Abstract
Cisplatin (CP) as an important anti-tumor drug causes nephrotoxicity mainly by oxidative stress and renin-angiotensin system (RAS). Since flavonoids have high antioxidant activity and probable role in the inhibition of RAS, this study was designed to investigate the protective effect of hydroalcoholic extract and flavonoid fraction of Morus alba leaves on cisplatin-induced nephrotoxicity in rat. Extracts of Morus alba leaves were prepared and analyzed Phytochemically. Male rats (160-200 g) were used in this study (n=7-9). Normal group received 0.2 ml normal saline intraperitoneally (i.p.) once daily for ten days. Control animals received CP on the third day and saline in the remaining days. Other groups received either hydroalcoholic extract (200, 400 and 600 mg/kg, i.p.) or flavonoid fraction (50, 100 and 200 mg/kg, i.p.) for two days before CP administration and thereafter until tenth day. Serum concentrations of blood urea nitrogen (BUN), creatinine (Cr) and nitric oxide were measured using standard methods. Also left kidneys were prepared for pathological study. The serum levels of BUN and Cr increased in animals received CP. Hydroalcoholic extract was ineffective in reversing these alterations but flavonoid fraction (50 and 100 mg/kg) significantly inhibited CP-induced increases of BUN and Cr. None of the treatments could affect serum concentration of nitric oxide. Flavonoid fraction could also prevent CP-induced pathological damage of the kidney. It seems that concurrent use of flavonoid fraction of Morus alba with CP can protect kidneys from CP-induced nephrotoxicity.
Keywords: Morus alba, Cisplatin, Nephrotoxicity, Flavonoid fraction
INTRODUCTION
Cisplatin (CP) is an important drug in the treatment of solid tumors, but nephrotoxicity as a side effect limits its use. Nephrotoxicity, as a result of proximal tubule injury, occurs with a high prevalence in the initial days of CP chemotherapy. Recent studies reported that the mechanism of CP nephrotoxicity might be related to oxidative stress, apoptosis or an inflammatory response (1–3) and therefore several chemical and natural compounds with antioxidant and/or anti-inflammatory activity have been examined for their protective effects against CP-induced nephrotoxicity (4). It has also been reported that activation of the renin-angiotensin system (RAS) causes oxidative stress and inhibitors of RAS may improve renal function in CP-treated rats (5–7). Quercetin as a well known flavonoid has been shown to inhibit RAS and oxidative stress (8,9) and as a result limits nephrotoxicity of CP (10,11). Several flavonoids including quercetin and some other polyphenols are found in Morus alba leaves (12). Morus alba L. or white mulberry (Toote Sefid in Persian) is a small sized tree from Moraceae family. It is widely distributed and cultivated in Iran, India, China, southern Europe and North America (13,14). Its dried or fresh berries as functional berries are eaten and very popular in traditional and folk Iranian medicine (13,15). Its leaves in the forms of infusion and decoction are well known in different parts of the world and Iran and used for preventing or treating of some metabolic (13,14), respiratory, urinary and rheumatic disorders (14,15) like diabetes, hypertension and arthritis (16,17). The chemical composition of M. alba has been the subject of several experiments. Flavonoids and other polyphenols (12), stilbenes and benzofuran derivatives, alkaloids (14,17), polysaccharides, proteins, minerals (13,17), vitamins and organic acids (17) are the most important components of its leaves. Several pharmacological, biological and clinical properties including antibacterial, antiviral (12), antitussive, hypoglycemic (16,18), hypotensive (13), antiatherogenic, antihyperlipi-demia (16,17), diuretic, astringent, antioxidant (14,19) and α-amylase inhibitory effects (16) have been reported for M. alba leaves.
MATERIALS AND METHODS
Pharmacognosy
Plant material
The leaves of M. alba were collected from Najafabad located in Isfahan province of Iran in May 2011. The plant material was confirmed by Dr. Iraj Mehregan in the Herbarium Department of Science and Research Branch, Tehran Islamic Azad University, Tehran, Iran. The voucher specimen of M. alba is in the herbarium department of Isfahan University, Isfahan, Iran with the number of 15619.
Preparation of extracts
The plant extracts were prepared as described before with minor modifications (20,21). Using percolation method and ethanol:water mixtures (70:30 v/v) as the extracting solvent, total hydroalcoholic extract of dried and powdered M. alba leaves was obtained after 72 h.
For preparation of the flavonoid-rich extract, M. alba leaves were extracted by ethanol:water (90:10 v/v) and ethanol:water (50:50 v/v) in two non-continuous steps for 72 h separately. The extracts were combined and evaporated and the consequent extract was cleared of rubbish non-polar compounds by extraction with chloroform in a separating funnel for several times to produce an aqueous solvent-extracted layer, containing the flavonoid-rich compounds. Hydroalcoholic and flavonoid-rich extracts were filtered and evaporated separately in a rotovapor under reduced pressure and then freeze-dried until dry powders yielded.
Phytochemical screening of different extracts
The different qualitative and preliminary micro-chemical tests are to be performed for establishing profile of the M. alba leaves extracts for its nature of chemical constituents. The phytochemical experimental tests were achieved to detect the presence of alkaloids, anthraquinones, tannins, cardiac glycosides, essential oils, flavonoids and saponins in the mulberry leaves (22,23).
The extracts were then analyzed by thin layer chromatography (TLC). The analysis was carried out on aluminum silica gel 60 F254 coated plates, with the layer thickness of 250 μm (Merck, Darmstadt, Germany) and developed in different systems where ethyl acetate:formic acid:glacial acetic acid: purified water (100:11:11:26, v/v/v/v) gave the highest resolution. All of the TLC solvents were of analytical grade and Merck brand. The bands were visualized under UV light and natural product reagent (24,25). The identified flavonoid is presented in results section.
Total phenol assay of the extract
Phenolic compounds in the mulberry leaves were assayed by Folin-Ciocalteau method previously described (21,26). Results are given as gallic acid equivalent (GAE) per gram of the lyophilized powder.
Pharmacology
Chemicals
Cisplatin was purchased from Sigma Chemical Company (St. Louis, MO, USA). BUN and Cr kits (Pars Azmoon, Iran) and nitric oxide kit (Promega, USA) were used for biochemical assays.
Animals
Male Wistar rats weighing 160-200 g, were used. The animals were fed with rat chaw and tab water ad libitum and kept in a temperature of 22 ± 3°C with a 12 h light/dark cycle. All procedures were approved by the Ethical Committee of the Isfahan University of Medical Sciences, (Isfahan, Iran) and conducted in accordance with the internationally accepted principles for laboratory animal use and care.
After one-week adaptation with the environmental conditions, orbital sinus blood sampling was performed for determination of the baseline values of biochemical parameters. The samples were centrifuged at 1000 rpm for 20 min, then the serum removed and stored at -20°C until analysis.
Experimental procedure
Rats were randomly divided into groups of 7-9 in each. One group received normal saline (0.2 ml, i.p) from the beginning to tenth day (end of the study). The second group received saline (0.2 ml, i.p.) for 2 days and in the third day CP (7 mg/kg, i.p) was injected. Other groups received either hydroalcoholic extract (200, 400 and 600 mg/kg, i.p.) or flavonoid fraction (50, 100 and 200 mg/kg, i.p.) for two days before CP administration and thereafter until the tenth day. In the last day, rats were anesthetized with ketamine (100 mg/kg) and then sacrificed. Blood samples were collected, centrifuged at 1000 rpm for 20 min, serum was removed and stored at -20°C until analysis. Both kidneys were removed and weighed. The left kidneys were prepared for pathological examinations.
Measurement of biochemical parameters
Serum creatinine concentration was measured by the Jaffe method and BUN (blood urea nitrogen) by Berthelot method using commercial kits. Serum nitric oxide was measured by a quantitative diagnostic kit (a colorimetric method). Osmolality of serum samples was determined by an Osmometer.
Histopathological examination
The left kidneys of animals halved through a coronal section after removal from the body. Then the two halves were fixed by a 10% solution of formalin for several days. After processing, they were embedded in paraffin and cut into 3-4 micrometer slices. The slices were mounted on glass slides and stained with hematoxylin and eosin (H & E) for light microscopic analysis. The assessment was conducted by a pathologist in a blinded way. The pathologic changes of the kidneys were recorded using a grading scale of 0-4 which was based on a subjective impression of the extent of cortical changes as follows:
0 = indistinguishable from controls
1 = minimal, ≤ 25% cortex affected
2 = mild, > 25% and ≤ 50% cortex affected
3 = moderate, > 50% and ≤ 75% cortex affected
4 = severe, >75% cortex affected
This grading scale is adapted from Goering and coworkers (27) with minor modification.
Statistical analysis
The analysis of data was performed with SPSS statistical analysis software (version 15.0). Data was reported as the mean ± SEM. All results except for pathologic findings were analyzed using one way analysis of variations (ANOVA) followed by Tukey post hoc test. Pathological data were analyzed by Kruskal-Wallis method followed by Mann-Whitney U test. The level of significance was considered as P<0.05.
RESULTS
Pharmacognosy
Hydroalcoholic and flavonoid-rich extracts gave dry powders yielded 21.9% and 10.7 % (w/w) respectively. Powders were stored in a refrigerator. The M. alba leaves extract was subjected to phytochemical screening for preliminary detection of its natural active constituents. The result of phytochemical screening of extracts revealed that flavonoids and tannins were present in M. alba leaves.
TLC separations were used to identify some more flavonoid components in the mulberry leaves. Quercetin as the most important part of the mulberry leaves showed a bright spot in the top of chromatogram (Rf=0.92).
The total phenols determined by Folin-Ciocalteau method showed 440 mg GAE per gram of the lyophilized powder.
Pharmacology
Treatment of animals with CP significantly increased serum concentrations of BUN and Cr. Hydroalcoholic extract (200-600 mg/kg, i.p.) could not reverse the elevation of these biochemical parameters (Figs. 1, 2). Flavonoid fraction at doses of 50 and 100 mg/kg inhibited CP-induced increases of BUN and Cr (Figs. 3, 4).
Fig. 1.
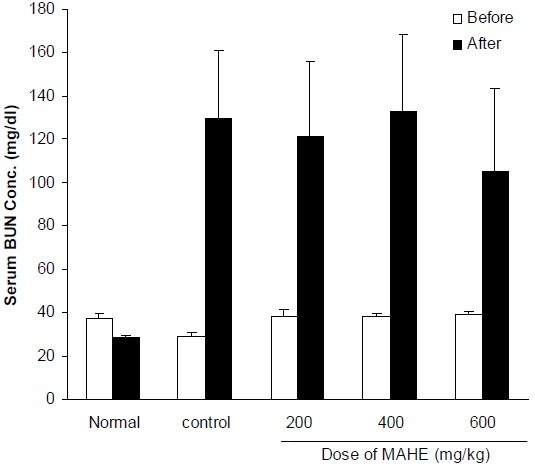
Effect of different doses of hydroalcoholic extract of M. alba on serum BUN concentration in cisplatin-treated rats. Normal group received normal saline (0.2 ml, i.p.) once daily for 10 days. Control group received saline (0.2 ml, i.p.) for 2 days and in the third day cisplatin (7 mg/kg, i.p) was injected. Other groups received hydroalcoholic extract (200, 400 and 600 mg/kg, i.p.) for two days before cisplatin administration and thereafter until tenth day. Data represent mean ± S.E.M of serum Blood urea nitrogen (BUN) concentration of 7-9 animals in each group. MAHE; M. alba hydroalcoholic extract.
Fig. 2.
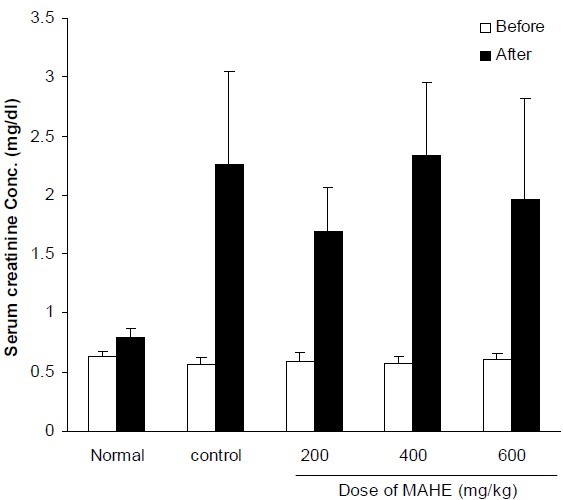
Effect of different doses of hydroalcoholic extract of M. alba on serum creatinine concentration in cisplatin-treated rats. Normal group received saline (0.2 ml, i.p.) once daily for 10 days. Control group received saline (0.2 ml, i.p.) for 2 days and in the third day cisplatin (7 mg/kg, i.p) was injected. Other groups received hydroalcoholic extract (200, 400 and 600 mg/kg, i.p.) for two days before cisplatin administration and thereafter until tenth day. Data represent mean ± S.E.M of serum creatinine concentration of 7-9 animals in each group. MAHE; M. alba hydroalcoholic extract.
Fig. 3.
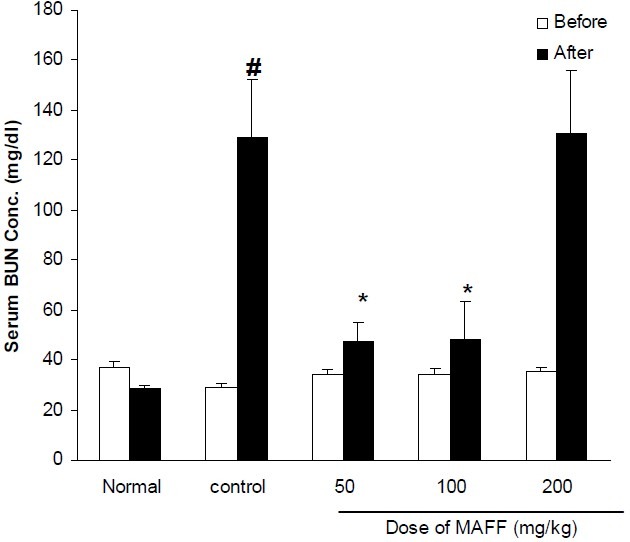
Effect of different doses of flavonoid fraction of M. alba on serum BUN concentration in cisplatintreated rats. Normal group received saline (0.2 ml, i.p.) once daily for 10 days. Control group received saline (0.2 ml, i.p.) for 2 days and in the third day cisplatin (7 mg/kg, i.p) was injected. Other groups received flavonoid fraction (50, 100 and 200 mg/kg, i.p.) for two days before cisplatin administration and thereafter until tenth day. Data represent mean ± S.E.M of serum BUN concentration of 7-9 animals in each group. MAFF; M. alba flavonoid fraction. * P< 0.05 compared to cisplatin (control) group; # P< 0.01compared to normal group.
Fig. 4.
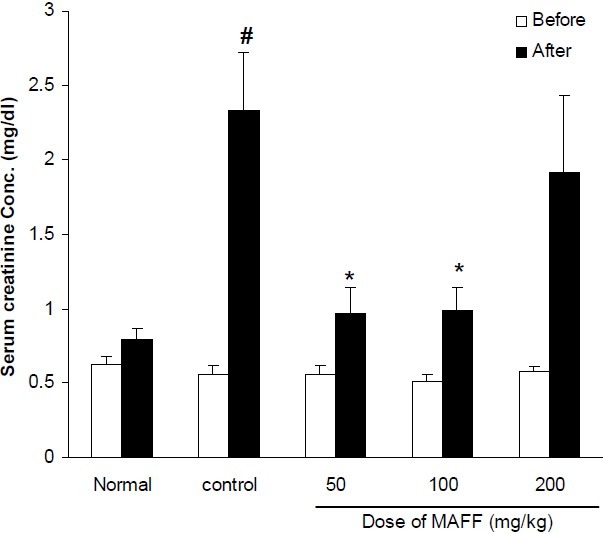
Effect of different doses of flavonoid fraction of M. alba on serum creatinine concentration in cisplatintreated rats. Normal group received saline (0.2 ml, i.p.) once daily for 10 days. Control group received saline (0.2 ml, i.p.) for 2 days and in the third day cisplatin (7mg/kg, i.p) was injected. Other groups received flavonoid fraction (50, 100 and 200 mg/kg, i.p.) for two days before cisplatin administration and thereafter until tenth day. Data represent mean ± S.E.M of serum creatinine concentration of 7-9 animals in each group. MAFF; M. alba flavonoid fraction. P< 0.05 compared to cisplatin (control) group; # P< 0.01compared to normal group.
As it is seen in Table 1, both hydroalcoholic extract and flavonoid fraction could not change serum osmolality and nitric oxide concentration.
Table 1.
Effect of different doses of M. alba hydroalcoholic extract and flavonoid fraction on kidney weight , serum osmolality and nitric oxide (NO) concentration in male rats.
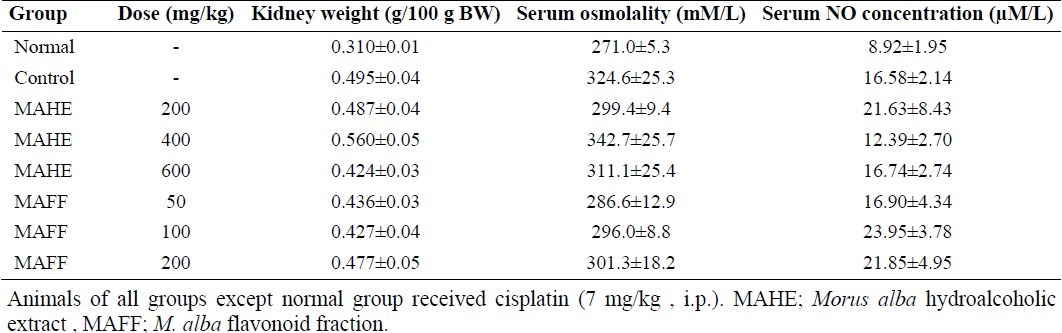
The weight of rat kidneys received CP was significantly greater than those of normal rats. Treatment of animals with different doses of hydroalcoholic extract or flavonoid fraction of M. alba leaves could not prevent the increase of CP-induced kidney weight. The pathological findings clearly showed that CP significantly damaged the kidney tissue and only flavonoid fraction could improve the CP-induced pathological scores (Table 2 and Fig. 5).
Table 2.
Effect of different doses of M. alba hydroalcoholic extract and flavonoid fraction on pathological scores
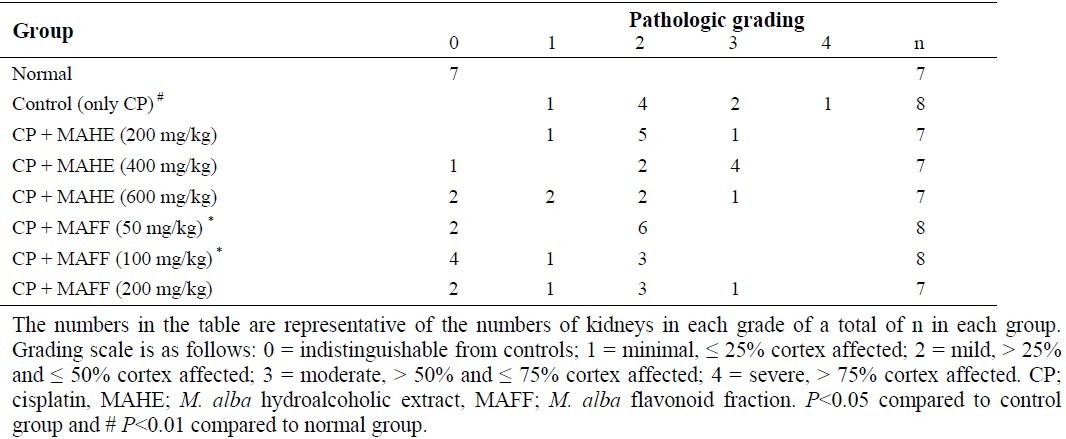
Fig. 5.
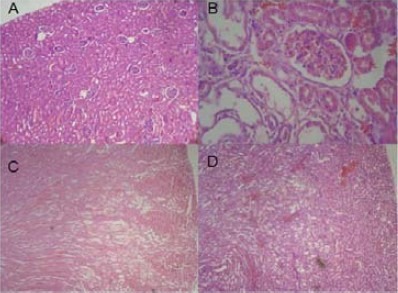
Effect of M. alba polyphenolic extract on the kidney histological changes induced by cisplatin in rat. A: normal group; B: cisplatin-treated group; C: M. alba flavonoid fraction (100 mg/kg) + cisplatin; D: M. alba flavonoid fraction (200 mg/kg) + cisplatin. Cisplatin treatment was associated with a loss of tubular architecture, and the integrity and hypercellularity of the interstitium (panel B). In contrast, M. alba flavonoid fraction prevented the damage (C and D).
DISCUSSION
Cisplatin nephrotoxicity is very complex and several mechanisms including accumulation of the drug in renal epithelial cells, attack of the drug to nuclear and mitochondrial DNA, initiation of a severe inflammatory response, induction of oxidative stress have been proposed (4). Based on these pathways, several in vitro and in vivo researches have been performed and a relatively large number of chemicals and natural compounds have been tested for their potential protection against CP-induced nephrotoxicity (4).
In the present study, we attempted to investigate the effect of hydroalcoholic and flavonoid rich extracts of M. alba leaves on CP- induced nephrotoxicity in rats. Our results clearly showed that flavonoid fraction of M. alba inhibited the CP-induced increases of kidney damage biomarkers (BUN and creatinine). Pathological findings also confirmed the protective effect of the flavonoid fraction. Flavonoid fraction showed significant protective effect at doses of 50 and 100 mg/kg and unexpectedly the dose of 200 mg/kg was ineffective. It seems that the dose of M. alba flavonoid fraction is detrimental and higher doses lose activity. At present we do not have explanation for it and further studies are required to find out the reason. According to the previous studies and our results in the current study, the leaves of M. alba are good source of flavonoid compounds such as quercetin. The findings of many researches confirmed that these compounds are responsible for useful effects in oxidative stress (18,28). On the other hand, several studies reported that the mechanism of CP nephrotoxicity can be associated with oxidative stress (1–4). As a result, the protective effect of flavonoid fraction of M. alba on CP-induced nephrotoxicity might be partly due to antioxidant potential of these compounds. Some investigators have reported that CP-induced nephrotoxicity has an inflammatory component and pro-inflammatory cytokines are involved in its pathogenesis. Flavonoid extracts of many plants have shown anti-inflammatory activity (29–31). Consistent with this idea, Choi and Hwang (32) reported that M. alba leaf extract reduced production of PGE2, nitric oxide and also TNF-alpha in a mouse macrophage cell line and since these mediators are involved in inflammatory process, it seems that at least a part of the protective effect of M. alba leaf extract might be due to its anti-inflammatory potential. Our results also showed that the protective effect of flavonoid fraction is not related to changes in serum concentration of nitric oxide.
CONCLUSIONS
In conclusion the mechanism of CP-induced nephrotoxicity is very complex and this drug affects several pathways in kidney cells. On the other hand, plant flavonoids also show anti-inflammatory, anti-apoptotic, antioxidant and several other biological activities. Based on these facts, further studies are needed to find out the exact mechanism of the protective effect of M. alba leaf extract against CP-induced nephrotoxicity.
ACKNOWLEDGMENT
We are grateful to Dr. Iraj Mehregan for confirming of the plant scientific name. This study was funded by Research Council at the Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 3.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy. 2009;55:399–406. doi: 10.1159/000262453. [DOI] [PubMed] [Google Scholar]
- 6.Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 2008;31:175–184. doi: 10.1291/hypres.31.175. [DOI] [PubMed] [Google Scholar]
- 7.Deegan PM, Nolan C, Ryan MP, Basinger MA, Jones MM, Hande KR. The role of the renin-angiotensin system in cisplatin nephrotoxicity. Ren Fail. 1995;17:665–674. doi: 10.3109/08860229509037634. [DOI] [PubMed] [Google Scholar]
- 8.Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J cardiovasc pharmacol. 2008;51:239–245. doi: 10.1097/FJC.0b013e318162011f. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61:67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- 10.Francescato HD, Coimbra TM, Costa RS, Bianchi Mde L. Protective effect of quercetin on the evolution of cisplatin-induced acute tubular necrosis. Kidney Blood Press Res. 2004;27:148–158. doi: 10.1159/000078309. [DOI] [PubMed] [Google Scholar]
- 11.Behling EB, Sendão MC, Francescato HDC, Antunes LMG, Costa RS, de Lourdes P, et al. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep. 2006;58:526–532. [PubMed] [Google Scholar]
- 12.Wang J, Wu FA, Zhao H, Liu L, Wu QS. Isolation of flavonoids from mulberry (Morus alba L.) leaves with macroporous resins. African J Biotechnol. 2008;7:2147–2155. [Google Scholar]
- 13.Emami A, Shams Ardekani MR, Mehregan I. Color atlas of medicinal plants. Vol. 198. Tehran: ITMRC publications; 2004. [Google Scholar]
- 14.Yang Y, Gong T, Liu Ch, Chen RY. Four new 2-arylbenzofuran derivatives from leaves of Morus alba L. Chem Pharm Bull. 2010;58:257–260. doi: 10.1248/cpb.58.257. [DOI] [PubMed] [Google Scholar]
- 15.Ebn-e Sina A. In: Ghanoon dar teb. Sharafkandi A, translator. Vol. 2. Tehran: Soroosh Press; 2006. pp. 328–329. [Google Scholar]
- 16.Nickavar B, Mosazadeh G. Influence of three Morus species extracts on á-amylase activity. Iranian J Pharm Res. 2009;8:115–119. [Google Scholar]
- 17.Varposhti MH, Asghari GR. Herbal medicine. Isfahan: Chaharbagh Press; 2007. pp. 140–143. [Google Scholar]
- 18.Naowaboot J, Pannangpetch P, Kukongviriyapan V, Kongyingyoes B, Kukongviriyapan U. Antihyperglycemic, antioxidant and antiglycation activities of mulberry leaf extract in streptozotocin-induced chronic diabetic rats. Plant Foods Hum Nutr. 2009;64:116–121. doi: 10.1007/s11130-009-0112-5. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Miyazawa M, Kojima T. The use of Morus alba L.(mulberry) and Eucommia ulmoides (Tochu) leaves as functional foods: a promising approach in the management of hyperlipidemia. J Trad Med. 2010;27:227–230. [Google Scholar]
- 20.Hajhashemi V, Ghanandi A, Mousavi S. Antinociceptive study of extracts of Platanus orientalis leaves in mice. Res Pharm Sci. 2011;6:23–128. [PMC free article] [PubMed] [Google Scholar]
- 21.Minaiyan M, Ghanandi A, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Ghannadi A. Phytochemical investigations on flavonoids and constituents of volatile oil of Stachys lavandulifolia. J Pharm Sci (J Tabriz Fac Pharm) 1998;4:138–146. [Google Scholar]
- 23.Ghannadi A, Rabbani M, Ghaemmaghami L, Malekian N. Phytochemical screening and essential oil analysis of one of the Persian sedges; Cyperus rotundus L. Int J Pharm Sci Res. 2012;3:424–427. [Google Scholar]
- 24.Males Z, Medic-Saric M. Optimization of TLC analysis of flavonoids and phenolic acids of Helleborus atrorubens Waldst. Et Kit. J Pharm Biomed Anal. 2001;24:353–359. doi: 10.1016/s0731-7085(00)00455-6. [DOI] [PubMed] [Google Scholar]
- 25.Sajeeth CI, Manna PK, Manavalan R, Jolly CI. Quantitative estimation of gallic acid, rutin and quercetin in certain herbal plants by hptlc method. Der Chem Sin. 2010;1:80–85. [Google Scholar]
- 26.Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (quine) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 27.Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ. Mercury induces regional and cell-specific stress protein expression in rat kidney. Toxicol Sci. 2000;53:447–457. doi: 10.1093/toxsci/53.2.447. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, Gao JJ, Lee WC, Ryu KS, Lee KR, Kim YC. Antioxidative flavonoids from the leaves of Morus alba. Arch Pharm, Res. 1999;22:81–85. doi: 10.1007/BF02976442. [DOI] [PubMed] [Google Scholar]
- 29.Ghannadi A, Hajhashemi V, Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8:488–493. doi: 10.1089/jmf.2005.8.488. [DOI] [PubMed] [Google Scholar]
- 30.Hajhashemi V, Ghannadi A, Pezeshkian SK. Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J Ethnopharmacol. 2002;82:83–87. doi: 10.1016/s0378-8741(02)00137-x. [DOI] [PubMed] [Google Scholar]
- 31.Hajhashemi V, Ghannadi A, Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003;89:67–71. doi: 10.1016/s0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 32.Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005;76:608–613. doi: 10.1016/j.fitote.2005.05.006. [DOI] [PubMed] [Google Scholar]


