Abstract
Alcoholic extracts of 8 different types of seaweeds from Iran’s Persian Gulf were tested for their antimalarial and acetylcholinesterase enzyme (AChE) inhibitory activities for the first time. A modified Ellman and Ingkaninan method was used for measuring AChE inhibitory activity in which galanthamine was used as the reference. The antimalarial assay was performed using microculture radioisotope technique. Mefloquine and dihydroartemisinin were uased as the standards. The extract of Sargassum boveanum (Sargasseae family) showed the highest AChE inhibitory activity (IC50 equals to 1 mg ml-1) while Cystoseira indica (Cystoseiraceae family) exhibited the least activity (IC50 of 11 mg ml-1). The species from Rhodophyta (Gracilaria corticata and Gracilaria salicornia) also showed moderate activities (IC509.5, 8.7 mg ml-1, respectively). All extracts were inactive in antimalarial assay.
Keywords: Acetylcholinesterase inhibitor, Antimalarial, Sargassum, Cystoseira, Gracilaria, Padina
INTRODUCTION
Alzheimer’s disease (AD) is a progressive and degenerative problem in brain regions, mainly campus and neocortex that are responsible for mental functions. This disease can lead to the loss of memory, behavior abnormalities and also cognitive disturbances (1). An estimated 24 million people worldwide are suffering from dementia, which two-thirds of them live in the developing countries, with no suitable treatment, the population of people suffering from AD will be more than 80 million by 2040 (2). In the cholinergic hypothesis, serious loss of cholinergic neuro-transmitter acetylcholine (ACh) in the CNS contributes significantly to the symptoms of AD (3). The inhibition of acetylcholinesterase (AChE) enzyme, which catalyzes the breakdown of ACh, plays a key role in enhancing cholinergic transmission in the brain, resulting to the treatment of AD (4).
Malaria represents the world’s greatest public health problem in terms of number of people affected and levels of morbidity and mortality. The malaria protozoan parasites (Plasmodium spp.) are transmitted by infected female mosquitoes when feeding on blood. Parasites soon enter liver cells, and after several days of multiplication, are released into the blood stream where further cycles of asexual repro-duction occur, giving rise to the clinical symptoms of malaria. This parasite has become increasingly resistant to standard antimalarials such as chloroquine and antifolates. Consequently, new drugs or drug combinations are urgently needed today for the treatment of malaria (5).
Ninety percent of the world’s biomass exist in the oceans which comprising approximately half of the total global biodiversity (6,7). This wide diversity of organisms is being recognized to have a potent reservoir of secondary metabolites such as terpenes, alkaloids and polyphenolics which many of these compounds are halogenated. (7,8). Among marine organisms, marine algae have been identified as an under-exploited plant resources (9,10).
This group of marines can be classified into three classes based on their pigmentation, namely brown, red, and green algae, which are referred to as Phaeophyceae, Rhodophyceae, and Chlorophyceae, respectively (11). Researchers have reported that algal originated compounds exhibit various biological activities such as anticoagulant (12,13), anti-viral (14,15), antioxidant (16–18), anti-allergic (19), anti-cancer (20), anti-inflammatory (21) and anti-obesity (22–24). Furthermore, several scientific studies have provided insight into neuroprotective and antiprotozoal properties of marine algae (25). The oceans, with their unique and wide range of biodiversity, producing unusual metabolites, also emerge as a good candidate for new pharmaceutical agents.
In recent years, biological activities, nutritional value, and potential health benefits of marine algae have been intensively investigated and reviewed. This survey, however, focuses specifically on the antimalarial and AChE inhibitory activity of marine algae of Persian Gulf, Iran for the first time and looking for their potential application as future pharmaceutical candidates to be used for the treatment of AD and malaria.
MATERIALS AND METHODS
Plant material
Eight different types of seaweeds were collected from Persian Gulf, Bushehr Province in the south part of Iran during the autumn (October and November 2010). The species were Cystoseira indica, Cystoseira merica (Cystoseiraceae family), Sargassum angosti-folium, Sargassum oligocystum and Sargassum boveanum (Sargasseae family) and Padina australis (Dictyotaceae family) which belong to Phaeophyta and also Gracilaria corticata and G. salicornia (Gracilariaceae family) belonging to Rhodophyta. They were transported to the laboratory within 24 h of collection, cut in to small pieces and air-dried under shade at room temperature (30°C). Voucher specimens (codes: 2660 to 2667) from all collected seaweeds were made and deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences (Isfahan, Iran) and were identified by Agricultural and Natural Resources Research Center of Bushehr.
Extraction and isolation
The dried materials were macerated in methanol for 3 days and filtered. The filtrate was evaporated under reduced pressure until dryness. The residue from the filtration was macerated in methanol again for 3 days and filtered. The filtrate was evaporated with the same procedure and combined with the extract from the first extraction.
Microplate assay for acetylcholinesterase enzyme activity
The assay for measuring AChE activity was modified from the assay described by Ellman and coworkers (26) and Ingkaninan and coworkers (27). Briefly, 125 μl of 3 mM Ellman’s reagent 5,5’-dithiobis-(2-nitrobenzoic acid) or DTNB], 25 μl of 15 mM ATCI (acetylthiocholine iodide), 50 μl of phosphate buffer, and 25 μl of sample were dissolved in buffer containing not more than 10% methanol and added to the wells followed by addition of 25 μl of 0.28 U/ml AChE. The blank for such a run consists of buffer, substrate, and DTNB solutions. The microplate was then read out at 405 nm every 5 sec for 2 min by a CERES UV 900C microplate reader (Bio-Tek Instrument, USA). The velocities of the reactions were measured. Enzyme activity was calculated as a percentage of the velocities compared to that of the assay using buffer without any inhibitor. Inhibitory activity was calculated from hundred subtracted by the percentage of enzyme activity. Every experiment was performed in triplicate. Various concentrations of seaweed extracts were taken for the study. IC50 values were determined by plotting a percent inhibition versus concentration curve, in which the concentration of sample required for 50% inhibition was determined and expressed as IC50 values.
Antimalarial test
The targeted parasite was Plasmodium falciparum (K1 multidrug resistant strain), which was maintained in Roswell Park Memorial Institute 1640 (RPMI 1640) medium containing 20 mM N-2-hydroxyethyl piperazine- N-2-ethanesulfonic acid (HEPES), 32 mM NaHCO3, and 10% heat activated human serum (28). Quantitative assessment of in vitro antimalarial activity was determined by means of the microculture radioisotope technique based upon the method previously described by Desjardins and coworkers (29). The assay uses the uptake of [3H] hypoxanthine by parasites as an indicator of viability. Continuous in vitro cultures of asexual erythrocytic stages of Plasmodium falciparum were maintained. Extracts were tested on K1 strain. Initial concentration of the extracts was 10 μg/ml. Mefloquine and dihydroartemisinin were used as positive references. In brief, a 200 μl mixture of 1.5% of erythrocytes with 1% parasitemia at an early ring state was exposed to 25 μL of RPMI medium containing tested sample in a serial dilution (doped with DMSO, 0.1% final concentration). This was inoculated at 37◦C under a 5% CO2 atmosphere for 24 h. A 25 μl of [3H] hypoxanthine in the RPMI medium was added to each well and the plate was incubated for additional 24 h. Detection of incorporated [3H] hypoxanthine was performed on a TopCount microplate scintillation counter. The active sample was able to cause 50% inhibition in growth and activity was reported in IC50 scale.
RESULTS
Of the 8 seaweeds representing 4 different families, the most active extracts with AChE activity as shown in Table 1 were made from S. boveanum (IC501 mg ml-1) and S. oligocystum (IC502.5 mg ml-1) while the least active extracts were made from C. indica (IC5011 mg ml-1), G. corticata (IC509.5 mg ml-1) and G. salicornia (IC508.7 mg ml-1). The three remaining showed medium activities (Fig. 1). Galanthamine, the pure compound used as a reference, had an IC50 of 0.0007 mg ml-1. Mixture of Ellman’s reagent, ATCI and phosphate buffer was used as negative controls.
Table 1.
In Vitro quantitative inhibition of acetylcholinesterase by the crude extracts of different seaweeds of Persian Gulf
Fig. 1.
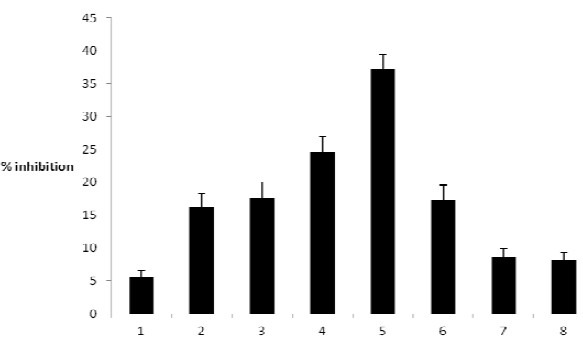
Percent inhibition activity of acetylcholinesterase by the crude extracts of different seaweeds (mean ± S.D. of three assays). Concentrations of samples: (100 μg/ml), 1. Cystoseira indica, 2. Cystoseira meric, 3. Sargassum angostifolium, 4. Sargassum oligocystum, 5. Sargassum boveanum, 6. Padina australis, 7. Gracilaria corticata, 8. Gracilaria salicornia
With regard to the antimalarial activity (Table 2), the solution of 0.1% DMSO was used as negative control while dihydro-artemisinin and mefloquine were used as the positive ones with the IC50 of 2.17 nM and 0.0324 μM, respectively. The extracts could not inhibit 50% growth in the final concentration of 10 μg/ml.
Table 2.
Antimalarial activity of crude extracts of different seaweeds of Persian Gulf
DISCUSSION
Many species of marine algae have long been used in food diets in Eastern countries and more recently in Europe and America. Also various activities of this group of marines especially cytotoxic, antibacterial, antioxidant and antifungal activities have been widely screened. Only a few studies have been reported regarding the AChE inhibitory and antimalarial activities of this diverse group of organisms. Also in a study for screening the AChE inhibitory of 11 seaweeds from South Indian coastal areas, the IC50 s of methanolic extracts were similarly from 1 to 10 mg ml-1 for most of the seaweeds although some of them showed less activity. The above mentioned study also represents that time of collection may be an important factor affecting this activity due to some changes in secondary metabolites (30). In the present study, it was found that Sargassum was the most active genus among all of different genera tested. Studies have shown that the genus Sargassum which is a widely distributed algae that represents various activities such as antioxidants, antibacterial, immune-stimulating and also AChEI activity while S. boveanum, the most active species, has previously shown antioxidant, antifungal and phytotoxic effects (16,31) but it has not yet been tested for AChE inhibitory activity. Besides, major classes of compounds reported to have such an activity are the alkaloids, terpenoids, glycosides and coumarins (32). During the search for anticholinesterase compounds from marine organisms, two plastoquinones, sargaquinoic acid and sargachromenol were isolated from S. sagamianum (33). Both compounds unexpec-tedly showed moderate AChE inhibitory activity in a micromole range. These two compounds were also previously isolated from S. serratifolium (34). In the light of aforementioned considerations, further work is in progress to identify the compounds responsible for this activity which may exist in S. boveanum and S. oligocystum, the two species which indicated more activities.
There are several antimalarial molecules that can efficiently integrate the panel of lead compounds isolated from marine sources with new chemical backbones and sometimes with unique functional groups (35). For instance, some seaweeds have been found to contain a powerful class of natural substances that can effectively destroy the malaria parasite. Researchers believe that bromophycolide compounds, are substances produced by the seaweeds as a chemical defense against marine fungi attacks, but they also appear to be effective against the malaria parasite (36). Although the extracts of seaweeds tested in this work were not active against malaria but work on the other seaweeds from Persian Gulf may lead to the isolation and structure elucidation of a number of exciting new pharma-cophores.
Whereas the southern coast of Iran bears a luxuriant and unique growth of seaweeds, but there are only limited publications about their pharmaceutical abilities (37). This was the first report of the selected species from Persian Gulf of Iran tested for AChE inhibitory and antimalarial activity and shows that a potential source of AChE inhibitors is certainly provided by seaweeds.
CONCLUSION
The methanolic extracts of seaweeds from Persian Gulf, Iran were screened for inhibitory activity on AChE and malaria for the first time. Of 8 seaweed extracts tested, the extracts from Sargassum boveanum showed the highest AChE inhibitory activity at the concentration of 0.1 mg/ml. All extracts were inactive about malaria. These seaweed extracts and their active components could emerge as natural and alternative anticholinesterase drugs or serve as starting points for synthesizing more effective AChE inhibitors.
ACKNOWLEDGMENT
This research project numbered 389176 was fully sponsored by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Pietrini P, Alexander G, Furey M, Hampel H, Guazzelli M. The neurometabolic landscape of cognitive decline: In vivo studies with positron emission tomography in Alzheimer’s disease. Int J Psychophysiol. 2000;37:87–98. doi: 10.1016/s0167-8760(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 2.Melzer D, Ely M, Brayne C. Cognitive impairment in elderly people: Population-based estimate of the future in England, Scotland and Wales. Br Med J. 1997;315:462. doi: 10.1136/bmj.315.7106.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 4.Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease. Age Ageing. 2006;35:336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood B M, Bojang K, Whitty C J, Targett G A. Malaria Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Wijesekara I. Development and biological activities of marine-derived bioactive peptides: A review. J Funct Foods. 2010;2:1–9. [Google Scholar]
- 7.Swing J. What future for the oceans. Foreign Aff? 2003;82:139–152. [Google Scholar]
- 8.Alonso D, Castro A, Martinez A. Marine compounds for the therapeutic treatment of neurological disorders. Expert Opin Ther. Patents. 2005;15:1377–1386. [Google Scholar]
- 9.Heo S J, Hwang J Y, Choi J I, Han J S, Kim H J, JHeo Y J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent [alpha]-glucosidase and [alpha]-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009;615:252–256. doi: 10.1016/j.ejphar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Pangestuti R, Kim S. Neuroprotective Effects of Marine Algae. Mar Drugs. 2011;9:803–818. doi: 10.3390/md9050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan S, Kong C, Kim J, Kim S. Protective effect of Amphiroa dilatata on ROS induced oxidative damage and MMP expressions in HT1080 cells. Biotech Bioproc Eng. 2010;15:191–198. [Google Scholar]
- 12.Matsubara K, Matsuura Y, Hori K, Miyazawa K. An anticoagulant proteoglycan from the marine green alga, Codium pugniformis. J Appl Phycol. 2000;12:9–14. [Google Scholar]
- 13.Athukorala Y, Lee K, Kim S, Jeon Y. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol. 2007;98:1711–1716. doi: 10.1016/j.biortech.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Artan M, Li Y, Karadeniz F, Lee S, Kim M, Kim S. Anti-HIV-1 activity of phloroglucinol derivative, 6, 6’-bieckol, from Ecklonia cava. Bioorgan Med Chem. 2008;16:7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 15.Huheihel M, Ishanu V, Tal J, Arad S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J Biochem Biophys Meth. 2002;50:189–200. doi: 10.1016/s0165-022x(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 16.Hwang P A, Wu C, Gau S, Chien S U, Hwang D F. Antioxidant and immune- stimulating activities of hot-water extracts from seaweed Sargassum hemiphyllum. J Mar Sci Tech. 2010;18:41–46. [Google Scholar]
- 17.Park P, Heo S, Park E, Kim S, Byun H, Jeon B, Jeon Y. Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. J Agr Food Chem. 2005;53:6666–6672. doi: 10.1021/jf050582+. [DOI] [PubMed] [Google Scholar]
- 18.Zou Y, Qian Z, Li Y, Kim M, Lee S, Kim S. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agr Food Chem. 2008;56:7001–7009. doi: 10.1021/jf801133h. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Lee S, Le Q, Kim M, Kim S. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc RI. J Agr Food Chem. 2008;56:12073–12080. doi: 10.1021/jf802732n. [DOI] [PubMed] [Google Scholar]
- 20.Kong CS, Kim JA, Yoon NY, Kim SK. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem Toxicol. 2009;47:1653–1658. doi: 10.1016/j.fct.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Rajapakse N, Kim S. Anti inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF B transcription factor in RAW 264.7 cells. Phytother Res. 2009;23:628–634. doi: 10.1002/ptr.2674. [DOI] [PubMed] [Google Scholar]
- 22.Maeda H, Hosokawa M, Sashima T, Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay Mice. J Agr Food Chem. 2007;55:7701–7706. doi: 10.1021/jf071569n. [DOI] [PubMed] [Google Scholar]
- 23.Tsukui T, Konno K, Hosokawa M, Maeda H, Sashima T, Miyashita K. Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KKAy obese/diabetic mice. J Agr Food Chem. 2007;55:5025–5029. doi: 10.1021/jf070110q. [DOI] [PubMed] [Google Scholar]
- 24.Kong C, Kim J, Ahn B, Vo T, Yoon N, Kim S. 1-(3, 5- Dihydroxyphenoxy) -7- (2, 4, 6-trihydroxyphenoxy)- 2, 4, 9- trihydroxydibenzo -1, 4- dioxin inhibits adipocyte differentiation of 3T3-L1 fibroblasts. Mar Biotechnol. 2010;12:299–307. doi: 10.1007/s10126-009-9224-z. [DOI] [PubMed] [Google Scholar]
- 25.Zarros A. In which cases is neuroprotection useful. Adv Altern Think Neurosci. 2009;1:3–5. [Google Scholar]
- 26.Ellman GL, Lourtney DK, Andres V, Gmelin G. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm. 1997;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Ingkaninan K, de Best CM, Irth H, van der Heijden R, Hofte AJP, Karabatak B, Tjaden UR, van der Greef J, Verpoorte R. High performance liquid chromatography with on-line coupled UV-mass spectrophotometric-biochemical detection for identification of acetyl cholinesterase inhibitors from natural products. J Chromatogr A. 2000;872:61–73. doi: 10.1016/s0021-9673(99)01292-3. [DOI] [PubMed] [Google Scholar]
- 28.Trager W, Jensen JB. Human malaria parasites in Continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 29.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1997;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stirke WA, Reinecke DL, van Staden J. Seasonal variation in antifungal, antibacterial and acetyl-cholinesterase activity in South African seaweeds. J Appl Phycol. 2007;19:271–276. [Google Scholar]
- 31.Rizvi MA, Shameel M. Pharmaceutical Biology of Seaweeds from the Karachi Coast of Pakistan. Pharm Biol. 2005;43:97–107. [Google Scholar]
- 32.Mukherjeea PK, Kumarb V, Malb M, J. Houghton P. Acetylcholinesterase inhibitors from plants. Phytomed. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Choi BW, Ryu G, Park SH, Kim ES, Shin J, Roh SS, et al. Anticholinesterase Activity of Plasto-quinones from Sargassum sagamianum: Lead Compounds for Alzheimer’s Disease Therapy. Phytother Res. 2007;21:423–426. doi: 10.1002/ptr.2090. [DOI] [PubMed] [Google Scholar]
- 34.Kusumi T, Shibata Y, Ishitsuka M, Kinoshita T. Structure of new plastoquinones from the brown algae Sargassum serratifolium. Chem Lett. 1979;12:277–278. [Google Scholar]
- 35.Fattorusso Eand, Taglialatela-Scafati O. Marine Antimalarials. Mar Drugs. 2009;7:130–152. doi: 10.3390/md7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin AS, Paige Stout E, Prudhomme J, Roch KL, Fairchild CR, Franzblau SG, et al. Bioactive bromophycolides R-U from the Fijian red alga Callophycus serratus. J Nat Prod. 2010;73:275–278. doi: 10.1021/np900686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanavi M, Nabavi M, Sadati N, Shams Ardekani M, Sohrabipour JB, Nabavi SM, et al. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43:31–37. [PubMed] [Google Scholar]


