Abstract
Cardiovascular disease is the common cause of mortality in diabetic subjects. Recently, it is indicated that peroxisome proliferator-activated receptors (PPARs) agonists have beneficial effect on cardiovascular system especially on angiogenesis. PPARs have three isotypes: PPARα, PPARβ/δ and PPARγ. In this study, we evaluated the effect of bezafibrate as pan PPAR agonist on myocardial capillary density in type I diabetic rats. Eighteen male wistar rats were randomly divided into three groups (n=6 each): control, diabetic and diabetic+bezafibrate (400 mg/kg/day) by gavage every day. Diabetes was induced by a single dose of streptozotocin (55 mg/kg), intraperitoneally. After 21 days, capillary density in the myocardial tissue was evaluated by immunohistochemical staining and reported as capillaries per mm2. Blood samples were taken before and after the experiment. Diabetes was associated by lower serum nitric oxide (NO) concentration and reduced myocardial capillary density compared to control group (121.71 ± 13.32 vs. 158.78 ± 11.08 /mm2; P<0.05). Administration of bezafibrate significantly increased serum NO level and improved angiogenesis in myocardial tissue of diabetic animals (170.24 ± 15.76 vs.121.71 ± 13.32 /mm2; P<0.05). There was a positive correlation between serum NO concentration and myocardial capillary density (r=0.90). Activation of all isotypes of PPAR by bezafibrate improves heart capillary density in diabetic animals and it seems that it can be considered for treatment or prevention of coronary heart disease in diabetic subjects.
Keywords: Diabetes, PPAR, Capillary density, Angiogenesis
INTRODUCTION
One of the most important causes of mortality and morbidity in diabetes in progressed society is coronary heart disease (CHD) which impose enormous economic burden (1,2). In general population, several long-term complications of vasculopathy in diabetes are characterized by impaired or increased growth of new blood vessels and alterations in vascular homeostasis (3–5).
Peroxisome proliferator-activated receptors (PPARs) are members of nuclear receptors. After activation by appropriate ligand, they constitute heterodimer with retinoid x receptor (RXR) and bind to specific PPAR responsive elements to regulate target gene expression (6,7). Three subtypes of PPAR are designated: PPARα, PPARβ/δ and PPARγ. PPARs are involved in lipid metabolism, inflammation, angiogenesis and glycaemic control (6,8). It is indicated that bezafibrate has affinity to three PPAR isoforms and act as pan PPAR (9). It uses clinically for the treatment of dyslipidemia and prevention of diabetes mellitus in subjects with coronary artery disease (9,10).
Since, recent studies have demonstrated the role of PPARs during angiogenesis process (8), the aim of the present study was to evaluate the changes of serum nitric oxide (NO) concentration and myocardial capillary density during diabetes and the effect of bezafibrate on coronary angiogenesis in male diabetic rats.
MATERIALS AND METHODS
Animals
Male Wistar rats were provided by Pasteur Institute of Iran weighing between 240-300 g used in this study. They were housed two per cages with 12 h light:dark cycle at temperature between 20-25°C. The animals were fed a standard rat diet and water ad libitum. All experimental procedures were approved by the ethical committee of the Isfahan University of Medical Sciences.
Experimental protocol
The study was done on 18 male rats and they were randomly divided into three groups: non-diabetics (control), diabetic and diabetic treated with bezafibrate. Bezafibrate (Sigma Co., USA) was dissolved in corn oil and administered 400 mg/kg/day by gavage (11). Diabetes was induced by single intraperitoneal injection of streptozotocin (Sigma Co., USA) at a dose of 55 mg/kg. After 48 h, blood glucose levels were measured. The animals with blood glucose concentrations higher than 16.7 mmol/L were considered diabetic (12). The blood glucose levels were measured every week. The treatment was continued for 21 days.
Serum nitric oxide measurement
Blood sample were taken before and after experiment. The samples were centrifuged at 3000 rpm for 20 min and serums were collected in separate eppendorf tubes and kept at -70°C for further analysis. Serum NO concentration was measured using an Elisa kit (Promega Corp, USA; Cat#G2930) using available reagents.
Measurement of capillary density
At the time of sacrifice, left ventricular samples were fixed in 10% formalin overnight, embedded in paraffin and cut in 5μm sections. Then, the sections were deparaffinized with xylosine, rehydrated and endogenous peroxidase was blocked with 3% H2O2 in dark.
Subsequently, for detection of CD31, the sections were incubated with rat monoclonal antibody against murine CD31 (Abcam, Cambridge UK) in a 1:50 dilution for 45 min and subsequent antibody for 45 min (13). Finally, capillaries were visualized by 3-3-diaminobezedine (DAB) and counterstained using hematoxilin. Then, the microvessels were counted in ten random microscopic fields using ×400 objective by two independent observers having no knowledge of characte-ristics of the samples and reported as the number of capillaries per square millimeter.
Statistical analysis
All values are reported as mean ± SEM. One way ANOVA was used to compare the means between groups. Paired t-test was used for the data analysis before and after experi-ment. Bivariate correlations were calculated using Pearson’s correlation coefficient. P <0.05 was considered statistically significant.
RESULTS
Blood samples and body weight
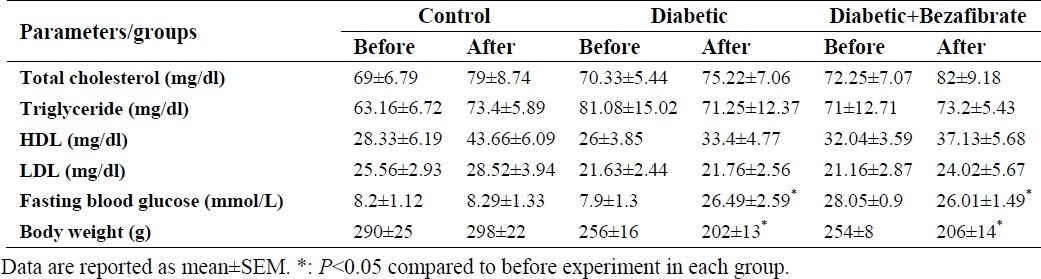
Blood glucose concentration, body weight and serum lipid profile before and after the experiment are shown in Table 1. Blood glucose concentrations were maintained over 16.7 mmol/L in diabetic animals throughout the study. Bezafibrate did not affect blood glucose level in diabetic animals (P>0.05). Body weight in diabetic animals was significantly reduced during the experiment (P<0.05) which did not change by bezafibrate administration.. Results also showed that there were no significant differences in serum lipid profile between groups before and after experiment (P>0.05).
Table 1.
Serum lipid profile, body weight and fasting blood glucose in experimental groups.
Serum nitric oxide concentration
Results showed that diabetic animals had lower serum NO concentration compared to control (4.73 ± 0.45 vs. 5.68 ± 0.79 μmol/L). Bezafibrate increased serum NO concentration in diabetic animals (4.73 ± 0.45 vs. 8.68 ± 1.34 μmol/L).
Myocardial capillry density
Capillary density (expressed as number of CD31 positive cells per mm2) in ventricular muscle was lower in diabetic animals compared to control (121.71 ± 13.32 vs. 158.78 ± 11.08 /mm2;P<0.05). Administration of bezafibrate improved myocardial capillary density in diabetic rats (170.24 ± 15.76 vs.121.71 ± 13.32 /mm2; P<0.05) (Fig. 1). Samples of immunohistochemical staining by anti-CD31 antibody in ventricular muscles are presented in Fig. 2.
Fig. 1.

Effect of diabetes and administration of pan PPAR agonist, bezafibrate, on myocardial capillary density. n=6 each group; *: P<0.05 compared to diabetic group.
Fig. 2.

Representative photographs of immunohistochemical staining (×400) of myocardial tissue with anti-CD31 monoclonal antibody in experimental groups. Arrows indicate CD31 positive cells.
Correlation between serum nitric oxide concentration and capillary density
In the correlation analysis, there was a positive correlation between serum NO concentration and myocardial capillary density (r=0.90) (Fig. 3).
Fig. 3.
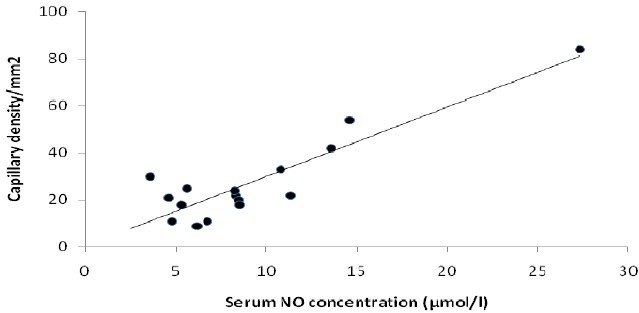
Correlation between serum NO concentration and myocardial capillary density (r=0.9).
DISCUSSION
This study reported the changes of myocardial capillary density in type I diabetic animals. We found that diabetes was associated with reduced serum NO concentration and angiogenesis in ventricular muscle and bezafibrate improved them independent of changing in blood glucose or serum lipid profile. Cardiovascular disease is one of the most important causes of morbidity and mortality in diabetic subjects (3). It is indicated that impaired growth of collateral artery in diabetes leads to reduced blood flow to myocardium and elevated death (14,15). A possible explanation for impaired collateral formation in myocardium is lower expression of angiogenic factors such as vascular endothelial growth factor (VEGF) and its receptors (VEGFR-2) and NO in diabetic state compare to euglycemic subjects (16–19). Thus, development of new therapeutic strategies for stimulating angiogenesis can improve tissue perfusion and organ function in coronary and peripheral ischemia in diabetes (20).
Bezafibrate, a well-known pan PPAR agonist, uses clinically for improving dyslipidemia, hyperglycemia and insulin sensitivity (21,22). Also, it induces atherosclerotic plaques regression and prevents heart injury resulting from ischemic conditions (23). In the present study, we found that treatment with bezafibrate improved angiogenesis in myocardium of diabetic animals. A clinical study on a subset of 164 diabetic subjects showed that administration of bezafibrate was related to reduced incidence of coronary artery diseases (24). Another clinical study demonstrated that bezafibrate prevents myocardial infraction in subjects with metabolic syndrome (25). In our laboratory, we recently found that bezafibrate restored neovascularization in ischemic skeletal muscle of diabetic rats in hind limb ischemia model possibly by increasing angiogenic factors specially NO (26). In this study, we found reduced serum NO con-centration in diabetic animals and improvement of serum NO level after bezafibrate treatment and interestingly, myocardial capillary density had a positive correlation with serum NO concentration. NO has beneficial effects on cardiovascular system specially improvement of angiogenesis (27). Thus, increased serum NO concentration may be a candidate for increased capillary density after bezafibrate administration.
One of the limitations of this study was the evaluation of myocardial capillary density in normoxic condition. Since, hypoxia is the most important stimulator of angiogenesis, it is suggested that this experiment is done in ischemic condition such as after coronary artery ligation. In our study, bezafibrate imp-roved capillary density in heart muscle even in normoxic condition and it seems that it will be able to act the same in hypoxic condition.
CONCLUSIONS
In conclusion, bezafibrate improves coronary angiogenesis in diabetic animals and can be considered in prevention or treatment of cardiovascular complications in diabetes. Further studies can help to clarify whether a sufficient developed vasculature will result from long-term use of pan PPAR agonist therapy in diabetic patients. D Elucidation of exact functional mechanisms of bezafibrate are also imperative.
ACKNOWLEDGMENTS
This study was financially supported by the Isfahan University of Medical Sciences (grant No: 188138).
REFERENCES
- 1.Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab. 2001;12:225–230. doi: 10.1016/s1043-2760(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 2.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 3.Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed. J Am Coll Cardiol? 2005;46:835–837. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Silvestre JS, Levy BI. Molecular basis of angiopathy in diabetes mellitus. Circ Res. 2006;98:4–6. doi: 10.1161/01.RES.0000200396.90220.41. [DOI] [PubMed] [Google Scholar]
- 5.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 6.Jones AB. Peroxisome proliferator-activated receptor (PPAR) modulators: diabetes and beyond. Med Res Rev. 2001;21:540–552. doi: 10.1002/med.1025. [DOI] [PubMed] [Google Scholar]
- 7.Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vascul Pharmacol. 2006;45:19–28. doi: 10.1016/j.vph.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Biscetti F, Straface G, Pitocco D, Zaccardi F, Ghirlanda G, Flex A. Peroxisome proliferator-activated receptors and angiogenesis. Nutr Metab Cardiovasc Dis. 2009;19:751–759. doi: 10.1016/j.numecd.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Tenenbaum A, Motro M, Fisman EZ. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc Diabetol. 2005;4:14. doi: 10.1186/1475-2840-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, et al. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steato-hepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci U S A. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: molecular mechanisms of delayed angiogenesis in diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Kondo T, Numaguchi Y, Kobayashi K, Aoki M, Inoue N, et al. Role of bradykinin, nitric oxide, and angiotensin II type 2 receptor in imidapril-induced angiogenesis. Hypertension. 2008;51:252–258. doi: 10.1161/HYPERTENSIONAHA.107.097394. [DOI] [PubMed] [Google Scholar]
- 14.Hammes HP. Pathophysiological mechanisms of diabetic angiopathy. J Diabetes Complications. 2003;17:S16–S19. doi: 10.1016/s1056-8727(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 15.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 16.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 17.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 18.Himadri R. Vascular Endothelial Growth Factors (VEGFs); Role in perivascular Therapeutic Angio-genesis and Diabetic macrovascular Disease. 2006 [Google Scholar]
- 19.Waltenberger J. VEGF resistance as a molecular basis to explain the angiogenesis paradox in diabetes mellitus. Biochem Soc Trans. 2009;37:1167–1170. doi: 10.1042/BST0371167. [DOI] [PubMed] [Google Scholar]
- 20.Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- 21.Tenenbaum A, Motro M, Fisman EZ, Adler Y, Shemesh J, Tanne D, et al. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J. 2005;26:2032–2038. doi: 10.1093/eurheartj/ehi310. [DOI] [PubMed] [Google Scholar]
- 22.Tenenbaum A, Fisman EZ, Boyko V, Benderly M, Tanne D, Haim M, et al. Attenuation of progression of insulin resistance in patients with coronary artery disease by bezafibrate. Arch Intern Med. 2006;166:737–741. doi: 10.1001/archinte.166.7.737. [DOI] [PubMed] [Google Scholar]
- 23.Ayaori M, Momiyama Y, Fayad ZA, Yonemura A, Ohmori R, Kihara T, et al. Effect of bezafibrate therapy on atherosclerotic aortic plaques detected by MRI in dyslipidemic patients with hypertrigly-ceridemia. Atherosclerosis. 2008;196:425–433. doi: 10.1016/j.atherosclerosis.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, et al. Cardiovascular outcomes in type 2 diabetes A double-blind placebo-controlled study of bezafibrate: the St Mary's, Ealing, Northwick Park Diabetes Cardio-vascular Disease Prevention (SENDCAP) Study. Diabetes Care. 1998;21:641–648. doi: 10.2337/diacare.21.4.641. [DOI] [PubMed] [Google Scholar]
- 25.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 26.Khazaei M, Salehi E, Rashidi B. Pan-PPAR agonist, bezafibrate, restores angiogenesis in hindimb ischemia in normal and diabetic rats. Int J Pept. 2012;2012:637212. doi: 10.1155/2012/637212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke JP. Nitric oxide and angiogenesis. Circulation. 2003;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]


