Abstract
Nontoxic naturally occurring compounds, especially those from dietary sources, are receiving increasing consideration for prevention and treatment of diseases including cancer. There is a growing need for innovative anticancer therapies and therefore search for natural compounds with novel biological activities or antineoplastic potential is currently an important area in drug discovery. Support for this interest also comes from increasing concern over the efficacy and safety of many conventional therapies, especially those that run over a long course of time. Laboratory studies in different in vitro and in vivo systems have shown that many natural compounds possess the capacity to regulate response to oxidative stress and DNA damage, suppress angiogenesis, inhibit cell proliferation and induce autophagy and apoptosis. This review discusses the induction of apoptosis and autophagy as a mechanism of cancer prevention by some of the most studied naturally occurring dietary compounds.
Keywords: Apoptosis, Autophagy, Chemoprevention, Cancer, Dietary, Agents
Introduction
Natural dietary agents have demonstrated their potential to prevent the occurrence and/or spread of cancer in various research studies, either alone or in combination with the chemotherapeutic agents [1, 2]. Consumption of vegetables, fruits, whole grains, and beverages, a wealth of biologically potent chemicals, have remarkable anti-inflammatory, antiviral, and anticancer activity and have been used in traditional medicines for thousands of years. Dietary agents have been shown to modulate cellular processes, exhibit chemopreventive and/or chemotherapeutic effects and induce apoptosis and autophagy against cancer [1, 2]. Cellular processes play a major role in cell survival and apoptosis. These events are essential for tissue homeostasis and the maintenance of proper growth and development of multicellular organisms [3]. Apoptosis is a genetically restricted process that plays an important role in embryogenesis, cellular homeostasis, and allows a sophisticated mechanism to remove infected, damaged, or mutated cells [3]. The two major pathways that initiate apoptosis are extrinsic pathway or death receptor-mediated and the intrinsic pathway or mitochondrial-mediated. The death receptor pathway involves transmembrane receptor-mediated interactions that include the tumor necrosis factor receptor (TNFR) gene superfamily [4]. In this pathway, the death domain transmits death signal from the cell surface to the intracellular signaling pathways. These death receptors (DR) and their ligands include FasL/FasR, TNF α/TNFR1, Apo3L/DR3, Apo2L/DR4 and Apo2L/DR5 [5–9]. Post ligand binding, the cytoplasmic adapter proteins are recruited and corresponding death domains bind with the receptors. For example, binding of Fas ligand to Fas receptor results in the binding of the adapter protein FADD and the binding of TNF ligand to TNF receptor results in the binding of the adapter protein TRADD with concomitant recruitment of FADD and RIP [10, 11]. FADD then associates with procaspase-8 and a death-inducing signaling complex (DISC) is formed which results in activation of procaspase-8 [12]. Death receptor mediated apoptosis can be inhibited by c-FLIP a protein that binds to FADD and caspase-8 which subsequently inactivates them [13, 14]. The intrinsic pathway or mitochondrial-initiated apoptosis includes stimuli that produce intracellular signals that initiate events within the cell. These signals cause changes in the inner mitochondrial membrane that results in an opening of the mitochondrial permeability transition (MPT) pore, loss of the mitochondrial transmembrane potential and release two groups of pro-apoptotic proteins from the intermembrane space into the cytosol [15]. The first group contains cytochrome c, second mitochondria-derived of activator of caspase (Smac)/direct IAP binding protein with low pI (DIABLO), and the serine protease HtrA2/Omi [16, 17]. These proteins activate the caspase dependent mitochondrial pathway. Cytochrome c binds and activates Apaf-1 as well as procaspase-9, forming an “apoptosome” [18, 19]. It is reported that Smac/DIABLO and HtrA2/Omi promote apoptosis by inhibiting IAP (inhibitors of apoptosis proteins) activity [20, 21]. The second group of pro-apoptotic proteins consists of AIF, endonuclease G and CAD and is released late from the mitochondria during apoptosis. Bcl-2 family of proteins, control and regulate apoptotic mitochondrial events [22]. The tumor suppressor gene, p53, has a role in regulation of the Bcl-2 family; however the exact mechanisms is not known yet [23]. The Bcl-2 family also control mitochondrial membrane permeability and can be pro-apoptotic such as Bcl-10, Bax, Bak, Bid, Bad, Bim, Bik, and Blk or anti-apoptotic that include Bcl-2, Bcl-x, Bcl-xL, Bcl-xS, Bcl-w, BAG. These proteins play a significant role in determining cell fate. The Bcl-2 family regulates cytochrome c release from the mitochondria by altering the permeability of mitochondrial membrane. Apoptotic pathways (the death-receptor pathway and the mitochondrial pathway), under certain circumstances may “cross talk” to enhance apoptosis. One example is the mitochondrial damage in the Fas pathway of apoptosis that is mediated by the caspase-8 cleavage of Bid [24]. Bad is associated with a family of multifunctional phosphoserine binding molecules. When phosphorylated, it is localized in the cytosol and upon unphosphorylation, it translocates to the mitochondria to release cytochrome c. Another role for Bad, is to heterodimerize with Bcl-xL or Bcl-2 and prevent their protective effect and promote cell death [25]. Bcl-2 and Bcl-xL inhibit the release of cytochrome c from the mitochondria when they are not bound with Bad. It is suggested that Bcl-2 and Bcl-xL inhibit apoptotic death primarily by controlling the activation of caspase proteases [26]. In addition, the protein “Aven” binds to both Bcl-xL and Apaf-1 and prevents activation of procaspase-9 [27]. There is evidence suggesting a reciprocal regulation between these two proteins and overexpression of either Bcl-2 or Bcl-xL down-regulates the other.
The execution phase, which is considered the final phase of apoptosis, involves the activation of the execution caspases. Execution caspases activate cytoplasmic endonucleases and proteases, which degrade nuclear material and nuclear and cytoskeletal proteins, respectively. Caspase-3, caspase-6, and caspase-7 function as effector or “executioner” caspases. They cleave various substrates including cytokeratin, PARP, the plasma membrane cytoskeletal protein alpha fodrin, the nuclear protein NuMA and others. All these events cause morphological and biochemical changes typically seen in apoptotic cells [28]. Caspase-3 is the most important of the effector caspases and is activated by the initiator caspases 8, -9 and -10. Caspase-3 activates the endonuclease CAD. In proliferating cells CAD is complexed with its inhibitor ICAD. In apoptotic cells, activated caspase-3 cleaves ICAD to release CAD [29]. CAD causes chromatin condensation by degrading chromosomal DNA within the nuclei. Caspase-3 also causes reorganization of the cytoskeletal and disintegration of the cell into apoptotic bodies. Gelsolin, an actin binding protein, has been identified as one of the key substrates of activated caspase-3.
Evidence suggests that mitochondria and endoplasmic reticulum (ER) play a critical role in death receptor-mediated apoptosis. Consequently deregulation of these pathways may contribute to drug resistance [30]. Imbalance in survival and apoptosis pathways may lead to the development of several diseases including cancer. Cancer is an example of impaired cell cycle regulation, with either an overproliferation of cells and/or decreased removal of cells [31]. Apoptosis suppression plays a critical role in the development and progress of some cancers during carcinogenesis [32]. There are a variety of molecular mechanisms that tumor cells use to suppress apoptosis either by expressing anti-apoptotic proteins such as Bcl-2 or by down regulation or mutation of pro-apoptotic proteins such as Bax. Both Bcl-2 and Bax expression are regulated by p53 tumor suppressor gene [33]. Molecules involved in cell death pathways are potential therapeutic targets in cancer (Figure 1). Therefore, understanding the molecular mechanisms of cell survival and apoptotic pathways is essential for the treatment and prevention of human diseases.
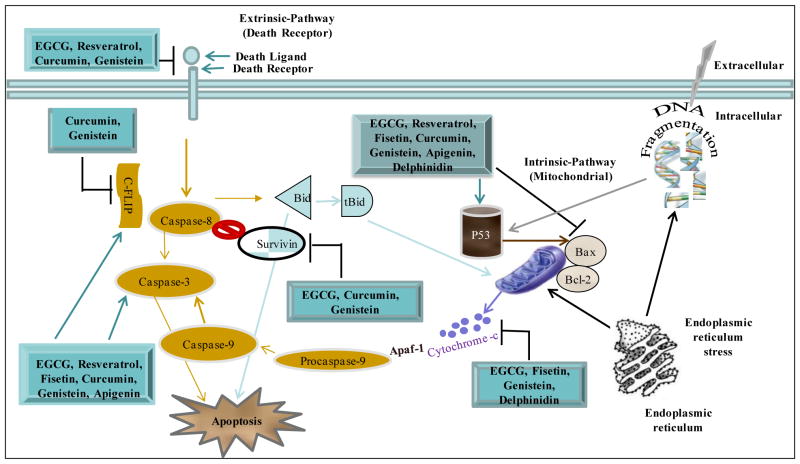
Figure 1.
Effects of dietary compounds on extrinsic and intrinsic pathways of apoptosis. The extrinsic pathway is triggered by death receptors and involves activation of the initiator caspase-8, which directly activates caspase-3 causing apoptosis. The intrinsic pathway is activated by different apoptotic stimuli that lead to release of cytochrome c from mitochondria and activation of caspase-9. The endoplasmic reticulum is triggered by stress and stimulates mitochondria to release cytochrome c and cause DNA damage. Many dietary agents affect molecules at several steps to initiate removal of unwanted cells by inducing apoptosis. EGCG is known to affect both the intrinsic as well as the extrinsic pathways and induces apoptosis in many cancer types.
Numerous natural agents have been investigated in cancer prevention. Many regulate the response to oxidative stress and others affect tumor proliferation and/or apoptosis. In the following sections, we discuss merits of a few popular naturally occurring dietary agents that have shown promise for treatment and/or prevention of cancer essentially by modulating one or more of above mentioned pathway (Figure 1; Table 1).
Table 1.
Mechanisms Of Dietary Compounds That Have Been Shown To Induce Apoptosis In Cancer Cells.
| Dietary compound | Target/Mechanisms | Ref # |
|---|---|---|
| EGCG | Activation of caspases and inhibition of NF-κB | [34] |
| Enhancement of TRAIL-induced apoptosis and activation of Fas | [35] | |
| Activation of p53 | [36] [38] |
|
| Activating capase-3/7 and inhibition of Bcl-2, survivin and XIAP | [39] | |
| Induction of Fas. Decreased phosphorylation of Akt and Erk1/2 | [40] | |
| Increase in Bax, and decrease in Bcl-2 | [42] | |
| ROS generation; and decreased Bax/Bcl-2 ratio | [46] | |
| Resveratrol | Activation of Fas, FADD and procaspase-8 | [47] |
| Caspase-3-dependent pathway | [49], [50] | |
| Activation of p53, release of cytochrome c, and alteration in Bax/Bcl-2 ratio | [52] | |
| Induction of ROS | [53] | |
| Cell cycle arrest at G2/M phase | [57] | |
| Fisetin | Release of cytochrome c from mitochondria into cytosol, downregulation of XIAP, and upregulation of Smac/DIABLO. Activation of caspase-3, -8 and -9. Induction of Bax, and decrease in Bcl-2. Inhibition of PI3K, and phosphorylation of Akt | [67] |
| Activation of p53. Inhibition of NF-κB activity | [68] | |
| Inhibition of VEGF. Cell cycle arrest at G1 and G2/M phases. Activation of p53 | [69] | |
| Decreased DR3 and increased the IkBalpha expression, and NF-κB inhibitor | [71] | |
| Genistein | Enhancement of TRIAL-induced apoptosis | [76] |
| Activation of caspase-9 and -3 release of cytochrome c |
[77] | |
| Activation of p53 Induction of G2/M phase |
[79] | |
| Downregulation of the expression of survivin and downregulation of EGFR, HER2, and ERα expression | [80] | |
| Increase in Bax/Bcl-2 ratio. Overexpression of calpain, caspase-12, and caspase-3, and apoptosis-inducing-factor | [81] | |
| Curcumin | Enhancement of TRAIL-induced apoptosis. Inhibition of NF-κB | [83] |
| Upregulation of caspase-3 and -9 activity | [88] | |
| Increased levels of cleaved caspase-3, caspase-7 and induction of ROS | [89] | |
| Apigenin | Induced Bid cleavage and the activation of caspases-8, -10, -9, and -3 | [91] |
| Reduced expression of phospho-JAK1 and phospho-STAT3 and activated NF-κB | [94] | |
| Delphinidin | Activation of caspases. Increase in Bax, Bid, Bak. Decrease in Bcl-2 and Bcl-xL | [95] |
| Inhibition of NF-κB | [99] | |
| Reduced HER2 and MAPK signaling | [101] |
I: (−)-Epigallocatechin-3-gallate (EGCG)
Many dietary compounds have been found to induce apoptosis of cancer cells without affecting normal cells. An example of this type of compound is epigallocatechin-3-gallate (EGCG), the major constituent of green tea (Table 2). At physiologically achievable concentrations, it causes induction of apoptosis and cell cycle arrest in many types of cancer cells without affecting normal cells [34]. Treatment of human prostate cancer cells LNCaP, PC-3, and CWR22Rν1 with EGCG combined with Cox-2 inhibitor resulted in enhanced cell growth inhibition, activation of caspases, apoptosis induction and inhibition of NF-κB [35]. EGCG was reported to sensitize TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis [36].
Table 2.
Structure And Sources Of Dietary Compounds Discussed.
| Compound | Sources | Structure |
|---|---|---|
| EGCG | Green tea |
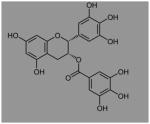
|
| Resveratrol | Grapes, Peanuts, Cocoa |
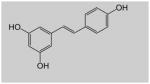
|
| Fisetin | Strawberries, Apples, Persimmon, Onions, Lotus root, Kiwis |
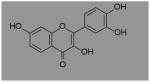
|
| Genistein | Lupin, Fava beans, Soybeans, Kudzu, Psoralea |
|
| Curcumin | Turmeric |
|
| Apigenin | Widely distributed in many fruits and vegetables, Parsley, Onions, Oranges, tea, Chamomile |
|
| Delphinidin | Pigmented fruits and vegetables; Cranberries, Concord grapes, Pomegranates |
|
Pretreatment of cells with EGCG resulted in the modulation of death-inducing signaling cascade complex involving DR4/TRAIL R1, Fas associated protein with death domain (FADD) and FLICE-inhibitory protein. Also, EGCG was observed to inhibit the invasion and migration of LNCaP cells through inhibition in the protein expression of vascular endothelial growth factor, urokinase plasminogen activator (uPA) and angiopoietin 1 and 2 [36]. Using isogenic cell lines, EGCG was observed to activate growth arrest and apoptosis in prostate carcinoma cells primarily via a p53-dependent pathway that involved the function of both p21 and Bax. Down-regulation of either p21 or Bax conferred a growth advantage to the cells [37]. Green tea polyphenols (GTPs) also functioned as histone deacetylases (HDACs) inhibitors and enhanced the p53 transcription activity and acetylation by inhibiting class 1 HDACs in LNCaP prostate cancer cells [38]. Also, GTPs induced the transcription activity of p21/waf1, and Bax enhanced the proteasomal degradation of class 1 HDACs and increased acetylation of histone H3, which led to cell cycle arrest and apoptosis of prostate cancer cells [39].
The self-renewal capacity of cancer stem cells (CSCs) was inhibited by EGCG in experiments with prostate cancer cells. EGCG induced apoptosis by activating capase-3/7 and inhibiting the expression of Bcl-2, survivin and XIAP in CSCs [40]. Also, EGCG prevented epithelial-mesenchymal transition by inhibiting the expression of vimentin, slug, snail and nuclear β-catenin, and the activity of LEF-1/TCF responsive reporter, and similarly retarded CSC’s migration and invasion. This indicated the EGCG’s ability to block the signaling molecules involved in early metastasis [40]. Treatment of human breast cancer MCF-7 cells with EGCG noticeably inhibited heregulin (HRG)-β-1 dependent induction of Fas mRNA and protein. Moreover, EGCG decreased the phosphorylation of Akt and Erk1/2 that were demonstrated as selected downstream HRG-β1-responsive kinases required for Fas expression using dominant-negative Akt, PI3K inhibitors or MEK inhibitor. Additionally, growth inhibition of HRG-β1-treated cells paralleled the suppression of Fas by EGCG [41]. Pretreatment of breast cancer cells with EGCG resulted in a decrease in both the mRNA as well as protein expression of survivin [42].
It is well known that the cell death through apoptosis depends on the balance between proteins that mediate and those that oppose cell death. Treatment with EGCG and GTP triggered Bax to increase, Bcl-2 to decrease and resulted in PARP cleavage in MDA-MB-231 human breast cancer cells [43]. EGCG treatment of human epidermoid carcinoma A431 cells resulted in significant activation of caspases, as evident by the dose- and time-dependent increase in DEVDase activity, and protein expression of caspase-3, -8 and -9. EGCG-mediated induction of apoptosis was blocked by the caspase inhibitor in these cells [44]. In multiple myeloma cells, EGCG treatment caused cell growth arrest and apoptosis by inducing the expression of death associated protein kinase 2, Fas ligand, Fas, and caspase-4, positive regulators of apoptosis and NF-κB activation and cyclin-dependent kinase inhibitors [45]. Several apoptosis signals convert mitochondrial permeability transition pore (MTP), a multiprotein complex, into an unspecific pore and activate mitochondria for the cellular self-destruction process. In human pancreatic cancer cells, EGCG induced Bax oligomerization, depolarization of mitochondrial membranes to facilitate cytochrome c release into cytosol and caspase-dependent apoptosis [46]. In human tongue squamous carcinoma CAL-27 cells, both green and black tea polyphenols generated reactive oxygen species (ROS) and a decrease in the Bax/Bcl-2 ratio. This induced mitochondrial permeability transition with consequent activation of caspase-3, which caused cellular apoptosis [47].
II: Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene), a type of natural phenol and a phytoalexin found in grape skin and various other food products (Table 2), is believed to have multiple bioactivities including antitumor and anti-inflammatory, and is able to produce chemopreventive effects in experimental models. The mechanisms by which resveratrol might mediate cell death include necrosis, apoptosis, autophagy, and others. Resveratrol has the ability to induce the clustering of Fas and its redistribution in cholesterol and sphingolipid-rich fractions of SW480 cells, together with FADD and procaspase-8. This redistribution may have contributed to the molecule’s ability to trigger apoptosis in colon cancer cells [48]. Resveratrol-induced apoptosis of acute monocytic leukaemia was independent of the Fas/FasL signaling pathway and, even more, resveratrol did not induce differentation of THP-1 cells which may serve as a chemotherapeutic agent for the control of acute monocytic leukaemia [49]. Resveratrol was shown to induce apoptosis in human breast cancer cells primarily through the caspase-3-dependent pathway [50]. The same findings were found for bladder cancer cells, which suggested that resveratrol triggered apoptosis through activation of caspase--9 and caspase-3 in a variety of human cancers [51]. PI3K inhibitors abolished the effect of trans-resveratrol, the other isoform of RSV, on p53 activation [52]. Treatment of resveratrol to mouse’s skin tumors induced the expression of p53 and Bax, with a concomitant decrease in Bcl-2. This alteration in Bax/Bcl-2 ratio, release of cytochrome c and induction of apoptotic protease activating factor 1 (Apaf-1) resulted in apoptosis [53]. In human pancreatic cancer cells, resveratrol caused damage of mitochondrial function that led to increased ROS, apoptosis and possibly intracellular drug accumulation via inhibition of proteins involved in multi-drug resistance [54]. Trans-resveratrol inhibited cell proliferation without cytotoxicity and was able to induce apoptosis in HT-29 colorectal carcinoma via a ROS-dependent apoptosis pathway [55]. Resveratrol induced the apoptotic death of U251 glioma cells via multiple signaling pathways. This included activation of caspase-3 and increased the cleavage of the downstream caspase substrate, poly (ADP-ribose) polymerase, induced cytochrome c release from mitochondria to the cytoplasm and activation of caspase-9. Also, resveratrol increased expression of proapoptotic Bax and its translocation to the mitochondria, inhibited U251 proliferation, and induced G0/G1 growth arrest [56]. These findings are potentially significant since it is difficult to treat and manage glioma.
Resveratrol enhances the antitumor efficacy of Melphalan (MEL), a breast cancer chemotherapeutic agent. The treatment of MCF-7 and MDA-MB-231 cells with MEL combined with resveratrol induced the activation of p53 level, decreased the procaspase 8 and led to the activation of caspases 7 and 9. Moreover, this combined treatment caused MCF-7 cells to arrest in S phase of the cell cycle, increased the levels of the p-Chk2, and decreased the level of cyclin A. Although the levels of cyclin dependent kinase 2 (CDK2) remained unchanged by treatments, its active form (Thr 160) -phosphorylated CDK2) was decreased by treatment with resveratrol and by the combination of resveratrol and MEL. All these results indicated that resveratrol could be used as an adjuvant agent during breast cancer therapy with MEL [57]. The anti-proliferative properties of trans-resveratrol in different colon cancer cell lines indicated that this polyphenol blocks the cell cycle at the transition S to G2/M [58]. Trans-resveratrol inhibited Wnt signaling pathway, and affected β-catenin intracellular localization and the expression of genes encoding proteins involved in this process [59, 60]. In the colon-derived cell lines RKO and NCM460 which do not have a basally activated Wnt pathway, trans-resveratrol at low concentrations inhibited Wnt signaling when this pathway was previously stimulated [59]. A therapeutic role was suggested for trans-resveratrol, based on the finding that it acted as a SIRT1 deacetylases activator. Supporting this hypothesis it was observed that the expression of SIRT1 was increased in Caco-2 cells after incubations during 24 hour with this polyphenol [61].
III: Fisetin
Fisetin (3, 3′,4′,7-tetrahydroxy flavone), is a natural flavonoid found in many fruits and vegetables such as strawberries, blueberries and the skin of cucumbers [62] (Table 2). Studies have shown that fisetin exhibits a wide variety of bioactivity including neurotrophic [63], anti-oxidant [64], anti-inflammatory [65], and anti-angiogenic [66, 67] effects. Treatment of prostate cancer cells with fisetin resulted in induction of mitochondrial release of cytochrome c into cytosol, downregulation of XIAP, and upregulation of Smac/DIABLO [68]. Moreover, there was significant activation of caspase-3, -8 and -9 upon treatment of cells with fisetin. This phenomenon was inhibited by pretreatment of the cells with caspase inhibitor; because it blocked fisetin induced caspases activation [68]. Also, fisetin treatment resulted in induction of apoptosis, PARP cleavage, increase in Bax, decrease in Bcl-2, modulation in the expressions of Bcl-2-family proteins, and inhibition of PI3K, and phosphorylation of Akt at Ser473 and Thr308 [68].
Fisetin-induced apoptosis in human bladder cancer was found to be mediated through up-regulation of p53 and down-regulation of NF-κB activity, which caused a change in the ratio of pro- and anti-apoptotic proteins [69]. Fisetin inhibited the T24 and EJ cells proliferation by induction of apoptosis and blockage of cell cycle progression in the G0/G1 phase. Also, fisetin increased protein expressions of p53 and p21, and decreased levels of cyclin D1, cyclin A, CDK4 and CDK2, which contributed to cell cycle arrest [69]. In addition, fisetin increased Bax and Bak expression and decreased the levels of Bcl-2 and Bcl-xL. These events subsequently triggered the mitochondrial apoptotic pathway [69].
In human umbilical vein endothelial cells (HUVECs), fisetin inhibited vascular endothelial growth factor (VEGF) in a dose- and time-dependent manner. Also, fisetin treatment led to arrest of cells at the G1 and G2/M phases of the cell cycle, decreased cyclin D1, and increased p53 levels [70]. Moreover, fisetin decreased survivin expression, increased cleaved forms of caspases-3 and -7 and PARP, and increased Bax/Bcl-2 ratio. All these effects culminate in the apoptotic death of the cells. Furthermore, fisetin inhibited capillary-like tube structure on matrigel and further inhibited migration of the HUVEC cells. In the in vivo matrigel plug assay conducted in mice fisetin treatment significantly decreased the size, vascularization and hemoglobin content of the plug [70].
Treatment of caspase-3-deficient breast cancer MCF-7 cells with fisetin resulted in induction of caspase-7-associated apoptosis and inhibition of autophagy [71]. Fisetin was found to induce apoptosis and inhibit the invasion of chemoresistant pancreatic cancer AsPC-1 cells via suppression of DR3-mediated NF-κB activation. At a transcriptional level, fisetin decreased DR3 expression and increased the IkBalpha expression, an NF-κB inhibitor [72]. Down-regulation of DR3 in pancreatic cancer cells was found to down regulate activated pNF-κB/p65, pIκBalpha/beta kinases (pIKK’s), MMP9 and XIAP that mostly impart chemoresistance in cancer of the pancreas [72].
IV: Genistein
Genistein (5,7,4′-trihydroxyisoflavone), an isoflavone is a naturally occurring phenolic compound, which is present in many plants including soybeans, legumes, peas, lentils, and beans (Table 2). Genistein has been reported to inhibit the growth of various cancer cells through altering cell signaling pathways, cell cycle, and apoptosis [73]. Also, it has been reported to inhibit the proliferation and induce apoptosis of DU145 and HeLa cells with minimal effects on normal L-O2 cells [74]. Genistein induced apoptosis via endoplasmic reticulum stress and mitochondrial damage in human hepatoma Hep3B cells [75] and Ca2+-mediated calpain/caspase-12-dependent apoptosis in breast cancer MCF-7 cells [76]. The combination of genistein and TRAIL treatment sensitized TRAIL-resistant AGS gastric adenocarcinoma cells to TRAIL-mediated apoptosis. Also, activation of DR5 and induction of caspase-3 activity were observed with fisetin treatment [77]. Genistein induced apoptosis in acute promyelocytic leukemia derived NB4 cells, which was mediated by activation of caspase-9 and -3. This was associated with a decrease in mitochondrial transmembrane potential and cytosolic release of cytochrome c [78] as well as mitochondrial damage with involvement of the MTP in T lymphoma cells [79]. Genistein induced G2/M phase cell cycle arrest, apoptosis, and increase in p53 and p21 levels in human malignant glioma cell lines [80]. Tamoxifen and genistein combination synergistically induced apoptosis in human breast cancer BT-474 cells in part by synergistic downregulation of the expression of survivin and downregulation of EGFR, HER2, and ERα expression [81].
Genistein combined with N-(4- hydroxyphenyl) retinamide reduced cell viability, caused subG1 accumulation, increased caspase-3 activity in vitro and reduced tumor growth in vivo in human malignant neuroblastoma. Also, this combination therapy down regulated Id2 to induce differentiation, increased pro-apoptotic Bax and decreased anti-apoptotic Bcl-2 leading to an increase in Bax/Bcl-2 ratio. Increased mitochondrial Bax level, caused mitochondrial release of Smac/DIABLO, down regulation of the baculovirus inhibitor-of-apoptosis repeat containing (BIRC) proteins and activation of calpain and caspase-3 in SH-SY5Y xenografts mice [82]. Apoptosis-inducing-factor accumulation in cytosol and increase in caspase-4 activation suggested involvement of mitochondrial pathway and endoplasmic reticulum (ER) stress, respectively, for apoptosis in SH-SY5Y xenografts. Moreover, N-(4- hydroxyphenyl) retinamide and genistein treatment, resulted in overexpression of calpain, caspase-12, and caspase-3, and apoptosis-inducing-factor, suggesting induction of mitochondrial caspase-dependent and caspase independent pathways for apoptosis [82].
V: Curcumin
Curcumin (diferulolylmethane), is the major chemical component of turmeric (Table 2). In the traditional system of medicine it has been used for the treatment of a variety of inflammatory conditions and other diseases. There are various mechanisms by which curcumin causes inhibition of tumorigenesis and these include anti-inflammatory, anti-oxidant, immunomodulatory, pro-apoptotic, and anti-angiogenic effects via pleiotropic effects on genes and cell-signaling pathways at multiple levels [2]. Curcumin inhibited growth of LNCaP xenografts in nude mice by inducing apoptosis and sensitized these tumors to undergo apoptosis by TRAIL. Also, curcumin upregulated the expression of TRAIL-R1/DR4, TRAIL-R2/DR5, Bax, Bak, p21/WAF1, and p27/KIP1, and inhibited the activation of NF-κB in xenografted tumors. Moreover, Curcumin reduced the number of blood vessels in tumors, and also the circulating endothelial growth factor receptor 2-positive endothelial cells in mice [83]. Curcumin induced apoptosis in human melanoma cells through a Fas receptor/caspase -3 and -8 pathway independent of p53 and blocked the NF-kB cell survival pathway [84]. Curcumin also, resulted in translocation of Bax and p53 to mitochondria, production of ROS, and activation of caspase-3 and induction of apoptosis in LNCap cells [85]. Treatment of HepG2 cells challenged with curcumin for 1 h showed a transient elevation of the mitochondrial membrane potential followed by cytochrome c release into the cytosol [86]. The anti-apoptotic Bcl-2 and survivin protein were downregulated by curcumin treatment together with enhancement of the Bax and p53 expression in human bladder cancer cells [87]. Curcumin and a dietary compound, garcinol resulted in a synergistic effect against pancreatic cancer. The combination of garcinol and curcumin significantly inhibited cell viability and caused induction of apoptosis via upregulation of caspase-3 and -9 activity in BxPC-3 and Panc-1 cell lines [88]. A BRCA-mutated ovarian cancer cell treated with STAT3 inhibitor HO-3867, a novel curcumin analog, exhibited a significant degree of apoptosis with increased levels of cleaved caspase-3, caspase-7 and PARP, and induced ROS. Also, HO-3867 treatment resulted in decreased expression of pTyr705 and its downstream targets cyclin D1, Bcl-2 and survivin. Furthermore, overexpression of STAT3 cDNA provided resistance to HO-3867-induced apoptosis [89].
VI: Apigenin
Apigenin (4′,5,7-trihydroxyflavone), a flavone found in many plants (Table 2), has been observed to contribute to the chemopreventive action of vegetables and fruits [90]. Apigenin and TRAIL at suboptimal concentrations induced Bid cleavage and the activation of caspases-8, -10, -9, and -3 in malignant tumor cells [91]. Apigenin is a potent inhibitor of CYP2C9, an enzyme responsible for the metabolism of many pharmaceutical drugs in the body [92]. It was shown that apigenin induces autophagy and possesses chemopreventive property. However, at the same time it induces resistance against chemotherapy [93]. Apigenin inhibited the proliferation of MCF-7 vec and MCF-7 HER2 cells in breast cancer. Apigenin induced upregulation in the levels of cleaved caspase-8 leading to cleavage of PARP subsequent apoptotic cell death. Also, apigenin was found to induce apoptosis through a p53-dependent pathway. Moreover, apigenin reduced the expression of phospho-JAK1 and phospho-STAT3 and activated NF-κB signaling pathway in MCF-7 vec and MCF-7 HER2 cells [94].
VII: Delphinidin
Delphinidin 3-(6-p-coumaroyl) glucoside, is an anthocyanidin that impart color to many pigmented fruits and vegetables [95] (Table 2). Delphinidin significantly reduced apoptosis elicited by actinomycin D [96] and the protective effect of delphinidin was abolished by inhibitors of nitric oxide-synthase and was associated with an increased endothelial NOS expression mediated by a MAP kinase pathway. Using human immortalized HaCaT keratinocytes and SKH-1 hairless mouse skin, it was observed that pretreatment of cells with delphinidin protected against UVB mediated decrease in cell viability and induction of apoptosis. Delphinidin pretreatment of HaCaT cells inhibited UVB-mediated increase in PARP cleavage, activation of caspases, increase in Bax, Bid and Bak and a decrease in Bcl-2 and Bcl-xL [95].
Delphinidin and other anthocyanidins exhibited strong growth inhibitory effects against human hepatoma HepG(2). Delphinidin induced apoptotic cell death characterized by internucleosomal DNA fragmentation and a rapid induction of caspase-3 activity. These effects were associated with an increase in the c-Jun and JNK phosphorylation expression. Dephinidin induced DNA fragmentation was blocked by N-acetyl-l-cysteine and catalase, suggesting that the death signaling was triggered by oxidative stress [97]. It was reported that pomegranate fruit extract (PFE) caused an increase in the protein expression of Bax and decrease in Bcl-2 in a dose-dependent fashion in PC-3 cells [98]. There was also a significant dose-dependent shift in the ratio of Bax to Bcl-2 after PFE treatment, indicating the induction of an apoptotic process [98].
Delphinidin’s apoptotic effects were also investigated in human colon cancer HCT116 cells. Treatment of cells with delphinidin resulted in cleavage of PARP, activation of caspases-3, -8, and -9 and an increase in Bax with a concomitant decrease in Bcl-2 protein [99]. Because NF-κB activation contributes to neoplastic transformation and allows malignant cells to resist apoptosis-based tumor surveillance mechanisms, delphinindin treatment of HCT116 cells was associated with inhibition of phosphorylation of NF-κB/p65 at Ser(536), its nuclear translocation and subsequent DNA binding activity [99].
Evidence was provided that delphinidin could be developed as a novel agent against human prostate cancer. Human prostate cancer cells LNCaP, C4-2, 22Rν1, and PC3 were treated with delphinidin and found to result in a dose-dependent inhibition of cell growth and induction of apoptosis and at the same time sparing normal human prostate epithelial cells. This induction of apoptosis was mediated via activation of caspases because N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluromethylketone significantly reduced apoptosis induced by delphinidin [100].
The effects of delphinidin were determined in vitro in established human breast cancer cell lines of varying molecular subtypes and compared to non-transformed breast epithelial cells. Compared with vehicle control, delphinidin inhibited proliferation, blocked anchorage-independent growth and induced apoptosis of estrogen receptor (ER)-positive, triple negative; and HER2-overexpressing breast cancer cell lines with limited toxicity to non-transformed breast epithelial cells. MAPK signaling was partially reduced in triple negative cells and ER-negative chemically transformed MCF10A cells after treatment with delphinidin. In addition, delphinidin induced a significant level of apoptosis in HER2-overexpressing cells in association with reduced HER2 and MAPK signaling. However, combination studies suggested a potential antagonism between delphinidin and HER2-directed treatments raising concerns regarding potential drug antagonism when used in combination with existing targeted therapies in HER2-overexpressing breast cancer [101]. These observations suggested potential usefulness of delphinidin against breast cancer and at the same time cautioned its use as an adjuvant with conventional therapeutic regimens.
3: Conclusion
Natural agents that can target important carcinogenic pathways without demonstrating discernible adverse effects could serve as ideal cancer chemopreventive agents. The continued identification of dietary compounds and their derivatives with chemopreventive and/or chemotherapeutic properties offers an alternative and complementary approach to prevention and treatment of cancer. During the last decade several natural compounds (e.g., EGCG, Resveratrol, Fisetin, Curcumin, Genisten and Apigenin) were tested for their potential to modulate the expression and/or activity of specific genes, transcription factors in an attempt to identify their anti-carcinogenic potential. Many of the compounds were observed to affect the process of apoptosis, alter proinflammatory-mediated signaling pathways including inflammation-promoted cell proliferation, angiogenesis, invasion and metastasis. It is also promising that such valuable agents can serve as an adjuvant to enhance the efficacy of other known chemotherapeutics regimens. Altogether, these findings underline the fact that natural compounds could be considered as a promising innovative strategy for cancer treatment and prevention.
Acknowledgments
The original work from the author’s (H. Mukhtar) laboratory outlined in this paper was supported by United States Public Health Service Grant RO1 CA 160867.
References
- 1.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 3.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 4.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 5.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 7.Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–51. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 8.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–33. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 9.Rubio-Moscardo F, Blesa D, Mestre C, Siebert R, Balasas T, Benito A, et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood. 2005;106:3214–22. doi: 10.1182/blood-2005-05-2013. [DOI] [PubMed] [Google Scholar]
- 10.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF- kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 11.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 12.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, et al. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–42. [PubMed] [Google Scholar]
- 14.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 15.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–74. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 16.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 17.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 18.Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. Embo J. 2004;23:2134–45. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Loo G, van Gurp M, Depuydt B, Srinivasula SM, Rodriguez I, Alnemri ES, et al. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002;9:20–6. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- 21.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–90. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 22.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 23.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–8. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 24.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 25.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 26.Newmeyer DD, Bossy-Wetzel E, Kluck RM, Wolf BB, Beere HM, Green DR. Bcl-xL does not inhibit the function of Apaf-1. Cell Death Differ. 2000;7:402–7. doi: 10.1038/sj.cdd.4400665. [DOI] [PubMed] [Google Scholar]
- 27.Chau BN, Cheng EH, Kerr DA, Hardwick JM. Aven, a novel inhibitor of caspase activation, binds Bcl-xL and Apaf-1. Mol Cell. 2000;6:31–40. [PubMed] [Google Scholar]
- 28.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–6. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 29.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–9. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6:2953–61. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 31.King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–17. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 32.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–805. [PubMed] [Google Scholar]
- 34.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 35.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–63. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 37.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. Faseb J. 2005;19:789–91. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 38.Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41:353–61. doi: 10.3892/ijo.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–84. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan MH, Lin CC, Lin JK, Chen WJ. Tea polyphenol (−)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J Agric Food Chem. 2007;55:5030–7. doi: 10.1021/jf070316r. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Zhao DY, Elliott S, Zhao W, Curiel TJ, Beckman BS, et al. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol. 2007;31:705–11. [PubMed] [Google Scholar]
- 43.Thangapazham RL, Passi N, Maheshwari RK. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol Ther. 2007;6:1938–43. doi: 10.4161/cbt.6.12.4974. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate- mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 45.Shammas MA, Neri P, Koley H, Batchu RB, Bertheau RC, Munshi V, et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood. 2006;108:2804–10. doi: 10.1182/blood-2006-05-022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–67. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 47.Mohan KV, Gunasekaran P, Varalakshmi E, Hara Y, Nagini S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Biol Int. 2007;31:599–608. doi: 10.1016/j.cellbi.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem. 2003;278:41482–90. doi: 10.1074/jbc.M304896200. [DOI] [PubMed] [Google Scholar]
- 49.Tsan MF, White JE, Maheshwari JG, Bremner TA, Sacco J. Resveratrol induces Fas signalling- independent apoptosis in THP-1 human monocytic leukaemia cells. Br J Haematol. 2000;109:405–12. doi: 10.1046/j.1365-2141.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- 50.Alkhalaf M, El-Mowafy A, Renno W, Rachid O, Ali A, Al-Attyiah R. Resveratrol-induced apoptosis in human breast cancer cells is mediated primarily through the caspase-3-dependent pathway. Arch Med Res. 2008;39:162–8. doi: 10.1016/j.arcmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Lin X, Wu G, Huo WQ, Zhang Y, Jin FS. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int J Urol. 2012 doi: 10.1111/j.1442-2042.2012.03024.x. [DOI] [PubMed] [Google Scholar]
- 52.Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80:134–43. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 53.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–58. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Sun W, Wang W, Kim J, Keng P, Yang S, Zhang H, et al. Anti-cancer effect of resveratrol is associated with induction of apoptosis via a mitochondrial pathway alignment. Adv Exp Med Biol. 2008;614:179–86. doi: 10.1007/978-0-387-74911-2_21. [DOI] [PubMed] [Google Scholar]
- 55.Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56:4813–8. doi: 10.1021/jf800175a. [DOI] [PubMed] [Google Scholar]
- 56.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–61. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 57.Casanova F, Quarti J, da Costa DC, Ramos CA, da Silva JL, Fialho E. Resveratrol chemosensitizes breast cancer cells to melphalan by cell cycle arrest. J Cell Biochem. 2012;113:2586–96. doi: 10.1002/jcb.24134. [DOI] [PubMed] [Google Scholar]
- 58.Liang YC, Tsai SH, Chen L, Lin-Shiau SY, Lin JK. Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem Pharmacol. 2003;65:1053–60. doi: 10.1016/s0006-2952(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 59.Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52 (Suppl 1):S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulrich S, Loitsch SM, Rau O, von Knethen A, Brune B, Schubert-Zsilavecz M, et al. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–54. doi: 10.1158/0008-5472.CAN-05-2777. [DOI] [PubMed] [Google Scholar]
- 62.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–50. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 63.Maher P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic Res. 2006;40:1105–11. doi: 10.1080/10715760600672509. [DOI] [PubMed] [Google Scholar]
- 64.Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47:3164–77. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 65.Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M, et al. Fisetin, a flavonol, inhibits TH2- type cytokine production by activated human basophils. J Allergy Clin Immunol. 2003;111:1299–306. doi: 10.1067/mai.2003.1456. [DOI] [PubMed] [Google Scholar]
- 66.Fotsis T, Pepper MS, Montesano R, Aktas E, Breit S, Schweigerer L, et al. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab. 1998;12:649–66. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 67.Touil YS, Seguin J, Scherman D, Chabot GG. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445–55. doi: 10.1007/s00280-010-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H, et al. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol. 2011;108:84–93. doi: 10.1111/j.1742-7843.2010.00613.x. [DOI] [PubMed] [Google Scholar]
- 70.Bhat TA, Nambiar D, Pal A, Agarwal R, Singh RP. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo--implications for angioprevention. Carcinogenesis. 2012;33:385–93. doi: 10.1093/carcin/bgr282. [DOI] [PubMed] [Google Scholar]
- 71.Yang PM, Tseng HH, Peng CW, Chen WS, Chiu SJ. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol. 2012;40:469–78. doi: 10.3892/ijo.2011.1203. [DOI] [PubMed] [Google Scholar]
- 72.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci. 2004;9:2714–24. doi: 10.2741/1430. [DOI] [PubMed] [Google Scholar]
- 74.Yuan-Jing F, Nan-Shan H, Lian X. Genistein synergizes with RNA interference inhibiting survivin for inducing DU-145 of prostate cancer cells to apoptosis. Cancer Lett. 2009;284:189–97. doi: 10.1016/j.canlet.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ, Wang SW, et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782–92. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 76.Sergeev IN. Genistein induces Ca2+-mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2004;321:462–7. doi: 10.1016/j.bbrc.2004.06.173. [DOI] [PubMed] [Google Scholar]
- 77.Jin CY, Park C, Cheong J, Choi BT, Lee TH, Lee JD, et al. Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett. 2007;257:56–64. doi: 10.1016/j.canlet.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 78.Ng AP, Nin DS, Fong JH, Venkataraman D, Chen CS, Khan M. Therapeutic targeting of nuclear receptor corepressor misfolding in acute promyelocytic leukemia cells with genistein. Mol Cancer Ther. 2007;6:2240–8. doi: 10.1158/1535-7163.MCT-06-0705. [DOI] [PubMed] [Google Scholar]
- 79.Baxa DM, Luo X, Yoshimura FK. Genistein induces apoptosis in T lymphoma cells via mitochondrial damage. Nutr Cancer. 2005;51:93–101. doi: 10.1207/s15327914nc5101_13. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt F, Knobbe CB, Frank B, Wolburg H, Weller M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep. 2008;19:1061–6. [PubMed] [Google Scholar]
- 81.Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007;46:534–42. doi: 10.1002/mc.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karmakar S, Choudhury SR, Banik NL, Ray SK. Induction of Mitochondrial Pathways and Endoplasmic Reticulum Stress for Increasing Apoptosis in Ectopic and Orthotopic Neuroblastoma Xenografts. J Cancer Ther. 2011;2:77–90. doi: 10.4236/jct.2011.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–14. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 85.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–18. [PubMed] [Google Scholar]
- 86.Cao J, Liu Y, Jia L, Zhou HM, Kong Y, Yang G, et al. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic Biol Med. 2007;43:968–75. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Tian B, Wang Z, Zhao Y, Wang D, Li Y, Ma L, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008;264:299–308. doi: 10.1016/j.canlet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 88.Parasramka MA, Gupta SV. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J Oncol. 2012;2012:709739. doi: 10.1155/2012/709739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tierney BJ, McCann GA, Cohn DE, Eisenhauer E, Sudhakar M, Kuppusamy P, et al. HO-3867, a STAT3 inhibitor induces apoptosis by inactivation of STAT3 activity in BRCA1-mutated ovarian cancer cells. Cancer Biol Ther. 2012;13:766–75. doi: 10.4161/cbt.20559. [DOI] [PubMed] [Google Scholar]
- 90.Ferreira CV, Justo GZ, Souza AC, Queiroz KC, Zambuzzi WF, Aoyama H, et al. Natural compounds as a source of protein tyrosine phosphatase inhibitors: application to the rational design of small-molecule derivatives. Biochimie. 2006;88:1859–73. doi: 10.1016/j.biochi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther. 2006;5:945–51. doi: 10.1158/1535-7163.MCT-05-0431. [DOI] [PubMed] [Google Scholar]
- 92.Si D, Wang Y, Zhou YH, Guo Y, Wang J, Zhou H, et al. Mechanism of CYP2C9 inhibition by flavones and flavonols. Drug Metab Dispos. 2009;37:629–34. doi: 10.1124/dmd.108.023416. [DOI] [PubMed] [Google Scholar]
- 93.Ruela-de-Sousa RR, Fuhler GM, Blom N, Ferreira CV, Aoyama H, Peppelenbosch MP. Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy. Cell Death Dis. 2010;1:e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seo HS, Choi HS, Kim SR, Choi YK, Woo SM, Shin I, et al. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFkappaB signaling in HER2-overexpressing breast cancer cells. Mol Cell Biochem. 2012;366:319–34. doi: 10.1007/s11010-012-1310-2. [DOI] [PubMed] [Google Scholar]
- 95.Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–32. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 96.Martin S, Giannone G, Andriantsitohaina R, Martinez MC. Delphinidin, an active compound of red wine, inhibits endothelial cell apoptosis via nitric oxide pathway and regulation of calcium homeostasis. Br J Pharmacol. 2003;139:1095–102. doi: 10.1038/sj.bjp.0705347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeh CT, Yen GC. Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J Agric Food Chem. 2005;53:1740–9. doi: 10.1021/jf048955e. [DOI] [PubMed] [Google Scholar]
- 98.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yun JM, Afaq F, Khan N, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol Carcinog. 2009;48:260–70. doi: 10.1002/mc.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozbay T, Nahta R. Delphinidin Inhibits HER2 and Erk1/2 Signaling and Suppresses Growth of HER2-Overexpressing and Triple Negative Breast Cancer Cell Lines. Breast Cancer (Auckl) 2011;5:143–54. doi: 10.4137/BCBCR.S7156. [DOI] [PMC free article] [PubMed] [Google Scholar]