Abstract
Plasma hemoglobin (Hb) scavenges endothelium-derived nitric oxide (NO), producing systemic and pulmonary vasoconstriction in many species. We hypothesized that i.v. administration of murine cell-free Hb would produce pulmonary vasoconstriction and enhance hypoxic pulmonary vasoconstriction (HPV) in mice.
To assess the impact of plasma Hb on basal pulmonary vascular tone in anesthetized mice we measured left lung pulmonary vascular resistance (LPVRI) before and after infusion of Hb at thoracotomy. To confirm the findings obtained at thoracotomy, measurements of right ventricular systolic pressure (RVSP) and systemic arterial pressure (SAP) were obtained in closed-chest wild-type mice. To elucidate whether pretreatment with Hb augments HPV we assessed the increase in LPVRI before and during regional lung hypoxia produced by left mainstem bronchial occlusion (LMBO) in wild-type mice pretreated with Hb.
Infusion of Hb increased SAP but did not change pulmonary arterial pressure (PAP), left lung pulmonary arterial flow (QLPA) or LPVRI in either wild-type or diabetic mice with endothelial dysfunction. Scavenging of NO by plasma Hb did not alter HPV in wild-type mice. Inhibition of NO synthase with L-NAME did not change the basal LPVRI, but augmented HPV during LMBO.
Our data suggest that scavenging of NO by plasma Hb does not alter pulmonary vascular tone in mice. Therefore, generation of NO in the pulmonary circulation is unlikely to be responsible for the low basal pulmonary vascular tone of mice.
Keywords: hemoglobin, endothelium, hypoxia, vasoconstriction, nitric oxide
Introduction
In mammals and most vertebrates hemoglobin (Hb) is contained within the red blood cell (RBC). Packaging Hb in this manner may prevent the toxicity and tissue injury produced by circulating cell-free Hb [1; 2]. Cell-free Hb scavenges endothelium-derived nitric oxide (NO) [3], causing systemic vasoconstriction [4] and contributing to pathogenic mechanisms including thrombosis [5] and inflammation [6].
Solutions of Hb polymers have been studied for decades as circulating oxygen carriers to substitute for red blood cells in animal models and patients [7; 8]. Infusing Hb polymers can produce systemic vasoconstriction in humans [4; 9]. Furthermore, infusion of cell-free oxyhemoglobin (oxyHb) and heme-based oxygen carriers produces pulmonary vasoconstriction in several species including pigs, dog, sheep and humans [9; 10; 11; 12]. Mammals produce haptoglobin (Hp) to neutralize cell-free Hb and, thereby, prevent inflammatory damage and systemic vasoconstriction. Data from Hp knockout mice suggest that Hp also attenuates Hb-mediated oxidative organ damage [13; 14]. However, mice have low baseline Hp levels [15], which could easily be depleted by cell-free Hb challenge.
The vascular endothelium modulates pulmonary artery tone by producing several vasoactive mediators, including the potent vasodilators prostacyclin (PGI2) and NO. Synthesis and release of NO from pulmonary endothelial cells leads to pulmonary vasodilation [16]. Uncoupling of nitric oxide synthase 3 (NOS3) by reduced co-factors (NADPH, tetrahydrobiopterin) or low levels of L-arginine results in formation of superoxide instead of NO [17]. In humans, impaired NO production or availability can result in pulmonary hypertension [18]. Systemic endothelial dysfunction is frequently associated with metabolic disorders such as diabetes [19] and is characterized by impaired generation of NO by endothelial cells [20]. We have previously reported that endothelial dysfunction in diabetic (db/db) mice augments the systemic vasoconstrictor response to infusion of cell-free Hb [21].
NO produced by pulmonary endothelium also modulates hypoxic pulmonary vasoconstriction (HPV) – a physiological mechanism unique to the pulmonary vasculature ensuring the optimal oxygenation of arterial blood. The precise mechanisms involved in the control of pulmonary vascular tone are complex, incompletely understood, and vary significantly between species [22]. Studies of NOS inhibition in rats [23], rabbits [24], dogs [25] and cats [26] all demonstrate that pharmacological NOS inhibition with NG-nitro-L-arginine methylester (L-NAME) enhances HPV. However, we did not know whether scavenging of NO by Hb affects pulmonary vascular tone in mice. Mice are widely studied in various experimental models, due to the great possibilities of altering their genetic composition. The interaction between Hb, NO and pulmonary vasculature is critical to our understanding of the effects of NO scavenging on pulmonary blood flow distribution, gas exchange and oxygen delivery during regional lung hypoxia.
The aim of this study was to elucidate the effects of plasma Hb on the pulmonary vascular tone of anesthetized and ventilated mice. In order to precisely assess pulmonary vascular resistance [27], we obtained dynamic simultaneous measurements of pulmonary arterial pressure and blood flow at thoracotomy. As in other species we hypothesized that i.v. infusion of Hb would produce pulmonary vasoconstriction in wild-type (WT) mice. We also hypothesized that the endothelial dysfunction of diabetic (db/db) mice [21], which sensitizes these mice to Hb-produced systemic vasoconstriction might enhance Hb-induced pulmonary vasoconstriction. In addition, we hypothesized that i.v. infusion of cell-free Hb, by scavenging NO and reducing NO-mediated vasodilation, would enhance the vasoconstrictor response of the pulmonary vasculature to regional hypoxia, thereby augmenting HPV. Surprisingly, we learned that scavenging of NO by cell-free oxyHb in mice did not change either the basal pulmonary vascular tone or the degree of HPV.
Methods
All animal experiments were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, Boston, MA. We studied 64 eight to ten week old male C57BL6 (WT) mice weighing 27±1 g and six BKS.Cg-Dock7m+/+Leprdb/J (db/db) mice weighing 60±1g on a C57BL6 background (Jackson Laboratory, Bar Harbor, ME). See Tables 1 and 2 for the numbers of mice studied in each group.
Table 1.
Hemodynamic measurements in WT mice at thoracotomy.
| Treatment Group | HR (b·min−1) | SAP (mmHg) | PAP (mmHg) | QLPA (μl·min−1·g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Post-inf. | Baseline | Post-inf. | Baseline | Post-inf. | Baseline | Post-inf. | |
| Hb | 6 | 601±8 | 573±13* | 99±5 | 109±6* | 21±1 | 20±1 | 82±3 | 93±6 |
| WB | 6 | 565±11 | 551±11 | 95±3 | 102±3 | 20±1 | 21±1 | 84±2 | 90±6 |
| L-NAME | 7 | 580±11 | 547±11* | 92±6 | 133±6†* | 21±1 | 19±1 | 104±1 | 90±2* |
| U46619 | 6 | 525±18 | 512±11 | 91±3 | 158±4†* | 21±1 | 27±1†* | 100±3 | 56±7†* |
Hemodynamic measurements at baseline and 3 minutes after treatment of WT mice with Hb, WB, L-NAME and U46619.
P<0.05 differs vs. Hb-treated WT mice.
P<0.05 value differs vs. baseline of the same parameter in the same group. Values are means ± SE.
Table 2.
Hemodynamic measurements in closed-chest WT mice.
| HR (b·min−1) | SAP (mmHg) | RVSP (mmHg) | |||||
|---|---|---|---|---|---|---|---|
| Treatment | n | Baseline | Post-inf. | Baseline | Post-inf. | Baseline | Post-inf. |
| Hb | 4 | 602±4 | 542±7* | 105±1 | 127±4* | 23±1 | 22±2 |
| WB | 4 | 613±12 | 613±8 | 107±2 | 109±1 | 23±1 | 23±2 |
Hemodynamic measurements at baseline and 3 minutes after infusion of Hb or WB in anesthetized closed-chest WT mice.
P<0.05 value differs vs. baseline. Values are means ± SE.
Reagents
We studied the thromboxane agonist U46619 (Cayman Chemical Company, Ann Arbor, MI) and the non-selective NO synthase (NOS) inhibitor NG-nitro-L-arginine methylester (L-NAME; Sigma-Aldrich, St. Louis, MO).
Preparation of cell-free Hb solution
Murine cell-free Hb solution (4 g·dl−1, methemoglobin ≤ 2%) for i.v. injection was prepared as previously described [28].
Measurements of plasma Hb and methemoglobin (metHb) concentration
In a separate group of WT mice (n=6 per group), whole blood was heparinized and collected via cardiac puncture at baseline, and at 15 and 30 min after i.v. infusion of cell-free Hb. Plasma was obtained by centrifuging whole blood at 1699g for 8 min at 4°C, and was stored at −20°C. Cell-free Hb and metHb concentrations were determined by the cyanmethemoglobin method [29]. Absorption was measured at 540 nm and 630 nm with a spectrophotometer (Biomate 3; Thermoelectron Corporation, Waltham, MA).
Surgical preparation of anesthetized, open-chest mice
Surgical preparation of animals and the measurement of left lung pulmonary vascular resistance indexed to body weight (LPVRI) were performed as described previously [30]. Briefly, mice were anesthetized with an intraperitoneal (i.p.) injection of ketamine (120 mg·kg−1) and fentanyl (0.09 mg·kg−1). Following tracheostomy, pancuronium (2 mg·kg−1) was injected i.p. to induce muscle relaxation and mice were subjected to a median thoracotomy. Volume-controlled ventilation was provided at a respiratory rate of 100 breaths·min−1, a tidal volume of 10 ml·kg−1 and inspired O2 fraction (FIO2) of 1.0 (Mini Vent 845; Harvard Apparatus, Holliston, MA).
Invasive hemodynamic measurements in anesthetized mice at thoracotomy
For hemodynamic measurements, fluid-filled PE 10 catheters were inserted into the right carotid artery and the main pulmonary artery, and a 0.5 mm VB-HSE flow probe (Transonic Systems Inc., Ithaca, NY) was placed around the left pulmonary artery. Heart rate (HR), systemic arterial pressure (SAP), pulmonary arterial pressure (PAP), and left pulmonary arterial blood flow (QLPA) were continuously measured and recorded. For some experiments the left atrial pressure (LAP) was measured via a fluid-filled PE 10 catheter placed in the left atrium. Cardiac output (CO) was estimated by measuring lower thoracic aortic flow (QLTAF) with a flow probe. To estimate LPVRI, the inferior vena cava (IVC) was partially occluded to transiently reduce QLPA to 50%. LPVRI was calculated from the slope of the PAP/QLPA relationship.
Total systemic vascular resistance (TSVR) was estimated using dynamic measurements of SAP and QLTAF. These measurements were performed during partial occlusion of the IVC to transiently reduce QLTAF to 50%. TSVR was calculated from the slope of the SAP/QLTAF relationship. After obtaining hemodynamic measurements, arterial blood was sampled from the right carotid artery. Arterial blood gas tensions and pHa were measured using an ABL800 FLEX analyzer (Radiometer America Inc., Westlake, OH).
Administration of cell-free Hb or syngeneic whole blood (WB) to anesthetized mice at thoracotomy
Plasma Hb (0.48 g·kg−1) or an equal volume of fresh WB was administered i.v. at 0.1 ml·min−1 via a PE 10 catheter placed in the jugular vein. We have previously reported that i.v. administration of plasma Hb at 0.48 g·kg−1 produced immediate and prolonged systemic vasoconstriction in both awake and anesthetized mice [28]. In the current study, each mouse was given a Hb or WB topload of 16% of blood volume (approximately 0.3 ml in a 25 g mouse). In order to maintain a constant blood volume and avoid volume overload, an equal volume of WB was withdrawn from the jugular vein at 0.1 ml·min−1 prior to administration of either Hb or WB. LPVRI was measured before and 3 minutes after administration of Hb or WB (Figure 1A). We chose to measure LPVRI at 3 minutes after administration of Hb or WB due to the evidenced scavenging of NO expressed in immediate systemic hypertension following infusion of Hb.
Figure 1.
(A) Experimental design of hemodynamic measurements before and 3 minutes after infusion of Hb, WB, L-NAME or U46619 in WT mice. (B) Experimental design of hemodynamic measurements in WT mice pretreated with normal saline, Hb or L-NAME 30 minutes before LMBO. IVC, inferior vena cava.
Invasive hemodynamic measurements in anesthetized closed-chest mice
Hemodynamic measurements in anesthetized closed-chest mice were performed in order to confirm the results observed in mice at thoracotomy. Mice were anesthetized, intubated and mechanically ventilated at FIO2 of 1.0. A fluid-filled polyethylene catheter (PE 10, 0.28-mm ID, 0.61-mm OD; Becton Dickinson, Franklin Lakes, NJ) was introduced into the left carotid artery to monitor HR and SAP using a pressure transducer (Deltran II; Utah Medical Products, Midvale, UT). A second PE 10 catheter was inserted into the left jugular vein to administer infusions. A 1.2F high-fidelity pressure catheter (FTS-1211B-0018, Scisense Inc, London, Ontario, Canada) was advanced into the right ventricle via the right jugular vein to measure right ventricular systolic pressure (RVSP). All signals were recorded using Chart 5 software and analyzed using PVAN software (both ADInstruments, Colorado Springs, CO).
Effects of NOS inhibition on pulmonary vascular tone
LPVRI was measured at baseline and 3 minutes after i.v. administration of L-NAME dissolved in 0.9% saline solution at a dose of 100 mg·kg−1 in WT mice at thoracotomy. This dose was chosen based on a previous study in mice [31].
Effects of the thromboxane A2 mimetic U46619 on the pulmonary vasculature
We confirmed the ability of the pulmonary vasculature to vasoconstrict in anaesthetized mice by i.v. injection of the potent smooth muscle constrictor and thromboxane agonist U46619 [32]. The LPVRI was measured at baseline and 3 minutes after i.v. administration of U46619 dissolved in 0.9% saline solution at a dose of 0.15 μmol·kg−1·min−1 in WT mice at thoracotomy. The dose of U46619 was chosen based on results from a previous study in mice [33].
Measurements of HPV at thoracotomy
To assess HPV in anesthetized and ventilated WT mice during unilateral left lung hypoxia, LPVRI was estimated using methods described previously [30]. Unilateral left lung hypoxia was induced by reversibly occluding the left main stem bronchus (LMBO) with a microvascular clip. Complete collapse of the left lung was visually observed to commence within one minute and confirmed by transient hyperinflation of the right lung. We chose to measure LPVRI at 5 minutes after LMBO because we observed total atelectasis of the collapsed left lung at this time. We have chosen to use LMBO in order to produce regional unilateral left lung hypoxia because LMBO prevents systemic hypoxia as compared to HPV models which use hypoxic gas mixtures. Metabolic acidosis during systemic hypoxia may affect HPV and therefore confound the results [22].
Effects of Hb and L-NAME on HPV in WT mice
Thirty minutes before LMBO we administered cell-free Hb (0.48 g·kg−1), L-NAME (100 mg·kg−1) dissolved in normal saline, or an equal volume of normal saline to WT mice via the jugular vein at 0.1 ml·min−1. LPVRI at baseline and 5 minutes after LMBO was calculated from the pressure-flow curve created by transient IVC occlusion. LMBO was produced thirty minutes after pretreatment with Hb, L-NAME or normal saline, due to the surgical preparation of the animals, which takes thirty minutes (Figure 1B).
Evaluation of superoxide production
To search for other possible causes of enhanced HPV we assessed superoxide levels in murine lung homogenates obtained from wild-type mice (n=6). Chemiluminescence measurements were performed as described previously [34]. Briefly, both lungs were collected from WT mice 5 minutes after LMBO. Superoxide production was measured in lung homogenates using a chemiluminescence assay supplemented with β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NADPH) and lucigenin in the presence or absence of L-NAME (1 or 10 mM). Chemiluminescence was recorded for 60 s (Centro XS3 LB 960 Microplate Luminometer, Berthold Technologies U.S.A. LLC, Oak Ridge TN) and was reported as relative light units (RLU). The background chemiluminescence level was subtracted.
Statistical analysis
All values are expressed as mean ± SE. P values < 0.05 were considered statistically significant. Statistical analyses were performed using Prism 5 software (GraphPad Software Inc., La Jolla, CA). Data were analyzed using a one-way ANOVA with post hoc Bonferroni tests (two-tailed) for normally distributed data or using a Kruskal-Wallis test (two-tailed) with a post hoc Dunn’s test for data that were not normally distributed. Measurements within the same experimental group were compared with a paired t-test. If the normality test failed, the Mann-Whitney rank sum test was applied. The effects of L-NAME on NOS-derived superoxide generation in the lung were compared using paired t-tests.
Results
Invasive hemodynamic measurements in anesthetized open-chest WT mice: Administration of WB or cell-free Hb
Invasive hemodynamic measurements were performed at thoracotomy, both before and 3 minutes after i.v. infusion of cell-free Hb or WB. We chose 3 minutes for the observation point because there is a maximal increase of systemic arterial pressure at 3 minutes after i.v. Hb infusion. The HR, SAP, PAP, QLPA and LPVRI were similar at baseline before transfusion in mice receiving either WB or Hb (n=6 per group). Infusion of WB did not change any of these hemodynamic parameters. In contrast, infusion of Hb significantly increased the SAP and decreased HR without altering the PAP, QLPA or LPVRI (Table 1, Figure 2).
Figure 2.
Left lung pulmonary vascular resistance (LPVRI) at baseline and 3 minutes after i.v. challenge with either hemoglobin (Hb), whole blood (WB), L-NAME or U46619 in WT mice at thoracotomy. Values are mean ± SE. *P<0.009 differs vs. baseline value of LPVRI in the same treatment group.
Invasive hemodynamic measurements in anesthetized closed-chest WT mice
To confirm the findings obtained in mice at thoracotomy, measurements of RVSP, SAP and HR were performed in anesthetized and ventilated closed-chest WT mice (n=8) by catheterizing the right ventricle via the jugular vein. At baseline, hemodynamic parameters did not differ between mice that received WB or Hb. Infusion of WB did not change HR, SAP, or RVSP. In contrast, infusion of Hb increased SAP and decreased HR, without affecting RVSP (Table 2).
Hemodynamic effects of L-NAME infusion on the pulmonary vascular tone of WT mice at thoracotomy
We studied the hemodynamic effects of acute inhibition of NOS by L-NAME on the pulmonary vasculature (n=7). Infusion of L-NAME (100 mg·kg−1) decreased HR (580±11 vs. 547±11 beats·min−1, P=0.049) and markedly increased SAP at 3 minutes (92±6 vs. 133±6 mmHg, P=0.0001). Pulmonary arterial pressure did not change and QLPA decreased slightly after treatment with L-NAME, however LPVRI was unchanged when compared to untreated animals (67±5 vs. 67±4 mmHg·min·g·ml−1).
Hemodynamic effects of U46619 infusion on the pulmonary vascular tone of WT mice at thoracotomy
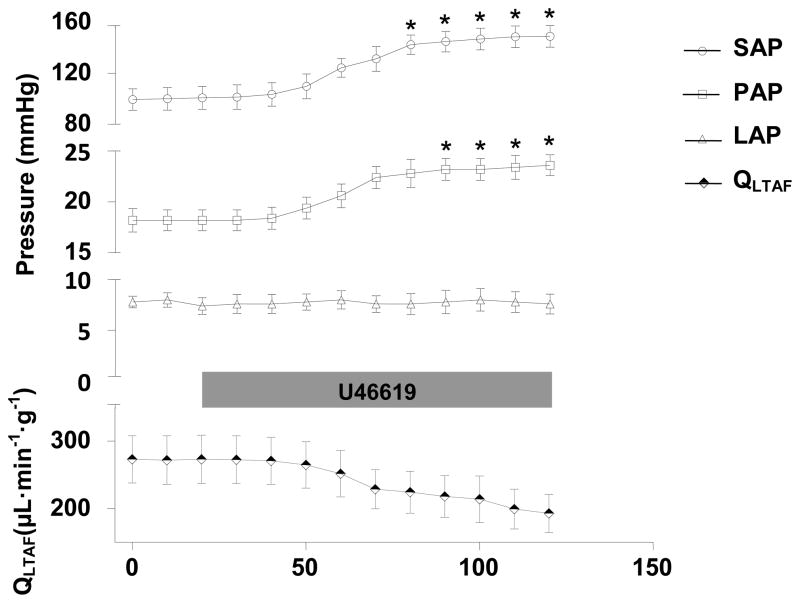
To confirm the ability of the pulmonary vasculature to vasoconstrict in anesthetized mice a potent vasoconstrictor, the thromboxane agonist U46619, was infused i.v. at 1.5 μmol·kg−1·min−1 for 2 minutes. Administration of U46619 to WT mice (n=6) markedly increased SAP, PAP, and LPVRI and decreased QLPA (Table 1, Figures 2 and 3). In additional experiments (n=5), we measured QLTAF and LAP before and after infusion of U46619 and calculated an estimate of TSVR and pulmonary vascular resistance (PVR). Administration of U46619 markedly increased TSVR (249±14 vs. 899±9 mmHg·min·g·ml−1, P=0.001) and PVR (36±3 vs. 103±10 mmHg·min·g·ml−1, P=0.01) and decreased QLTAF without changing LAP (Figure 3).
Figure 3.
Hemodynamic measurements in WT mice (n=5) at baseline and during an i.v. U46619 infusion (0.15 μmol·kg−1·min−1). SAP, mean systemic arterial pressure; PAP, pulmonary arterial pressure; LAP, left atrial pressure; QLTAF, lower thoracic aortic blood flow. Values are mean ± SE. *P<0.05 differs vs. baseline value of the same parameter.
Administration of cell-free Hb to diabetic (db/db) mice at thoracotomy
To explore whether endothelial dysfunction produced by diabetes, which sensitizes the systemic circulation to the NO scavenging effects of Hb [21], would alter the pulmonary vascular response to i.v. infusion of Hb in mice, we measured LPVRI before and 3 minutes after infusion of Hb in db/db mice breathing at FIO2 1.0. Infusion of Hb markedly increased SAP from 93±6 to 154±4 mmHg (P=0.001) in db/db mice (n=5) at 3 minutes, but did not change PAP, HR, and QLPA (data not shown) or LPVRI (Figure 4).
Figure 4.
Left lung pulmonary vascular resistance (LPVRI) at baseline and 3 minutes after i.v. infusion of Hb in diabetic (db/db) mice (n=5). Db/db mice weighed approximately twice as much as WT mice. Thus their indexed LPVRI is doubled. Values are mean ± SE.
Administration of cell-free Hb, L-NAME or saline solution to WT mice 30 minutes before producing unilateral left lung hypoxia by LMBO
To determine the impact of infusing Hb on HPV in mice, we examined the changes of LPVRI induced by LMBO at thoracotomy. We studied a total of 13 mice pretreated with Hb, L-NAME or a saline solution 30 min after cannulation but before LMBO. The plasma concentration of cell-free Hb increased from 51±7 mg·dl−1 (7.9±1 μM) at baseline to 729±29 mg·dl−1 (113±4 μM) at 30 minutes after i.v. administration of Hb. Levels of metHb were less than 1% in WB and 16% of plasma Hb at 30 minutes after the i.v. administration of Hb, perhaps indicating scavenging of NO by cell-free Hb. Infusion of Hb or L-NAME increased SAP at 30 min after infusion when compared to saline-treated mice (Table 3). LMBO decreased the QLPA and increased LPVRI without affecting the HR, SAP, or PAP in mice pretreated with Hb, L-NAME, or saline (Table 3, Figure 5). The increase of LPVRI during LMBO in mice pretreated with Hb or saline was similar. In contrast, pretreatment with L-NAME resulted in a greater increase of LPVRI during LMBO as compared to Hb-pretreated animals (Figure 5). During LMBO the arterial partial pressure of oxygen (PaO2) did not differ between mice pretreated with Hb, L-NAME or saline (data not shown).
Table 3.
Hemodynamic measurements at baseline and 5 minutes after LMBO in WT mice.
| HR (b·min−1) | SAP (mmHg) | PAP (mmHg) | QLPA (μl·min−1·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | Baseline | LMBO | Baseline | LMBO | Baseline | LMBO | Baseline | LMBO |
| vehicle | 5 | 576±6 | 547±16 | 86±5 | 92±6 | 20±1 | 22±1 | 94±2 | 53±1* |
| Hb | 4 | 547±30 | 571±33 | 112±5‡ | 112±5‡ | 20±1 | 21±1 | 99±3 | 49±1* |
| L-NAME | 4 | 484±8 | 493±17 | 112±3‡ | 112±1‡ | 19±1 | 21±1 | 98±2 | 43±5* |
Hemodynamic measurements during LMBO at 30 minutes after infusion of saline, Hb or L-NAME in WT mice.
P<0.05 value differs vs. vehicle-pretreated mice.
P<0.05 value differs vs. baseline of the same parameter in the same group. Values are means ± SE.
Figure 5.
Increase of left lung pulmonary vascular resistance (LPVRI) during LMBO at 30 minutes after pretreatment with saline, Hb or L-NAME in WT mice. Values are mean ± SE. *P<0.01 value differs vs. both Hb and saline treated mice.
Effects of NOS inhibition on superoxide generation in lung tissue
The observation that in vivo pretreatment of mice with L-NAME but not with plasma Hb augmented HPV indicated the possible presence of a NOS-derived mediator, which affects HPV. It has been reported that NOS3 can produce superoxide instead of NO [17]. To investigate whether L-NAME could inhibit NOS3-derived superoxide generation in murine lung tissue we measured superoxide production of lung homogenates, using lucigenin-enhanced chemiluminescence, in the presence and absence of L-NAME. Superoxide production was inhibited in a dose-dependent manner in lung homogenates of WT mice in the presence of L-NAME (Figure 6). There was no difference in the relative reduction of superoxide generation by L-NAME in the homogenates of right lungs ventilated at FIO2 1 as compared to homogenates of left lungs exposed to hypoxia produced by LMBO (data not shown). A combination of superoxide dismutase (SOD) and Tiron (a non-enzymatic scavenger of superoxide) markedly inhibited chemiluminescence (>90%), confirming that luminescence was attributable to superoxide generation (Figure 6).
Figure 6.

Reduction of NOS-derived superoxide production in normoxic (right) (A) and hypoxic (left) (B) lung homogenates from WT mice (n=6). Superoxide production in freshly isolated lung tissue was detected by using lucigenin-enhanced chemiluminescence in the absence or presence of L-NAME (1mM and 10mM) or SOD/Tiron. Values are mean ± SE. *P<0.05 differs vs. levels of superoxide generation in lung homogenates in the presence of normal saline.
Discussion
We investigated the effects of i.v. infusion of cell-free Hb on the pulmonary vascular tone of anesthetized and ventilated mice. Plasma oxyHb destroys endothelium-derived NO by the NO dioxygenation reaction [35] and is known to produce systemic and pulmonary vasoconstriction in many species [3; 10; 11; 36]. Surprisingly, i.v. infusion of cell-free Hb did not alter pulmonary hemodynamic parameters from baseline levels during normal ventilation. In addition, during regional hypoxia caused by LMBO, HPV was not enhanced by Hb infusion. In contrast, SAP consistently increased after i.v. administration of cell-free Hb. We were surprised by this finding, as we expected NO scavenging by plasma Hb to cause pulmonary vasoconstriction. Thus, we explored another method of reducing NO levels. Administration of L-NAME caused significant systemic arterial hypertension but did not produce pulmonary vasoconstriction or hypertension in WT mice. However, acute inhibition of NOS by L-NAME enhanced HPV, and reduced superoxide generation in the lungs. The latter finding may be the cause of the enhanced HPV after L-NAME administration. The findings of the present study suggest that pulmonary NO signaling does not play a major role in the control of pulmonary vascular tone during mechanical ventilation or during regional hypoxia in mice.
Intravenous administration of cell-free Hb acutely increases pulmonary arterial pressure due to pulmonary vasoconstriction in rabbits, pigs, sheep and humans [11; 36; 37; 38]. In humans, nitric oxide, synthesized by endothelial cells in the lung’s vasculature, contributes to the low pressure and resistance of the intact pulmonary circulation [39; 40]. Scavenging of NO by plasma Hb appears to be the underlying mechanism of murine systemic vasoconstriction in response to Hb, since i.v. infusion of Hb does not cause systemic vasoconstriction in mice with a congenital absence of NOS3 [28]. In the present study, administration of Hb had no effect on the baseline pulmonary pressure-flow relationship or RVSP of mice but significantly increased their SAP. In a previous study we have shown this systemic hypertension in mice to be due to systemic vasoconstriction [28]. Thus, scavenging of NO by plasma oxyHb, at concentrations that produce profound systemic vasoconstriction, did not alter pulmonary vascular tone of mice. To investigate the contribution of NO to the regulation of pulmonary vascular tone in intact mice, we studied the effects of inhibition of NOS by L-NAME. It has been reported that i.v. L-NAME administration acutely increases PVR in isolated and perfused lungs of sheep, pigs, and humans, but not in isolated and perfused lungs of rats and dogs [41; 42; 43]. Liu et al. reported that PAP and LPVR do not differ in anesthetized NOS3−/− and WT mice breathing at FIO2 1 [44], supporting the hypothesis that NO generated by NOS3 does not regulate basal pulmonary vascular tone in mice. In the present study i.v. administration of L-NAME did not alter the pulmonary vascular resistance, confirming previous reports in anesthetized mice [31]. In contrast, infusion of the thromboxane A2 analog U46619, markedly increased PAP and LPVRI, confirming the ability of anesthetized and ventilated WT mice to undergo profound pulmonary vasoconstriction. Taken together, these findings indicate that NO production in the pulmonary circulation is not primarily responsible for the low basal pulmonary vascular tone of anesthetized mice.
Endothelial dysfunction is associated with a variety of disorders, including hypertension and diabetes [20], and is characterized by a reduction of NO synthesis by endothelial cells. We have previously shown that diabetic mice with endothelial dysfunction have a greater systemic vasoconstrictor response to an i.v. infusion of cellfree Hb than do WT mice [21]. In the present study, we also observed that infusion of oxyHb induced a larger increase in SAP in db/db mice than in WT mice, in contrast the pulmonary vascular tone of db/db mice was not affected by administration of plasma Hb. It is possible that endothelial dysfunction in db/db mice is limited to the systemic vasculature. However, diabetic rats were found to have endothelial dysfunction in pulmonary arteries, associated with reduced bioavailability of NO [45].
Hypoxic pulmonary vasoconstriction diverts blood flow away from hypoxic lung regions, thereby matching perfusion with ventilation of the lung [46; 47]. In previous investigations HPV was commonly assessed by breathing hypoxic mixtures and measured by the increase of total pulmonary resistance in isolated buffer-perfused lung models [48]. Studying our in vivo model, we assessed HPV by obtaining dynamic measurements of PAP and QLPA during transient inferior vena cava occlusion at thoracotomy. Examining this murine model of acute unilateral lung hypoxia, we were able to study the in vivo effects of regional hypoxia on pulmonary vascular tone and systemic oxygenation, avoiding systemic hypoxia. We report that i.v. infusion of cell-free Hb did not increase HPV in mice. However, non-selective inhibition of all three isoforms of NOS by L-NAME augmented HPV.
There are several possible explanations for the observation that inhibition of NOS with L-NAME but not the scavenging of NO by cell-free Hb enhances HPV. It is possible that scavenging of NO by Hb is compensated by increased production of NO via several NOS isoforms, resulting in unaffected HPV. Conversely, acute inhibition of all three NOS isoforms by L-NAME could potentially lead to a vasodilator/vasoconstrictor imbalance that augments HPV. Alternatively, it is known that NOS3 can produce superoxide instead of NO [17]. Reactive oxygen species (ROS), specifically superoxide, can modulate pulmonary vascular tone and are reported to be key mediators of HPV [22; 49]. However, there is considerable controversy concerning the precise roles of ROS in HPV signaling with some investigators reporting that hypoxia was associated with reduced levels of ROS generation [50; 51] and others reporting that hypoxia increased ROS production [52; 53]. We have previously demonstrated that HPV is preserved in septic mice that are treated with ROS scavengers, emphasizing the contribution of ROS to the regulation of HPV [54]. In the present study, L-NAME markedly inhibited superoxide production by the lungs of WT mice in vitro. This finding indicates that inhibition of NOS by L-NAME in intact mice is associated with reduced superoxide production by the lung, which could alter the vasoconstrictor/vasodilator balance in the pulmonary circulation and augment HPV. On the contrary, plasma Hb does not inhibit NOS and therefore NOS-derived superoxide generation remains unchanged, which helps to explain the unaffected HPV in mice pretreated with Hb.
In conclusion, we have demonstrated that i.v. infusion of cell-free Hb did not alter basal murine pulmonary vascular tone or the response of the pulmonary vasculature to acute regional hypoxia. The pulmonary vascular tone of mechanically ventilated db/db mice was not affected by i.v. administration of plasma oxyHb. Pharmacological inhibition of NOS by L-NAME in WT mice did not affect basal pulmonary vascular tone but augmented HPV, likely by reducing NOS-derived superoxide generation during hypoxia and favoring vasoconstriction. Therefore, in mice NO may not be involved in the regulation of basal pulmonary vascular tone or HPV. The results of the present study emphasize both the marked species differences of mediators affecting basal pulmonary vascular tone and the species variation of the pulmonary vascular response to NO scavenging by plasma hemoglobin.
Highlights.
We investigated the impact of plasma Hb on murine pulmonary vascular tone
Scavenging of NO by plasma Hb does not alter pulmonary vascular tone in mice
Infusion of Hb does not change the pulmonary vasoconstrictor response to regional hypoxia
Acknowledgments
The authors would like to thank Patricio Leyton, M.D. (Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts) for providing advice on the lucigenin chemiluminescence assay.
Grants: This study was supported by funds of the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts. Dr. Kenneth D. Bloch was supported by a National Institute of Health R01 grant (HL074352), Bethesda, Maryland.
Footnotes
Disclosures: Dr. Zapol receives royalties from patents on inhaled nitric oxide licensed by Massachusetts General Hospital to Linde Corporation, Munich, Germany, and Ikaria Corporation, Clinton, New Jersey. Dr. Bloch has received grants from Ikaria to study inhaled nitric oxide. The remaining authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–79. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nat Med. 2010;16:381–2. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savitsky JP, Doczi J, Black J, Arnold JD. A clinical safety trial of stroma-free hemoglobin. Clin Pharmacol Ther. 1978;23:73–80. doi: 10.1002/cpt197823173. [DOI] [PubMed] [Google Scholar]
- 5.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–72. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winslow RM. In: αα-Crosslinked Hemoglobin. Winslow RM, editor. Blood Substitutes; Academic Press: 2006. pp. 386–398. [Google Scholar]
- 9.Dube GP, Vranckx P, Greenburg AG. HBOC-201: the multi-purpose oxygen therapeutic. EuroIntervention. 2008;4:161–5. doi: 10.4244/eijv4i1a26. [DOI] [PubMed] [Google Scholar]
- 10.Krieter H, Hagen G, Waschke KF, Kohler A, Wenneis B, Bruckner UB, van Ackern K. Isovolemic hemodilution with a bovine hemoglobin-based oxygen carrier: effects on hemodynamics and oxygen transport in comparison with a nonoxygen-carrying volume substitute. J Cardiothorac Vasc Anesth. 1997;11:3–9. doi: 10.1016/s1053-0770(97)90243-3. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR, MacDonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol. 1993;74:1769–78. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 12.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary Hypertension in Lambs Transfused with Stored Blood Is Prevented by Breathing Nitric Oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagoonee S, Gburek J, Hirsch E, Marro S, Moestrup SK, Laurberg JM, Christensen EI, Silengo L, Altruda F, Tolosano E. Plasma protein haptoglobin modulates renal iron loading. Am J Pathol. 2005;166:973–83. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–7. [PubMed] [Google Scholar]
- 15.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–47. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- 19.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 21.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–94. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archer SL, Tolins JP, Raij L, Weir EK. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of the synthesis of an endothelium derived relaxing factor. Biochem Biophys Res Commun. 1989;164:1198–205. doi: 10.1016/0006-291x(89)91796-8. [DOI] [PubMed] [Google Scholar]
- 24.Deem S, Swenson ER, Alberts MK, Hedges RG, Bishop MJ. Red-blood-cell augmentation of hypoxic pulmonary vasoconstriction: hematocrit dependence and the importance of nitric oxide. Am J Respir Crit Care Med. 1998;157:1181–6. doi: 10.1164/ajrccm.157.4.9707165. [DOI] [PubMed] [Google Scholar]
- 25.Sander M, Welling KL, Ravn JB, Boberg B, Amtorp O. Endogenous NO does not regulate baseline pulmonary pressure, but reduces acute pulmonary hypertension in dogs. Acta Physiol Scand. 2003;178:269–77. doi: 10.1046/j.1365-201X.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 26.Shirai M, Shimouchi A, Kawaguchi AT, Ikeda S, Sunagawa K, Ninomiya I. Endogenous nitric oxide attenuates hypoxic vasoconstriction of small pulmonary arteries and veins in anaesthetized cats. Acta Physiol Scand. 1997;159:263–4. doi: 10.1046/j.1365-201X.1997.106357000.x. [DOI] [PubMed] [Google Scholar]
- 27.Versprille A. Pulmonary vascular resistance. A meaningless variable. Intensive Care Med. 1984;10:51–3. doi: 10.1007/BF00297557. [DOI] [PubMed] [Google Scholar]
- 28.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition) J Clin Pathol. 1996;49:271–4. doi: 10.1136/jcp.49.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichinose F, Ullrich R, Sapirstein A, Jones RC, Bonventre JV, Serhan CN, Bloch KD, Zapol WM. Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest. 2002;109:1493–500. doi: 10.1172/JCI14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 32.Coleman RA, Humphrey PP, Kennedy I, Levy GP, Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 1981;73:773–8. doi: 10.1111/j.1476-5381.1981.tb16814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3:157–63. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaporidi K, Francis RC, Bloch KD, Zapol WM. Nitric oxide synthase 3 contributes to ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L150–9. doi: 10.1152/ajplung.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 36.Koch T, Duncker HP, Heller A, Schaible R, van Ackern K, Neuhof H. Effects of stroma-free hemoglobin solutions on pulmonary vascular resistance and mediator release in the isolated perfused rabbit lung. Shock. 1994;1:146–52. doi: 10.1097/00024382-199402000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology. 2009;110:113–22. doi: 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy JH, Goodnough LT, Greilich PE, Parr GV, Stewart RW, Gratz I, Wahr J, Williams J, Comunale ME, Doblar D, Silvay G, Cohen M, Jahr JS, Vlahakes GJ. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124:35–42. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 39.Celermajer DS, Dollery C, Burch M, Deanfield JE. Role of endothelium in the maintenance of low pulmonary vascular tone in normal children. Circulation. 1994;89:2041–4. doi: 10.1161/01.cir.89.5.2041. [DOI] [PubMed] [Google Scholar]
- 40.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–40. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- 41.Cremona G, Wood AM, Hall LW, Bower EA, Higenbottam T. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol. 1994;481(Pt 1):185–95. doi: 10.1113/jphysiol.1994.sp020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaacson TC, Hampl V, Weir EK, Nelson DP, Archer SL. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J Appl Physiol. 1994;76:933–40. doi: 10.1152/jappl.1994.76.2.933. [DOI] [PubMed] [Google Scholar]
- 43.Barnard JW, Wilson PS, Moore TM, Thompson WJ, Taylor AE. Effect of nitric oxide and cyclooxygenase products on vascular resistance in dog and rat lungs. J Appl Physiol. 1993;74:2940–8. doi: 10.1152/jappl.1993.74.6.2940. [DOI] [PubMed] [Google Scholar]
- 44.Liu R, Evgenov OV, Ichinose F. NOS3 deficiency augments hypoxic pulmonary vasoconstriction and enhances systemic oxygenation during one-lung ventilation in mice. J Appl Physiol. 2005;98:748–52. doi: 10.1152/japplphysiol.00820.2004. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Lopez JG, Moral-Sanz J, Frazziano G, Gomez-Villalobos MJ, Flores-Hernandez J, Monjaraz E, Cogolludo A, Perez-Vizcaino F. Diabetes induces pulmonary artery endothelial dysfunction by NADPH oxidase induction. Am J Physiol Lung Cell Mol Physiol. 2008;295:L727–32. doi: 10.1152/ajplung.90354.2008. [DOI] [PubMed] [Google Scholar]
- 46.Naeije R, Brimioulle S. Physiology in medicine: importance of hypoxic pulmonary vasoconstriction in maintaining arterial oxygenation during acute respiratory failure. Crit Care. 2001;5:67–71. doi: 10.1186/cc989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brimioulle S, LeJeune P, Naeije R. Effects of hypoxic pulmonary vasoconstriction on pulmonary gas exchange. J Appl Physiol. 1996;81:1535–43. doi: 10.1152/jappl.1996.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 48.Mazmanian GM, Baudet B, Brink C, Cerrina J, Kirkiacharian S, Weiss M. Methylene blue potentiates vascular reactivity in isolated rat lungs. J Appl Physiol. 1989;66:1040–5. doi: 10.1152/jappl.1989.66.3.1040. [DOI] [PubMed] [Google Scholar]
- 49.Archer SL, Peterson D, Nelson DP, DeMaster EG, Kelly B, Eaton JW, Weir EK. Oxygen radicals and antioxidant enzymes alter pulmonary vascular reactivity in the rat lung. Journal of applied physiology. 1989;66:102–11. doi: 10.1152/jappl.1989.66.1.102. [DOI] [PubMed] [Google Scholar]
- 50.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circulation research. 1993;73:1100–12. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 51.Mohazzab KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. The American journal of physiology. 1995;269:L637–44. doi: 10.1152/ajplung.1995.269.5.L637. [DOI] [PubMed] [Google Scholar]
- 52.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. American journal of respiratory cell and molecular biology. 1996;15:633–44. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 53.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circulation research. 2001;88:1259–66. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 54.Baboolal HA, Ichinose F, Ullrich R, Kawai N, Bloch KD, Zapol WM. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology. 2002;97:1227–33. doi: 10.1097/00000542-200211000-00028. [DOI] [PubMed] [Google Scholar]