Abstract
Dried aceton-chloroform extract of aerial parts of Euphorbia spinidens Bornm. ex Prokh. endemic to Iran, yielded two new triterpenoids, lup-20(29)-ene-33, 28 diol commonly known as betulin and (3β,23E)-Cycloarta-23-ene-3,25-diol. The structures of the isolated compounds were elucidated by 13C- and 1H-NMR as well as 2D-NMR, IR and by the aid of mass fragmentation pattern. In phagocyte chemiluminescence assay, different concentrations of compounds were incubated with the human whole blood in triplicate and the chemiluminescence activity of phagocytic cells were measured by using serum opsonized zymosan and luminol. For lymphocyte proliferation assay, peripheral human blood lymphocytes were incubated with different concentrations of the test compound in supplemented Roswell Park Memorial Institute (RPMI) 1640 medium along with 5.0 μg/ml phytohemagglutinin (PHA) at 37°C in CO2 environment for 72 h and proliferation level was determined by Beta-scintillation counter. In phagocyte chemiluminescence assay, betulin showed moderate inhibitory effect on the oxidative burst in the neutrophils, while addition of betulin triterpene was able to stimulate the proliferation of the phytohemagglutinin (PHA) treated human peripheral blood lymphocytes (hPBLs).
Keywords: Euphorboa spinidens, lupane type triterpenes, cycloartane, Immunomodulation, hPBLs, Iran
INTRODUCTION
Recent studies in the natural product sources have resulted in many compounds to treat immunosuppressive disorders including immune response in organ transplantation and autoim-mune diseases. These natural compounds also are used to treat some other non-autoimmune inflammatory diseases like long term allergic asthma control (1). The genus Euphorbia is traditionally used in the folk medicine to treat inflammations and cancer tumors (2). Ayatollahi and coworkers have identified the cycloartanes, with marked immunoinhibitory effects on hPBLs (3). Lupane type triterpene like betulinic acid also show the anti-inflammatory activities without affecting the normal cells and cytotoxicity on the peripheral blood lymphoblasts, the human astrocytes and the human dermal fibroblasts (4–6). In lupane type triterpenes, different C-28 derivativeswith changed nucleophilicity, strength of hydrogen bonding and/or acidity at this position have different biological effects and there are reports about the chemical modification of these compounds to produce derivatives with enhanced effects (4). The current study aimed to evaluate the tri-terpenoidal contents of the acetone-chloroform extract of Euphorbia spinidens Bornm. ex Prokh. The immuno-modulatory activity of one of these compounds was also investigated in the search to be applied in the further pharmacoimmunologic researches.
MATERIALS AND METHODS
General experimental procedures
The NMR spectra were recorded on a Bruker Avance AV 400, using CDCl3 as solvent. HPLC was carried out on a waters 515 using a Pack-Sil column (250×20 mm i.d.) packed with 5 μm silica (YMC Co., Ltd., Kyoto, Japan). Hexane: Ethyle acetate was used as the mobile phase. Chromatographic materials were silica gel (Merck Co., Germany), and Sephadex LH-20 (Pharmacia, Inc., Piscataway, NJ, USA). Thin layer chromatography detection was achieved by spraying the silica gel plates with cerium sulfate in 10% aqueous H2SO4, followed by heating.
Materials
Calcium chloride, magnesium sulphate, Ficoll, and Luminol (3-aminophthalhydrazide) purchased from Sigma-aldrich company (St Louis, MO, USA), serum opsonized zymosan (Saccharomyces cerevisiae origin) from the Fluka (Buchs, Switzerland), thymidine [3H] from the Amersham company (Bucking-hamshire, UK), and RPMI-1640 medium from the Gibco Laboratories (Grand Island, NY). All solvents used were of analytical grade and purchased from Merck Company (Germany).
Plant material
Aerial flowering parts of Euphorbia spinidens (Euphorbiaceae) were collected in June 2011 from the populations growing in the Northern Khorasan province (Iran). Plant material was identified by Herbaceous Science Research Center at Ferdowsi University, Mashhad (Iran) and a voucher specimen (# 43033) was deposited.
Extraction and isolation
Air-dried plant material (3 kg) was macerated with methanol at room temperature for 5 days. Filtration and in vacuo concentration resulted in a green gum which was partitioned between aqueous. methanol and hexane. The defatted methanolic extract was concentrated, dissolved in water, and extracted sequentially with chloroform and n-butanol. Chloroform fraction (150 g), was subjected to the silica gel column chromatography (hexane/dichloromethane, 0→100 and dichloromethane : methanol, 0→20) to yield several fractions of Fr1-Fr5. Fraction Fr.4 eluted with hexane/dichloromethane (70%) was purified on sephadex LH-20 to remove chlorophylls. Using CC (hexane/EtOAc, 0→50) afforded Fr4a-Fr4j. Inferred from1H-NMR spectra, Fr4e containing triterpenes was further purified using HPLC on a YMC-Pak-Sil column (250 × 20 mm) to yield compound 1 (20 mg) and compound 2 (100 mg).
Phagocytic chemiluminescence assay
Formation of the reactive oxidants in whole blood during the oxidative burst was measured by the Luminol-enhanced chemiluminescence assay procedure (3). In brief, three concen-trations of each compound (0.5, 5 and 50 μg/ml) were prepared in 25 μl of Hank’s buffered salt solution (HBSS++) in 96 well flat bottomed plates for a final incubation volume of 100 μl. Then 25 μl of whole blood diluted 1:50 in suspension of HBSS++with calcium chloride and magnesium sulphate were added. Positive and negative control and blank wells were included in the assay. Positive and negative controls are activated and non-activated cells in the absence of compounds, respectively. Cells and compounds were incubated for 30 min at 37°C, then 25 μl luminal was added into each well and 25 μl serum opsonized zymosan was added except for negative and blank wells. The phagocytosis kinetic was monitored with luminometer for 50 min in the repeated scan mode. Peak and total integral chemiluminescence reading were expressed in the relatively light unit.
Isolation of peripheral blood mononuclear cells (PBMC)
Venous blood (50 ml) was aseptically taken from the healthy human volunteer in heparin-containing tubes. The blood was diluted with Hank’s balance salt solution Ca and Mg-free (HBSS) buffer, pH 7.4 and layered onto Ficoll paque carefully. After centrifugation at 400 x g for 50 min, the lymphocytes at the junction of the two layers were separated and washed three times with the RPMI incomplete media. The cells were resuspended in RPMI incomplete media and stored on ice (7).
Lymphocyte proliferation assay
Peripheral human blood lymphocytes were incubated with different concentrations of the test compounds (0.5, 5, and 50 μg/ml in duplicates) in supplemented RPMI-1640 along with phytohemagglutinin (PHA) at 37°C in CO2 environment for 72 h. Thymidine (3H) was added and incubated for 18 h. Then cells were harvested by cell harvester and the proliferation level was determined by the radioactivity count as count per min (CPM) recorded from the β scintillation counter (7).
Statistical analysis
All samples were presented as mean ± SD for three measurements. Significance was attributed to P- values (P<0.05) and the probability values obtained by the student t-test between sample and control data.
RESULTS
Chemistry
Two known triterpenes for the first time were isolated from the chloroform extract of plant with the following NMR and spectroscopic properties (Fig. 1).
Fig. 1.
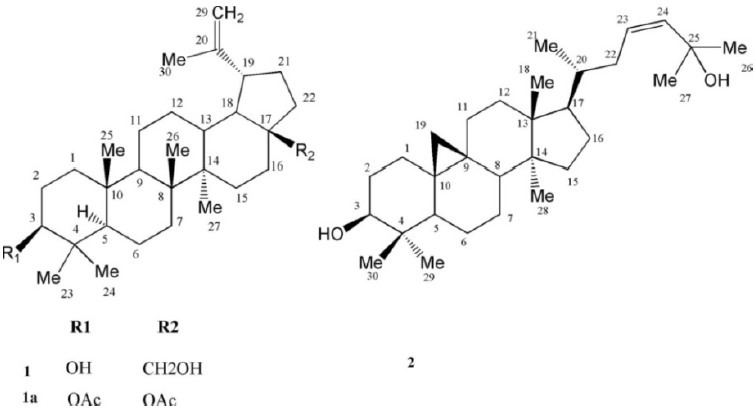
Triterpenes structure isolated from Euphorbia spinidensProkh.
Betulin (1)
White powder; MW(g/mol): 442;m.p.(˚C): 255-258; yield: 0.007%; FT-IR (KBr) cm-1: 3402 (O-H, str.), 2974, 2925, 1635, 1605, 1456, 1219, 1032, 883, 771 cm-1;1H-NMR (CDCl3, 400 MHz) δH, mult, J in Hz 4.66 (1H, d, J=2.1, H-29a), 4.55 (1H, dd, J =2.10, 1.20 Hz, H-29b), 3.78 (1H, brd, J=10.80 Hz, H-28a), 3.30 (1H, brd, J=10.80 Hz, H-28b), 3.16 (1H, dd, J =5.10, 10.50 Hz, H-3), 2.37 (1H, dt, J= 6.00, 10.80 Hz, H-19), 1.66 (3H, s, H-30), 1.02 (3H, s, H-27), 0.96 (3H, s, H-26), 0.95 (3H, s, H-23), 0.80 (3H, s, H-25), 0.74 3H, s, H-24;13C-NMR (CDCl3, 100 MHz) δc:39.1 (C-1), 27.8 (C-2), 79.4 (C-3), 39.3 (C-4), 55.7 (C-5), 18.7 (C-6), 34.6 (C-7), 41.3 (C-8), 50.8 (C-9), 37.6 (C-10), 21.2 (C-11), 25.6 (C-12), 37.7 (C-13), 43.1 (C-14), 27.5 (C-15), 29.6 (C-16), 48.2 (C-17), 49.2 (C-18), 48.2 (C-19), 150.9 (C-20), 30.2 (C-21), 34.4 (C-22), 28.4 (C-23), 15.7 C-24, 16.5 (C-25), 16.4 (C-26), 15.2 (C-27), 61.0 (C-28), 110.1 (C-29), 19.5 (C-30); EI-MS m/z 442 [M]+, 427, 424, 411, 399, 384, 288, 234, 207, 205, 189, 135, 121, 107, 105, 95, 81.
Cycloart-23Z-ene-3β,25-diol(2)
White crystals; MW(g/mol): 442; m.p. .(˚C): 255-258; yield: 0.033%;1H-NMR (CDCl3, 400 MHz): δH5.62 (1H, dd, J=4.80, 3.20 Hz, H-23), 5.62 1H, brs, H-24, 3.30 (1H, dd, J=4.00, 11.2 Hz, H-3), 1.33 (2 × 3H, s, H-26, H-27), 0.99 (2× 3H, s, H-18, H-30), 0.90 (3H, s, H-28), 0.89 (3H, d, J=6.40 Hz, H-21), 0.83 (3H, s, H-29), 0.57, 0.35 (each 1H, d, J=4.40 Hz, H-19a, b));13C-NMR (CDCI3 , 100 MHz) δc: 32.0 (C-1), 30.4 (C-2), 78.8 (C-3), 40.5 (C-4), 47.1 (C-5), 21.1 (C-6), 28.1 (C-7), 48.0 (C-8), 20.0 (C-9), 26.1 (C-10), 26.0 (C-11), 32.77 (C-12), 45.3 (C-13), 48.8 (C-14), 35.6 (C-15), 26.4 (C-16), 52.0 (C-17), 18.1 (C-18), 30.1 (C-19), 36.4 (C-20), 18.3 (C-21), 39.0 (C-22), 125.6 (C-23), 139.3 C-24, 70.8 (C-25), 29.9 (C-26), 19.3 (C-27), 61.0 (C-28), 14.0 (C-29), 25.4 (C-30). EI-MS m/z: 442 [M]+, 427, 424, 381, 363, 355, 315, 313, 302, 269, 175.
In the phagocytic chemiluminescence assay where activation of the phagocytic cells induces the release of reactive oxygen free radicals, as illustrated in Fig. 2, betulin showed moderate inhibitory effect on oxidative burst in neutrophils with 30.84 ± 2.86 % at the higher concentration (50 μg/ml). The results are consistent with other reports on the suppressive effects of betulin on superoxide generation in the human neutrophils and inhibiting ROS (reactive oxygen species; such as superoxide) agents produced by HepG2 cells and ethanol-activated hepatic satellite cells (8–10).
Fig. 2.
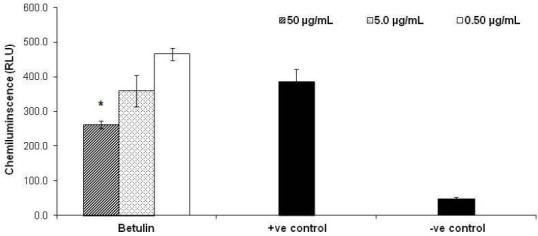
Effects of betulin on oxidative burst by phagocyte cells. Three different concentrations of betulin (0.5, 5 and 50 μg/ml) were incubated with the human whole blood in triplicate and the chemiluminescence activity of phagocytic cells was measured by using serum opsonized zymosan and luminol. Positive (+ve) and negative (– ve) controls are activated and non-activated cells in the absence of compound, respectively. Results are expressed as mean ± SD. *, P<0.05 versus control.
In the lymphocyte proliferation assay, the antiproliferative action was tested against the peripheral blood T-lymphocytes (PBLs). Addition of betulin triterpene to PHA stimulated hPBLs at the concentration ranges 0.5, 5 and 50 μg/ml resulted in stimulation of lymphocyte proliferation at 50 μg/ml by 63.13 ± 2.4% (Fig. 3).
Fig. 3.
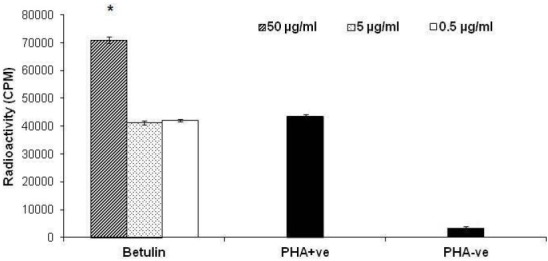
Peripheral blood lymphocyte proliferation assay on betulin. PBLs were stimulated by phytohemagglutinin (PHA) in the presence of three different concentrations of compounds. Stimulated (PHA +ve) or unstimulated T-cells (PHA – ve) in the absence of compounds were used as controls. Results are expressed as mean ± SD. *, P<0.05 versus control.
DISCUSSION
Compound 1 , was identified as C30H50O2 on the basis of positive EI-MS of m/z 442,13C-NMR, and DEPT spectral data. IR spectrum showed peak of free hydroxyls at 3402 cm-1, olefinic absorption at (1635, and 1605 cm-1) with lack of carbonyl groups. Six singlet methyls at δH1.66, 1.02, 0.96, 0.95, 0.80, and 0.74 along with a pair of olefinic protons as part of an exocyclic-methylene group at δH4.66 and 4.56, two gemineal oxymethylene at δH3.77 and 3.31 together with one oxymethine proton at δH3.16, and a doublet of triplet of one proton at δH2.37 (J=10.8, 6.0 Hz), characteristic for lupane triterpenes (11), were observed in1H-NMR spectrum. Taken together, the spectra data of 1 are consistent with the literature for betulin (12,13). Using acetic-anhydride/pyridine, compound 1 was acetylated to give diacetyl derivative (1a) with EI-MS m/z 526 which confirmed the structure of betulin (Fig. 1).
Compound 2 , showed the molecular formula of C30H50O2 based on positive EI- MS m/z 442 and in accordance with the number and the multiplicity of13C-NMR spectra (BB and DEPT).1H-NMR revealed six tertiary methyls at δH1.33 (2 × 3H, s, H-26, H-27), 0.99 (2× 3H, s, H-18, H-30), 0.90 (3H, s, H-28) and 0.83 (3H, s, H-29), one secondary methyl group at δH0.89 (3H, d, J0=6.4 Hz, H-21), and a pair of doublets in the up-field area at δH0.57 and 0.35 (each 1H, d, J=4.4 Hz, H-19a, b), characteristic of cycloartane cyclo-propane ring. Carbinolic proton at δH3.30 (1H, dd, Jax, eq=4.0, Jax, ax=11.2 Hz, H-3) with reference to its axial and α-orientation, assigned the hydroxyl group as 3 β-OH. Olefinic protons at δH5.62 (1H, dd, J=4.8, 3.2 Hz, H-23), 5.62 1H, brs, H-24, with low coupling constants allowed assignment of Z-geometry to the Δ2324. Downfield chemical shifts of two singlet methyl protons of the side chain atoms at δH1.33 (2 × 3H, s, H-26, H-27) was in accordance with the second hydroxyl group on C-25 at δC70.8. EI-MS fragmen-tation pattern, also detected m/z 355 and 302, typical ions of 4,4’ dimethyl 9:19 cycloesterols and related tetracyclic triterpens (3) as well as m/z 315 [M - sidechain], m/z 381 [M-H2O-C3H7] and m/z 355 [M-H2O-C5H9]. Therefore based on the aforementioned data and complete agreement of13C- and1H-NMR with other reported data in the literature (14), compound 1 was identified as cycloart-23Z-ene-3β,25-diol (Fig. 1).
The stimulatory effect seen by betulin agreed with another report on increasing cellular and the humoral immunity by one of the betulin analogues, betulinic acid, that stimulated the proliferation of mice thymocytes, splenocytes and human peripheral blood mononuclear cells in a time and dose-dependent manner (15).
Another report by Shahlaei and coworkers on the immunomodulatory effets of lupan type triterpenes shows that position 28, on these compounds has two major interactions including hydrophobic and hydrophilic interaction with amino acids at the active site of the receptor. Substitution with a group of both hydrophilic and hydrophobic characteristics such as ethyl betulinate or hydrophobic group alone like lupeol inhibits the lymphocyte proliferations. While, substitution with only hydrophilic group at this position like betulinic acid stimulate the lymphocyte proliferations (11).
CONCLUSION
Similar to betulinic acid, the results showed that betulin has moderate inhibitory effects on the oxidative burst in the neutrophils which are initiated by NADPH oxidase enzyme through protein kinase C. In addition, betulin has shown encouraging stimulatory effect on the proliferation of human peripheral blood lymphocytes activated by PHA. Therefore, since betulin shows concomitant proliferation of T cells with anti-inflammatory actions (10), once its mechanism of action is elucidated, it could be used as a lead molecule for the new generation of drugs in cancer and infectious treatment, particularly in boosting up the immune system .
ACKNOWLEDGMENT
The authors are grateful to the Isfahan University of Medical Sciences, for financial supporting the project.
REFERENCES
- 1.Mesaik MA. Department of Microbiology and HEJ Research Institute of Chemistry University of Karachi, Pakistan. 2003. Studies on Some Natural Compounds for their Immunomodulatory Activity Thesis for PhD in microbiology. Retrieved from http://eprints.hec.gov.pk/566 . [Google Scholar]
- 2.Jassbi AR. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006;67:1977–1984. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Ayatollahi AM, Ghanadian M, Afsharypuor S, Mesaik MA, Abdella MO, Shahlaei M, et al. Cycloartanes from Euphorbia aellenii Rech f and their Antiproliferative Activity Iran. J Pharm Res. 2011;10:105–112. [PMC free article] [PubMed] [Google Scholar]
- 4.Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 5.Einzhammer DA, Xu ZQ. Betulinic acid: A promising anticancer candidate. Idrugs. 2004;4:359–373. [PubMed] [Google Scholar]
- 6.Domínguez-Carmona DB, Escalante-Erosa F, García-Sosa K, Ruiz-Pinell G, Gutierrez-Yapu D, et al. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine. 2010;17:379–382. doi: 10.1016/j.phymed.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen MB, Gerwien J, Nielsen M, Geisler C, Ropke C, Svejgaard A. MHC class II ligation induces CD58 (LFA-3)-mediated adhesion in human T cells. Exp Clin Immunogenet. 1998;15:61–68. doi: 10.1159/000019055. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita K, Lu H, Lu J, Chen G, Yokoyama T, Sagara Y, et al. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin Chim Acta. 2002;325:91–96. doi: 10.1016/s0009-8981(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 9.Szuster-Ciesielska A, Pilipów K, Kandefer-Szerszeñ M. Protective effect of betulin and betulinic acid on acetaminophen and ethanol-induced cytotoxicity and reactive oxygen species production in HepG2 cells. Journal of pre-clinical and clinical research. 2010;4:96–100. [Google Scholar]
- 10.Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-á, TGF-â) production and by influencing intracellular signalling. Toxicology. 2011;280:152–163. doi: 10.1016/j.tox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Shahlae M, Ghanadian SM, Ayatollahi AM, Mesaik MA, Abdalla OM, Afsharypour S, et al. Molecular modeling, structure activity relationship and immunomodulatory properties of some lupeol derivatives. Med Chem Res. 2012 DOI 10.1007/s00044-012-0183-y. [Google Scholar]
- 12.Murthy YLN, Sree PA. Characterisation of Lupeol (A Tri Terpene) Esters As Liquid Crystals. Mol Cryst Liq Cryst. 1997;30:93–97. [Google Scholar]
- 13.Arshad k. Thesis for PhD in chemistry of HEJ research institute of chemistry, University of Karachi, Karachi, Pakistan. 2001. Studies in the chemical constituents of Andrachne aspera spreng (Euphorbiaceae) Retrived from http://eprints.hec.gov.pk/427 . [Google Scholar]
- 14.Khan MT, Khan SB, Ather A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure-activity relationship. Bioorg Med Chem. 2006;14:938–943. doi: 10.1016/j.bmc.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Mashitoh AR, Yeap SK, Ali AM, Faujan A, Suhaimi M, Ng MK, et al. Immunomodulatory Effects of Betulinic Acid Isolation from the Bark of Melaleuca cajuputi. Pertanika J Trop Agric Sci. 2012;35:293–305. [Google Scholar]


