L.D.; drafting the article or revising it critically for important intellectual content: Z.Y. and J.C. All authors approved the final version of the manuscript. The authors declare no conflict of interest.
Funding
This work is supported by NIH grants MH84233 and MH85774 (Z.Y.).
Reference
- Anastasio NC, Xia Y, O’Connor ZR, Johnson KM. Differential role of N-methyl-D-aspartate receptor subunits 2A and 2B in mediating phencyclidine-induced perinatal neuronal apoptosis and behavioral deficits. Neuroscience. 2009;163:1181–1191. doi: 10.1016/j.neuroscience.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosc Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Govea R, Du J. Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors. J Neurosci. 2011;31:12727–12737. doi: 10.1523/JNEUROSCI.6558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Shinozaki H, Bockaert J, Fagni L. The metabotropic glutamate receptor types 2/3 inhibit L-type calcium channels via a pertussis toxin-sensitive G-protein in cultured cerebellar granule cells. J Neurosci. 1994;14:7067–7076. doi: 10.1523/JNEUROSCI.14-11-07067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Minger SL, Honer WG, Whiteheart SW. Organization of the secretory machinery in the rodent brain: distribution of the t-SNAREs, SNAP-25 and SNAP-23. Brain Res. 1999;831:11–24. doi: 10.1016/s0006-8993(99)01371-2. [DOI] [PubMed] [Google Scholar]
- Chung SH, Polgar J, Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. J Biol Chem. 2000;275:25286–91. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X. Basic pharmacology of NMDA receptors. Curr Pharm Des. 2012;18:1558–1567. doi: 10.2174/138161212799958521. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signalling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Wei J, Yan Z. Regulation of N-methyl-D-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J Biol Chem. 2012;287:10265–10275. doi: 10.1074/jbc.M111.325175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harich S, Gross G, Bespalov A. Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology. 2007;192:511–519. doi: 10.1007/s00213-007-0742-y. [DOI] [PubMed] [Google Scholar]
- Hu C, Hardee D, Minnear F. Membrane fusion by VAMP3 and plasma membrane t-SNAREs. Exp Cell Res. 2007;313:3198–3209. doi: 10.1016/j.yexcr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM. Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol Cell Biol. 2003;23:4892–900. doi: 10.1128/MCB.23.14.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J, Javitt DC. Glutamatergic transmission in schizophrenia: from basic research to clinical practice. Curr Opin Psychiatry. 2012;25:96–102. doi: 10.1097/YCO.0b013e32835035b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T, Tamori Y, Kanda H, Yoshikawa M, Tateya S, Nishino N, Kasuqa M. The t-SNAREs syntaxin4 and SNAP23 but not v-SNARE VAMP2 are indispensable to tether GLUT4 vesicles at the plasma membrane in adipocyte. Biochem Biophys Res Commun. 2010;391:1336–41. doi: 10.1016/j.bbrc.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Klein J, Reymann KG, Riedel G. Activation of phospholipases C and D by the novel metabotropic glutamate receptor agonist tADA. Neuropharmacology. 1997;36:261–263. doi: 10.1016/s0028-3908(96)00174-8. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–693. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein Kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–90. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Li L, Omata W, Kojima I, Shibata H. Direct interaction of Rab4 with syntaxin 4. J Biol Chem. 2001;276:5265–5273. doi: 10.1074/jbc.M003883200. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mohanasundaram P, Shanmugam MM. Role of syntaxin 4 in activity-dependent exocytosis and synaptic plasticity in hippocampal neurons. Sci Signal. 2010;3:jc7. doi: 10.1126/scisignal.3144jc7. [DOI] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Steckler T, Wettstein JG, Mackie C, Spooren W. Metabotropic glutamate receptor modulation, translational methods, and biomarkers: relationships with anxiety. Psychopharmacology. 2008;199:389–402. doi: 10.1007/s00213-008-1096-9. [DOI] [PubMed] [Google Scholar]
- O’Connor V, Augustine GJ, Betz H. Synaptic vesicle exocytosis: Molecules and models. Cell. 1994;75:785–787. doi: 10.1016/0092-8674(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW., 2nd Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acas Sci U S A. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993a;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993b;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–3444. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV, Dolly JO. Dendritic SNAREs add a new twist to the old neuron theory. Proc Natl Acas Sci U S A. 2011;108:19113–19120. doi: 10.1073/pnas.1017235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Consolandi O, Memo M, Spano PF. Activation of multiple metabotropic glutamate receptor subtypes prevents NMDA-induced excitotoxicity in rat hippocampal slices. Eur J Neurosci. 1996;8:1516–1521. doi: 10.1111/j.1460-9568.1996.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB. Cholestrol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J Biol Chem. 2005;280:37130–37138. doi: 10.1074/jbc.M505659200. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Wenthold RJ. N-Methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem. 2004;279:9673–9676. doi: 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Roman-Vendrell C, Yu YJ, Yudowski GA. Fast Modulation of mu-opioid receptor (MOR) recycling is mediated by receptor agonists. J Biol Chem. 2012;287:14782–14791. doi: 10.1074/jbc.M111.319616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Ryden Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuro-psychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Laureto PP, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxin block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1993;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoepp DD, Salhoff CR, Wright RA, Johnson BG, Burnett JP, Mayne NG, Belagaje R, Wu S, Monn JA. The novel metabotropic glutamate receptor agonist 2R,4R-APDC potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5-dihydroxyphenylglycine: evidence for a synergistic interaction between group 1 and group 2 receptors. Neuropharmacology. 1996;35:1661–1672. doi: 10.1016/s0028-3908(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Schwab Y, Mouton J, Chasserot-Golaz S, Marty I, Maulet Y, Jover E. Calcium-dependent translocation of synaptotagmin to the plasma membrane in the dendrites of developing neurones. Mol Brain Res. 2001;96:1–13. doi: 10.1016/s0169-328x(01)00244-3. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Bechtold AG, Tham RC, Bonham AC. A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J Neurosci. 2009;29:11807–11816. doi: 10.1523/JNEUROSCI.2617-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Denis JF, Cabaniols JP, Cushman SW, Roche PA. SNAP-23 participates in SNARE complex assembly in rat adipose cells. Biochem J. 1999;338:709–15. [PMC free article] [PubMed] [Google Scholar]
- Ster J, Mateos JM, Grewe BF, Coiret G, Corti C, Corsi M, Helmchen F, Gerber U. Enhancement of CA3 hippocampal network activity by activation of group II metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2011;108:9993–9997. doi: 10.1073/pnas.1100548108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nature Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL, Taylor A, Jones DN, Emson PC, Southam E. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse. 2009;63:836–846. doi: 10.1002/syn.20665. [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J Physiol. 2004;554:765–777. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan VV, Puri N, Roche PA. The last exon of SNAP-23 regulates granule exocytosis from mast cells. J Biol Chem. 2001;276:25101–25106. doi: 10.1074/jbc.M103536200. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M MH. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]


