Abstract
This study was conducted to evaluate the burn wound healing and antioxidant activity of methanolic and aqueous extracts of Galium odoratum (L.) Scop. in rats. Second degree burn wounds were induced in six groups of six rats each. Groups 1 and 2 received eucerin and silver sulfadiazine as control and reference standard and groups 3, 4, 5 and 6 were given methanolic and aqueous extracts of 15% and 30% (w/w in eucerin base) respectively. The topical treatment was done daily for 14 days. The percentage of wound contraction and histology parameters of healed wounds were observed. The antioxidant potential of both extracts was assessed by 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) assay. There was statistically significant improvement in wound contraction of animals treated with extracts in comparison to control (p < 0.001). The healed wounds in extracts-treated animals contained less inflammatory cells and had better reepithelialization. Wound contraction and histology parameters were relatively better in aqueous extract (90.68 ± 6.13% and 97.18 ± 4.37% for aqueous extracts of 15% and 30% in comparison to 79.29 ± 9.16% and 91.94 ± 4.14% for methanolic extracts of 15% and 30% respectively). In DPPH assay, both methanolic and aqueous extracts displayed significant antioxidant activity with IC50 values of 148 μg/ml and 83 μg/ml, respectively. In conclusion, both extracts had desirable antioxidant potential plus experimentally and histologically ascertained burn wound healing activity, relatively better for aqueous extract.
Keywords: DPPH-scavenging activity, Galium odoratum, Second degree burn, Wound healing
INTRODUCTION
Burn is a skin tissue injury caused by a variety of factors such as extreme heat, chemicals or electricity and categorized based on its depth into first, second and third degree. In deep partial thickness (second-degree) burns, the epidermis and the deep dermis are mainly affected. Burn wound healing involves dynamic interaction between cytokine cells and extracellular matrix. This process is divided into three phases including inflammation, proliferation and tissue remodeling. Re-epithelialization and wound contracture are two processes that occur during the wound repair, whereby the epithelial layers are regenerated and the area of the wound shrinks (1). Healing of burn is still a challenge in modern medicine and there are a few drugs capable of accelerating wound healing. As an alternative plants are rich sources to survey (2). Galium odoratum (L.) Scop. (Syn Asperula odorata L.) from Rubiaceae family, commonly known as sweet woodruff, is naturally distributed in Northern and Central Europe, Siberia, Northern Africa and various regions in the north of Iran. The plant contains coumarin (0.4-1%), asperuloside (0.05-0.3%), monotropein (0.04%), tannins, iridoids, anthra-quinones, flavonoids and traces of nicotinic acid. Aerial parts and flowering tops are used as a treatment for nervous agitation, jaundice, hemorrhoids, circulation and venous disorders traditionally and the crushed leaves have been used topically to reduce swelling and to accelerate wound healing (3,4). Previous studies suggested that this plant may have anti-inflammatory activity (5) and an anthra-quinone derivative in this plant showed an inhibitory effect against thymidine kinase of herpes simplex virus1 (HSV-1) (6). Based on this ethno-pharmacological profile, we were interested to examine the burn wound healing potential of aqueous and methanolic extract of G. odoratum and their 1,1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging antioxidant activity.
MATERIALS AND METHODS
Plant material
The aerial parts of Galium odoratum were collected from Mazandaran Province, Iran in June 2010 and authenticated by Yousef Ajani (Department of Pharmacognosy, Institute of Medicinal Plants, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran). A voucher specimen of the plant (No. Ajani 1455) has been deposited in Central Herbarium of Medicinal Plants (ACECR), Tehran, Iran.
Chemicals
All reagents and solvents were of analytical grade or of pure quality which were purchased from Merck Company (Germany) with the exception of silver sulfadiazine which was purchased from Behvazan Company (Iran).
Preparation of extracts and phytochemical screening
The aerial parts were air-dried and pulverized to a coarse powder in a mechanical grinder. The extraction procedure was done in two perculators at room temperature. Distilled water and methanol/H2O (80:20 v/v) were used as solvents for extraction of 100 g and 300 g coarse powder, respectively. The proce-dure involved three successive extractions of 48 h using new solvent each time. The extracts were concentrated under vacuum below 35o C using a rotary evaporator to obtain dry extracts. Then the aqueous extract was lyophilized to obtain a dry powder extract. This dry powder was stored at -20o C. An attempt was also made to observe the presence or absence of different phytochemical constituents in both extracts qualitatively.
Extracts and standards used
Two types of cream formulations (methanolic extract cream (MEC) and aqueous extract cream (AEC)) with different concentrations of the plant extracts (15% and 30% w/w) were prepared. Eucerin was used as cream basis. Silver sulfadiazine (SS) 1% and eucerin were used as reference standard and control respectively.
1,1-diphenyl-2-picryl-hydrazyl free radical scavenging activity
The DPPH scavenging activity was measured using the method described by Sanchez-Moreno and coworker with some modification (7). Briefly 2 ml of a methanolic solution of DPPH (0.004%) was mixed with 1 ml of serial dilutions (50, 100, 300 and 500 μg/ml) of G. odoratum extracts, 1 ml of Butylated Hydroxy Anisole (BHA) solution (0.001%) and 1 ml of methanol. Then the absorbance was recorded immediately at 517 nm and its decline was measured every 5 min up to 30 min, using a UV spectro-photometer. All of solutions were kept in the darkness and at room temperature during this period. BHA and methanol were used as reference standard and blank respectively. To eliminate the innate absorption effect of the extracts and methanol, two solutions with 1 ml of each extract (1 mg/ml) and 2 ml of methanol were used as control. All tests were carried out in triplicate. Finally the radical scavenging activity was calculated as percentage of DPPH discolor-ation using the equation below:
Scavenging DPPH free radical or Inhibition (%) = 100 × [1-((AE-AC)/AD)]
where, AE is the absorbance of the DPPH solution, when extract has been added at a particular level, AD is the absorbance of the DPPH solution with methanol (blank, without extract) and AC is the absorbance of the control solution.
Pharmacological activity
Animals used
Male Wistar rats weighing 200-250 g were used to do this experiment. They were housed individually (one rat per cage) in standardized environmental conditions (23 ± 1o C and light dark cycles of 12 h and 12 h, respectively), fed with normal diet and water ad libitum. This study was undertaken after obtaining the approval of institute review board of Tehran University of Medical Sciences (TUMS).
Creation of burn
Thirty-six rats were shaved on the back and anaesthetized by injection of pentobarbital (50 mg/kg, IP). Then a deep circular 20 mm diameter burn wound (314 mm2) was created on their dorsal parts, using an electrical heater (110o C for 10 sec). The underlying skin was cleaned with normal saline (8).
Experiment protocol
In the experiment, the rats bearing the deep partial thickness second degree burn wound, were divided into six groups (n=6): group 1 was the control group which received eucerin (314 mm2), group 2 was treated with reference standard (SS 1% w/w) (314 mm2), groups 3 and 4 received MEC 15% and 30% (w/w) (314 mm2) respectively, groups 5 and 6 received AEC 15% and 30% (w/w) (314 mm2) respectively. Eucerin, MEC, AEC and SS were used topically with 24 h intervals on wound created on the dorsal back of rats. The day of burn creation was assumed as zero and treatment procedure was started 24 h after burn creation for 14 days.
Rate of wound contraction
The rate of wound contraction was measured as the percentage of reduction in the original wound size at every 4 days interval from second day of treatment, by taking from second day of treatment, by taking picture with digital camera at an equal distance from the burn and right angle to its surface. Before taking picture, the wounds were rinsed by normal saline to cleaning the wound surface and removing debris. The captured images were examined by Image Mixle software to evaluate the wound size. The percentage of wound contraction was calculated using this equation (9) :
Wound contraction (%) = 100 × [(zero day wound size - specific day wound size)/zero day wound size]
Histological evaluation
Granulated tissue was collected on the last day and preserved in 10% buffered formalin. Series of 3-4 μm thickness sections were prepared and stained with hematoxylin and eosin and photographed under 100 or 400 × magnification. These samples were evaluated by a blind histologist.
Statistical analysis
All values were expressed as mean ± S.D. Data were analyzed by one-way ANOVA, followed by Tukey’s post hoc test. The results were considered significantly different at P <0.05.
RESULTS
Preparation of extracts and phytochemical screening
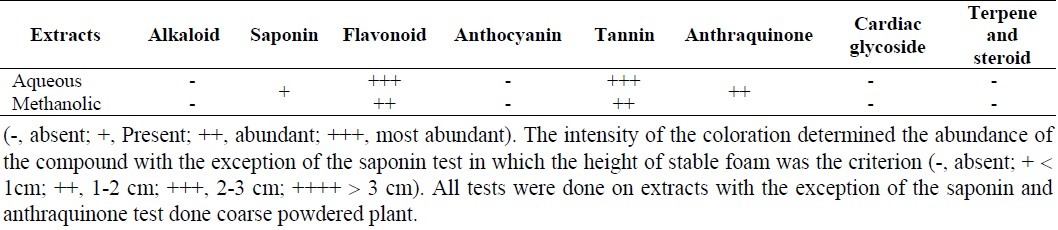
The extraction procedure yielded 22.57% and 29.83% (w/w) for aqueous and methanolic extracts, respectively. The results of preliminary phytochemical screening of the aqueous and methanolic extracts are shown in Table 1. Both extracts contained flavonoids and tannins. Moreover, the tests of saponin and anthraquinone were positive for plant powder.
Table 1.
Preliminary phytochemical screening of extracts of G. odoratum
DPPH free radical scavenging activity
Comparison of the antioxidant activity of two extracts in four concentrations (50, 100, 300, 500 μg/ml) after 30 min is shown in Fig 1. Both extracts exhibited a good and notable dose dependent inhibition of the DPPH activity, significantly higher for aqueous extract (P <0.05). BHA inhibition activity at the end of 30 min was significantly lower than both extracts in 300 and 500 μg/ml (P <0.05). The inhibitory concentrations 50 (IC50) for plant extracts, defined as the concentration causing 50% inhibition in DPPH absorbance were measured from the concentration-inhibition curve and were 148 μg/ml and 83 μg/ml for methanolic and aqueous extracts respectively.
Fig. 1.
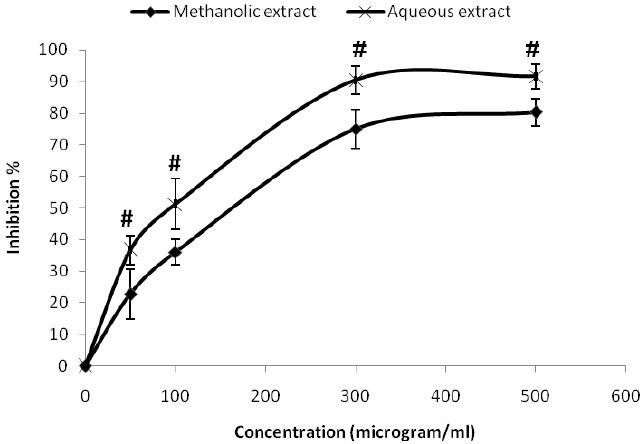
Anti radical activity of methanolic and aqueous extracts of G.odoratum in 30th min determined by DPPH assay. Values are mean ± S.D. of triplicate determinations. # P<0.05 compared to methanolic extract.
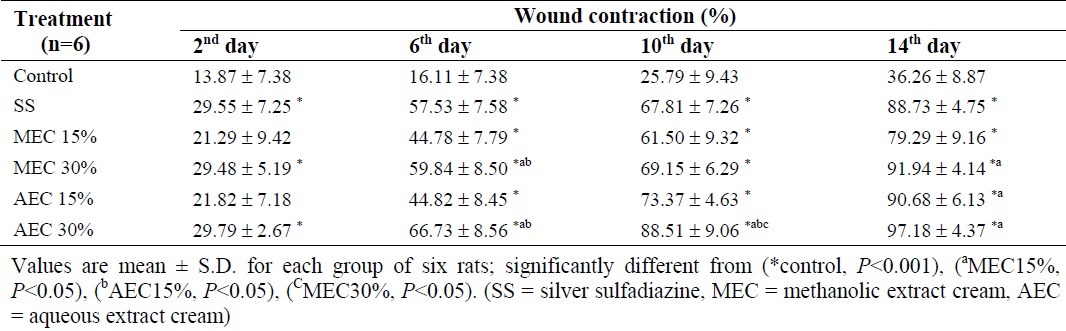
Rate of wound contraction
Table 2 shows the percentage of wound contraction for each group. The percentage of wound contraction increased in all treated groups during 14 days. The wound area was found to decrease significantly in all treated groups when compared to control on the day 6, 10 and 14 (P <0.001). On the day 6, the wound contraction was significantly greater in higher concentrations for both extracts (P <0.05). On the day 10, the wound contraction of ACE 30% was significantly greater than other treatments (P <0.05). On the 14th day, there was a significant difference between the wound contraction of MEC 15% and MEC 30% (P <0.05).
Table 2.
Percentage reduction of wound size in control and treated rats
Histological study
Histological evaluation was performed on samples of the last day. Hematoxylin and eosin stained sections of tissues were evaluated for inflammatory cells and epidermal regeneration. Comparison of granulation tissue section from the extracts-treated rats with control group showed significant improvement in the wound healing in the extracts-treated groups. The microscopic photographs are shown in Fig. 2. Sample of control group showed burn wound with a lot of necrotic areas and severe hemorrhage in dermis. There was no epidermal regeneration. A large number of infiltrated polymorphonuclear (PMN) cells were seen, indicating severe inflammation (Fig. 2A). In SS group, the focal epidermal regeneration had been done but the epithelial layers were fewer than normal and disorganized. The PMN infiltration had been reduced but there were some mast cells in dermis layer indicating mild inflammation (Fig. 2B). In MEC group of higher concentration, the epidermal layer had been regenerated relatively and infiltrated PMN cells were fewer than control significantly (Fig. 2C and 2D), but in lower concentration, the epidermal regeneration and PMN reduction was weaker (focal re-epithelialization) and the epidermal layers were disorganized. In AEC group of both concentrations there was a complete epidermal regeneration (full thickness re-epithelialization) and epidermal layers were well-organized. The PMN infiltration was very slight. The view of dermis layer was completely normal and dermis papillae were clearly seen. The boundary layer between the epidermis and the dermis was clear (Figs. 2E and 2F).
Fig. 2.
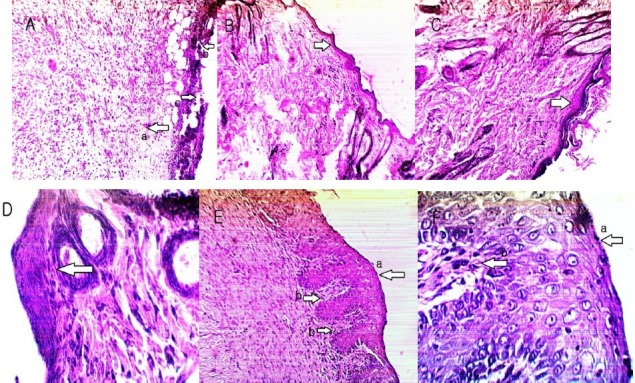
Microscopic panel of burns on the 14th day of treatment in rats. *A) Eucerin treated burn as control; (a) A lot of PMN cells and (b) necrotic areas are seen with (c) no re-epithelialization, * B) SS treated burn as reference standard; Focal and disorganized re-epithelialization is seen. *,•D) MEC treated burn; Focal re-epithelialization is seen. *,•F) AEC treated burn; (a) Full thickness re-epithelialization and (b) dermal papillae are seen. PMN; polymorphonuclear, SS; silver sulfadiazine, MEC; methanolic extract cream, AEC; aqueous extract cream. *100× or •400× magnification.
DISCUSSION
Burn healing is a complex process that degenerates dermal and epidermal tissues. It stimulates some physiological events at its site, including an inflammatory response started quickly after injury and followed by an intense formation of tissue for several days (10). This acute inflammation is mediated by granulocytes or PMN leucocytes. During this phase, they generate free radicals at the inflamed site which can cause damage to tissues and impair wound healing (11).
Thus, a free radical scavenger can help to reduce inflammation and as a result, the tissue formation, re-epithelization and differentiation of epidermis can be accelerated. Another key point in healing process is preventing some frequent fatal infections on the wound area. The antibacterial activity can partially control growth of pathogens over the skin and boost wound healing. Therefore, the antioxidant, anti-inflammatory and antimicrobial effects of natural products can play a major role in burn healing (12–17).
In an in vivo evaluation of anti-inflammatory effect in rats, G. odoratum inhibited carrageenan-induced rat paw edema by 25% which was favorably compared with reference standard, indomethacin (5). Further-more, sweet woodruff has general antimicrobial effect (18) and its desirable antioxidant activity was confirmed in present study by DPPH antioxidant assay which is based on the decolorization property of DPPH near antioxidants.
Therefore, beneficial effects of G. odoratum in burn healing acceleration and inflammation reduction can be explained partially by its antibacterial, anti-inflammatory and antioxidant activities.
Phytochemical constituents may be responsible for these effects. According to previous studies and our phytochemical screening, coumrins, flavonoids and tannins are of important components of G. odoratum. The coumarins in this plant are of unsubstituted coumarin, umbelliferone and scopoletin types. Moreover, flavonoid glycosides like rutin have been recognized in this plant (19).
Phenolic phytochemicals like coumarins, flavonoids and tannins are described as major groups of antimicrobial secondary metabolites (20). Tannins can accelerate wound healing by different mechanism such as antioxidant, astringent, antibacterial and angiogenic activity (21). Coumarins can reduce tissue swelling and inflammation by enhancing the destruction of the accumulated extracellular proteins and inhibiting prostaglandin synthesis. They have positive effect on free radical-mediated injury by their antioxidant capacity, as can flavonoids do due to different mechanisms such as direct radical scavenging (22,23).
Flavonoids possess in vivo anti-inflammatory activity because of their antioxidative effect and regulation of the inflammation-related cells (24). Besides, their lipid peroxidation reduction can prevent cell damage and necrosis, increase vascularity and circulation and improve collagen viability and strength (25).
According to these explanations, relatively higher effect of aqueous extract in burn healing can be related to its better antioxidant and anti-inflammatory activities but it is not the only reason.
The qualitative phytochemical screening showed greater content of flavonoids in aqueous extract. Therefore, the greater anti-oxidant activity of aqueous extract can be explained by its higher content of flavonoids. However, for more precise evaluation, further quantitative experiments are required.
CONCLUSION
Both extracts of G. odoratum showed a good potential for acceleration of burn wound healing in rats, which was further confirmed in the histological assessment. This effect might be due to several mechanisms such as increasing rate of re-epithelialization and vascularization, inhibition of destructive free radicals, edema and inflammation reduction and control of infection by the effects of antioxidant, anti-inflammatory and antimicrobial components of this plant. Further studies with purified constituents are needed to understand the complete mechanism of G. odoratum burn healing activity.
ACKNOWLEDGMENT
This research was financially supported by Tehran University of Medical Sciences grant (Grant Number: 89-04-33-11844).
REFERENCES
- 1.Brunicardi F, Anderson D, Billiar T, Dunn D, Hunter J, Pollock RE, et al. Schwartz’s principles of surgery. 9th ed. New York: McGraw-Hill Companies, Inc.; 2010. pp. 223–296. [Google Scholar]
- 2.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Gruenwald J, Brendler T, Jaenicke C. PDR for herbal medicine. 4th ed. Montvale: Medical economics company; 2007. p. 835. [Google Scholar]
- 4.Soltani A. Encyclopedia of traditional medicine (Medicinal plants) Tehran: Arjmand publication; 2004. pp. 290–291. [Google Scholar]
- 5.Mascolo N, Autore G, Capasso F, Menghini A, Fasulo MP. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res. 1987;1:28–31. [Google Scholar]
- 6.Rivola G, Guicciardi A. New antiviral anthraquinone from Asperula odorata L. Eur J Pharm Sci. 1994;2:128. [Google Scholar]
- 7.Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. [Google Scholar]
- 8.Mohajeri Gh, Masoudpour H, Heidarpour M, FatemehKhademi E, Ghafghazi Sh, Adibi Sh, Akbari M. The effect of dressing with fresh kiwifruit on burn wound healing. Surgery. 2010;148:963–8. doi: 10.1016/j.surg.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Abbas AK, Fausto F, Mitchel RN. Robbin’s basic pathology. 8th ed. Philadelphia: Sunders; 2007. pp. 31–59. [Google Scholar]
- 12.De Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12:249–255. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 13.Chah KF, Eze CA, Emuelosi CE, Esimone CO. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol. 2006;104:164–167. doi: 10.1016/j.jep.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 14.Fan ZF, Wang JH, Li ZQ, Yi CH. Influence of sea water immersion on inflammation and healing of the wounds in scalded rats. Chinese Journal of Burns. 2006;22:215–217. [PubMed] [Google Scholar]
- 15.Shukla A, Rasik AM, Dhawan BN. Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytother Res. 1999;13:50–54. doi: 10.1002/(SICI)1099-1573(199902)13:1<50::AID-PTR368>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Abdolghaffari AH, Mahdaviani P, Fallah-Bonekohal S, Ghasemi-Niri SF, Hagiaghaei R, Banan-Khojaste SM, et al. Wound healing effect of Rosemary and Chamomile combination in rat. Pharmacologyonline. 2010;3:139–145. [Google Scholar]
- 17.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 2009;8:2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 18.Duke JA, Bogerschutz MG, Ducellier J, Duke PAK. Handbook of medicinal herbs. 2nd Ed. Florida: CRC Press; 2002. p. 794. [Google Scholar]
- 19.Wagner H, Bladt S. Plant drug analysis: A thin layer chromatography atlas. Berlin: Springer; 1996. pp. 125–148. [Google Scholar]
- 20.Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of medicinal plants research. 2010;4:104–111. [Google Scholar]
- 21.Li K, Diao Y, Zhang H, Wang S, Zhang Z, Yu B, et al. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. Promote cutaneous wound healing in rats. BMC Complementary and Alternative Medicine. 2011;11:86–94. doi: 10.1186/1472-6882-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadjipavlou-Litina DJ, Litinasb KB, Kontogiorgisa C. The anti-inflammatory effect of coumarin and its derivatives. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2007;6:293–306. [Google Scholar]
- 23.Nijveldt RJ, Nood EV, Hoorn DV, Boelens BP, Norren KV, Leeuwen PV. Flavonoids : a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 24.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 25.Getie M, Gebre MT, Reitz R, Neubert RH. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae) Pharmazie. 2002;57:320–322. [PubMed] [Google Scholar]


