Abstract
Sirtuin1 (SIRT1) is an enzyme that deacetylates histones and several nonhistone proteins including p53 during stress and plays an important role in the survival of tumor cells. Hereby, this study describes the potency of salermide as a SIRT1 inhibitor to induce apoptosis in the MCF-7 and MRC-5 cell lines. MCF7 and MRC-5 cell lines were cultured in RPMI-1640 and treated with or without salermide at concentration of 80.56 μmol/L, based on the half-maximal inhibitory concentration (IC50) index at different times (24, 48 and72 h). The IC50 value was established for the salermide in MCF-7. The percentage of apoptotic cells was measured by flow cytometry. Real-time quantitative RT-PCR was performed to estimate the mRNA expression of sirtuin1 in MCF-7 and MRC-5 with salermide at different times. ELISA and Bradford protein techniques were used to detect endogenous levels of total and acetylated p53 protein generated in MCF-7 and MRC-5 cells. Our findings indicated that salermide can induce apoptosis in MCF-7 significantly more effective than MRC-5 cells. We showed that the expression of SIRT1 was dramatically down-regulated by increasing the time of salermide treatment in MCF-7 but not MRC-5 and that the acetylated and total p53 protein levels were increased more in MCF-7 than MRC-5. Salermide, by decreasing the expression of sirtuin1 gene, can induce acetylation of P53 protein and consequently induce significant cell death in MCF-7 that was well tolerated in MRC-5.
Keywords: Cancer, Apoptosis, Salermide, Sirtuin1, MCF-7, MRC-5
INTRODUCTION
Cancer is one of the most important cause of mortality in the world population and the second leading cause of the death in developing countries (1). Breast cancer is the most common cancer among females worldwide (2). Each year, as estimated, 1.15 million cases of females worldwide are diagnosed with breast cancer while about 502,000 die from the disease (3). While more than half of the cancer cases often have mutations in P53 gene (the most important tumor suppressor gene), but alterations in tumor suppressor genes are not always due to mutations. They may also be due to epigenetic alteration (4).
In some diseases such as cancer, often there is an imbalance between the expression of histone acetyltransferase (HATs) and histone deacetylase (HDACs) families. HDACs comprise a super family of enzymes involved in regulating the lifespan which include regulation of transcription (5). HDACs are divided into four different classes (I-IV) based on their homology to yeast enzymes (6). Class III HDACs were discovered more recently and this group of deacetylases were named ’sirtuins’ (7). The sirtuins have a nicotine adenine dinucleotide (NAD) as a unique cofactor to this family that is necessary for the removal of the acetyl group from the lysine residues (deacetylases function) (8). Other HDACs (classic HDACs) use Zn2+ ion for deacetylation in a different reaction, although the details of this unique biochemical reaction is not yet fully understood at the molecular level. In human structures of seven different NAD dependent deacetylase genes, SIRT1 (silent information regulator1) to SIRT-7, have been recognized (9) and are implicated in the control of cellular responses through the deacetylation of important regulatory proteins. The p53 tumor suppressor protein plays a major role in cellular response to DNA damage and other genomic aberrations (10). Activation of p53 can lead to cell cycle arrest, DNA repair and apoptosis. Following DNA damage, Human p53 becomes acetylated at Lys382. SIRT1 mediates deacetylation of p53 and negatively regulates the activity of this protein (11).
In normal cells, p53 is a short-lived protein due to the activity of Mdm2 (its negative regulator), as a ubiquitin ligase, which inhibits and destabilizes p53, so p53 levels will be undetectable and inactive to induce apoptosis (12). In response to various types and stress levels which cause DNA damage, HAT family mediate acetylation of p53 in C terminus and blocks some of the major p53 ubiquitination sites by Mdm2 (13). This function leads to p53 protein stabilization and significant increase in the amount and activity of p53 protein in human cells (14). It seems that SIRT1 is able to deacetylase and inhibit p53 activity and suppress the induction of apoptosis and prolongs cellular survival in response to DNA damage in some cancer cells (15).
The balance between p53 acetylation (positively regulates p53 activity) and deacetylation (negatively regulates its activity) is mediated by the HATs and SIRT1, and is usually well regulated, but the balance is often upset in diseases such as cancer (16). Studies suggest that pharmacologic inhibition of SIRT1 may promote apoptosis by direct acetylation of p53 in some cells and can be used as an anticancer strategy (17). Some reports suggest that SIRT1 probably mediates p53 deacetylation and inhibits p53, thereby prevents apoptosis in response to various types of stress in some of malignancies (18). These effects can be reversed in cancer cells by inactivation of SIRT1. The human breast carcinoma cell line MCF-7 has wild-type p53 (19) but this tumor suppressor gene due to epigenetic events, is not functional and is unable to induce apoptosis (20). Some researches have shown that in certain type of cancerous cell lines SIRT1 inhibitors could induce p53 acetylation as an antitumor effect (21).
We assumed that the apoptotic effect of this drug is different in normal and cancer cells. However, little is known about the mechanisms of cellular targets of sirtuin1 gene (22). In this study we investigated apoptotic effects of salermide, as the inhibitor of sirtuin1 in MCF-7 (breast adinocarsinoma) and MRC-5 (lung fibroblasts as non-tumorigenic control) cell lines.
MATERIALS AND METHODS
Cell lines, drug treatment and culture condition
Human breast carcinoma cell line (MCF-7) and human lung fibroblasts cell line (MRC-5) were purchased from the National Cell Bank of Iran-Pasteur Institute. Salermide (N-{3-[2-hydroxynaphthalen-1- ylmethylene )- amino ]-phenyl} -2-phenylpropionamidea) (Fig. 1) as the inhibitor of sirtuin1, was purchased from Sigma (USA). All Cell lines used in the present study were cultured in RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma) and 1% penicillin-streptomycin (Sigma), at 37°C and in humidified atmosphere containing 5% CO2. Salermide was dissolved in stock solutions and for treatments; the compounds were diluted in DMSO to appropriate concentrations according to the reported procedures (23). When cells became >80% confluent and growing exponentially in 10 cm diameter culture dishes, 105 cells (MCF-7 or MRC-5) were counted and plated in 3-cm diameter culture dishes for 24 h in RPMI-1640 culture medium before they were incubated with certain concentrations of salermide, based on IC50 index, at different times (24, 48 and 72 h). Photography was done for cultures before and after treatment with salermide at different times using inverted microscope (Nikon, TE 2000-U, Japan) (24).
Fig. 1.
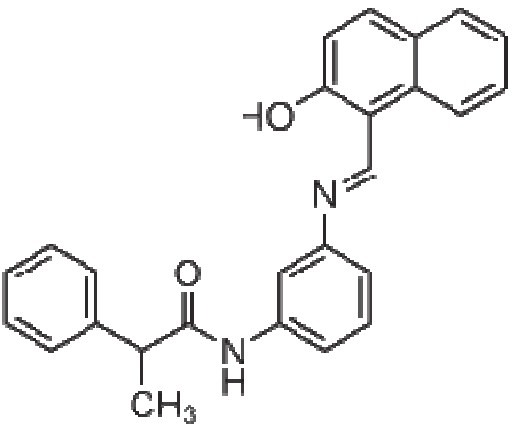
Structures of Salermide (N-{3-[(2-hydroxy-naphthalen-1-ylmethylene) - amino] -phenyl}-2-phenyl-propionamide)
IC50 assay
After 24 h of the treatment, the IC50 value for salermide in MCF-7 group was determined. Briefly, 104 Cells (MCF-7) were counted and placed into each well of a 24-well micro plate and were treated with various drug concen-trations (0, 6.25, 12.5, 25, 50, 100, 150, 200 μM ) for 24 h and the MTT survival assay was then carried out for the evaluation of the cell viability with different drug concentration. A plot of viability versus drug concentration was used to calculate IC50 values for MCF-7 cell line (5).
Flowcytometric analysis
The percentage of apoptotic cells was measured by flow cytometry following AnnexinV (FL1-H) and PI (FL2-H) labeling.. A minimum of 5Χ105cells/ml were analyzed for each sample. Cells were treated with salermide (80.56 µg/ml) for 24, 48 and 72 h, then washed in PBS and resuspended in Binding buffer (1x). After the additionof 5 μl AnnexinV-FITC to 195 μl cell suspension the analysis was carried out according to the manufacturer’s protocol (BMS500F1/100CE AnnexinV-FITC, eBiscience, USA), Finally the apoptotic cells were counted by FACScan flow cytometry (Becton Dickinson, Heidelberg, Germany). These experiments were carried out in triplicate and independently repeated at least three times (25).
Reverse transcription and real-time PCR analysis
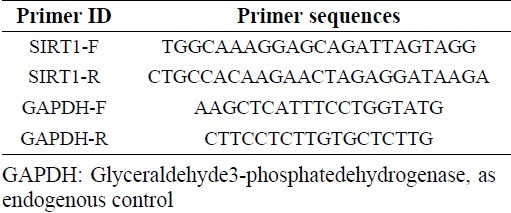
Real-time quantitative RT-PCR was performed to quantitatively estimate the mRNA expression of sirtuin1 in MCF-7 and MRC-5 cells before and after treatment with salermide at different times. Total RNA was isolated by RNeasy mini kit (Qiagen), treated by RNase free DNase (Qiagen) to eliminate the genomic DNA. The RNA concentration was determined using a Biophotometer (Eppendorf). Total RNA (100 ng) was reverse-transcribed to cDNA by using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer’s instructions. Real-time RT-PCR was performed by the Maxima SYBR Green Rox qPCR master mix kit (Fermentas). Primer sequences are shown in Table 1. Real-time PCR reactions were performed using the Steponeplus (Applied Biosystem). The PCR amplification conditions consisted of 10 min at 95°C followed by 40 cycles of denaturation step at 95°C for 15 sec and annealing and extension for 1 min at 60 °C. Data were analyzed using the comparative Ct (ΔΔct) method, the relative expression level of SIRT1 was calculated by determining a ratio between the amount of SIRT1 and that of endogenous control. Melting curve analysis (60°C → 95°C increment at 0.3°C) was used to determine melting temperature of specific amplification products and primer dimmers. These experiments were carried out in triplicate and independently repeated at least three times (26).
Table 1.
Primers used in real-time PCR
Bradford protein assay
Total (intracellular) protein concentration was determined by Bradford method. This method was carried out before the ELISA assay. Total Protein was extracted from MCF-7 and MRC-5 cells before and after salermide treatment as described in ELISA assay. Bovine serum albumin (BSA) was used to prepare protein standards at nine concentrations (0.25, 0.5, 1, 1.5, 2, 3, 4, 5 and 6 mg/ml). Absorbance was measured at 595 nm using a spectro-photometer (Bausch & lomb70, Germany) (27).
Acetylated and total p53 sandwich ELISA assay
Enzyme linked immunosorbent assay (ELISA) was used to detect specifically endogenous levels of total and acetylated p53 protein generation in MCF-7 and MRC-5 cells in presence or absence of salermide at different incubation times (24, 48 and 72 h). Acetylated and total p53 ELISA Kit were obtained from Cell Signaling Technology (Cat.No.7236 & 7370). To extract the proteins cells were lysed first. Briefly, after treatment with salermide cells were harvested and media was removed. Cells were then rinsed with ice-cold PBS and after removal of PBS 0.5 ml of ice-cold Cell Lyses Buffer ( CST.Cat.No. 9803 ) with 1 mM phenylmethylsulfonyl fluoride (PMSF) was added to each plate and incubated on ice for 5 min. Cells were scraped off the plate and transferred to an appropriate tube and a freeze-and-thaw cycle was performed three times to further disrupt the cell membrane. Tubes were then centrifuged for 10 min at 4°C and the supernatant was transferred to a new tube. This supernatant was the cell lysate and was used for the ELISA and protein assays. Sandwich ELISA was performed according to the manufacturers’ protocol. Absorbance was measured using an ELISA reader (Hyperion, Germany) at 450 nm. The absorbance of each sample was divided by the absorbance of the untreated cell with the same incubation time to calculate a control index. All experiments were carried out in triplicate (28).
Statistical analysis
All the quantitative data were presented as the mean ± standard deviations. Kolmogorov-Simonov test was used for assessing normal distribution of variables. One-way-analysis of variance (ANOVA) with LSD post hoc test was performed to determine statistical significance among different groups by using SPSS software package16.0. Significance was accepted at P<0.05.
RESULTS
IC50
After the treatment of MCF-7 cells with MTT solution, the dark blue formazan crystals were seen in the cells, which indicated their metabolic activity. The reduction in the number of cells was dependent on the cell type as shown by the IC50 index (half-maximal inhibitory concentration). The IC50 values for the salermide were established (Fig. 2). The results showed that the essential salermide concentration to achieve the IC50 in MCF-7 cells at 24 h was 80.56 μmol/L (Fig. 1).
Fig. 2.
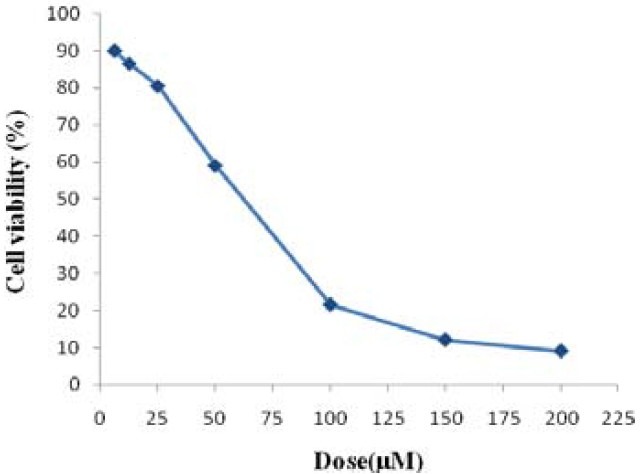
IC50 assay for the analysis of half-maximal inhibitory concentration of Salermide in MCF-7 cancer cell lines after 24-h of treatment. Cells were incubated with or without Salermide using 0, 6.25, 12.5, 25, 50, 100, 150 and 200 µM doses and the relative amount of viable cells were estimated by measuring the absorbance of the cell suspension after incubation with MTT. The graph of viability versus drug concentration was used to calculate IC50 values base on trend line equation (Y=-0.260X + 87.06) for MCF-7 cell line (80.56 µM)
Flow cytometry
To establish the anticancer potential of salermide, we first investigated the effects of this SIRT1 inhibitor on the proliferation of the breast carcinoma cell line (MCF-7). The flow cytometry results showed that based on IC50 index salermide at concentration of 80.56 μmol/L could significantly induce apoptosis in MCF-7 cells that increased with time (Fig. 3A, 3B. Salermide treatment arrested MCF-7 cell proliferation (≥95% of inhibition)
Fig. 3.
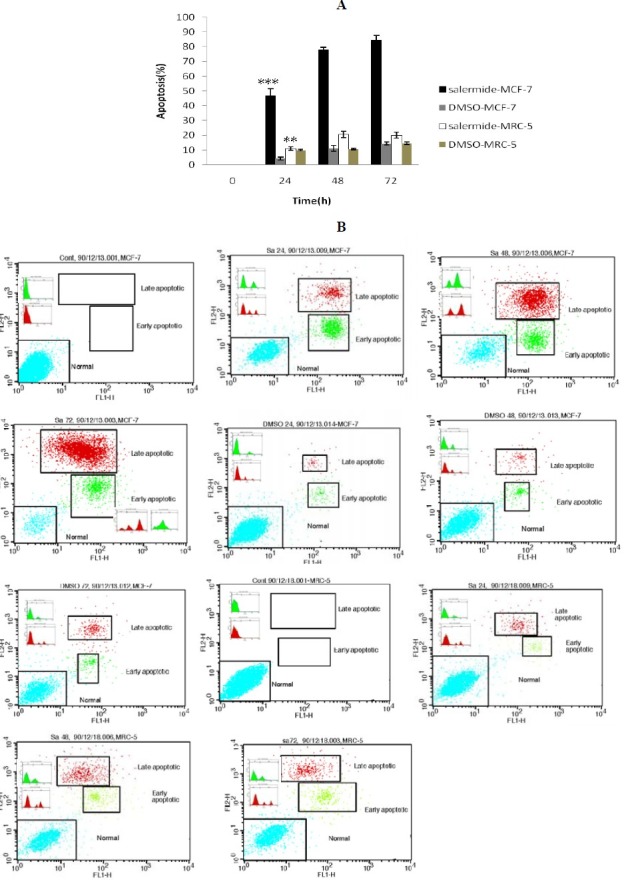
Relative levels of apoptotic cells in MCF-7 and MRC-5cells treated with 80.56 µM/L Salermide for different times. Cells incubated with the vehicle (DMSO) were used as a control. A. The percentage of apoptotic cells was measured using the AnnexinV FITC and PI assay. ***P<0.001 vs. All other groups of MCF-7 cells treated with Salermide. **P<0.05 vs. 0, 24 and 48 h groups of MRC-5 Cells. *P<0.05 vs. All other groups of MCF-7 Cells incubated with the vehicle (DMSO). B. MCF-7and MRC-5 cells were treated with 80.56 µM/L of Salermide, and apoptosis was measured by flow cytometry following AnnexinV (FL1-H) and PI (FL2-H) staining. Cells that are AnnexinV-positive and Propidium iodide negative are in early apoptosis, as phosphatidylserine (PS) translocation has occurred, although the plasma membrane remains intact. Cells that are positive for both AnnexinV and PI are either in the late stages of apoptosis, as PS translocation has occurred and the loss of plasma membrane integrity is visible.
Real-time PCR
To examine the hypothesis that apoptotic induction in cancer cell line by salermide required the suppression of sirtuin1 gene expression, we used two cell lines, MCF-7 as cancer cell line and MRC-5 as noncancerous cell line. We examined the inhibitory effect of 80.56 μmol/L salermide on the mRNA expression of Sirtuin1 in MCF-7 and MRC-5 cells using real-time PCR. The SIRT1 gene expression was dramatically down-regulated by salermide treatment with time in MCF-7 cells, in particular, at 72-h treatment its expression was fully suppressed (Fig. 4), P<0.01). In MRC-5 cells the expression of SIRT1 was also significantly reduced at all treatment times but the difference in the expression among different times were not statistically significant (P>0.05) (Fig. 5). However, the effect of salermide treatment on down-regulation of SIRT1 expression was significantly higher in MCF-7 cells in comparison with MRC-5 cells (Fig. 5).
Fig. 4.
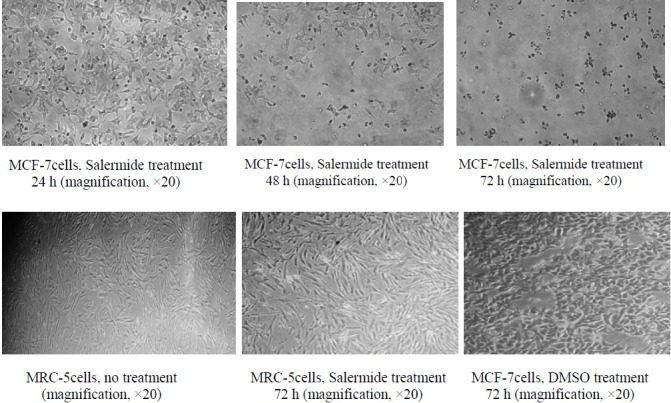
Inverted microscopy images of MCF-7and MRC-5 cells before and after treatment with 80.56 µmol/L Salermide and control DMSO at different times. Magnification, × 20
Fig. 5.
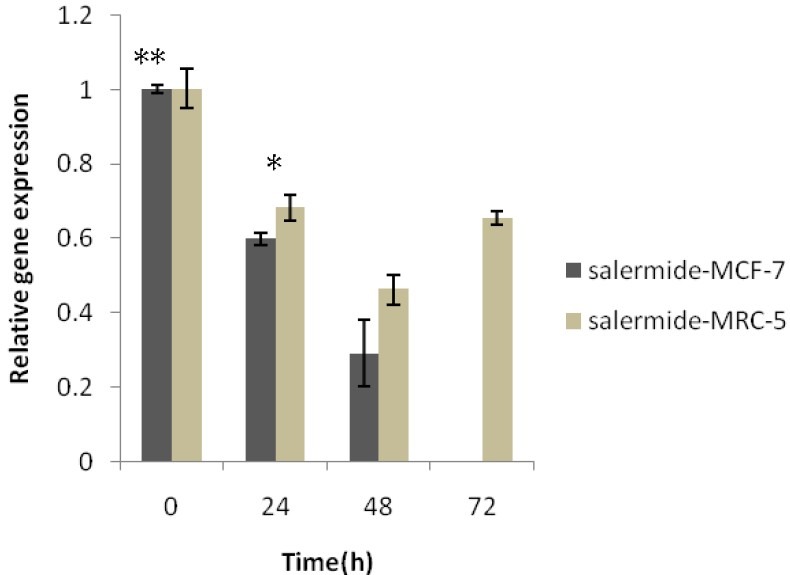
Results of Real-time quantitative PCR on the Sirtuin1 mRNA expression in MCF-7 and MRC-5 cells before and after Salermide treatment at different times. Relative expression levels of each gene were obtained by using the Comparative Ct (ÄÄct) method. SIRT1 inhibitor caused epigenetically silenced sirtuin. 1Values were the means of triplicate experiments * P<0.001 vs. Control (non-treated Salermide) and other MCF-7 groups. *P<0.05 vs. MRC-5, 24h and Control group.
Bradford protein
The Bradford assay was carried out to determine the total protein concentration in MCF-7 and MRC-5 cell lysates at 48 h treatment with salermide. The standard curve was linear in the range of 0.25 to 6 mg/ml of BSA. The total protein concentration for MRC-5 and MCF-7 was respectively 4.48 and 5.17 μg/ml)
Acetylated and total p53 Sandwich ELISA
To investigate further the effects of salermide (SIRT1 inhibitor) on cell apoptosis, the ELISA analysis was performed in MCF-7 (wild-type p53) and MRC-5 cells. The cell were treated with 80.56 μmol/L salermide for different length of time (0, 24, 48 and 72 h), to study its effects on the acetylation status of the p53 as target of SIRT1.
The results of ELISA analysis was calculated based on control index. The results showed that, salermide could induce p53 acetylation in MCF-7 and MRC-5 cells and that significant increase in total protein levels with time was observed until 48 h of the treatment in MCF-7 cells but not in MRC-5 cells (P<0.05). Interestingly, between 48 and 72 h, a decrease of protein levels was observed in MCF-7 cells (Fig. 6A). After treatment by salermide at different times, the acetylated P53 protein levels in MCF-7 cells, was significantly higher than in MRC-5 cells (P<0.05) (Fig. 6A). We also performed the above method to examine the total p53 protein level in both cell lines. These results were similar to the above results of acetylated p53 except for a significant rise in total P53 protein levels in MRC-5 cells with increasing time until 48 h (P<0.05) (Fig. 6B). In control samples using DMSO (as salermide solvent) had a negligible effect on inducing total and acetylated p53 in both cell lines at the different time of study (Fig. 6A, 6B).
Fig. 6.
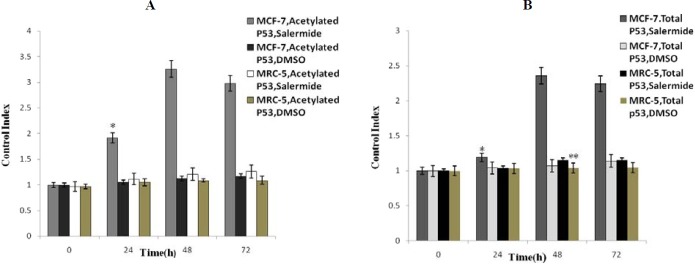
The results of ELISA assay of total and acetylated p53 protein generated in MCF-7 and MRC-5 based on control index. Cells were treated with 80.56 ìmol/L of Salermide for 0, 24, 48 and 72 h. Values are the means ± SD for triplicate experiments. (A) *P<0.001 vs. all other groups in different times. (B) *P<0.001 vs. all other groups in different times. *P<0.05vs. Total P53 in MRC-5 without Salermide treatment and with Salermide treatment at time 24 and 48 h. No significant difference was shown in total and acetylated p53 content of in other groups.
DISCUSSION
We have studied the potency and functional mechanism of the recently developed SIRT1 inhibitor (salermide) at a concentration according to IC50 on MCF-7 as the breast cancer cell line and MRC-5 as a non-tumorigenic control cells. Salermide IC50 result was similar to the previously published data on the efficiency of salermide on SIRT1 in breast cancer cells(MDA-MB-231) (5). Previous studies have shown that this compound can inhibit NAD-dependent deacetylases in mammalian cells (23). In recent years, several researchers have described the apoptotic effect on HDAC (class III) inhibitors and have shown that these effects depend on the type of cancerous cells (29),(30).
The role of SIRT1 is complex during stress and its inhibitory effect is likely to be cell context specific (31). Specific explanations regarding the molecular mechanisms for most of sirtuin inhibitors (particularly salermide) in different cancerous and normal cell lines and in relation to time is lacking. In this study we examined the apoptotic potency of salermide, using the MCF-7 breast carcinoma cell line because this cell line has substantial expression level of SIRT1, has mainly wild-type p53 genes and this type of cancer is the most prevalent malignancy in woman. In this study, treatment by 80.56 µM salermide at different times showed a time-dependent increase in apoptotic cells count of the cancerous cells but not in non-tumorigenic MRC-5 cells as measured by flow cytometric assay. On the other hand although salermide can effectively induce inhibition of SIRT1 and subsequent cancer cell death, it does not have such effect on the fibroblastic cells. These results revealed that the apoptotic sensitivity of MRC-5 cells to salermide was negligible. This is in accordance with findings of Lara and coworkers (5) who showed that sirtuin inhibitors can induce massive apoptosis in cancerous but not in nonmalignant cells. We observed the strongest apoptotic effect in MCF-7 cells after 72 h of incubation with salermide, in that only 10% of MCF-7 cells were viable while at this time MRC-5 cells showed only a slight increase in level of apoptosis (only 10-20%). It seems that this observation in MCF-7 cells is due to the presence of the wild-type p53, since MDA-MB-231 breast cancer cells (mutant-P53) shows only a small increase of apoptotic response to salermide (5).
Therefore, it seems that in MCF-7 cells SIRT1 promotes cell survival and salermide alone can induce apoptosis in this cancer cell with wild-type p53 in a time-dependent manner. Our results are in close agreement with the findings of Zou and coworkers (32) who have shown that TSA (HDAC inhibitor) can induce apoptosis in BGC-823 gastric cell line in a time-dependent manner. Also our results are consistent with the findings of Solomonand coworkers (33) that SIRT1 may be an important regulator of wild-type p53 function and small molecule inhibitors of SIRT1 may act as anticancer agents. We observed that salermide is ineffective in promoting P53 acetylation and hence is unable to activate P53 to induce apoptosis in fibroblastic cells. This finding is supported by morphological examination using inverted microscope.
Once we determined that salermide antitumor activity was primarily because of the promotion of apoptosis, we decided to study the molecular mechanisms involved in this process. We first studied the expression of SIRT1 in salermide-mediated apoptosis using Real time PCR. The results of Real time PCR assay indicated that salermide mediated inhibition of sirtuin1 expression in a time-dependent manner in MCF-7 cells. We also found that sirtuin1 expression levels in MRC-5 cells was low and after the treatment with salermide slightly decreased in a time dependent manner. Importantly, we found that in the noncancerous cells, SIRT1 silencing due to salermide was at least equivalent to MCF-7 breast cancer cells. Subsequently, we observed that SIRT1 silencing occurred particularly after 48 h of salermide treatment in MCF-7 cells. However, SIRT1 silencing had no apparent effect on the cell growth or viability of the noncancerous cells. This is in close agreement with the findings of other investigators (34–38) that, in the absence of applied stress, SIRT1 silencing induces growth arrest and/or apoptosis in human epithelial cancerous cells. In contrast, normal human epithelial cells and normal human diploid fibroblasts seem to be refractory to SIRT1 silencing. This is in apparent disagreement with the findings of Peck and coworkers (19) who described that knockdown of SIRT1 using siRNA-silencing assay did not affect the growth of the cancerous cells in comparison to the controls. However, these data can explain the failure of salermide to induce cell death in MRC-5 cells.
These results indicated that the constitutive function of SIRT1 differs in MCF-7 and MRC-5 cells and that SIRT1 enables MCF-7 cancer cell viability, but is not essential for the viability of lung fibroblast cells. These results are also similar to the study of Kojima and coworkers (26) who noted that the up-regulation of SIRT1 expression was observed in breast cancer MCF-7 cells and that treatment with a SIRT1 inhibitor, Sirtinol, induced inhibition of SIRT1 expression and subsequently inhibited cell growth in human prostate cancer cell lines. Importantly, in MRC-5 cells we observed that after 48h of treatment, SIRT1 mRNA levels started to rise so that the effect of 72-h of treatment of MRC-5 cells by salermide was similar to the non-treatment condition. Subsequently, we used real time PCR to evaluate the SIRT1 expression in both cell lines before the treatment and we observed over-expression of SIRT1 in MCF-7 cells compared to MRC-5 cells. This increased level of SIRT1expresion in MCF-7 cells presumably inhibit apoptosis and mediate survival in response to stress. This finding is in accordance with the findings of Sun and coworkers (39) that reported over expression of sirtuin1 in breast carcinoma. Our findings indicated that the induction of cell death by SIRT1 inhibitor required the inactivation of SIRT1 gene so these findings show its potential antitumor effects. A recent study by Calvanese and coworkers (40), has discussed the implications of similar findings on sirtuin inhibition based cancer treatment and application of sirtuin activation for anti‐aging therapy. A number of previous studies have reported that SIRT1 inhibition could induce (24,41) or not induce (42) p53 acetylation in cancer cell lines. We observed a direct correlation between total and acetylated P53 protein levels and salermide toxicity in the MCF-7 cell line. These results suggest that incubation of MCF-7 with salermide could induce hyperacetylation of p53 protein and apoptosis in MCF-7 cells. Our results indicated a slightly decrease of total and acetylated p53 at 72 h of incubation in MCF-7 cells. We presume that, although increase in total and acetylated p53 levels in response to SIRT1 silencing occurs at this time, this increase is undetectable by ELISA assay because of the release of proteases and activation of degradation processes inside the cancer cells after 48 h of cell death. These findings show that although in MCF-7cells P53 was wild-type, however, it was a target for deacetylation by SIRT1and so, could not induce apoptosis due to this aberrant epigenetic event.
These data are in accordance with the findings of Pruitt and coworkers (43) who reported that SIRT1 is a cancer-related gene and inhibits p53 function through epigenetic changes. Our findings suggest that acetylation of wild-type p53 as a tumor suppressor leads to activation of apoptotic program and is an integral part of cytotoxic activity of salermide to induce massive apoptosis in less than 24h treatment in MCF-7 cells (44). The role of SIRTs in drug resistance may be foremost related to their ability to target and modulate the activity of tumor suppressors, including p53. This finding further support the theoretical assumption that SIRT1 inactivation is required for the cell death induction and p53 acetylation in cancer cells only for wild-type p53 and salermide neither triggers cell death nor p53 acetylation in normal cells. Our results is also in agreement with the studies of Kamel and coworkers (45), Matsushita and coworkers (46) and Heltweg and coworkers (24) who suggested that SIRT inhibitors are targets for SIRT1and SIRT2 and p53 acetylation is important for the cell death induction. Huhtiniemi and coworkers (47) has shown that salermide is ineffective in inducing apoptosis in cells with functional p53 expression (wild-type p53) which is in contrast to our findings. Intriguingly, Salermide can induce apoptosis without increasing p53 acetylation in SW480 cells (5). Treatment with chemotherapeutic drugs also induce cell death indistinguishable from that triggered by silencing of SIRT1 (48) that is also accompanied by p53 acetylation suggesting that SIRT inhibitors, such as salermide, function through common pathways and mediated their cytotoxic effects through targeting p53 and its acetylation.
CONCLUSION
A hypothesis is formulated that SIRT1 is aberrantly recruited to proapoptotic proteins in cancer but not in normal cells and as a consequence of this; P53 is aberrantly repressed in MCF-7 cells. The treatment with salermide would induce apoptosis predominantly through the inhibition of SIRT1 and consequent hyperacetylation and reactivation of tumor suppressor P53 to induced cell death in MCF-7cells. In this study we describe that salermide represent a potential for cancer therapy that has a strong inhibitory activity on sirtuin1. It induces massive apoptosis in MCF-7 cancer cells but not in noncanceric cells. This makes it a promising novel class of antitumorigenic drugs that target acetylation of proteins that are epigenetically repressed by SIRT1 exclusively in cancer cells.
ACKNOWLEDGMENT
We would like to appreciate Dr. Ali Valiani, and Dr. Malekmasoud Ansar for their sincere help. Without their contributions this study could not be performed.
REFERENCES
- 1.Ahmedin J, Bray F, Ferlay M, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Autier P, Boniol M, Gavin A, Vatten LJ. Breast cancer mortality in neighboring European countries with different levels of screening but similar access to treatment: trend analysis of Who mortality database. BMJ. 2011;343:1–10. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montazeri A, Vahdaninia M, Iraj Harirchi, AM Harirchi, Sajadian A, Khaleghi F, et al. Breast cancer in Iran: need for greater women awareness of warning signs and effective screening methods. Asia Pac Fam Med. 2008;7:447–556. doi: 10.1186/1447-056X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res. 2011;3:166–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Lara E, Mai A, Calvanese V, Altucci L, Lopez p, Chantar ML, et al. Salermide, a sirtuin inhibitor with a strong cancer-specific proapoptic effect. Oncogene. 2009;28:781–791. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 6.Spratlin JL, Pitts TM, Kulikowski GN, Morelli MP, Tentler JJ, Serkovan NJ, et al. Synergistic Activity of Histone Deacetylase and Proteasome Inhibition against Pancreatic and Hepatocellular Cancer Cell Lines. Anticancer Res. 2011;31:1093–1104. [PMC free article] [PubMed] [Google Scholar]
- 7.Calvanese V, Fraga MF. Sirt1 brings stemness closer to cancer and aging. Aging. 2011;3:162–168. doi: 10.18632/aging.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linares AI, Vico RM, Moles MA. Potential role of HDAC inhibitors in cancer therapy: Insights into oral squamous cell carcinoma. Oral Oncol. 2010;46:323–329. doi: 10.1016/j.oraloncology.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Lawsona M, Uciechowskaa U, Schemiesb J, Rumpfb T, Jungb M, Sippla W. Inhibitors to understand molecular mechanisms of NAD+-dependent deacetylases (sirtuins) Biochim Biophys Acta. 2010;12:726–739. doi: 10.1016/j.bbagrm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Cazzalini O, Perucca P, Savio M, Necchi D, Stivala LA, Ducommun B, Scovassi AI, et al. Interaction of p21CDKN1A with PCNA regulates the histone acetyltransferase activity of p300. in nucleotide excision repair. Nucleic Acids Res. 2008;36:1713–1722. doi: 10.1093/nar/gkn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Cur Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Beckerman R, Prives C. Transcriptional Regulation by P53. Cold Spring Harb Perspect Biol. 2011;11:23–42. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knights DC, Catania J, Giovanni SD, Muratoglu S, Perez RR, Swartzbeck A, et al. Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. Cell Biol. 2006;173:533–545. doi: 10.1083/jcb.200512059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Scolnick DM, Trievel RC, Zhang HB, Berger SL, et al. P53 sites acetylated in vitro by PCAF and p300 in vivo response to DNA damage. Mol Cell Biol. 2000;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwahashi S, Ishibashi H, Utsunomiya T, Morine Y, Ochir TL, Hanaoka J, et al. Effect of histone deace-tylase inhibitor in combination with 5-fluorouracil on pancreas cancer and cholangio-carcinoma cell lines. J Med Invest. 2011;58:106–110. doi: 10.2152/jmi.58.106. [DOI] [PubMed] [Google Scholar]
- 16.Escande C, Chini CS, Nin V, Dykhouse KM, Novak CM, Levine J, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giahuai T, Shundong C, Yuehua M, Richard LP, Delong L. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. Hematoly oncoly. 2010;3:15–28. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endoc -Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 19.Peck B, Chen CY, Lam EW, Ho KK, Fruscia PD, Myatt SS, et al. SIRT Inhibitors Induce Cell Death and p53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol Cancer. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 20.Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene. 2005;24:4609–4623. doi: 10.1038/sj.onc.1208585. [DOI] [PubMed] [Google Scholar]
- 21.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Sang N. Histone deacetylase Inhibitors: The Epigenetic Therapeutics That Repress Hypoxia-Inducible Factors. J Biomed Biotechnol. 2011;10:197–211. doi: 10.1155/2011/197946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisser A, Wolzt M, Storka A. Salermide down-regulates sirtuin proteins to induce human cancer cell apoptosis. BMC Pharmacol. 2011;11:49–55. [Google Scholar]
- 24.Helweg B, Gatbonton T, Schuler AD. Antitumor Activity of a small - molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006;66:4368–4377. doi: 10.1158/0008-5472.CAN-05-3617. [DOI] [PubMed] [Google Scholar]
- 25.Vaziri H, Dessain SK, Eaton EN, Imai SI, Frye RA, Pandita TK, et al. hSIR2 and SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 26.Kojima K, Ohhashi R, Fujita Y, Hamada N, Akao Y, Nozawa Y, et al. A role for SIRT1in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun. 2008;373:423–442. doi: 10.1016/j.bbrc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Fermento ME, Gandini NA, Lang CA, Perez JE. Intracellular distribution of p300 and its differential recruitment to aggresomes in breast cancer. Exp Mol Pathol. 2010;88:256–264. doi: 10.1016/j.yexmp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Park MK, Kim JY, Chuong PD. Development of a sandwich ELISA for the detection of Listeria spp. Using specific flagella antibodies. J Vet Sci. 2005;6:41–46. [PubMed] [Google Scholar]
- 29.Kong S, Kim S, Sandal B, Lee M, Gao B, Zhang DD, et al. The Type III Histone Deacetylase Sirt1 Suppresses p300-mediated Histone H3 Lysine 56 Acetylation at Bclaf1 Promoter to Inhibit T Cell Activation. J Biol Chem. 2011;48:111–129. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JC, Kim DD, Lee YM, Kim TW, Cho DH, Kim MB, et al. Evaluation of novel histone deacetylase inhibitors as therapeutic agents for colorectal adinocarsinoma compared to established regimens with the histoculture drug response assay. Int J Colorectal Dis. 2009;24:209–218. doi: 10.1007/s00384-008-0590-1. [DOI] [PubMed] [Google Scholar]
- 31.Cen Y. Sirtuins inhibitors: the approach to affinity and selectivity. Biochim Biophys Acta. 2010;1804:1635–1644. doi: 10.1016/j.bbapap.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Zou XM, Li YL, Wang H, Cui W, Li XL, Fu SB, et al. Gastric cancer cell lines induced by trichostatin A. World J Gastroenterol. 2008;14:4810–4815. doi: 10.3748/wjg.14.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon JM, Pasupuleti R, Xu L, McDonagh T, DiStefano PS, Huber LJ. Inhibition of SIRT1 Catalytic Activity Increases p53 Acetylation but Does Not Alter Cell Survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enables human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–10463. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 35.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB, et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Chen L, Hou X, Li Z, Kabra N, Yand M, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 37.Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Tellez M, et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol. 2008;2:1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 38.Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN, et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2:40–47. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Sun D, Li F, Tian L, Li C, Li L, et al. Down regulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer. 2008;58:21–29. doi: 10.1016/j.lungcan.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Calvanese V, Fraga MF. Sirt1 brings stemness closer to cancer and aging. Aging. 2011;2:162–169. doi: 10.18632/aging.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13:454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 43.Pruitt K, Zinn RL, Ohm JE, Mcgarvey KM, Kang SH, Watkins DN, et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2:40–55. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olmos Y, Brosensb JJ, Lama EW. Interplay between SIRT proteins and tumor suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resistance. 2011;-14:35–44. doi: 10.1016/j.drup.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Kamel C, Abrol M, Jardine K, He X, Mcburney MW. Sirt1 fails to affect p53-mediated biological functions. Aging. 2006;-5:81–88. doi: 10.1111/j.1474-9726.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita N, Takami Y, Kimura M, Tachiiri S, Ishiai M, Nakayama T, et al. Role of NAD-dependent deacetylases SIRT1 and SIRT2 in radiation and cisplatin-induced cell death in vertebrate cells. Genes Cells. 2005;10:321–332. doi: 10.1111/j.1365-2443.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 47.Huhtiniemi T, Wittekindt C, Laitinen T, Leppanen J, Salminen A, Poso A, et al. Comparative and pharmacophore model for deacetylase SIRT1. J Comput Aided Mol Des. 2006;20:589–599. doi: 10.1007/s10822-006-9084-9. [DOI] [PubMed] [Google Scholar]
- 48.Langley E, Pearson M, Farettam M, Bauer UM, Frye RA, Minucci S, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]


