Abstract
Background and Aims
The clinical significance of colorectal sensori-motor evaluation in patients with slow transit constipation (STC) is unclear. We investigated whether colonic manometric evaluation is useful for characterizing colonic sensorimotor dysfunction and for guiding therapy in STC.
Methods
24-hour ambulatory colonic manometry was performed in 80 patients (70 females) with STC by placing a 6 sensor solid state probe, along with assessment of colonic sensation with barostat. Anorectal manometry was also performed. Manometrically, patients were categorized as having colonic neuropathy or myopathy based on gastrocolonic response, waking response and high amplitude propagated contractions (HAPC); and based on colonic sensation, as colonic hyposensitivity or hypersensitivity. Clinical response to pharmacological, biofeedback and surgical treatment was assessed at 1yr and correlated with manometric findings.
Results
59% of patients had abnormal colonic manometry with features suggestive of neuropathy (26%), and myopathy (33%); 41% had normal colonic manometry. 74% patients had abnormal colonic sensation and 61% had overlapping dyssynergic defecation. Patients with neuropathy were more likely to have colonic hyposensitivity. 64% of patients with colonic myopathy or normal manometry improved with medical/biofeedback therapy when compared to 15% with colonic neuropathy (p<0.01). Selected patients with colonic neuropathy had excellent response to surgery, but many developed bacterial overgrowth.
Conclusions
Colonic manometry demonstrates significant colonic sensori-motor dysfunction in STC patients and reveals considerable pathophysiological heterogeneity. It can be useful for characterizing the underlying pathophysiology and for guiding clinical management in STC, especially surgery.
Keywords: Colonic manometry, slow transit constipation, neuropathy, treatment outcomes, colectomy
INTRODUCTION
Slow transit constipation (STC) affects 13–37% of patients with chronic constipation.(1, 2) Alterations in colonic muscle or nerve activity, enteric neurotransmitters and loss of interstitial cells of Cajal (ICC) may cause this problem.(3, 4) Also, other mechanisms may play a role, because there is significant overlap of STC with other subtypes of constipation notably dyssynergic defecation (DD) and constipation-predominant irritable bowel syndrome (IBS-C).(5–7)
Prolonged manometry studies have revealed that colonic motor activity is complex, intermittent, and variable across colonic segments, and exhibits both temporal and spatial variation.(8–10) In patients with STC, while the circadian rhythm is preserved, gastrocolonic and waking responses, as well as high amplitude propagating contractions (HAPCs) are significantly diminished or absent. (11, 12) Based on these manometric features, in a pilot study, we described distinct pathophysiological subcategories, notably colonic myopathy or neuropathy.(11) However, the validity of such a classification in the evaluation and management of patients with STC is not known. Also, the relationship between colonic and anorectal sensorimotor disturbances is unclear, with a recent study reporting phenotypic variations in colonic function.(13) Whether patients with STC have colonic motor and/or sensory dysfunction and coexisting anorectal sensorimotor dysfunction has not been systematically examined.
Furthermore, the management of STC is not standardized, with failure of medical therapy usually forming the basis for surgical intervention that has a success rate of 39–100%.(14, 15) The variable success rate could be due to a lack of recognition of the heterogeneity of colonic dysfunction in STC. While colonic manometry is routinely used to guide therapy in children with chronic constipation, its role in the management of adults with STC is unclear.(16, 17)
We hypothesized that patients with STC exhibit manometric features of either colonic myopathy or neuropathy or mixed sensori-motor dysfunction, and that an ambulatory 24-hour colonic manometry study facilitates better characterization of pathophysiology and a rational approach to the management of these patients. Hence, our objectives were to: (a) investigate the clinical features and prevalence of colonic and anorectal sensorimotor dysfunction in patients with STC; (b) identify pathophysiological subsets of STC based on colonic manometric features; (c) investigate whether colonic manometry is useful in guiding clinical management.
METHODS
Patients
Adult patients referred to our tertiary care center from 1997 to 2011 for further management of ‘medically-refractory’ chronic constipation, many for consideration of colectomy, were evaluated in this retrospective cohort study. All patients fulfilled the Rome II or III diagnostic criteria for functional constipation and underwent standard investigations including a colonoscopy and blood tests to exclude secondary causes of constipation.(4, 18, 19) Each patient underwent a colonic transit study using one capsule (24 radio-opaque markers, Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) and one X-ray technique.(20) Patients were only included if they retained ≥5 markers at 120 hrs that suggested STC.(4)Patients with neurological problems or those who had previous abdominal, pelvic or anorectal surgery other than appendectomy and cholecystectomy were excluded. The manometric data were compared with those from healthy controls previously reported from our center using identical protocol.(21–23)
The study protocol was approved by the Institutional Review Board at University of Iowa.
Study Protocol
All patients underwent 24-hr ambulatory colonic manometry to examine the colonic motor function. Additionally, using the same probe, colonic sensation testing was performed in 58 patients, seen after 2004 using a barostat. Also, anorectal manometry, rectal sensation assessment and balloon expulsion test were performed to examine anorectal physiology. Studies were performed over 3 visits, using previously described methods that are summarized below.(11, 24)
1. Colonic manometry and sensory assessment
Manometry assembly
A 6-mm diameter flexible probe containing six strain-gauge pressure transducers (Koningsberg Instruments, Pasadena, CA) with a 10 cm long highly compliant balloon was placed under colonoscopic and fluoroscopic guidance such that the balloon was located in the mid-descending colon. The transducers when correctly positioned were approximately located at 10, 20, 35, 50, 70 and 90 cm from the anus, with the most proximal sensor near the hepatic flexure. The variable distances between transducers were chosen to optimize their location in the rectum, sigmoid, descending, and transverse colon.(8). The probe was attached to an ambulatory recorder (Type 7-MPR or Nanologger, Gaeltec Ltd., Isle of Syke, UK).
Experimental Design
Patients were asked to discontinue laxatives, enemas and prokinetics at least 72 hrs before the study. All subjects received a gallon of polyethylene glycol (Golytely®, Braintree Laboratories Inc., Braintree, MA), the day before the study. Thereafter, using previously described technique, the manometry probe was placed in the colon.(8) In brief, a silk thread tied to the tip of the probe was grasped by a polypectomy snare and using minimal sedation (Midazolam 3 to 5mg IV), the probe and the pediatric colonoscope (Olympus GIFC10, Japan) were advanced under direct vision up to the hepatic flexure. Once the location was confirmed by fluoroscopy, the silk thread was released, freeing the probe. Next, the probe was clipped to the colonic mucosa using mucosal clips (Olympus America Inc, Melville, NY).(25) The probe was taped securely to the gluteal region. Fluoroscopy was repeated at study completion to assess the location of each sensor and the total radiation exposure did not exceed 1144 μrads.
The recorder was placed in a shoulder bag and the subjects were free to ambulate and were allowed free access to water. At 3:00 PM they received a standardized 1000-Kcalorie meal. They slept overnight either at the Clinical Research Center or at home. The next morning, they were awakened at 6:00 AM. The motility recording was continued until 2:00 PM. The subjects used an event marker to record the time of eating, walking, and sleeping or symptoms such as abdominal pain, passing flatus etc., and described these in a diary.
Colonic sensory testing was performed on day 2 at the end of 24-hr colonic manometry recording. Briefly, sample distensions were performed to unfold the balloon and educate the subject, using a barostat (Distender II, G&J Electronics, Toronto, Canada). Next, ramp distensions were performed in 2 mm Hg increments, each distention maintained for 60s duration and separated by 60s rest interval up to a maximal distending pressure of 50 mm Hg. Pressure thresholds for first sensation, discomfort, and pain were recorded. Finally, the probe was removed and patient was discharged.
Colonic manometry- Data analysis
The pressure activity from each transducer was analyzed by observing the manometry tracings on a monitor and with the help of a software analysis program [AMBB or NanoLogger6P, Gaeltec Ltd, Dunvegan, Isle of Skye]. Pressure waves with an amplitude ≥8 mm Hg and a duration ≥3s were analyzed. Movement artifacts induced by walking or coughing were excluded. The manometric data from 9:00 AM to 2:00 PM were discarded to minimize the effects of sedation and probe placement. The 24 hr recording obtained from 2:00 PM on day 1 until 2:00 PM on day 2 was analyzed.
We measured the incidence and maximum amplitude of HAPCs, defined as pressure wave sequences that migrated aborad across three or more consecutive channels with an amplitude ≥105 mm Hg and duration ≥14s.(8, 26) The number of waves and area under the curve (AUC) of pressure waves were measured through automated analysis, for quantifying colonic pressure activity. The morning waking response and meal-induced gastocolonic response are regarded as hallmarks of colonic neuromuscular integrity.(11) We analyzed AUC for a one-hour period before and after waking and 1-hour pre-prandially with 1-hour and 2-hour post-prandially. Colonic manometry data were compared with data from healthy subjects in our lab.(8)
The pathophysiological subsets of STC were identified based on colonic manometry patterns. The absence of any 2 out of 3 physiologic manometric responses, such as HAPCs, meal-induced gastrocolonic response and waking response, was categorized as a manometric pattern suggestive of colonic neuropathy. If ≥2/3 aforementioned colonic motor responses were present but attenuated, defined as a pressure activity/area under curve less than 2 standard deviations (SD) of the normal colonic motor response, then the manometric pattern was subcategorized as suggestive of colonic myopathy. Patients were categorized as having a normal colonic motility if the 3 manometric responses were normally present, or there was a mild impairment of only one of three manometric responses.(11) These definitions are consistent with previous manometric classifications of STC in the adult and pediatric literature,(11, 27) though some previous studies have defined colonic myopathy as complete absence of pressure events.(28) Colonic hyposensitivity and hypersensitivity were defined as increased or decreased sensory pressure thresholds that were 2 SD outside the normal range, and for two or more colonic sensations (first sensation, discomfort/urge or pain) respectively.
2. Anorectal manometry and rectal sensory assessment
Anorectal manometry was performed using a 6-sensor, solid-state probe and 4 cm long balloon attached to a recorder (Gaeltec 7MPR; Dunvegan, Isle of Skye, UK) using previously described methodology.(22, 24) The manometric profiles during attempted defecation were classified as either normal or abnormal (Type I-IV).(22) Rectal sensation was evaluated by sequentially inflating the rectal balloon with air. The threshold volumes required to induce a first sensation, desire to defecate/discomfort and urgent desire/pain were noted. (22, 24)
3. Balloon expulsion test
The subject was asked to sit on a commode and expel a 50 mL balloon with warm water. The inability to expel the balloon in <60s was interpreted as abnormal.(24)
DD was defined based on previously defined clinical and objective physiologic criteria.(29) This included the presence of 2 of the following 4 criteria in patients with constipation: dyssynergic pattern of defecation on ARM, prolonged balloon expulsion, prolonged colonic transit time or abnormal defecography. Severe DD was defined as DD with prolonged balloon expulsion (>300s). Rectal hyposensitvity and rectal hypersensitivity were defined as increased or decreased rectal volume threshold that was 2 SD outside the normal range, and for two or more sensory responses (first, desire/discomfort, urgency/pain).
Treatment for STC
All patients were recommended pharmacological management, based on symptomatic response and tolerance.(30) These included fiber supplements, stool softeners, stimulant laxatives (senna, bisacodyl), osmotic laxatives (polyethylene glycol, magnesium gluconate), secretagogues (lubiprostone, colchicine) and/or prokinetic agents (erythromycin, tegaserod, cisapride, bethenachol).(31) All patients were advised lifestyle changes.
In addition, all patients with DD and/or rectal hyposensitivity were recommended biweekly biofeedback therapy and/or rectal sensory training using visual/auditory/verbal biofeedback techniques, followed by re-inforcement at 6, 12 and 24 weeks as needed.(29, 32)
Surgical intervention was recommended for patients who fulfilled the following criteria: (a) manometric features of colonic neuropathy based on colonic manometry, (b) failed conservative management (pharmacological treatment with or without biofeedback), (c) normal gastric emptying and 24-hr small bowel motility, and (d) no significant medical or psychological comorbidities.(11, 14, 23) If DD and rectal hyposensitivity were corrected, then subtotal colectomy was recommended. Patients with suspected colonic myopathy or normal manometry were advised against surgery. Patients who developed symptoms post-surgery were assessed for post-operative complications, including small intestinal bacterial overgrowth (SIBO) with glucose-breath test and/or small bowel aspirate culture.
Response to treatment
The response to treatment was assessed at 1 year either through follow up clinic visit or structured questionnaire administered via telephone by an investigator (SH) not involved with manometry or treatment. Treatment was considered successful if patients reported a sustained symptomatic global improvement in their constipation-related symptoms for at least 6 months, with either pharmacological, biofeedback or surgical intervention. Patients were also asked to rate their overall satisfaction with their bowel habit on a visual analogue scale (VAS), 0 to 10, with ‘0’ being completely dissatisfied and ‘10’ being completely satisfied.
Statistical Analysis
The data are reported as mean (± SD) or median (range) as appropriate. Quantitative parameters were compared by Student’s t-test and qualitative parameters were compared by chi-square test to assess differences between groups. Data between the three pathophysiological subsets of STC were analyzed using repeated measures analysis of variance (ANOVA). The primary clinician who made recommendations for treatment based on colonic manometry was not involved in the assessment of treatment outcome. Follow-up data were obtained by an investigator who did not have access to clinical data and was not involved in data analysis. P <0.05 was considered significant. All analyses were performed using the SPSS software (SPSS 19.0, Illinois, Chicago).
RESULTS
Clinical characteristics
A total of 90 patients with refractory STC and referred for possible colectomy, were evaluated with colonic manometry; 2 patients failed colonic manometry probe placement. Of these 10 patients were excluded from analysis based on exclusion criteria (spinal cord injury). Eighty patients (70 females, mean age 44.1 ± 14.5 yrs; median symptom duration 12 yrs, range 1–50 yrs) were included in the analysis.
The key clinical features of our patients are shown in Table 1. Additionally, 50% of patients reported having <1BM/wk; the median Bristol stool score, off laxatives, was 2 (range 1–4). All patients had delayed colonic transit, and on average, they retained ~2/3rds of transit markers on day 5. Fourteen patients (17.5%) had coexisting features of IBS-C (clinical features and rectal hypersensitivity on rectal barostat), and 49 patients (61%) had coexisting DD. Neither clinical symptoms nor transit marker retention/distribution predicted the presence of co-existing DD. Two patients had eating disorders and 11 patients had a history of physical or sexual abuse. None of the patients had Hirschsprung’s disease or megacolon or megarectum. Colonoscopy revealed melanosis coli in 20% (16/80) of patients and a redundant colon in 29% (23/80) of patients.
Table 1.
Distribution of clinical features and bowel symptoms present with ≥25% of bowel movements among patients with normal colonic manometry, colonic myopathy and colonic neuropathy, in adults with STC
| Clinical features | Normal colonic manometry (n=33) | Colonic myopathy (n=26) | Colonic neuropathy (n=21) |
|---|---|---|---|
|
| |||
| Age (in yrs) (Mean ± SD) | 45.8 ± 13.9 | 41.6 ± 15.7 | 44.9 ± 13.8 |
|
| |||
| Sex – Female/Male | 32/1 | 22/4 | 16/5* |
|
| |||
| Symptom duration (in yrs) [Median (range)] | 12 (1–35) | 13 (1–50) | 16 (1–50)* |
|
| |||
| Transit marker distribution [Mean % (SD)] | |||
| • R-colon | 21.7 (17.1) | 12.9 (12.6) | 17.9 (21.5) |
| • L-colon | 25.0 (17.1) | 24.0 (12.6) | 23.7 (14.2) |
| • Rectosigmoid | 20.0 (15.0) | 26.2 (15.9) | 24.2 (18.1) |
| • Total | 67.1 (28.0) | 62.5 (28.8) | 65.8 (30.5) |
|
| |||
| Symptoms | |||
| • <3 BMs/wk (%) | 89 | 94 | 95 |
| • Hard stools (%) | 94 | 92 | 97 |
| • Feeling of incomplete evacuation | 93 | 95 | 95 |
| • Straining (%) | 85 | 100 | 89 |
| • Anorectal blockage (%) | 52 | 68 | 63 |
| • Use of manual maneuvers (%) | 37 | 47 | 58 |
| • Laxative dependence (%) | 92 | 84 | 80 |
p=0.03, neuropathy v/s normal
Colonic manometry profiles
Based on manometric features, 47/80 patients with STC had abnormal colonic manometry; 21 patients (26.2%) were categorized as having colonic neuropathy and 26 patients (32.5%) as having colonic myopathy. Thirty three patients (41.2%) had normal colonic manometry.
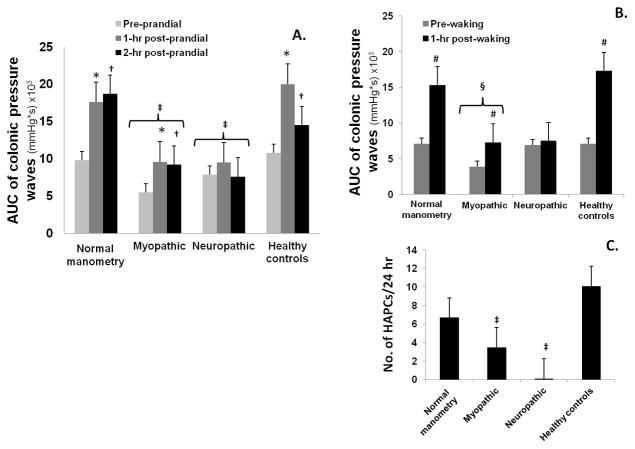
Figure 1 shows the distribution for the area under the curve of pressure waves for the 3 common types of manometric responses that were seen during colonic manometry in STC patients and healthy controls.(4) The gastrocolonic and waking responses in patients categorized as having normal colonic manometry were significantly higher than the pre-prandial and pre-waking state (p<0.05). Also, these profiles were comparable to the responses seen in healthy controls (Figure 1). In contrast, the gastrocolonic and waking responses were either absent or significantly attenuated in patients categorized as having colonic neuropathy. In patients categorized as having colonic myopathy, the gastrocolonic and manometric responses were present and higher than basal state, but the responses were significantly attenuated, when compared to healthy controls (Figure 1).
Figure 1.
This shows the distribution for area under the curve of pressure waves for the meal-induced gastrocolonic response (A), and waking response (B) and the number of HAPC over 24 hours (C) in the 3 subcategories of patients with STC, and in healthy controls
(*=p< 0.05, 1-hr post-prandial v/s pre-prandial; †=p<0.05, 2-hr post-prandial v/s pre-prandial; ‡=p≤0.05; healthy controls v/s neuropathic/myopathic; #=p<0.05, 1-hr post-waking v/s pre-waking; §=p<0.05, healthy controls v/s myopathic)
Patients with colonic neuropathy had longer duration of symptoms, when compared to patients with normal colonic manometry (p=0.03), but there was no relation between transit marker distribution and manometric subsets of STC. Importantly, none of the constipation-related symptoms could differentiate or predict manometric categorization of STC (Table 1).
Colonic sensation in STC
This was assessed in 58 patients. Approximately 74% (43/58) of patients had abnormal colonic sensation, with 31 patients exhibiting features of colonic hyposensitivity and 12 patients demonstrating colonic hypersensitivity (Table 2). In 21% (12/58) patients, colonic hyposensitivity was the only physiologic abnormality found during colonic and anorectal manometric evaluation. Patients with colonic neuropathy were more likely to have colonic hyposensitivity, than those with either normal colonic manometry or myopathy (Figure 2).
Table 2.
Colonic and rectal sensation in adults with STC. Data on healthy adults was obtained from previous studies in our lab.(21, 22)
| Colonic sensation Pressure threshold [Mean pressure (SEM)] | ||||
|---|---|---|---|---|
| Normosensitive (n=15) | Hyposensitive (n=31) | Hypersensitive (n=12) | Healthy adults (n=7) | |
| 1st sensation (mmHg) | 18 (2.5) | 36 (10.7) | 14 (4.0) | 16 (0.8) |
| Discomfort (mmHg) | 25 (2.2) | 42 (8.2) | 19 (4.8) | 29 (1.2) |
| Max tolerable pressure (mmHg) | 31 (1.4) | 47 (2.5) | 23 (3.6) | 35 (0.6) |
| Rectal sensation Volume threshold [Mean volume (SD)] | ||||
| Normosensitive (n=16) | Hyposensitive (n=37) | Hypersensitive (n=27) | Healthy adults (n=45) | |
| 1st sensation (cc) | 26 (19.6) | 78 (23.2) | 13 (5.8) | 20 (4.6) |
| Desire to defecate (cc) | 101 (38.8) | 220 (65.5) | 52 (25.6) | 106 (8.3) |
| Max tolerable volume (cc) | 215 (50.4) | 308 (22.8) | 125 (32.8) | 237 (11.2) |
Figure 2.
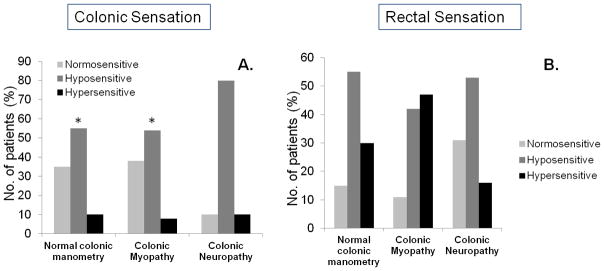
Distribution of the number of patients with normal or abnormal colonic (A) and rectal sensation (B) in the 3 manometries subcategories of patients with STC (* = p<0.05, colonic neuropathy v/s myopathy/normal colonic motility)
Anorectal manometry and rectal sensation
We found that 61% (49/80) of STC patients also had features of DD; 19 patients had severe features including an inability to expel the rectal balloon even after 5 minutes (Table 3). Altered rectal sensation was present in 80% (64/80) patients with STC, (37 with rectal hyposensitivity and 27 with rectal hypersensitivity). The rectal sensory dysfunction was equally distributed among the 3 manometric categories of STC. Also, they were unrelated to colonic sensory dysfunction (Table 2).
Table 3.
Distribution of DD among patients with normal colonic manometry, colonic myopathy and colonic neuropathy, in adults with STC
| Normal colonic manometry (n=33) | Colonic myopathy (n=26) | Colonic neuropathy (n=21) | |
|---|---|---|---|
|
| |||
| Dyssynergia – no./total (%) | 21/33 (64) | 15/26 (58) | 13/21 (62) |
|
| |||
| Type of dyssynergia | |||
| • Type 1 | 8/21 (38) | 5/15 (33) | 4/13 (31) |
| • Type 2 | 8/21 (38) | 4/15 (27) | 9/13 (69)* |
| • Type 3 | 5/21 (24) | 6/15 (40) | 0/13 (0) |
|
| |||
| Prolonged Balloon Expulsion – no./total (%) | 5/33 (15) | 4/26 (15) | 10/21 (48)* |
|
| |||
| Severe DD – no./total (%) | 5/21 (24) | 4/15 (27) | 10/13 (77)* |
p<0.05, neuropathy v/s myopathy/normal
Patients with colonic neuropathy were more likely to have a prolonged balloon expulsion time and severe DD, compared to patients with normal colonic manometry or colonic myopathy. Moreover, type 2 dyssynergia was significantly more common in patients with colonic neuropathy than other categories (Table 3).(29)
Clinical outcome in STC
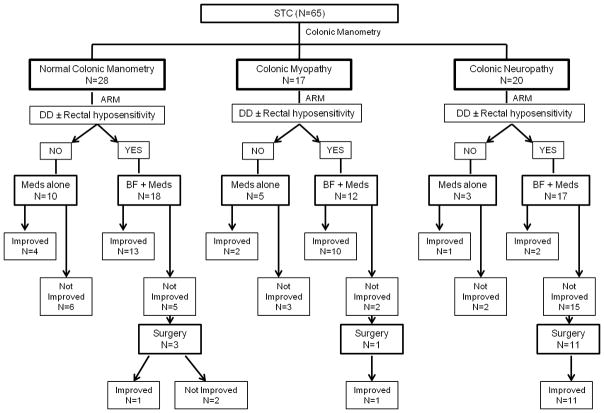
Sixty five patients (81%) (56 females, mean age 46 ±14.3 yrs) who completed a minimum of 1-yr follow up (median duration of follow up: 3 years; range 1–12 years) were assessed either through follow up clinic visit (65%) or via telephone questionnaire (35%). The remaining patients were lost to follow up or could not be contacted. Their demographic and clinical profiles were similar to the original group; 28 patients (43%) had normal colonic manometry, 17 (26%) had colonic myopathy and 20 (31%) colonic neuropathy (Figure 3). Forty seven patients (72.3%) underwent biofeedback therapy for DD and/or sensory retraining. Overall, 38.9% (7/18) patients responded to pharmacological therapy alone and 25/37 (53.2%) responded to pharmacological and biofeedback therapy. Fifteen patients (23.1%) underwent surgical intervention, and 13/15 (86.7%) improved.
Figure 3.
Treatment and clinical outcomes in the 3 manometric subcategories of patients with slow transit constipation (STC)
(ARM=Anorectal manometry & Rectal sensory test; Meds = Pharmacological therapy, BF = Biofeedback, DD =Dyssynergic defecation)
Response in patients with normal colonic manometry
In this group, 17 (60.7%) responded to conservative management. Of 11 patients who failed conservative therapy, 3 underwent surgery, but only 1 reported symptomatic improvement.
Response in patients with colonic myopathy
In this group, 12 (70.6%) responded to conservative management, 1 underwent surgery with good clinical response
Response in patients with colonic neuropathy
In this group, only 3 (15%) improved with conservative management. Thirteen patients were recommended surgery, and 11 underwent subtotal colectomy and reported symptomatic improvement. Four patients who failed conservative therapy were advised against surgical intervention due to comorbidities (3 had eating disorders, 1 had upper gastrointestinal dysmotility).
Post-surgical complications
Over time, 46.7% of patients (7/15) who underwent subtotal colectomy developed new onset of significant bloating, distension and flatulence. Further testing with glucose breath test and/or duodenal aspirate revealed that all of these patients had small intestinal bacterial overgrowth which responded to cyclical antibiotics.
VAS score
Among patients who improved with any treatment, the mean VAS score was 7.0 ± 1.7, and for those who did not improve, the mean VAS was 2.6 ± 2.2 (p=0.02). The mean VAS score for those who improved on pharmacological/biofeedback therapy was 6.6 ± 1.6, whereas for those who underwent surgery was 7.7 ± 2.0 (p=0.84).
DISCUSSION
Colonic manometry is recommended by the American Neurogastroenterology and Motility Society in selected patients with chronic STC who do not respond to medical therapy.(16, 33) In this current study using prolonged 24-hr colonic manometry under ambulatory conditions, and by assessing colonic responses to physiologic stimuli of sleep and meal, we have reaffirmed the presence of 3 distinct manometric categories of STC – colonic neuropathy (likely caused by damage to the neuronal circuitry within the colon with secondary muscle dysfunction), colonic myopathy (likely caused by significant end-organ/muscle damage but intact neuronal circuitry) and normal colonic manometry.(11) Previous studies have characterized the phenotypic variability of chronic constipation based on intraluminal colonic phasic and tonic motility assessment, but the manometric subsets have not been described in part because limited segments were evaluated, the study duration was short and subjects were non-ambulatory.(13) Recent pediatric studies have revealed a high prevalence of enteric neuropathology in both the resected colon and the residual colon implying the presence of a generalized neuromuscular pathology in patients with refractory STC undergoing colectomy.(34)
Colonic manometry patterns
We found that ~26% patients with STC had manometric features of colonic neuropathy, ~32% patients had features of colonic myopathy, and ~41% had normal colonic manometry. There was no difference in the distribution of regional or global transit marker distribution or in symptom patterns in these 3 subcategories of STC, reiterating the fact that clinical characteristics cannot reliably distinguish patients who have intact or severely abnormal neuromuscular dysfunction. Thus, in refractory STC patients, colonic manometry appears to be the most useful tool for identifying patients with significant colonic neuromuscular dysfunction.(4, 33) Interestingly, patients categorized as having colonic neuropathy were more likely to show prolonged balloon expulsion and type 2 DD characterized by impaired propulsion during defecation suggesting that this group has both impaired colonic and rectal neuronal circuitry, consistent with colorectal neuropathy.(35) This finding may explain why we found a limited symptomatic response (only 2/17 patients) with biofeedback in patients with colonic neuropathy, despite correction of dyssynergia in 96% of these patients.
Colonic sensory dysfunction
Abnormalities on colonic sensation testing were seen in 74% patients. This may be a consequence of long-standing functional constipation and laxative dependence, or may be due to an underlying sensory neuropathy. None of our patients had a megacolon. Altered colonic sensation was the only demonstrable abnormality in 21% of patients, suggesting that isolated sensory neuropathy may play a pathophysiologic role in STC. Colonic hyposensitivity was also more likely to be present in patients with colonic neuropathy than other subsets of STC, suggesting receptor/neuronally mediated afferent sensory dysfunction or CNS processing. Systemic small fiber sensory neuropathy has been described in ~40% patients with STC.(36) Rectal hyposensitivity is the only identifiable physiologic abnormality in ~50% patients with constipation, responsive to sensory retraining.(37) Therefore, it is conceivable that sensory training may improve constipation in a subset of patients with ‘isolated’ sensory impairment.
Overlap with dyssynergic defecation
Significant overlap (10–60%) between STC and DD and STC and IBS-C has been described.(5–7, 38) We found ~61% patients with DD and 17% with IBS-C, in our cohort of patients with STC. The development of DD in this group of patients could be attributed to the loss of “learned” defecation with failure of abdominal, pelvic and recto-anal coordination in this group of patients due to long-standing severe STC (median symptom duration 12 yrs) and laxative dependence.(39) Of the 47 patients with STC who underwent biofeedback therapy, 96% showed objective improvement of dyssynergia, but only 57% reported symptomatic improvement of constipation. Thus, in 43% of patients, the colonic neuromuscular dysfunction was responsible for severe constipation rather than DD alone. Our findings confirm the 50% response rate to biofeedback therapy in patients with ‘mixed STC and DD’, previously reported by Chiarioni et al.(40) These rates are below the typical 80% response for DD.(29, 41)
Management of slow transit constipation
Studies on optimal management and long term outcomes in adults with STC are lacking. While colonic motility testing is routinely performed by pediatric gastroenterologists and in children to optimize their medical and surgical treatment, its utility in adults with STC is not clear.(11, 13, 16) Most physicians use failed therapeutic trials as the indication for colectomy especially in those with documented slow colonic transit and normal evacuation.(16) Using prolonged ambulatory colonic manometry, we found that nearly 2/3rds of patients with normal colonic manometry or colonic myopathy responded to aggressive pharmacological and/or biofeedback therapy. With the advent of newer targeted pharmacological therapy such as prucalopride or linaclotide, the response rate is likely to improve.(31, 42) This may suggest that severe muscle dysfunction in patients with a myopathic pattern may be reversible, as opposed to permanent muscle damage. Unlike these, patients with colonic neuropathy responded poorly to conservative management with only 15% reporting improvement. Thus, after correcting any co-existing anorectal dysfunction, and excluding upper gut dysfunction or co-morbidities, selected STC patients with neuropathy are good candidates for subtotal colectomy. The highly variable 39–100% success rate reported in the literature for surgery is probably related to a failure of recognition of pathophysiologic heterogeneity, and/or inappropriate patient selection.(14, 15) Given the significant differences in response to therapy in patients with neuropathy and myopathy, efforts to distinguish these entities through a combination of colonic and anorectal sensorimotor function testing and histological correlation could be very important.
Interestingly, a small proportion (15%) of patients with severe colonic neuropathy improved with conservative management. Half of this small subgroup of patients had severe coexisting DD, and after receiving biofeedback therapy, their constipation symptoms improved. This improvement in STC patients may be consistent with changes in colonic neuronal plasticity as shown in animal experiments where reduced interstitial cells of Cajal (ICCs) (following partial small bowel obstruction, inflammation, surgical transection and/or anastomosis), normalized after resolution of the underlying problem.(43) This suggests that ICCs may have regenerative ability of ICC if the underlying problem is corrected. Hence, a short trial of aggressive conservative management should be tried in patients with colonic neuropathy, and this may include biofeedback therapy if the patient has co-existing DD.
However, despite strict selection of cases for surgery, there is some early and late postoperative morbidity, with ~40% reporting persistent abdominal symptoms.(15, 44) Also 47% of our patients who underwent subtotal colectomy developed small intestinal bacterial overgrowth. This problem responded well to cyclical antibiotics in all patients. A lack of an effective barrier between the small bowel and rectum may have caused this problem, and should be recognized and treated.
Limitations
Our study has some limitations. Assessment of fasting and postprandial colonic tone and compliance has been reported to be helpful in studying the phenotypic variation of chronic idiopathic constipation, but this was not examined in our study.(13, 28) Histological confirmation of neuropathy and myopathy would have been helpful, but unfortunately, most patients underwent surgery at centers outside our institution, and hence they could not be characterized. We were also limited in assessing response to therapy. There is potential for recall bias with a telephonic interview, although it was conducted by an independent investigator, who was not involved in patient care. Future prospective studies should focus on systematic assessment of response to therapy using stool and symptom diaries and quality of life questionnaires. Also, the current pan-colonic manometry tool used in our study with a 6 sensor probe sampling at approximately 15–20 cm levels in the colon may not provide complete information regarding colonic motor function. High-resolution colonic topography or high-sensor count fiber-optic manometry that have recently become available may improve spatial and temporal resolution at much closer distance, and may provide better characterization and more comprehensive assessment, enabling better understanding of colonic pathophysiology.(23, 45, 46)
Conclusion
In conclusion, our study shows that ambulatory prolonged colonic manometry with colonic sensory assessment is useful in characterizing the underlying sensorimotor dysfunction and pathophysiology of neuromuscular disturbances in patients with STC. This information can serve as the basis for an informed decision towards better management of patients with STC.
Acknowledgments
Grant Support: Dr. Rao was supported in part by Grant R01DK 57100-03 National Institute of Health and NIHNCRR 1UL1RR024979, 1KL2RR024980 & 1TL1RR024981 – University of Iowa Clinical and Translational Science Program. Portions of this work were presented at Digestive Disease Week and published as an abstract; Gastroenterology 2010;138:S225
The authors sincerely appreciate the excellent technical assistance of Mrs. Jessica Valestin, Mrs. Deborah Dickinson and the secretarial support of Mrs. Jann Leverett.
Abbreviations
- AUC
Area under curve of pressure waves
- DD
Dyssynergic defecation
- DI
Defecation Index
- HAPC
High amplitude propagated contractions
- IBS-C
Constipation-predominant Irritable bowel syndrome
- ICC
Interstitial Cells of Cajal
- PPW
Propagated pressure waves
- STC
Slow transit constipation
- VAS
Visual Analogue Scale
Footnotes
Competing Interests: The authors have no competing interests.
Author Contributions: SS - study concept and design; analysis and interpretation of data; statistical analysis, drafting of the manuscript; SH and ECA - acquisition of data; SSCR - study concept and design; study performance and probe placement; interpretation of data; critical revision of the manuscript for important intellectual content; overall supervision
Disclosures: None
References
- 1.Surrenti E, Rath DM, Pemberton JH, Camilleri M. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–1475. [PubMed] [Google Scholar]
- 2.Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40:273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 3.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am. 2007;36:687–711. x. doi: 10.1016/j.gtc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Karlbom U, Pahlman L, Nilsson S, Graf W. Relationships between defecographic findings, rectal emptying, and colonic transit time in constipated patients. Gut. 1995;36:907–912. doi: 10.1136/gut.36.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prather CM. Subtypes of constipation: sorting out the confusion. Rev Gastroenterol Disord. 2004;4 (Suppl 2):S11–16. [PubMed] [Google Scholar]
- 7.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol. 1999;94:609–615. doi: 10.1111/j.1572-0241.1999.922_a.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 9.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. Prolonged multi-point recording of colonic manometry in the unprepared human colon: providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol. 2001;96:1838–1848. doi: 10.1111/j.1572-0241.2001.03924.x. [DOI] [PubMed] [Google Scholar]
- 10.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am J Physiol. 1988;255:G660–664. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405–2416. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagger R, Kumar D, Benson M, Grundy A. Colonic motor activity in slow-transit idiopathic constipation as identified by 24-h pancolonic ambulatory manometry. Neurogastroenterol Motil. 2003;15:515–522. doi: 10.1046/j.1365-2982.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 13.Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladman MA, Knowles CH. Surgical treatment of patients with constipation and fecal incontinence. Gastroenterol Clin North Am. 2008;37:605–625. viii. doi: 10.1016/j.gtc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230:627–638. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 17.Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407–1411. doi: 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead WE, Wald A, Diamant NE, Enck P, Pemberton JH, Rao SS. Functional disorders of the anus and rectum. Gut. 1999;45(Suppl 2):II55–59. doi: 10.1136/gut.45.2008.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinton JM, Lennard-Jones JE. Constipation: definition and classification. Postgrad Med J. 1968;44:720–723. doi: 10.1136/pgmj.44.515.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao S, Mudipalli R, Paulson J, Brown C, Seaton K. Investigation of colonic and rectal sensory properties and compliance and its reproducibility in humans. American Journal of Gastroenterology. 2008;103:S465–S465. [Google Scholar]
- 22.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol. 2010;44:597–609. doi: 10.1097/MCG.0b013e3181e88532. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 25.Rao SS, Singh S, Sadeghi P. Is endoscopic mucosal clipping useful for preventing colonic manometry probe displacement? J Clin Gastroenterol. 2010;44:620–624. doi: 10.1097/MCG.0b013e3181d04899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook IJ, Furukawa Y, Panagopoulos V, Collins PJ, Dent J. Relationships between spatial patterns of colonic pressure and individual movements of content. Am J Physiol Gastrointest Liver Physiol. 2000;278:G329–341. doi: 10.1152/ajpgi.2000.278.2.G329. [DOI] [PubMed] [Google Scholar]
- 27.Villarreal J, Sood M, Zangen T, et al. Colonic diversion for intractable constipation in children: colonic manometry helps guide clinical decisions. J Pediatr Gastroenterol Nutr. 2001;33:588–591. doi: 10.1097/00005176-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Di Lorenzo C, Flores AF, Reddy SN, Hyman PE. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr. 1992;120:690–695. doi: 10.1016/s0022-3476(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 29.Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am. 2008;37:569–586. viii. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163–171. doi: 10.2147/cia.s8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Rao SS. Pharmacologic management of chronic constipation. Gastroenterol Clin North Am. 2010;39:509–527. doi: 10.1016/j.gtc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–338. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Zarate N, Knowles CH, Newell M, et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008;103:427–434. doi: 10.1111/j.1572-0241.2007.01675.x. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg MM, Di Lorenzo C, Mousa HM, Benninga MA, Boeckxstaens GE, Luquette M. Morphological changes of the enteric nervous system, interstitial cells of cajal, and smooth muscle in children with colonic motility disorders. J Pediatr Gastroenterol Nutr. 2009;48:22–29. doi: 10.1097/MPG.0b013e318173293b. [DOI] [PubMed] [Google Scholar]
- 35.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 36.Knowles CH, Scott SM, Wellmer A, et al. Sensory and autonomic neuropathy in patients with idiopathic slow-transit constipation. Br J Surg. 1999;86:54–60. doi: 10.1046/j.1365-2168.1999.00994.x. [DOI] [PubMed] [Google Scholar]
- 37.Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 38.Iantorno G, Cinquetti M, Mazzocchi A, Morelli A, Bassotti G. Audit of constipation in a gastroenterology referral center. Dig Dis Sci. 2007;52:317–320. doi: 10.1007/s10620-006-9486-5. [DOI] [PubMed] [Google Scholar]
- 39.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 40.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 43.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollen RM, Kuijpers HC, Claassen AT. Colectomy for slow-transit constipation: preoperative functional evaluation is important but not a guarantee for a successful outcome. Dis Colon Rectum. 2001;44:577–580. doi: 10.1007/BF02234332. [DOI] [PubMed] [Google Scholar]
- 45.Dinning PG, Arkwright JW, Gregersen H, o’grady G, Scott SM. Technical advances in monitoring human motility patterns. Neurogastroenterol Motil. 2010;22:366–380. doi: 10.1111/j.1365-2982.2010.01488.x. [DOI] [PubMed] [Google Scholar]
- 46.Giorgio V, Borrelli O, Smith VV, et al. High-resolution colonic manometry accurately predicts colonic neuromuscular pathological phenotype in pediatric slow transit constipation. Neurogastroenterol Motil. 2012 doi: 10.1111/nmo.12016. [DOI] [PubMed] [Google Scholar]